Abstract
The brown planthopper (BPH), Nilaparvata lugens Stål (Hemiptera: Delphacidae) is one of the most destructive rice pests in Asia. The microbial metabolite decoyinine (DCY) has been extracted from Streptomyces hygroscopicus. Recent studies have suggested that treating rice seeds with DCY could improve the growth and yield of rice. To further assess the effects of priming the seeds of different rice varieties with DCY on rice seedling resistance against BPH, an age-stage, two-sex life table and choice test were applied to investigate the individual fitness, population parameters, and preference behavior of BPHs in this study. The results indicated that feeding on rice seedlings grown from seeds primed with DCY significantly affected BPHs’ adult longevity, oviposition period, fecundity, as well as the net reproductive rate (R0), intrinsic rate of increase (rm), finite rate of increase (λ), doubling time (DT), and population size in terms of BPH. The honeydew excretions and the weights of BPHs fed on DCY-pretreated rice plants were remarkably lowered. The two-way ANOVA results also showed that there were significant differences in the biological parameters, honeydew excretions, and of BPH weights owing to DCY treatment, rice variety, and the interactions between DCY treatment and rice variety. Additionally, the feeding and oviposition preferences of BPHs for the pretreated rice plants were reduced. Our results imply that the priming of seeds with DCY can improve rice resistance against BPH, which could facilitate the utilization of seed priming as a new avenue for effective crop protection.
1. Introduction
Rice (Oryza sativa) is one of the most important global cereal crops and forms a staple food for more than 50% of the world’s human population. The brown planthopper (BPH, Nilaparvata lugens) is one of the most devastating rice pests in Asia, which not only damages rice plants by feeding on phloem sap and laying eggs in rice tissues but also transmits various rice viruses. BPH grows rapidly and has high fecundity under suitable conditions, thereby leading to serious losses in rice production [1,2,3]. The control of BPH mainly depends on spraying chemical pesticides. However, the extensive application of pesticides has caused BPH resistance and resurgence and also threatens the ecological environment [4]. Therefore, it is urgent to develop a cost-effective and eco-friendly method to control BPH.
Seed priming is a cost-effective and feasible technique to boost up the defense mechanism and thereby potential tolerance of plants towards biotic and abiotic stresses [5]. The priming of seeds results in ultrastructural modifications and the regulation of water content, the cell cycle, oxidative stresses, reserve mobilization, and protein synthesis, triggering an intricate signaling event [6]. Worrall et al. reported that the priming of tomato seeds with jasmonic acid (JA) showed significantly increased resistance against Manduca sexta L. (Lepidoptera: Sphingidae), Myzus persicae Sulzer (Hemiptera: Aphididae), and Tetranychus urticae Koch (Acari: Tetranychidae) [7]. In another similar study, Berglund et al. reported that spruce seedlings grown from seeds primed with JA displayed a significant reduction in seedlings infested by the pine weevil Hylobius abietis L. (Coleoptera: Curculionidae) [8]. Wheat seeds treated with calcium chloride primed a resistance to the aphid Schizaphis graminum Rondani (Hemiptera: Aphididae) [9]. Treating maize seeds with Bacillus pumilus affected the feeding behavior and host preference of the rootworm Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) [10]. Tobacco seeds treated with insect pathogenic fungus Beauveria bassiana showed enhanced resistance against microbial pathogens and insect pests [11]. Various studies have suggested that the priming of rice seeds increases growth and enhances tolerance to stresses including salinity, drought, heavy metals, temperature, and pathogens [12,13,14,15,16,17,18]. A recent study showed that rice seeds treated with the entomopathogenic bacterium Serratia marcescens promoted plant growth and increased resistance against BPH in rice seedlings [19]. These studies suggest that seed priming could enhance plant resistance against insect pests.
Decoyinine (DCY) is a secondary metabolite that was isolated from Streptomyces hygroscopicus [20]. DCY, also called angustmycin A, can inhibit guanosine monophosphate synthase [21]. DCY can suppress melanoma cell invasion and tumorigenicity in immunocompromised mice [22]. DCY is also known as a new plant growth regulator called Wugufengsu in China and studies have demonstrated that Wugufengsu can improve the growth and yield of maize and rice [23,24,25]. Seed treatment of the rice varieties Baixiang 139 and Guangliangxiang 2 with Wugufengsu significantly improved various rice growth indexes [24,25]. Shah et al. found that the fecundity of the small brown planthopper (SBPH, Laodelphax striatellus Fallén) that fed on the seedlings of the rice variety Wuyujing 3 grown from seeds pretreated with DCY was significantly reduced, and the activities of rice defense-related enzymes were also changed [26].
Our recent study proved that the priming of seeds of the rice variety Taichung Native 1 (TN1) with DCY could improve its resistance against BPH [27]. Building on the previous report, which evaluated a susceptible rice variety, the effects of DCY were further evaluated on commercial rice varieties in this study, indicating DCY’s potential as an alternative for pest control in rice production. Here, we used an age-stage, two-sex life table to determine the fitness traits and population parameters of BPHs reared on rice seedlings of two different varieties pretreated with DCY. The feeding behavior and feeding preference of BPHs were also applied to assess the effects of DCY-pretreated rice seedlings on BPH. The present study contributes to our understanding of rice resistance when seedlings are primed with DCY and provides a simple strategy to achieve improvements in insect resistance in rice.
2. Materials and Methods
2.1. Insect and Plant Treatment
The BPH colony was continuously reared on the rice variety TN1 (a susceptible rice variety, provided by China National Rice Research Institute, Hangzhou, China) at a temperature of 27 ± 1 °C, a 70 ± 5% relative humidity, and a 16 h:8 h (L: D) photoperiod in a chamber [27].
DCY was purchased from Shanghai Macklin Biochemical Co. Ltd. (Shanghai, China). The indica rice variety Liangyou566 and the japonica rice variety Nanjing9108, which are commercial rice varieties in Jiangsu and the neighboring provinces of China, were used as experimental rice materials for BPH feeding. The rice seeds (provided by Jiangsu Academy of Agricultural Sciences) were washed and soaked in the DCY solutions for 24 h at 28 °C with a photoperiod of 16 h:8 h (L:D) with the following concentrations: 0 mg/L (control), 25 mg/L (DCY25), 50 mg/L (DCY50), and 100 mg/L (DCY100), and they were then sprouted in the dark for 24 h [27]. After the rice seeds sprouted, they were grown in the growth chamber for one month, and then the rice seedlings were used for the subsequent experiments.
2.2. Survival, Development, and Reproduction
To assess the effects of DCY-pretreated rice plants of Liangyou566 and Nanjing9108 on the biological parameters of BPH, both the untreated and different concentrations of DCY-pretreated rice seedlings were used to feed the newly hatched BPH nymphs. One BPH nymph in a glass tube was considered as one replicate. The molting and death of each BPH nymph was recorded every day, and the rice plant of each tube was replaced weekly until the adult emerged. A female and a male of the same treatment were mated and placed into a new tube containing the corresponding rice plant. After BPH mating, the rice plants were replaced every day and the rice seedlings were dissected to observe whether eggs were laid to determine the pre-oviposition period. After the eggs were observed, the number of eggs was recorded, and then the rice seedlings were changed every three days to count the numbers of eggs laid until the female died.
2.3. Honeydew and Weight Measurement
Honeydew excretion is usually applied to evaluate a feeding index for the measurement of the susceptibility and resistance of plants to hemipteran pests [28]. A newly emerged BPH female adult was placed in a Parafilm® bag (Parafilm, Menasha, WI, USA) and then fixed in the leaf sheath of a 30-day-old rice plant for 24 h [27]. Then, the amount of honeydew secreted by female adults fed on the untreated rice plants and DCY100-pretreated plants of Liangyou566 and Nanjing9108 was measured. One female in a Parafilm® bag was considered as one replicate, and each treatment was performed with 30 replicates.
Newly hatched BPH nymphs were fed on the untreated rice plants and DCY100-pretreated plants of the two varieties. After feeding on these rice plants, the BPH nymphs grew up and emerged, and the females at 1 d, 3 d, 5 d, and 7 d after emergence were selected and weighed. Each group was performed with 21–30 replicates.
2.4. Feeding and Oviposition Choice Test
The method used to determine the feeding and oviposition preference behaviors of BPHs was described previously [27]. Three cups of untreated rice plants and three cups of DCY100-pretreated plants were placed in a cage and arranged in a circle. Thirty-five 3-day-old gravid BPH females were starved in a Petri dish for 1 h and then placed in the center of the circle. The number of BPHs settled on untreated rice plants and treated rice plants was recorded at different time points after release (2 h, 4 h, 8 h, 12 h, 24 h, and 48 h). The numbers of eggs laid in each rice plant were counted.
2.5. Life Table Analysis
Based on the data concerning daily survival and reproduction, the age-stage specific survival rate (sxj) (x = age, j = stage), age-stage specific life expectancy (exj), and age-stage reproductive value (vxj) were determined. sxj represents the probability that a newly born aphid will survive to age x and stage j, exj represents the expected survival period of an individual of age x and stage j, and vxj represents the devotion to future offspring at age x and stage j. The population life table parameters, including the net reproductive rate (R0 = ∑(lxmx)), mean generation time (T = ln(R0)/rm), intrinsic rate of increase (∑e−rm(x+1)lxmx = 1), finite rate of increase (λ = erm), and doubling time (DT = ln2/rm), were analyzed using the TWOSEX-MSChart program [29,30,31,32]. The bootstrap method with 100,000 re-samplings was applied to estimate the variances and standard errors of all population life table parameters, and the differences among the various treatments were calculated by a paired bootstrap test at the 5% significance level. The TIMING-MSChart program was used to project the population size in terms of BPH for 60 days under various DCY treatments. The population projection was begun with 10 individuals per treatment for comparative purposes [31].
