Effects of Priming Rice Seeds with Decoyinine on Fitness Traits and Virus Transmission Ability of the Small Brown Planthopper, Laodelphax striatellus
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice, Insects, and DCY Treatments
2.2. DCY Effects on SBPH Body Weight
2.3. DCY Effects on SBPH Honeydew
2.4. DCY Effects on SBPH Fecundity
2.5. DCY Effects on SBPH Survival
2.6. DCY Effects on Seedling Mortality Fed with SBPH
2.7. DCY Effects on RSV Transmission
2.7.1. DCY Effects on Horizontal RSV Transmission Rates by SBPH
- i.
- RSV acquisition/development (Transfer of RSV from infected rice to SBPH)
- ii.
- RSV inoculation (Transfer of RSV from virulent SBPH to healthy rice)
2.7.2. DCY Effects on Vertical RSV Transmission Rates by SBPH
2.8. DCY Effects on SBPH Feeding Behavior
2.9. Statistical Analysis
3. Results
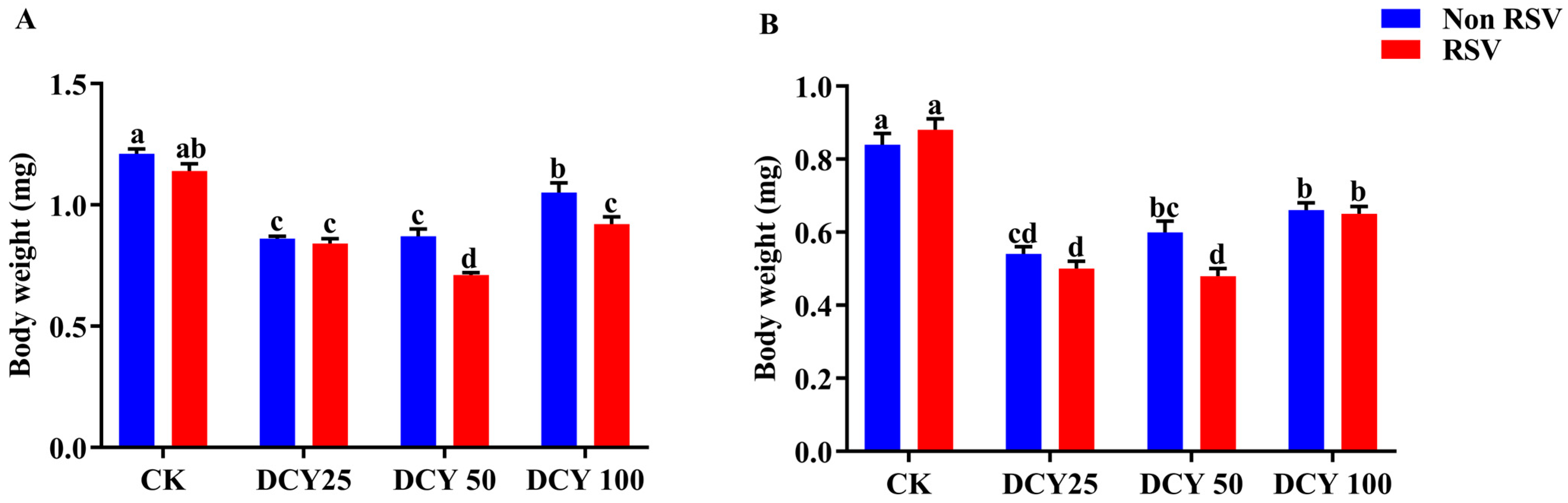
3.1. Body Weight of Different Populations of SBPH on DCY-Treated Rice
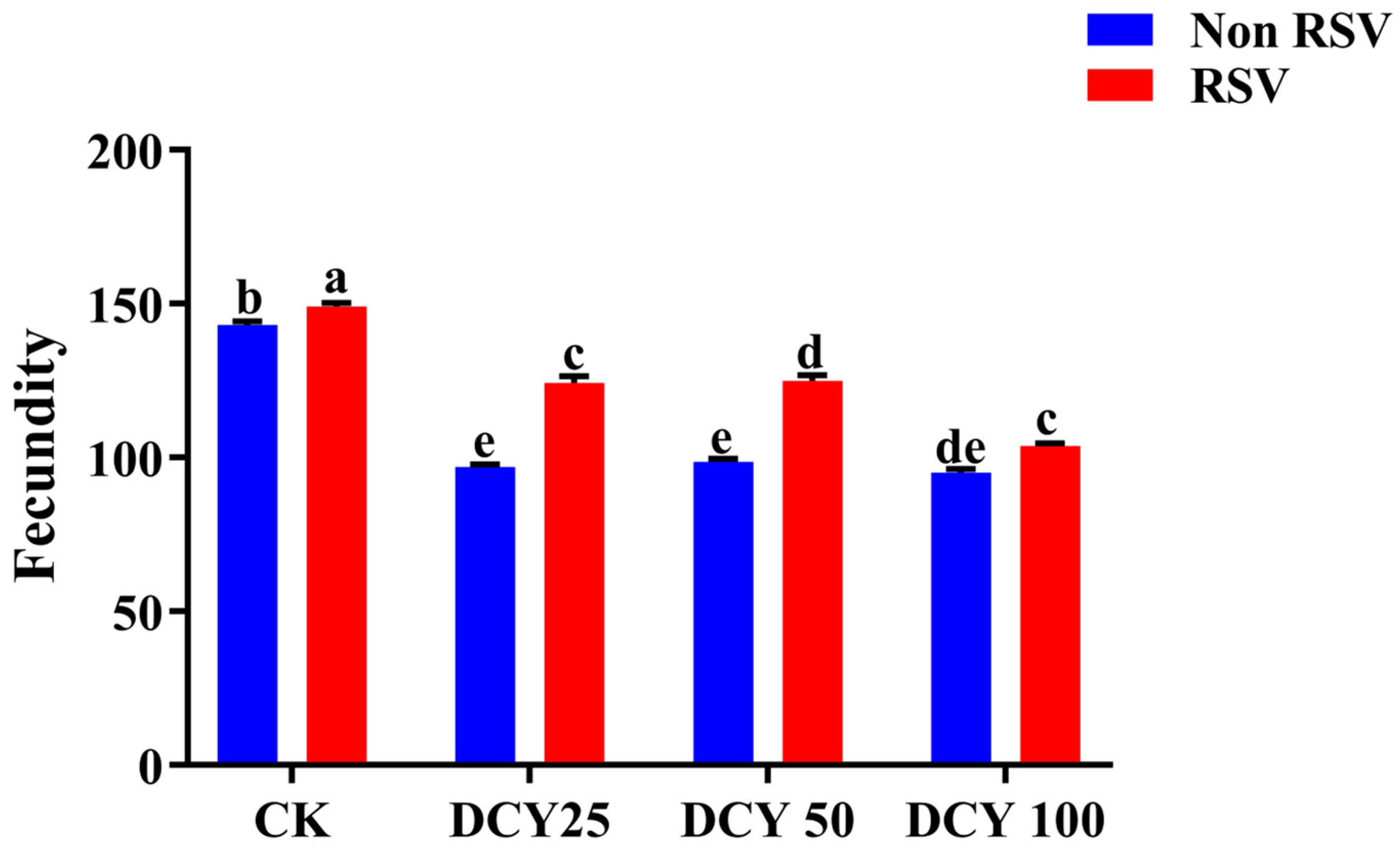
3.2. Effects of DCY on SBPH Fecundity
3.3. Nymphal Survival of Different Populations of SBPH on DCY-Treated Rice
3.4. Mortality of Rice Seedlings Fed with Different Populations of SBPH
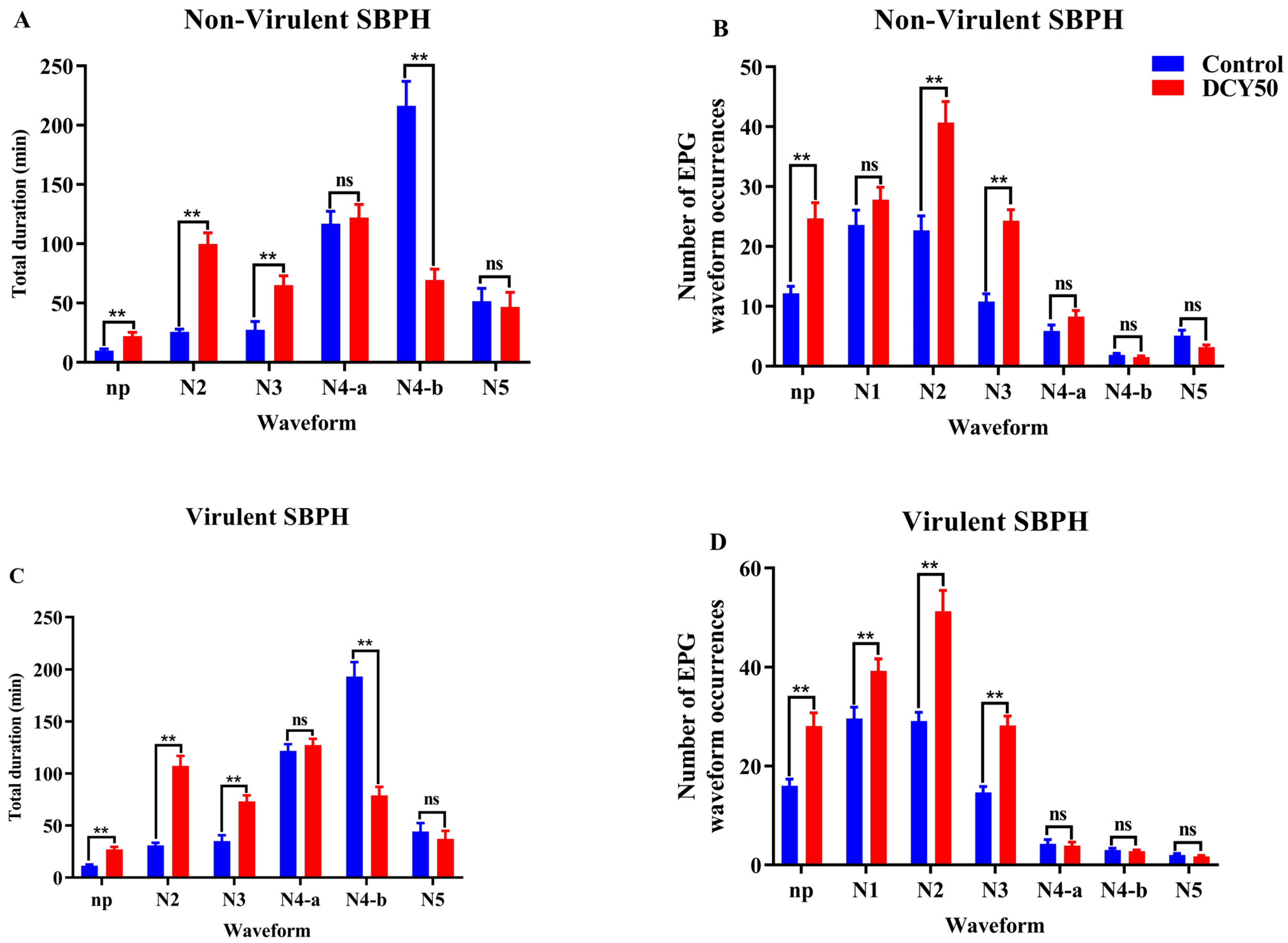
3.5. Effects of DCY on SBPH Feeding Behavior
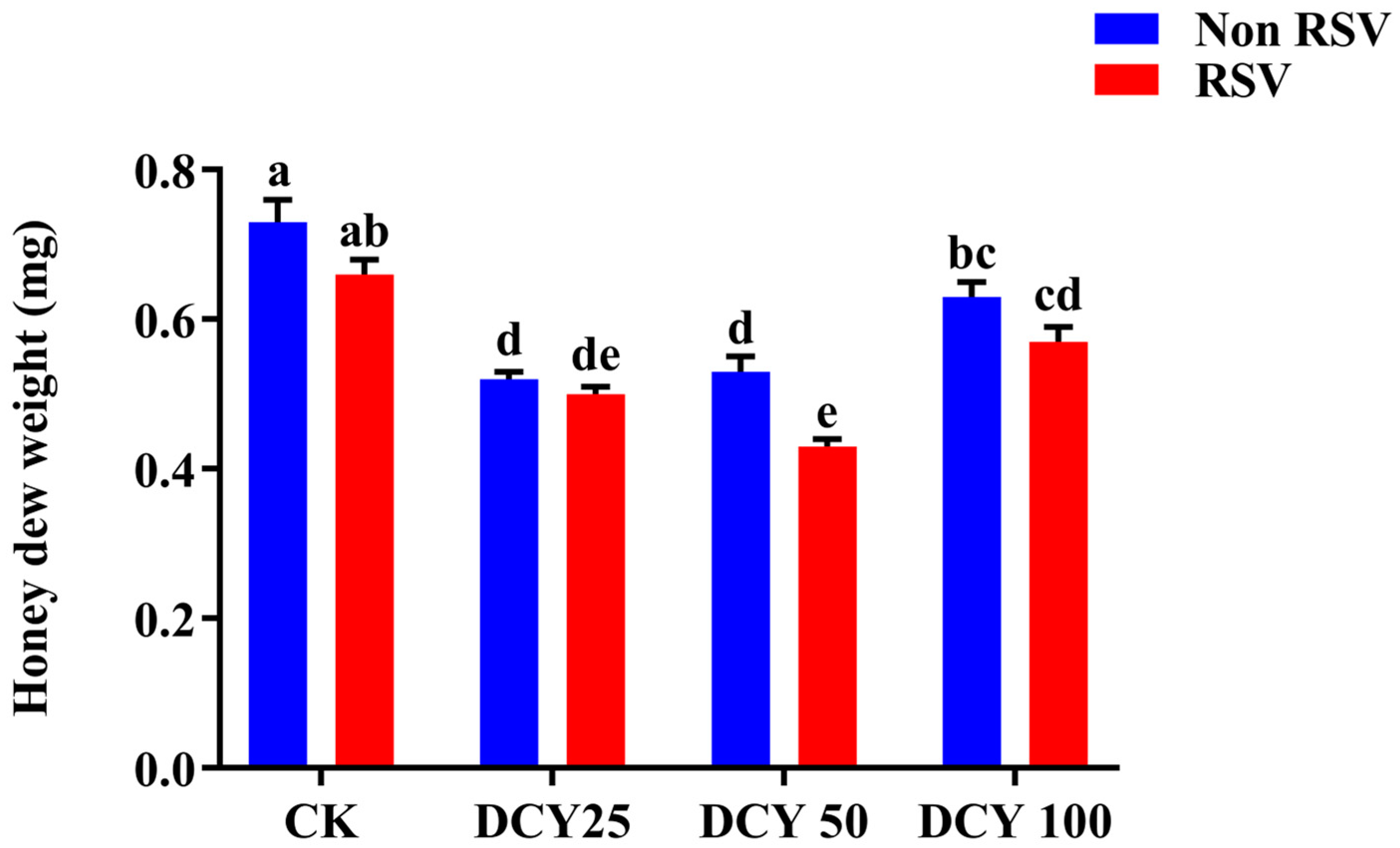
3.6. Honeydew Weight of Different Populations of SBPH on DCY-Treated Rice
3.7. Effects of DCY on RSV Transmission
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Strategies for developing green super rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef]
- Deng, J.; Li, S.; Hong, J.; Ji, Y.; Zhou, Y. Investigation on subcellular localization of Rice stripe virus in its vector small brown planthopper by electron microscopy. Virol. J. 2013, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, S.; Cheng, Z.; Zhou, T.; Fan, Y. Research advances in rice stripe disease in China. Jiangsu J. Agric. Sci. 2012, 28, 1007–1015. [Google Scholar]
- Li, S.; Wang, X.; Xu, J.; Ji, Y.; Zhou, Y. A simplified method for simultaneous detection of Rice stripe virus and Rice black-streaked dwarf virus in insect vector. J. Virol. Methods 2015, 211, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Yang, J.G.; Liao, F.L.; Gao, F.L.; Lu, L.M.; Zhang, X.T.; Li, F.; Wu, Z.J.; Lin, Q.Y.; Xie, L.H. Genetic diversity and population structure of rice stripe virus in China. J. Gen. Virol. 2009, 90, 1025–1034. [Google Scholar] [CrossRef]