2.6. Statistical Analysis
The biological parameters were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test (p < 0.05). The Student’s t-test was performed to determine statistical differences in the honeydew, weight, and oviposition preference between the control and DCY100 treatment (** p < 0.01). Feeding preference was analyzed using the binomial test (* p < 0.05, ** p < 0.01). Two-way ANOVA was performed to assess the effects of DCY treatment, rice variety, and the interactions between DCY treatment and rice variety on the biological parameters, honeydew, and weight (p < 0.05). All statistical analyses were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA), and the figures were visualized using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Biological Parameters
The biological parameters of BPHs fed on the control plants and DCY-pretreated plants of two rice varieties (Liangyou566 and Nanjing9108) are given in Table 1 and Table 2. Compared with the control groups, the two DCY-pretreated rice varieties had no significant effect on the total nymphal duration, pre-oviposition period, or egg duration of BPHs but did have an effect on the nymphal duration of several instars. However, the adult longevity, oviposition period, and fecundity were significantly affected.

Table 1.
Biological parameters of BPHs reared on Liangyou566 rice plants pretreated with various concentrations of decoyinine (DCY).

Table 2.
Biological parameters of BPHs reared on Nanjing9108 rice plants pretreated with various concentrations of decoyinine (DCY).
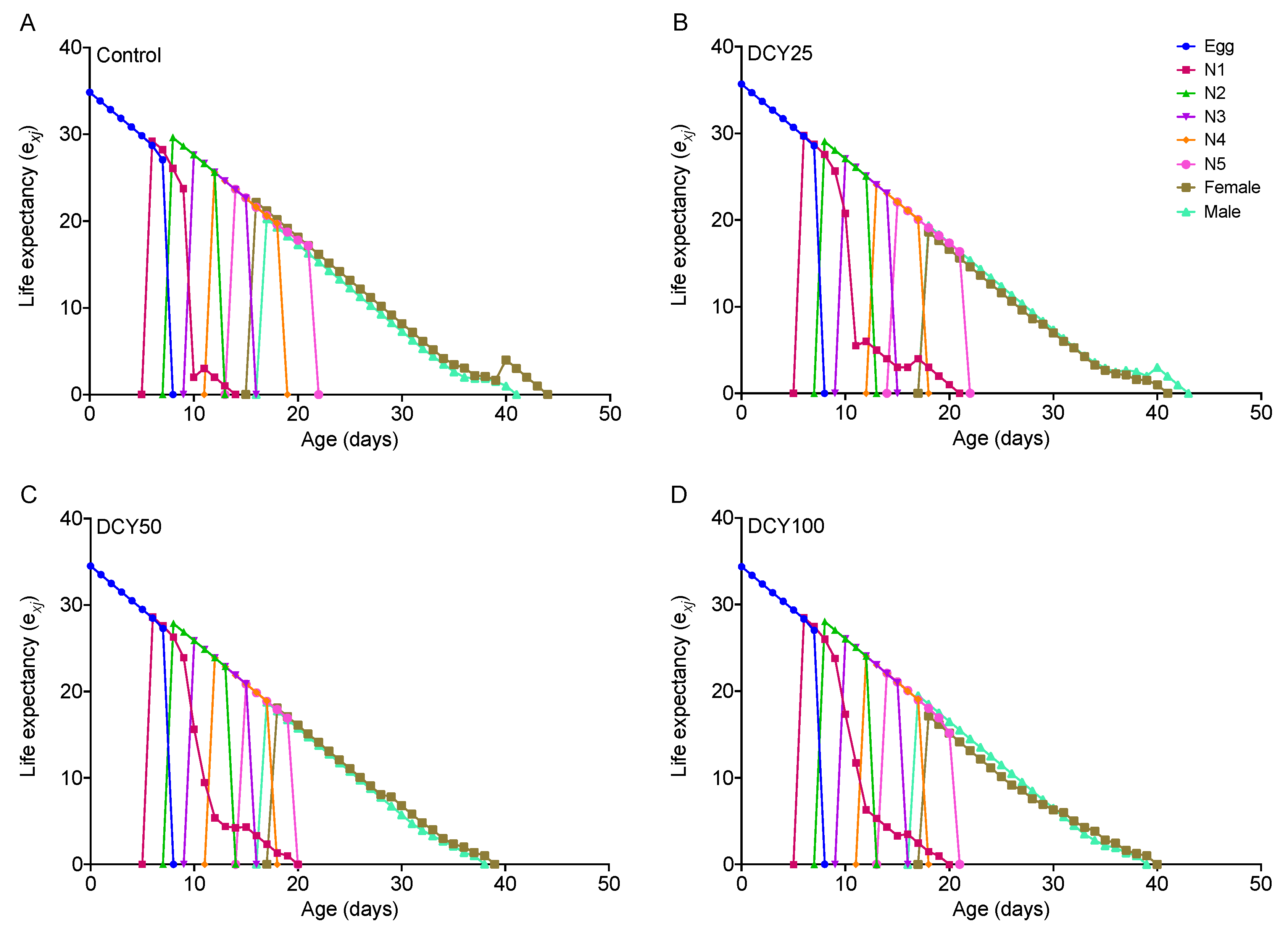
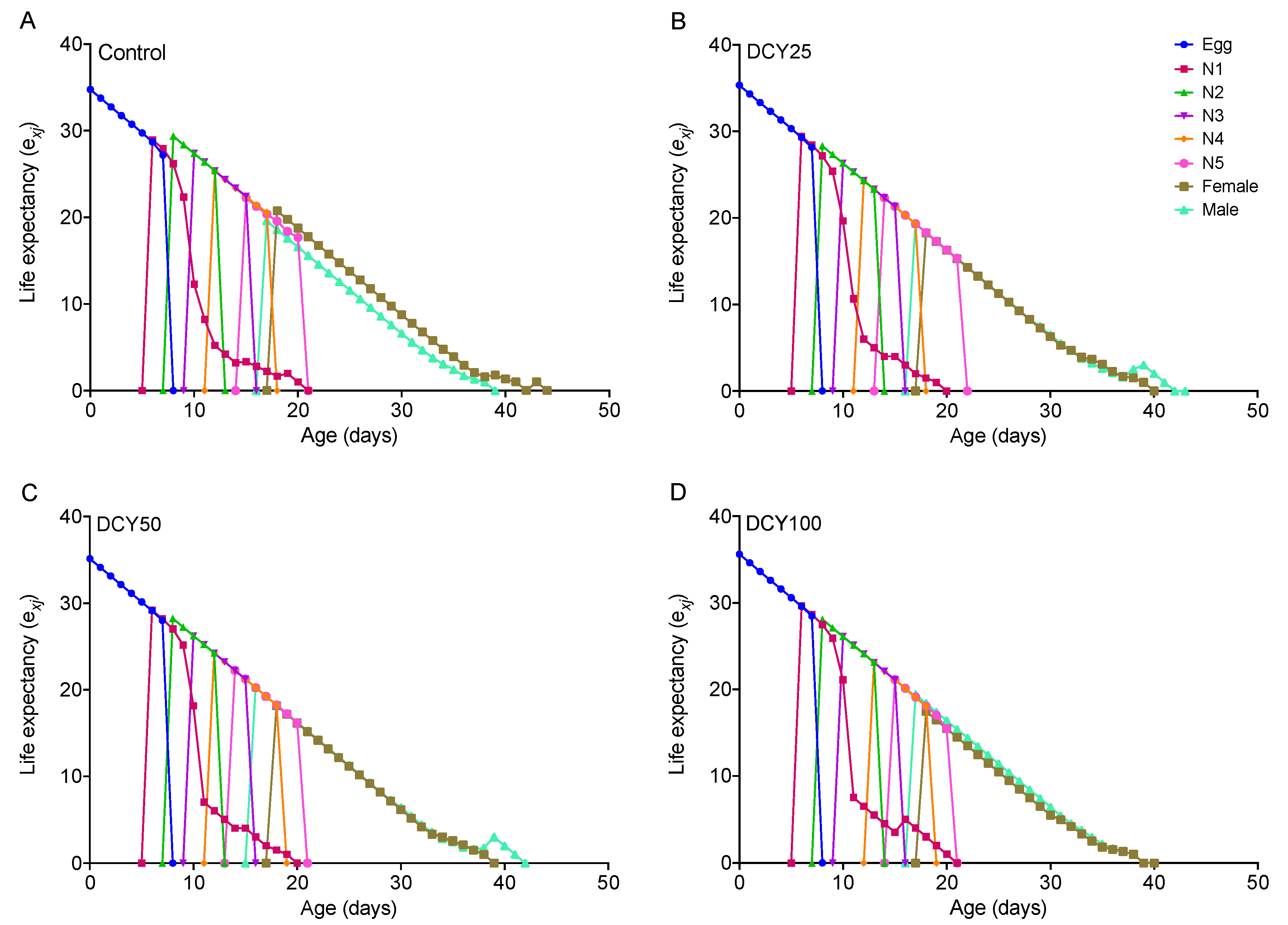
For BPHs fed on DCY-pretreated Liangyou566 plants, the male longevity and female longevity were both significantly shortened by DCY50 treatment, and the female longevity was also affected by DCY100 (Table 1). For Nanjing9108, the female longevity was significantly reduced under DCY treatments, but the male longevity was not affected (Table 2). These results were supported by the age-stage specific life expectancy (exj) curves (Figure 1 and Figure 2). The exj values for adult males on Liangyou566 were 20.29 d (control), 19.35 d (DCY25), 18.75 d (DCY50), and 19.5 d (DCY100). The exj values for females were 22.17 d (control), 18.62 d (DCY25), 18.09 d (DCY50), and 17.15 d (DCY100) (Figure 1). The exj values for males on Nanjing9108 were 19.60 d (control), 19.33 d (DCY25), 20.25 d (DCY50), and 19.40 d (DCY100), and the exj values for females were 20.76 d (control), 18.27 d (DCY25), 18.16 d (DCY50), and 17.48 d (DCY100) (Figure 2).
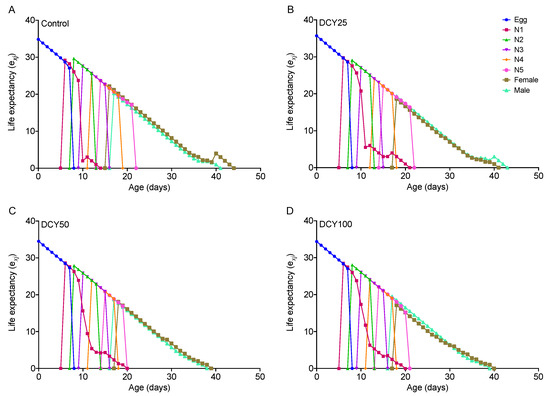
Figure 1.