- Cho, W.K.; Lian, S.; Kim, S.M.; Park, S.H.; Kim, K.H. Current insights into research on Rice stripe virus. Plant Pathol. J. 2013, 29, 223. [Google Scholar] [CrossRef]
- Fang, Y.; Choi, J.Y.; Park, D.H.; Park, M.G.; Kim, J.Y.; Wang, M.; Kim, H.J.; Kim, W.J.; Je, Y.H. Suppression of rice stripe virus replication in Laodelphax striatellus using vector insect-derived double-stranded RNAs. Plant Pathol. J. 2020, 36, 280. [Google Scholar] [CrossRef]
- Hibino, H. Biology and epidemiology of rice viruses. Annu. Rev. Phytopathol. 1996, 34, 249–274. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, W.; Zhang, F.; Chen, X.; Li, L.; Liu, Q.; Zhou, Y.; Wei, T.; Fang, R.; Wang, X. Transovarial transmission of a plant virus is mediated by vitellogenin of its insect vector. PLoS Pathog. 2014, 10, e1003949. [Google Scholar] [CrossRef]
- Wang, H.D.; Chen, J.P.; Wang, A.G.; Jiang, X.H.; Adams, M. Studies on the epidemiology and yield losses from rice black-streaked dwarf disease in a recent epidemic in Zhejiang province, China. Plant Pathol. 2009, 58, 815–825. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, H.; Yuan, P.; Zhang, X.; Chen, Q.; Jiang, X.; Zhou, Y. Development of a simplified RT-PCR without RNA isolation for rapid detection of RNA viruses in a single small brown planthopper (Laodelphax striatellus Fallén). Virol. J. 2017, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Dong, H.; Wang, X.; Zhou, G. Transmission by Laodelphax striatellus Fallen of Rice black-streaked dwarf virus from frozen infected rice leaves to healthy plants of rice and maize. J. Phytopathol. 2011, 159, 152–156. [Google Scholar] [CrossRef]
- Hunter, W.; Patte, C.; Sinisterra, X.; Achor, D.; Funk, C.; Polston, J. Discovering new insect viruses: Whitefly iridovirus (Homoptera: Aleyrodidae: Bemisia tabaci). J. Invertebr. Pathol. 2001, 78, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ni, Y.; Liu, H.; Rao, L.; Zhou, Y.; Zhou, X. Development and use of three monoclonal antibodies for the detection of rice black-streaked dwarf virus in field plants and planthopper vectors. Virol. J. 2013, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Yokozawa, M.; Nishimori, M.; Ueda, Y.; Yokosuka, T. How to analyze long-term insect population dynamics under climate change: 50-year data of three insect pests in paddy fields. Popul. Ecol. 2006, 48, 31–48. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yu, X.P.; Chen, J.M. High temperature effects on yeast-like endosymbiotes and pesticide resistance of the small brown planthopper, Laodelphax striatellus. Rice Sci. 2008, 15, 326–330. [Google Scholar] [CrossRef]
- Jing, P.; Huang, L.; Bai, S.; Liu, F. Effects of rice resistance on the feeding behavior and subsequent virus transmission efficiency of Laodelphax striatellus. Arthropod Plant Interact. 2015, 9, 97–105. [Google Scholar] [CrossRef]
- Sanada-Morimura, S.; Sakumoto, S.; Ohtsu, R.; Otuka, A.; Huang, S.-H.; Thanh, D.V.; Matsumura, M. Current status of insecticide resistance in the small brown planthopper, Laodelphax striatellus, in Japan, Taiwan, and Vietnam. Appl. Entomol. Zool. 2011, 46, 65–73. [Google Scholar] [CrossRef]
- Ranga Rao, G.; Rupela, O.; Rao, V.R.; Reddy, Y. Role of biopesticides in crop protection: Present status and future prospects. Indian J. Plant Prot. 2007, 35, 1–9. [Google Scholar]
- Pathak, D.; Yadav, R.; Kumar, M. Microbial pesticides: Development, prospects and popularization in India. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Berlin/Heidelberg, Germany, 2017; pp. 455–471. [Google Scholar]
- Desneux, N.; Fauvergue, X.; Dechaume-Moncharmont, F.-X.; Kerhoas, L.; Ballanger, Y.; Kaiser, L. Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol. 2005, 98, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Feng, C.; Guo, W.; Zhu, X.; Li, S.; Jiang, X.; Peng, Y. Effects of spraying pesticides at early flowering or grain filling stages on small brown planthoppers during wheat filling period and relevant biochemical analysis. Nongye Huanjing Kexue Xuebao 2007, 26, 985–989. [Google Scholar]
- Xu, G.; Gu, Z.; Xu, D.; Xu, X.; Shi, W. Impacts of five insecticides on the productivity of Laodelphax striatellus. J. Plant Prot. Res. 2008, 35, 361–366. [Google Scholar]