Age-stage specific life expectancy (exj) of BPHs reared on decoyinine (DCY)-pretreated Liangyou566 rice plants. (A) Control (0 mg/L); (B) DCY25 (25 mg/L); (C) DCY50 (50 mg/L); (D) DCY100 (100 mg/L).

Figure 2.
Age-stage specific life expectancy (exj) of BPHs reared on decoyinine (DCY)-pretreated Nanjing9108 rice plants. (A) Control (0 mg/L); (B) DCY25 (25 mg/L); (C) DCY50 (50 mg/L); (D) DCY100 (100 mg/L).
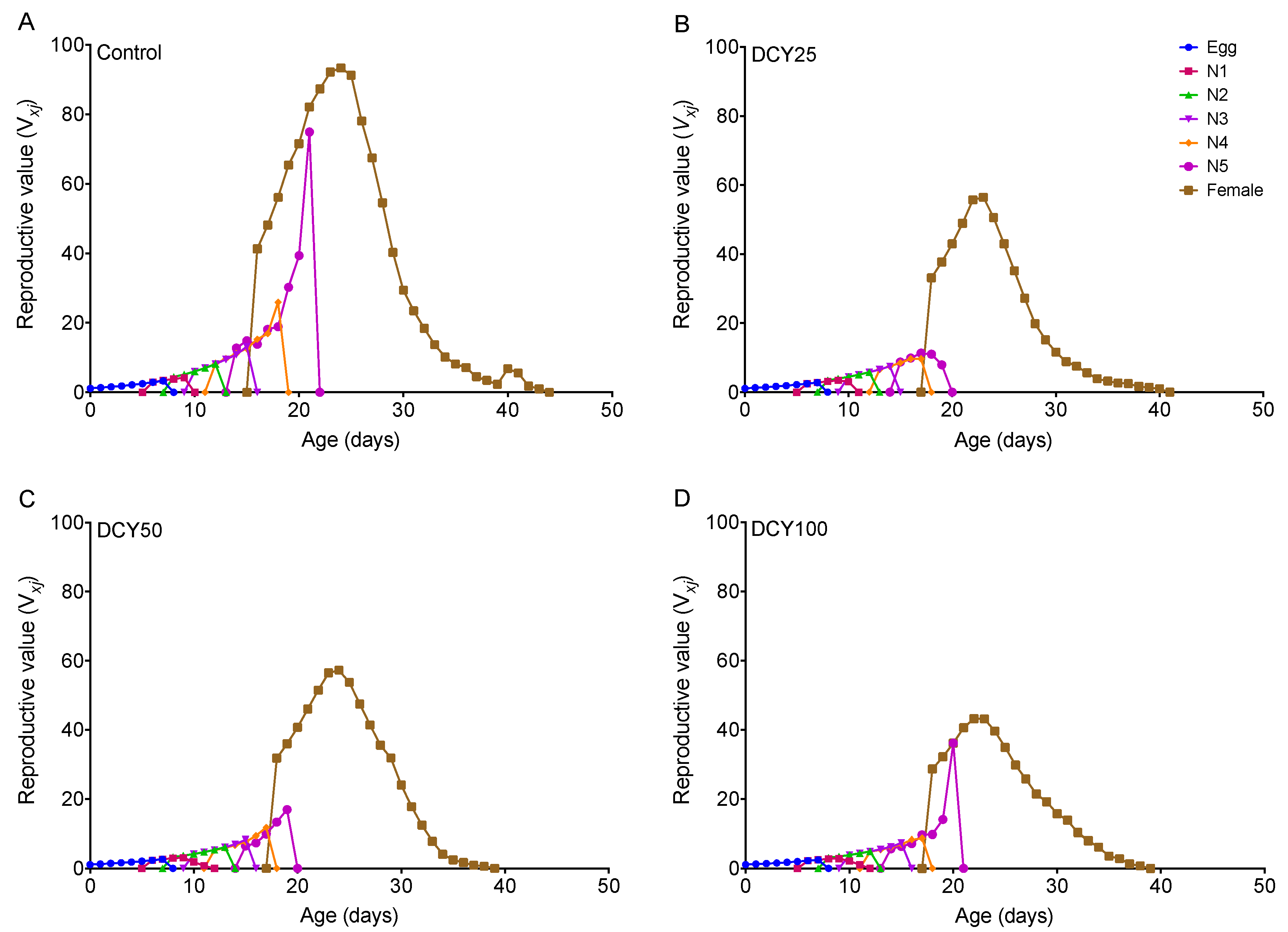
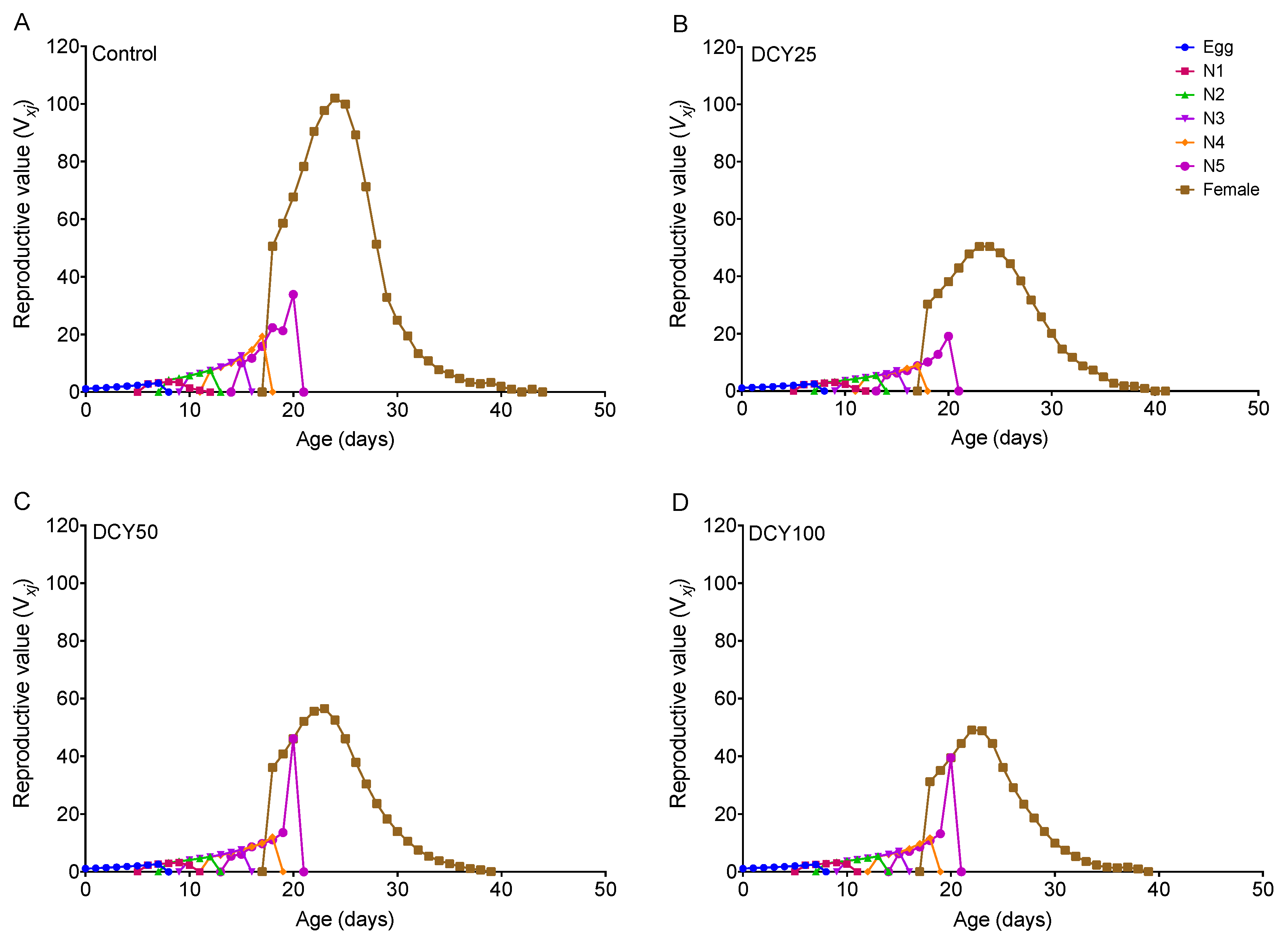
The oviposition periods of BPHs were shortened under different DCY treatments of Liangyou566 (except DCY25) and Nanjing9108 (Table 1 and Table 2). The fecundity was significantly decreased under all DCY treatments of Liangyou566 and Nanjing9108 (Table 1 and Table 2). The age-stage reproductive value (vxj) curves showed that the vxj values of BPHs were lower when they fed on DCY-pretreated rice plants (Figure 3 and Figure 4). For Liangyou566, the maximal vxj values of females were 93.35 (control), 56.47 (DCY25), 57.27 (DCY50), and 43.23 (DCY100) offspring/adult (Figure 3). For Nanjing9108, the maximal vxj values were 50.47 (DCY25), 56.61 (DCY50), and 49.14 (DCY100) offspring/adult, which were significantly lower than those of the control (102.00 offspring/adult) (Figure 4).
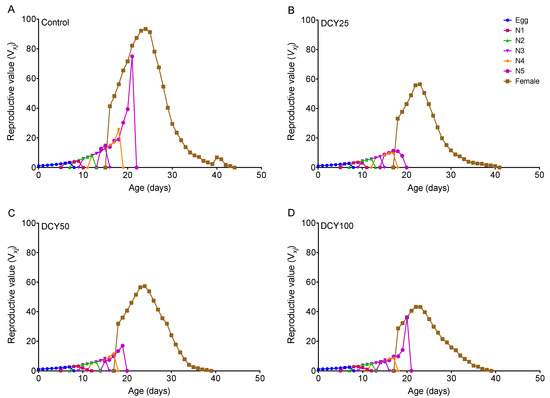
Figure 3.
Age-stage reproductive value (vxj) of BPHs reared on decoyinine (DCY)-pretreated Liangyou566 rice plants. (A) Control (0 mg/L); (B) DCY25 (25 mg/L); (C) DCY50 (50 mg/L); (D) DCY100 (100 mg/L).
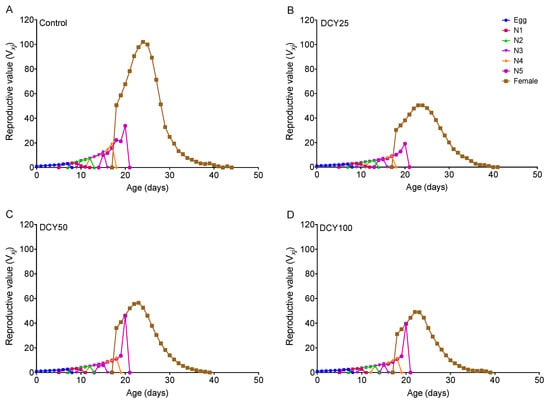
Figure 4.
Age-stage reproductive value (vxj) of BPHs reared on decoyinine (DCY)-pretreated Nanjing9108 rice plants. (A) Control (0 mg/L); (B) DCY25 (25 mg/L); (C) DCY50 (50 mg/L); (D) DCY100 (100 mg/L).
Meanwhile, two-way ANOVA was also used to analyze the effects of DCY treatment and rice variety on the biological parameters of BPHs (Table 3). The results showed that under DCY treatment, there were significant differences in the biological parameters, except for the nymphal duration of the 2nd and 4th instar, total nymphal duration of females, male longevity, and egg duration. Rice variety significantly affected all biological parameters except for pre-oviposition period and egg duration. Furthermore, the interactions between DCY treatment and rice variety had a significant effect on the nymphal duration of the 1st, 3rd, and 5th instars and the total nymphal duration of males and females. These results were also supported by the interaction plots (Figure S1). Through the above two-way ANOVA, it was found that there were certain interactive effects regarding DCY treatment and rice variety on the biological parameters of BPHs, but the main effects of DCY treatment or rice variety were greater, suggesting that the interactions may weaken their effects on the biological parameters to some extent.

Table 3.
Results of two-way ANOVA for the effects of decoyinine (DCY) treatment and rice variety on the biological parameters of BPHs.
In addition, the age-stage specific survival rate (sxj) indicated that no significant differences occurred between the control and DCY treatments (Figures S2 and S3).
3.2. Population Life Table Parameters
Two DCY-pretreated rice varieties significantly influenced the net reproductive rate (R0), intrinsic rate of increase (rm), finite rate of increase (λ), and doubling time (DT) (Table 4 and Table 5). The R0, rm, and λ values of BPHs fed on two varieties of rice plants were significantly decreased under DCY treatments, but their DT values were significantly increased, except for in the case of the DCY50 treatment of Nanjing9108. However, the mean generation time (T) of BPHs fed on the Liangyou566 plants was not significantly affected by DCY treatments (Table 4), and the T of BPHs fed on Nanjing9108 was shortened only with the DCY100 treatment (Table 5).

Table 4.
Population parameters of BPHs reared on Liangyou566 rice plants pretreated with various concentrations of decoyinine (DCY).

Table 5.
Population parameters of BPHs reared on Nanjing9108 rice plants pretreated with various concentrations of decoyinine (DCY).
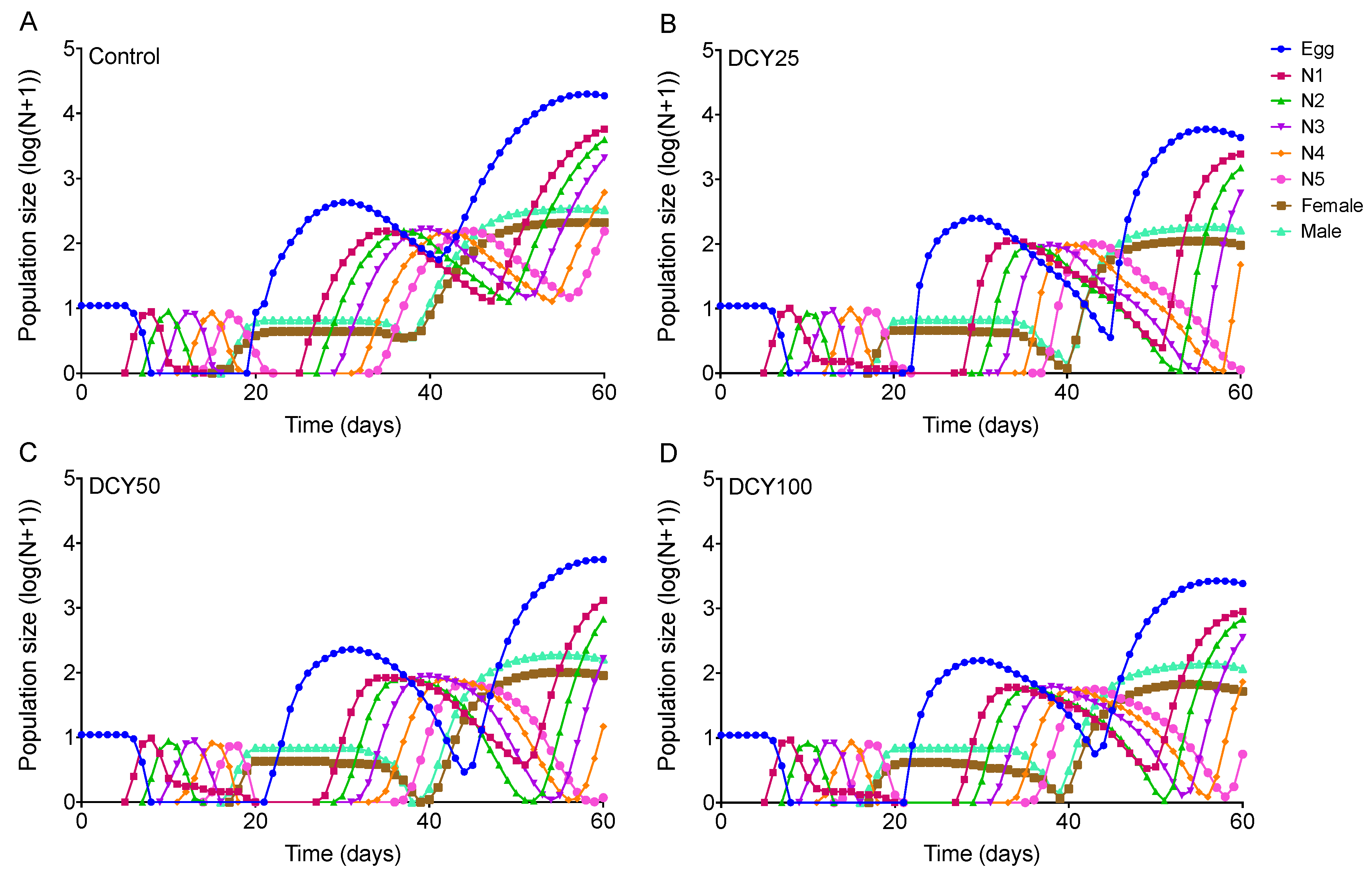
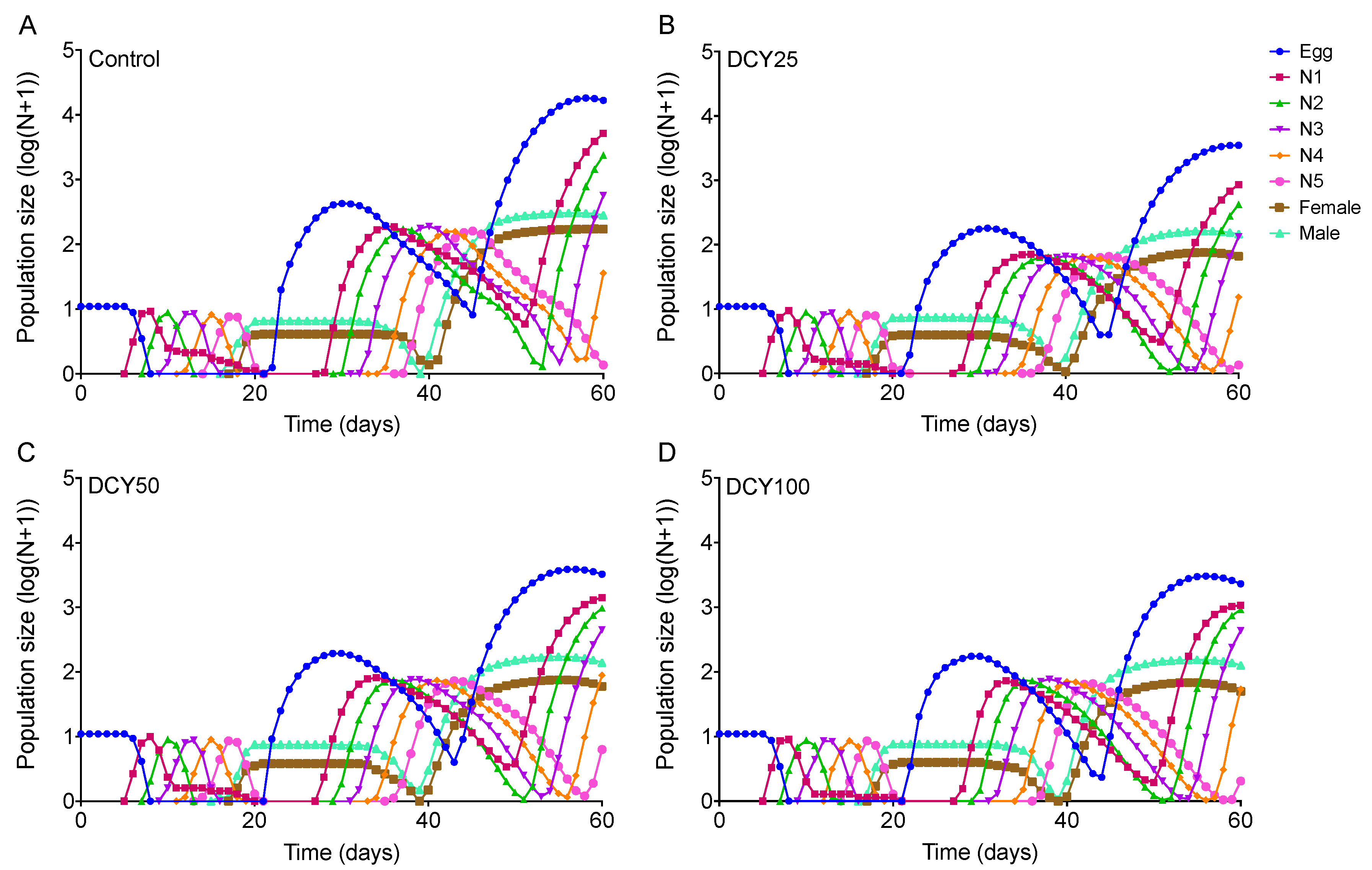
Differences in the population dynamics of BPHs reared on DCY-pretreated rice plants were marked over the 60-day simulation (Figure 5 and Figure 6). The projections in terms of population size were started from a population of 10 eggs. The curves for Liangyou566 showed that there were 18746 eggs, 208 females, and 329 males in the control; while there were 4452 eggs, 70 females, and 101 males in the DCY25 treatment; 5587 eggs, 89 females, and 161 males in the DCY50; and 2418 eggs, 51 females, and 116 males in the DCY100, after 60 days (Figure 5). For Nanjing9108, there were 16737 eggs, 171 females, and 281 males in the control; while there were 3515 eggs, 65 females, and 146 males in the DCY25 treatment; 3275 eggs, 89 females, and 139 males in the DCY50; and 2319 eggs, 49 females, and 126 males in the DCY100 (Figure 6).