- Kour, D.; Rana, K.L.; Yadav, N.; Yadav, A.N.; Kumar, A.; Meena, V.S.; Singh, B.; Chauhan, V.S.; Dhaliwal, H.S.; Saxena, A.K. Rhizospheric microbiomes: Biodiversity, mechanisms of plant growth promotion, and biotechnological applications for sustainable agriculture. In Plant Growth Promoting Rhizobacteria for Agricultural Sustainability; Springer: Berlin/Heidelberg, Germany, 2019; pp. 19–65. [Google Scholar]
- Kour, D.; Rana, K.L.; Yadav, N.; Yadav, A.N.; Singh, J.; Rastegari, A.A.; Saxena, A.K. Agriculturally and industrially important fungi: Current developments and potential biotechnological applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–64. [Google Scholar]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. [Google Scholar]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An overview of some biopesticides and their importance in plant protection for commercial acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.R., Jr.; Robins, R.K.; Robins, M.J. Purine nucleosides. XXII. Synthesis of angustmycin A (decoyinine) and related unsaturated nucleosides. J. Am. Chem. Soc. 1968, 90, 4993–4999. [Google Scholar] [CrossRef] [PubMed]
- Bloch, A.; Nichol, C.A. Inhibition of ribosephosphate pyrophosphokinase activity by decoyinine, an adenine nucleoside. Biochem. Biophys. Res. Commun. 1964, 16, 400–403. [Google Scholar] [CrossRef]
- Baugh, C.M.; Krumdieck, C.L. Biosynthesis of riboflavine in Corynebacterium species: The purine precursor. J. Bacteriol. Res. 1969, 98, 1114–1119. [Google Scholar] [CrossRef]
- Prisbe, E.J.; Smejkal, J.; Verheyden, J.P.; Moffatt, J.G. Halo sugar nucleosides. V. Synthesis of angustmycin A and some base analogues. J. Org. Chem. 1976, 41, 1836–1846. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, W.; She, Y.; Ma, H.; Cai, Y.-S.; Jiang, M.; Deng, Z.; Price, N.P.; Chen, W. Efficient biosynthesis of nucleoside cytokinin angustmycin A containing an unusual sugar system. Nat. Commun. 2021, 12, 6633. [Google Scholar] [CrossRef]
- Mitani, T.; Heinze, J.E.; Freese, E. Induction of sporulation in Bacillus subtilis by decoyinine or hadacidin. Biochem. Biophys. Res. Commun. 1977, 77, 1118–1125. [Google Scholar] [CrossRef]
- Tojo, S.; Hirooka, K.; Fujita, Y. Expression of kinA and kinB of Bacillus subtilis, necessary for sporulation initiation, is under positive stringent transcription control. J. Bacteriol. Res. 2013, 195, 1656. [Google Scholar] [CrossRef] [PubMed]
- Uratani, B.; Lopez, J.; Freese, E. Effect of decoyinine on peptidoglycan synthesis and turnover in Bacillus subtilis. J. Bacteriol. Res. 1983, 154, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bianchi-Smiraglia, A.; Wawrzyniak, J.; Bagati, A.; Marvin, E.; Ackroyd, J.; Moparthy, S.; Bshara, W.; Fink, E.; Foley, C.; Morozevich, G. Pharmacological targeting of guanosine monophosphate synthase suppresses melanoma cell invasion and tumorigenicity. Cell Death Differ. 2015, 22, 1858–1864. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Xue, Y.; Yang, W. Biotechnology and Sustainable Agriculture 2006 and Beyond: Proceedings of the 11th IAPTC&B Congress, 13–18 August 2006, Beijing, China; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Sun, K.X. Effects of a New Plant Growth Regulator, Wufengfengsu on Seed Germination, Growth, Yield and Quality of Nanning Rice Variety “Baixiang 139” in Guangxi Province; Northeast Agricultural University: Harbin, China, 2020. [Google Scholar]
- Shah, A.Z.; Ma, C.; Zhang, Y.; Zhang, Q.; Xu, G.; Yang, G. Decoyinine induced resistance in rice against small brown planthopper Laodelphax striatellus. Insects 2022, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, Y.; Gui, W.; Zhang, Q.; Xu, G.; Yang, G. Priming of rice seed with decoyinine enhances resistance against the brown planthopper Nilparvata lugens. Crop Prot. 2022, 157, 105970. [Google Scholar] [CrossRef]
- Ma, C.; Gui, W.; Zhang, Y.; Shah, A.Z.; Xu, G.; Yang, G. Combined physio-biochemical and transcriptome analyses illuminate the resistance response of rice priming with decoyinine against Nilaparvata lugens. Agronomy 2022, 12, 3098. [Google Scholar] [CrossRef]
- Xu, G.; She, S.; Gui, W.; Ma, C.; Zhang, Y.; Qian, M.; Yang, G. Seed priming of rice varieties with decoyinine improve their resistance against the brown planthopper Nilaparvata lugens. Agronomy 2023, 13, 72. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; Mannino, G.; Brillada, C.; Novero, M.; Dall’Osto, L.; Maffei, M.E. Biology of Two-Spotted Spider Mite (Tetranychus urticae): Ultrastructure, Photosynthesis, Guanine Transcriptomics, Carotenoids and Chlorophylls Metabolism, and Decoyinine as a Potential Acaricide. Int. J. Mol. Sci. 2023, 24, 1715. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, G.; Jiang, Y.; Ma, C.; Yang, G. Sublethal effects of Imidacloprid on fecundity, apoptosis and virus transmission in the small brown planthopper Laodelphax striatellus. Insects 2021, 12, 1131. [Google Scholar] [CrossRef]