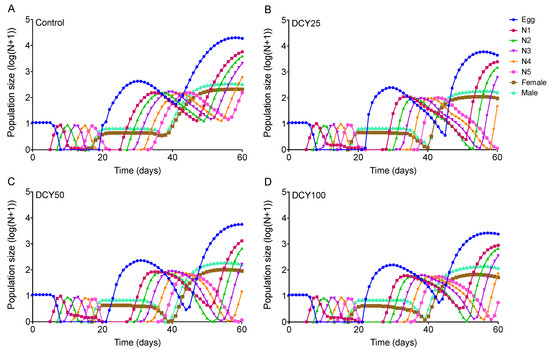
Figure 5.
BPH population prediction based on the data of life table when fed on decoyinine (DCY)-pretreated Liangyou566 rice plants over 60 days. (A) Control (0 mg/L); (B) DCY25 (25 mg/L); (C) DCY50 (50 mg/L); (D) DCY100 (100 mg/L).
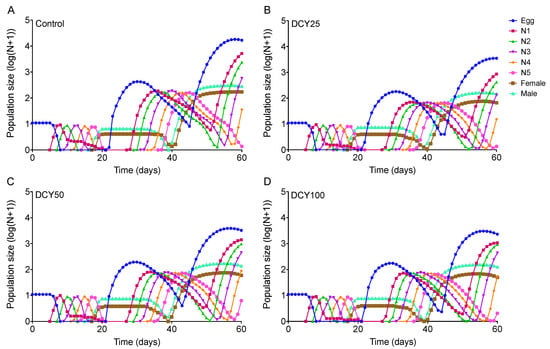
Figure 6.
BPH population prediction based on the data of life table data when fed on decoyinine (DCY)-pretreated Nanjing9108 rice plants over 60 days. (A) Control (0 mg/L); (B) DCY25 (25 mg/L); (C) DCY50 (50 mg/L); (D) DCY100 (100 mg/L).
3.3. Honeydew Production and BPH Weight
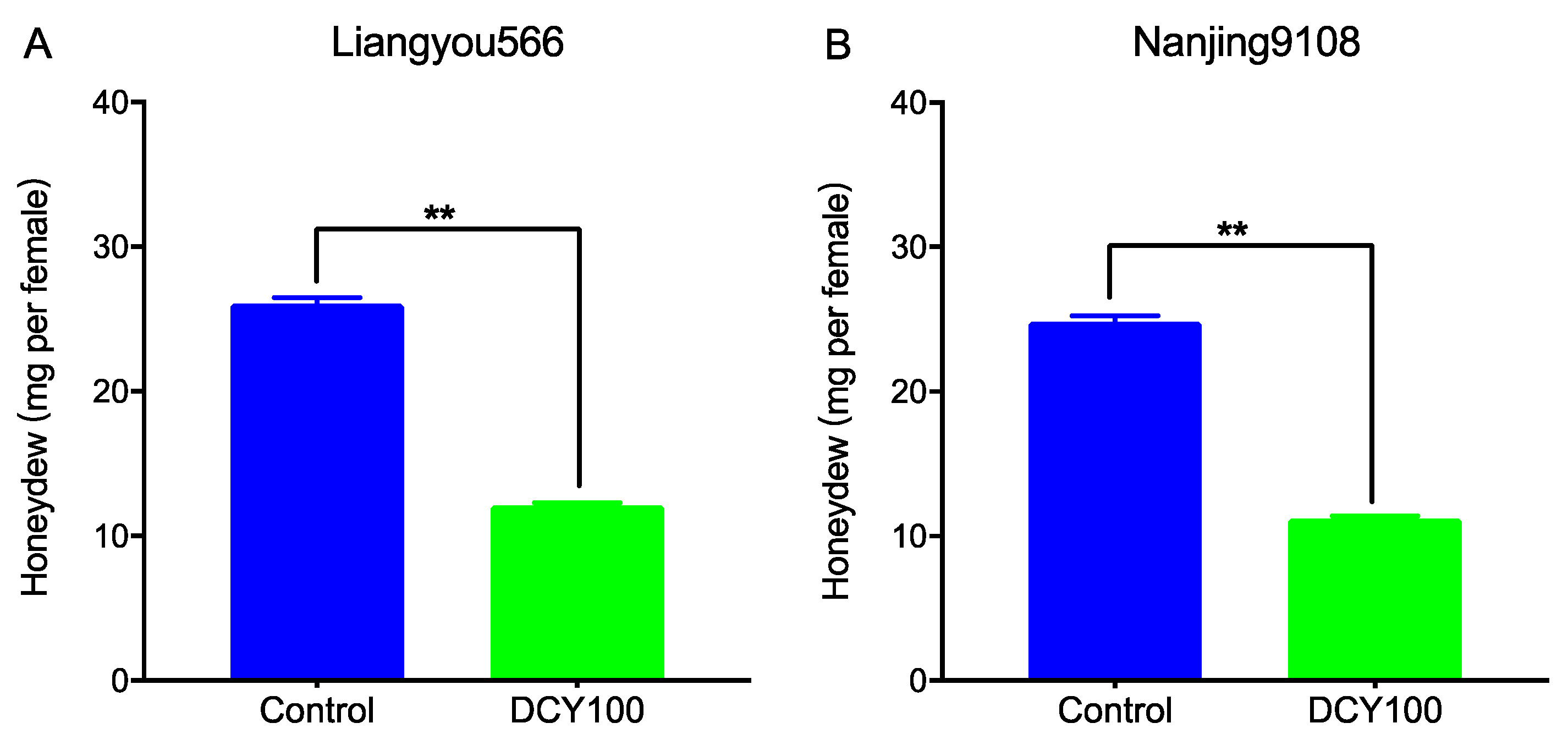
To investigate the effects of DCY-pretreated rice plants on the feeding behavior of BPHs, the honeydew excretions were determined. Compared with the control, the honeydew excretions of BPH female adults fed on DCY100-pretreated rice plants were remarkably reduced (Figure 7). When feeding on DCY100-pretreated Liangyou566 and Nanjing9108 plants, the amount of honeydew produced by BPH females was decreased by 53.5% (Figure 7A) and 54.8% (Figure 7B), respectively.
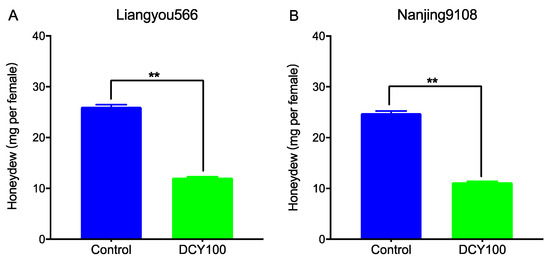
Figure 7.
The amount of honeydew produced by a newly emerged BPH female fed on the control rice plants and DCY100-pretreated plants after 24 h. (A) Liangyou566; (B) Nanjing9108. Asterisks indicate statistical differences between the control and treatment (** p < 0.05, Student’s t-test). Data represent means ± SE. DCY100, 100 mg/L decoyinine.