- Xu, G.; Jiang, Y.; Zhang, N.; Liu, F.; Yang, G. Triazophos-induced vertical transmission of rice stripe virus is associated with host vitellogenin in the small brown planthopper Laodelphax striatellus. Pest Manag. Sci. 2020, 76, 1949–1957. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Qian, M.; Zhang, Q.; Yang, G.; Xu, G. Comparative transcriptomic analysis of head in Laodelphax striatellus upon rice stripe virus infection. Agronomy 2022, 12, 3202. [Google Scholar] [CrossRef]
- Rashid, M.; Jahan, M.; Islam, K.S.; Latif, M. Ecological fitness of brown planthopper, Nilaparvata lugens (Stål), to rice nutrient management. Ecol. Process. 2017, 6, 15. [Google Scholar] [CrossRef]
- Pathak, P.; Saxena, R.; Heinrichs, E. Parafilm sachet for measuring honeydew excretion by Nilaparvata lugens on rice. J. Econ. Entomol. 1982, 75, 194–195. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Li, P.; Wen, L.; Hou, M. Silicon amendment to rice plants impairs sucking behaviors and population growth in the phloem feeder Nilaparvata lugens (Hemiptera: Delphacidae). Sci. Rep. 2017, 7, 1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, J.; Liu, B. Relative toxicity of insecticides to Laodelphax striatellus (Fallén) (Homoptera: Delphacidae) and the resistance of field populations from different areas of East China. Acta Entomol. Sin. 2008, 51, 930–937. [Google Scholar]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Wang, G.; Huang, X.; Cheng, Z.; Chen, Z.; Zhou, X. Immuno-detection of Rice stripe virus carried by brown planthopper. Jiangsu Agric. Sci. 2004, 1, 50–51. [Google Scholar]
- He, Y.; Chen, L.; Chen, J.; Zhang, J.; Chen, L.; Shen, J.; Zhu, Y.C. Electrical penetration graph evidence that pymetrozine toxicity to the rice brown planthopper is by inhibition of phloem feeding. Pest Manag. Sci. 2011, 67, 483–491. [Google Scholar] [CrossRef]