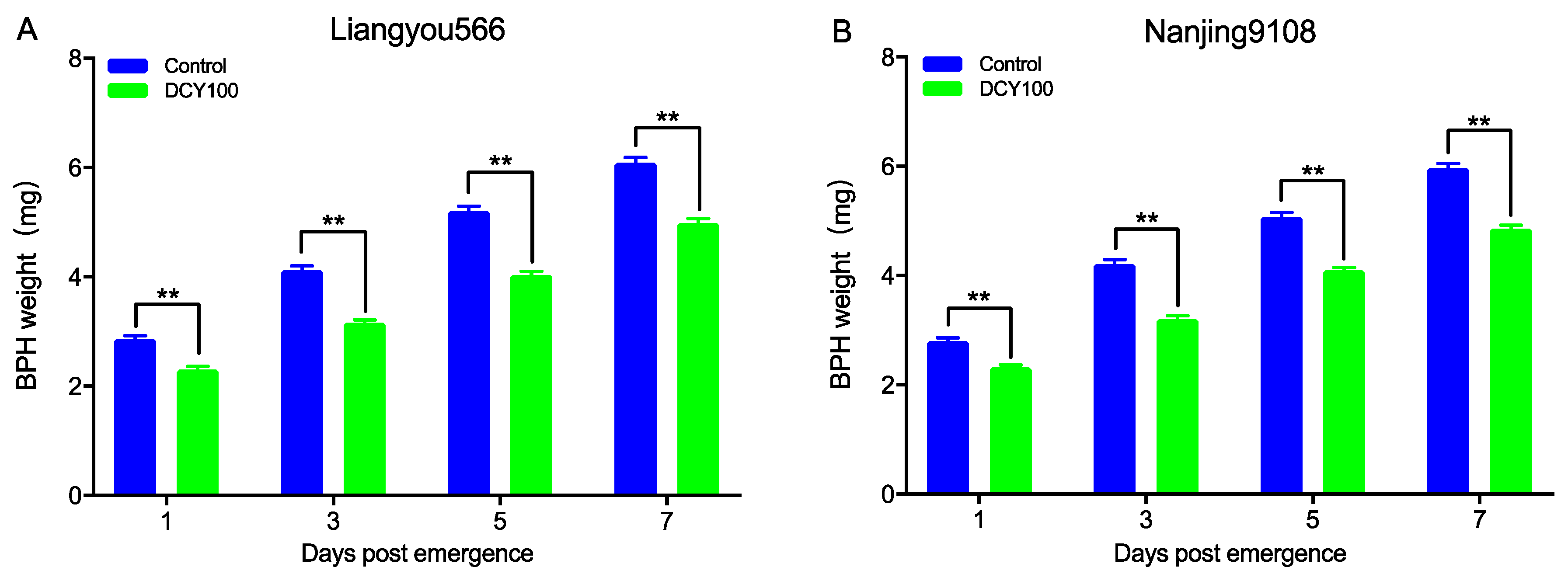
To further analyze the effects of DCY100-pretreated rice plants on the development of BPHs, the weight of females at 1d, 3d, 5d, and 7d post-emergence was measured. The results indicated that the weight of females fed on DCY100-pretreated plants was decreased significantly for the two rice varieties (Figure 8 and Table S1).
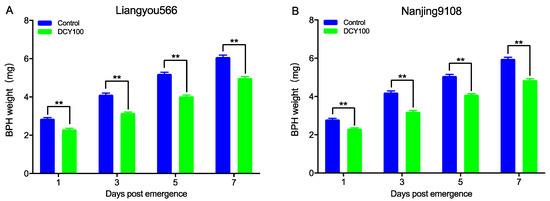
Figure 8.
The weight of BPH females fed on the control rice plants and DCY100-pretreated plants at 1 d, 3 d, 5 d, and 7 d post-emergence. (A) Liangyou566; (B) Nanjing9108. Asterisks indicate statistical differences between the control and treatment (** p < 0.01, Student’s t-test). Data represent means ± SE. DCY100, 100 mg/L decoyinine.
In addition, the two-way ANOVA results suggested that DCY treatment significantly affected the honeydew excretions and weight of BPH females. Rice variety affected the honeydew excretions, but there were no interactions between DCY treatment and rice variety on the honeydew excretions and weight (Table 6).

Table 6.
Results of two-way ANOVA for the effects of decoyinine (DCY) treatment and rice variety on the honeydew excretions and weight of BPHs.
3.4. Feeding and Oviposition Preference
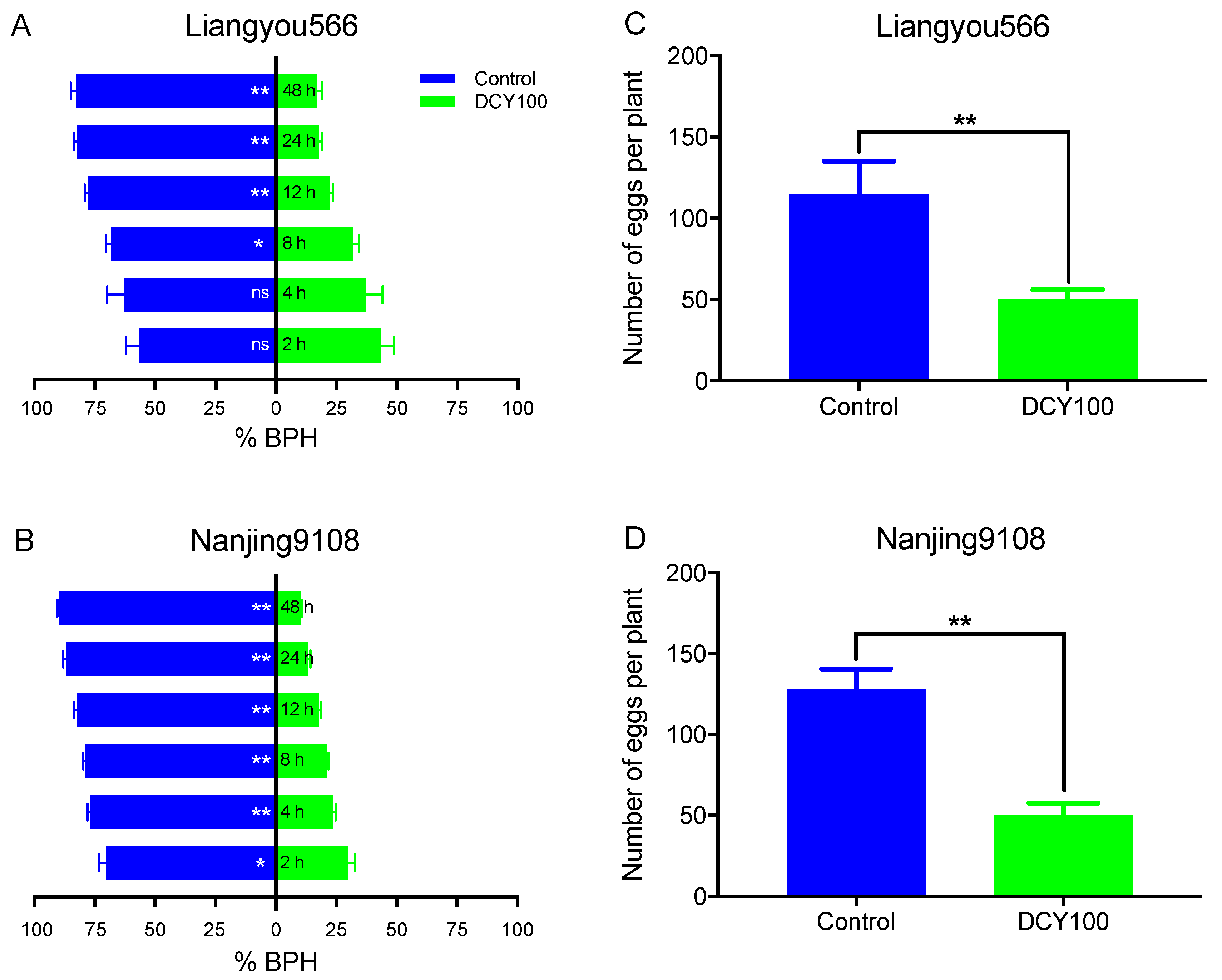
To further explore the influence of DCY100-pretreated rice plants on the preference behaviors of BPHs, we compared the percentages of BPH females settling on the control rice plants and DCY100-pretreated plants. The results suggested that BPH females preferred to feed on the Liangyou566 control rice plants except for at 2 h and 4 h post-release, and approximately 83% of BPHs chose the control plants at 48 h (Figure 9A and Table S2). For Nanjing9108, significantly fewer BPHs preferred the DCY100-pretreated rice plants at all time points post-release, and approximately 90% of BPHs chose the control plants at 48 h (Figure 9B and Table S2).
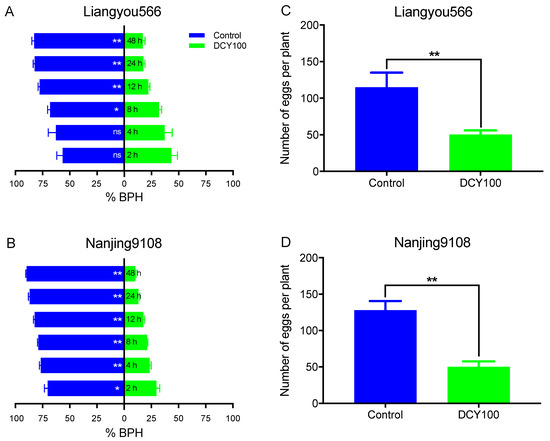
Figure 9.
Feeding and oviposition preference of BPHs. The percentage of gravid females choosing the untreated rice plants or DCY100-pretreated plants during 48 h. (A) Liangyou566; (B) Nanjing9108. The number of eggs laid by gravid females in the untreated rice plants or DCY100-pretreated plants. (C) Liangyou566; (D) Nanjing9108. Asterisks indicate statistical differences between the control and treatment (* p < 0.05, ** p < 0.01, binomial test for (A,B), Student’s t-test for (C,D), ns, no significant difference). Data represent means ± SE. DCY100, 100 mg/L decoyinine.
Next, we also counted the number of eggs laid in the control rice plants and DCY-pretreated plants. The results indicated that the number of eggs laid in the DCY100-pretreated rice plants was significantly lower than in the control plants for both rice varieties (Figure 9C,D).
4. Discussion
Bio-priming is a type of seed priming that involves the inoculation of seeds with living microorganisms, microbial metabolites, or antagonist microorganisms such as fungi and plant growth-promoting bacteria as priming agents [5,33]. Studies have indicated that seed bio-priming with the microbial metabolite DCY could improve the germination indexes, growth, development, and yield of rice varieties [24,25]. Recent studies have found that the seed priming of rice varieties with DCY improved their resistance against planthoppers, but the tested rice varieties were susceptible rice varieties [26,27]. Here, we analyzed the influence of priming rice seeds of two commercial varieties with DCY on rice seedling resistance against BPH, with this method possibly providing a simple and feasible way of achieving improvements to the insect resistance of rice.
A life table can facilitate a comprehensive understanding of the development, survival, and reproduction of an insect population, revealing fitness levels in abiotic and biotic environmental conditions [34]. Here, we used an age-stage, two-sex life table to investigate the fitness traits of BPHs fed on the rice plants pretreated with DCY. This method not only considers male individuals, which are generally ignored in female age-specific life tables, but also pays attention to the differences among individual insects and the death of several pre-adult individuals [29,30]. Our results indicated that feeding on the rice seedlings of two varieties (Liangyou566 and Nanjing9108) grown from seeds primed with DCY did not affect the total nymphal duration, pre-oviposition period, or egg duration of BPHs, but it had an significant effect on the nymphal duration of several instars, adult longevity, oviposition period, and fecundity (Table 1 and Table 2). The exj and vxj curve analyses also supported these results (Figure 1, Figure 2, Figure 3 and Figure 4). Shah et al. reported that DCY-pretreated rice variety Wuyujing3 affected one of the biological parameters in SBPHs, i.e., the fecundity was significantly reduced, and the activities of rice defense-related enzymes were induced [26]. Then, Ma et al. showed that DCY-pretreated rice variety TN1 remarkably decreased the fecundity and egg hatchability of BPHs [27]. In our study, in addition to the fecundity, DCY-pretreated rice varieties also affected the adult longevity and oviposition period. In addition, the two-way ANOVA results indicated that DCY treatment, rice variety, and the interactions between these two factors significantly affected the biological parameters of BPHs (Table 3). Therefore, it could be speculated that DCY pretreatment of different rice varieties may prime various plant defense responses against insect pests.
The population life table parameters R0, T, rm, λ, and DT are often used to evaluate the growth capacity for a given insect population under a specific environmental condition, and they often vary with host plants and local environmental conditions [35,36,37]. Our study showed that priming rice seeds with DCY significantly decreased the population parameters (R0, rm, and λ) and increased the DT of BPHs fed on the plants of Liangyou566 and Nanjing9108 (Table 4 and Table 5), suggesting that the population growth capacity of the DCY treatment groups was significantly lower than that of the control groups. A similar study demonstrated that the R0, rm, and λ values of BPHs reared on DCY50-pretreated TN1 rice plants were reduced significantly, while the DT was increased [27]. Meanwhile, another study reported that the R0, rm, and λ of SBPHs fed on DCY100-pretreated Wuyujing3 plants were significantly lower, but the DT was higher [26]. A population projection based on an age-stage, two-sex life table can reveal the changes in stage structures during population growth, and the TIMING-MSChart program is usually used for population prediction when analyzing the life tables of insects [31] such as Bemisia tabaci [38], Bactrocera dorsalis [39], and Spodoptera frugiperda [40]. Predicted population dynamic curves indicated that the simulated control population grew faster than the DCY-treated populations (Figure 5 and Figure 6), which also supported the results of the population life table parameters. Therefore, we inferred that DCY could prime the seeds of various rice varieties to suppress the population of planthoppers.
In BPH, the amount of food intake can be directly reflected by the amount of honeydew excretion [41]. Our results suggested that the honeydew from newly emerged BPH females fed on either DCY100-pretreated Liangyou566 or Nanjing9108 rice plants were both significantly reduced (Figure 7). Ma et al. demonstrated that feeding on DCY-pretreated TN1 rice plants significantly reduced the honeydew excretions of BPHs and also affected the electrical penetration graph responses [27]. In addition, the weights of female BPHs fed on DCY100-pretreated plants of two rice varieties were decreased significantly (Figure 8), and these results were consistent with those of a previous study [27]. Recent studies have indicated that treating seeds with triflumezopyrim enhanced rice resistance against BPH by inhibiting phloem ingestion, which resulted in the reduced fecundity of females and lowered the population in the field [42,43]. A two-way ANOVA suggested that the effect of DCY treatment on the honeydew excretions and weights of BPHs was greater than rice variety and the interactions between DCY treatment and rice variety (Table 6). Thus, it could be inferred that DCY-pretreated rice plants may disrupt the development of BPHs by affecting their feeding behavior, thus inhibiting their reproduction.
The interactions between plants and bio-priming agents may affect the survival and preference of insect pests via certain underlying mechanisms, such as the induction of systemic resistance or feeding deterrence [11,44]. Insect pests choose the host plants on which to feed and oviposit depending on their nutritional requirements and have evolved complex behavioral and sensory mechanisms to locate and select a preferred host for oviposition and avoid unsuitable plants [45,46,47]. Our study indicated that BPH females preferred to feed and oviposit on untreated rice plants rather than DCY100-pretreated plants of Liangyou566 and Nanjing9108 (Figure 9). Ma et al. also demonstrated that the numbers of BPH females and the number of eggs laid in TN1 rice seedlings grown from seeds pretreated with DCY were significantly lower than those of the control [27]. Disi et al. showed that D. v. virgifera larvae showed less attraction to plants grown from maize seeds treated with B. pumilus than the untreated plants [10]. Similarly, Niu et al. found that the inoculation of rice seeds with bacterial entomopathogen S. marcescen affected the preference of BPHs for rice seedlings and caused elevated levels of secondary metabolites [19]. In rice, treatment with Bacillus velezensis YC7010 could enhance defenses against BPH by inducing changes in secondary metabolites [48]. We therefore suggest that the reduced feeding and oviposition preference of BPHs may be driven by DCY-mediated resistance or changes in secondary metabolites in rice. Our study suggests that seed priming with DCY could improve the resistance of different rice varieties against BPH, but we did not investigate the underlying mechanism of DCY-mediated rice resistance, and the future work should focus on this.
5. Conclusions
Our study investigated the effects of priming the seeds of rice varieties with DCY on BPH using an age-stage, two-sex life table, measurements of honeydew excretion, and a choice test. The results showed that DCY applied as a seed treatment to rice varieties significantly affected the adult longevity, oviposition period, and fecundity of BPHs as well as population parameters (R0, rm, λ, and DT). The honeydew excretions and the weights of BPHs were also decreased. Meanwhile, the feeding and oviposition preference were significantly reduced. Based on the present study, we conclude that DCY can prime the seeds of rice varieties to resist the BPH, and that seed priming, as an environmental-friendly technique, has great potential in sustainable crop protection. The results of the two-way ANOVA showed that significant differences occurred in the biological parameters, honeydew excretions, and weights of BPHs under DCY treatment, rice variety, and the interactions between DCY treatment and rice variety, suggesting that rice variety could determine different responses to DCY treatment, highlighting the necessity of exploring the effects of DCY on different rice varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13010072/s1, Figure S1: The interaction plots for the effects of decoyinine (DCY) treatment and rice variety on the biological parameters of BPH; Figure S2: Age-stage specific survival rate (sxj) of BPH fed on DCY-pretreated Liangyou566 rice plants; Figure S3: Age-stage specific survival rate (sxj) of BPH fed on DCY-pretreated Nanjing9108 rice plants; Table S1: The statistical results of the weight of BPH female adults; Table S2: The statistical results of the feeding preference of the gravid BPH female adults.
Author Contributions
G.X. and G.Y. conceived and designed the research; S.S., W.G., C.M. and M.Q. conducted the experiments; S.S., W.G. and Y.Z. analyzed the date; G.X. and W.G. wrote the manuscript; G.X. and Y.Z. reviewed and edited the manuscript; funding acquisition, G.X. and G.Y.; software, G.X., W.G. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20220570), the Experimental Teaching Project of the Integration of Scientific Research and Education of Yangzhou University (KJRH202203), and the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB210010).
Data Availability Statement
Data are contained within the article or Supplementary Material.