- Yang, G.Q.; Gao, X.; Zhang, N.N.; Chen, D.Y.; Liu, F.; Xu, J.X.; Wu, J.C. Pesticide-induced changes in fecundity and rice stripe virus transmission ability in Laodelphax striatellus (Homoptera: Delphacidae). J. Asia Pac. Entomol. 2017, 20, 830–834. [Google Scholar] [CrossRef]
- Wu, J.C.; Xu, J.X.; Yuan, S.Z.; Liu, J.L.; Jiang, Y.H.; Xu, J.F. Pesticide-induced susceptibility of rice to brown planthopper Nilaparvata lugens. Entomol. Exp. Appl. 2001, 100, 119–126. [Google Scholar] [CrossRef]
- Wang, L.P.; Shen, J.; Ge, L.Q.; Wu, J.C.; Yang, G.Q.; Jahn, G.C. Insecticide-induced increase in the protein content of male accessory glands and its effect on the fecundity of females in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Crop Prot. 2010, 29, 1280–1285. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, S.C.; Jha, M.; Kant, R.; Upendra, S.; Kumar, P. Microbial pesticide: A boom for sustainable agriculture. Int. J. Sci. Eng. 2014, 5, 1394–1397. [Google Scholar]
- Wang, X.; Liu, X.; Liu, C.; Wen, S.; Xue, Y.; Jin, Y.; Zhang, G.; Xia, X. Effects of sublethal concentrations of cyantraniliprole on the biology and metabolic enzyme activities of Laodelphax striatellus (Fallén). Crop Prot. 2022, 156, 105964. [Google Scholar] [CrossRef]
- Wen, S.; Xue, Y.; Du, R.; Liu, C.; Wang, X.; Wang, Y.; Liu, C.; Wang, S.; Wang, J.; Xia, X. Toxicity and sublethal effects of triflumezopyrim on the development and detoxification enzymatic activities in the small brown planthopper (SBPH), Laodelphax striatellus (Fallen). Crop Prot. 2021, 150, 105813. [Google Scholar] [CrossRef]
- Wang, J.; Song, J.; Wu, X.B.; Deng, Q.Q.; Zhu, Z.Y.; Ren, M.J.; Ye, M.; Zeng, R.S. Seed priming with calcium chloride enhances wheat resistance against wheat aphid Schizaphis graminum Rondani. Pest Manag. Sci. 2021, 77, 4709–4718. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.-J.; Kim, K.-M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. 2020, 10, 14685. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Jiang, S.; Wang, W.; Li, G.; Tao, X.; Pan, W.; Sword, G.A.; Chen, F. Rice stripe virus counters reduced fecundity in its insect vector by modifying insect physiology, primary endosymbionts and feeding behavior. Sci. Rep. 2015, 5, 12527. [Google Scholar] [CrossRef]