Acknowledgments
The authors sincerely thank Zefeng Yang and Rujia Chen at the Agricultural College of Yangzhou University for their help regarding the two-way ANOVA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xue, J.; Zhou, X.; Zhang, C.X.; Yu, L.L.; Fan, H.W.; Wang, Z.; Xu, H.J.; Xi, Y.; Zhu, Z.R.; Zhou, W.W.; et al. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014, 15, 521. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.E.; Zheng, Y.Y.; Xu, C.X.; Jiao, Q.Q.; Ye, C.L.; Chen, T.T.; Yu, X.P.; Pang, K.; Hao, P.Y. Rice defense against brown planthopper partially by suppressing the expression of transferrin family genes of brown planthopper. J. Agric. Food Chem. 2022, 70, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Ang, L.; Weilin, Z. Current understanding of the molecular players involved in resistance to rice planthoppers. Pest Manag. Sci. 2019, 75, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Ge, L.Q.; Liu, F.; Song, Q.S.; Stanley, D. Pesticide-induced planthopper population resurgence in rice cropping systems. Annu. Rev. Entomol. 2020, 65, 409–429. [Google Scholar] [CrossRef]
- Sen, A.; Johnson, R.; Puthur, J.T. Seed priming: A cost-effective strategy to impart abiotic stress tolerance. In Plant Performance Under Environmental Stress: Hormones, Biostimulants and Sustainable Plant Growth Management; Husen, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 459–480. [Google Scholar]
- Paul, S.; Dey, S.; Kundu, R. Seed priming: An emerging tool towards sustainable agriculture. Plant Growth Regul. 2021, 97, 215–234. [Google Scholar] [CrossRef]
- Worrall, D.; Holroyd, G.H.; Moore, J.P.; Glowacz, M.; Croft, P.; Taylor, J.E.; Paul, N.D.; Roberts, M.R. Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol. 2012, 193, 770–778. [Google Scholar] [CrossRef]
- Berglund, T.; Lindström, A.; Aghelpasand, H.; Stattin, E.; Ohlsson, A.B. Protection of spruce seedlings against pine weevil attacks by treatment of seeds or seedlings with nicotinamide, nicotinic acid and jasmonic acid. Forestry 2015, 89, 127–135. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Wu, X.B.; Deng, Q.Q.; Zhu, Z.Y.; Ren, M.J.; Ye, M.; Zeng, R.S. Seed priming with calcium chloride enhances wheat resistance against wheat aphid Schizaphis graminum Rondani. Pest Manag. Sci. 2021, 77, 4709–4718. [Google Scholar] [CrossRef]
- Disi, J.O.; Kloepper, J.W.; Fadamiro, H.Y. Seed treatment of maize with Bacillus pumilus strain INR-7 affects host location and feeding by Western corn rootworm, Diabrotica virgifera virgifera. J. Pest Sci. 2018, 91, 515–522. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, X.; Huang, S.S.; Deng, J.; Li, X.B.; Luo, Z.B.; Zhang, Y.J. Pest management via endophytic colonization of tobacco seedlings by the insect fungal pathogen Beauveria bassiana. Pest Manag. Sci. 2021, 77, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Jisha, K.C.; Puthur, J.T. Seed priming with beta-amino butyric acid improves abiotic stress tolerance in rice seedlings. Rice Sci. 2016, 23, 242–254. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Moulick, D.; Santra, S.C.; Ghosh, D. Rice seed priming with Se: A novel approach to mitigate as induced adverse consequences on growth, yield and as load in brown rice. J. Hazard. Mater. 2018, 355, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.K.; Mukherjee, A.K.; Chakraborty, K. Seed priming alleviates stress tolerance in rice (Oryza sativa L.). In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer Singapore: Singapore, 2019; pp. 181–204. [Google Scholar]
- Dhillon, B.S.; Kumar, V.; Sagwal, P.; Kaur, N.; Singh Mangat, G.; Singh, S. Seed priming with potassium nitrate and gibberellic acid enhances the performance of dry direct seeded rice (Oryza sativa L.) in north-western India. Agronomy 2021, 11, 849. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Jiang, Q.; Wang, Z.; Gao, C.; Wang, W. Seed priming with calcium chloride enhances stress tolerance in rice seedlings. Plant Sci. 2022, 323, 111381. [Google Scholar] [CrossRef]
- Pal, G.; Mehta, D.; Singh, S.; Magal, K.; Gupta, S.; Jha, G.; Bajaj, A.; Ramu, V.S. Foliar application or seed priming of cholic acid-glycine conjugates can mitigate/prevent the rice bacterial leaf blight disease via activating plant defense genes. Front. Plant Sci. 2021, 12, 746912. [Google Scholar] [CrossRef]
- Niu, H.T.; Sun, Y.; Zhang, Z.C.; Zhao, D.X.; Wang, N.; Wang, L.H.; Guo, H.F. The endophytic bacterial entomopathogen Serratia marcescens promotes plant growth and improves resistance against Nilaparvata lugens in rice. Microbiol. Res. 2022, 256, 126956. [Google Scholar] [CrossRef]
- Yuntsen, H.; Yonehara, H.; Ui, H. Studies on a new antibiotic, angustmycin. I. J. Antibiot. 1954, 7, 113–115. [Google Scholar]
- Yu, L.; Zhou, W.; She, Y.; Ma, H.; Cai, Y.S.; Jiang, M.; Deng, Z.; Price, N.P.J.; Chen, W. Efficient biosynthesis of nucleoside cytokinin angustmycin A containing an unusual sugar system. Nat. Commun. 2021, 12, 6633. [Google Scholar] [CrossRef]
- Bianchi-Smiraglia, A.; Wawrzyniak, J.A.; Bagati, A.; Marvin, E.K.; Ackroyd, J.; Moparthy, S.; Bshara, W.; Fink, E.E.; Foley, C.E.; Morozevich, G.E.; et al. Pharmacological targeting of guanosine monophosphate synthase suppresses melanoma cell invasion and tumorigenicity. Cell Death. Differ. 2015, 22, 1858–1864. [Google Scholar] [CrossRef]
- Gong, L. Effect of Combination of Wugufengsu and Yuhuangjin on Maize Growth and Yield; Northeast Agricultural University: Harbin, China, 2019. [Google Scholar]
- Sun, K.X. Effects of a New Plant Growth Regulator, Wufengfengsu on Seed Germination, Growth, Yield and Quality of Nanning Rice Variety “Baixiang 139” in Guangxi Province; Northeast Agricultural University: Harbin, China, 2020. [Google Scholar]
- Zhou, R. Effects of WGF Soaking Seed on Growth and Yield of Direct Seedling Rice “Guangliangxiang 2”; Northeast Agricultural University: Harbin, China, 2021. [Google Scholar]
- Shah, A.Z.; Ma, C.; Zhang, Y.Y.; Zhang, Q.X.; Xu, G.; Yang, G.Q. Decoyinine induced resistance in rice against small brown planthopper Laodelphax striatellus. Insects 2022, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, Y.Y.; Gui, W.; Zhang, Q.X.; Xu, G.; Yang, G.Q. Priming of rice seed with decoyinine enhances resistance against the brown planthopper Nilparvata lugens. Crop Prot. 2022, 157, 105970. [Google Scholar] [CrossRef]
- Lin, Y.B.; Lin, X.H.; Ding, C.H.; Xia, M.; Xue, R.R.; Sun, Z.X.; Chen, D.Q.; Zhu-Salzman, K.Y.; Zeng, R.S.; Song, Y.Y. Priming of rice defense against a sap-sucking insect pest brown planthopper by silicon. J. Pest Sci. 2022, 95, 1371–1385. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. Timing of control based on the stage structure of pest populations: A simulation approach. J. Econ. Entomol. 1990, 83, 1143–1150. [Google Scholar] [CrossRef]
- Chi, H.; You, M.S.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Ozgokce, M.S.; Guncan, A.; Tuan, S.J.; Fu, J.W.; Xu, Y.Y.; et al. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Bose, B.; Kumar, M.; Singhal, R.K.; Mondal, S. Impact of seed priming on the modulation of physico-chemical and molecular processes during germination, growth, and development of crops. In Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer: Singapore, 2018; pp. 23–40. [Google Scholar]
- Li, X.M.; Zhao, M.H.; Huang, F.; Shang, F.G.; Zhang, Y.H.; Liu, C.M.; He, S.J.; Wu, G. Effects of elevated CO2 on the fitness of three successive generations of Lipaphis erysimi. Insects 2022, 13, 333. [Google Scholar] [CrossRef]
- Altaf, N.; Idrees, A.; Ullah, M.I.; Arshad, M.; Afzal, A.; Afzal, M.; Rizwan, M.; Li, J. Biotic potential induced by different host plants in the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 921. [Google Scholar] [CrossRef]
- Acharya, R.; Malekera, M.J.; Dhungana, S.K.; Sharma, S.R.; Lee, K.Y. Impact of rice and potato host plants is higher on the reproduction than growth of corn strain fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2022, 13, 256. [Google Scholar] [CrossRef]
- Zhang, Z.; Batuxi; Jiang, Y.N.; Li, X.R.; Zhang, A.H.; Zhu, X.; Zhang, Y.H. Effects of different wheat tissues on the population parameters of the fall armyworm (Spodoptera frugiperda). Agronomy 2021, 11, 2044. [Google Scholar] [CrossRef]
- Li, J.; Ding, T.B.; Chi, H.; Chu, D. Effects of Tomato chlorosis virus on the performance of its key vector, Bemisia tabaci, in China. J. Appl. Entomol. 2018, 142, 296–304. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Qi, F.J.; Tan, X.M.; Zhang, T.; Teng, Z.W.; Fan, Y.J.; Wan, F.H.; Zhou, H.X. Use of age-stage, two-sex life table to compare the fitness of Bactrocera dorsalis (Diptera: Tephritidae) on northern and southern host fruits in China. Insects 2022, 13, 258. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.F.; Zhang, L.; Liu, Y.Q.; Cheng, Y.X.; Su, J.Y.; Sappington, T.W.; Jiang, X.F. Population development, fecundity, and flight of Spodoptera frugiperda (Lepidoptera: Noctuidae) reared on three green manure crops: Implications for an ecologically based pest management approach in China. J. Econ. Entomol. 2022, 115, 124–132. [Google Scholar] [CrossRef]
- Zhu, J.H.; Liu, X.Q.; Zhu, K.M.; Zhou, H.Y.; Li, L.; Li, Z.X.; Qin, W.W.; He, Y.P. Knockdown of TRPV genes affects the locomotion and feeding behavior of Nilaparvata lugens (Hemiptera: Delphacidae). J. Insect Sci. 2020, 20, 9. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, G.; Chen, Y.; Yu, J.L.; Zhou, Y.K.; Shu, Z.L.; Ge, L.Q. Seed dressing with triflumezopyrim controls brown planthopper populations by inhibiting feeding behavior, fecundity and enhancing rice plant resistance. Pest Manag. Sci. 2021, 77, 2870–2886. [Google Scholar] [CrossRef]
- Xi, C.Y.; Ahmad, S.; Yu, J.L.; Zhang, J.Y.; Chen, Y.; Zhang, G.; Zhu, H.W.; Ge, L.Q.; Yu, X.Y.; Shu, Z.L. Seed coating with triflumezopyrim induces the rice plant’s defense and inhibits the brown planthopper’s feeding behavior. Agronomy 2022, 12, 1202. [Google Scholar] [CrossRef]
- Disi, J.; Simmons, J.; Zebelo, S. Plant growth-promoting rhizobacteria-induced defense against insect herbivores. In Field Crops: Sustainable Management by PGPR; Maheshwari, D.K., Dheeman, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 385–410. [Google Scholar]
- Bertea, C.M.; Casacci, L.P.; Bonelli, S.; Zampollo, A.; Barbero, F. Chemical, physiological and molecular responses of host plants to Lepidopteran egg-laying. Front. Plant Sci. 2020, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Renwick, J.; Chew, F. Oviposition behavior in Lepidoptera. Annu. Rev. Entomol. 1994, 39, 377–400. [Google Scholar] [CrossRef]
- Carroll, M.J.; Schmelz, E.A.; Meagher, R.L.; Teal, P.E.A. Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J. Chem. Ecol. 2006, 32, 1911–1924. [Google Scholar] [CrossRef]
- Harun-Or-Rashid, M.; Kim, H.J.; Yeom, S.I.; Yu, H.A.; Manir, M.M.; Moon, S.S.; Kang, Y.J.; Chung, Y.R. Bacillus velezensis YC7010 enhances plant defenses against brown planthopper through transcriptomic and metabolic changes in rice. Front. Plant Sci. 2018, 9, 1904. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).