- Attia, M.S.; El-Sayyad, G.S.; Abd Elkodous, M.; El-Batal, A.I. The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani-causing early blight disease in tomato plant. Sci. Hortic. 2020, 266, 109289. [Google Scholar] [CrossRef]
- Ranganathan, S.; Suvarchala, V.; Rajesh, Y.; Srinivasa Prasad, M.; Padmakumari, A.; Voleti, S. Effects of silicon sources on its deposition, chlorophyll content, and disease and pest resistance in rice. Biol. Plantarum. 2006, 50, 713–716. [Google Scholar] [CrossRef]
- Yang, L.; Li, P.; Li, F.; Ali, S.; Sun, X.; Hou, M. Silicon amendment to rice plants contributes to reduced feeding in a phloem-sucking insect through modulation of callose deposition. Ecol. Evol. 2018, 8, 631–637. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, P.; Mo, X.; Hu, L.; Jin, N.; Chen, X.; Yu, Z.; Meng, J.; Erb, M.; Shang, Z. Induction of defense in cereals by 4-fluorophenoxyacetic acid suppresses insect pest populations and increases crop yields in the field. Proc. Natl. Acad. Sci. USA 2020, 117, 12017–12028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, B.; Zhang, Y. Electrical penetration graphs indicate that tricin is a key secondary metabolite of rice, inhibiting phloem feeding of brown planthopper, Nilaparvata lugens. Entomol. Exp. Appl. 2015, 156, 14–27. [Google Scholar] [CrossRef]
| DCY | Concentration (mg L−1) | No. of Valid Insects | No. of Plants Acquiring RSV | Virus Transmission Rate (%) | Chi-Square (χ2) Test |
|---|---|---|---|---|---|
| 0 | 30 | 6 | 20.00 | χ2 = 4.40 | |
| 25 | 30 | 3 | 10.00 | df = 3 | |
| 50 | 30 | 3 | 10.00 | p = 0.22 | |
| 100 | 30 | 1 | 3.33 |
| DCY | Concentration (mg L−1) | No. of Valid Insects | No. of Nymphs Acquiring RSV | Virus Transmission Rate (%) | Chi-Square (χ2) Test |
|---|---|---|---|---|---|
| 0 | 50 | 2 | 4.00 | χ2 = 3.88 | |
| 25 | 50 | 1 | 2.00 | df = 3 | |
| 50 | 30 | 3 | 10.00 | p = 0.27 | |
| 100 | 51 | 5 | 9.81 |
| DCY | Concentration (mg L−1) | No. of Valid Insects | No. of Nymphs Acquiring RSV | Virus Transmission Rate (%) | Chi-Square (χ2) Test |
|---|---|---|---|---|---|
| 0 | 96 | 73 | 76.04 | χ2 = 2.70 | |
| 25 | 95 | 65 | 68.42 | df = 3 | |
| 50 | 95 | 70 | 73.68 | p = 0.44 | |
| 100 | 96 | 64 | 66.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, A.Z.; Zhang, Y.; Gui, W.; Qian, M.; Yu, Y.; Xu, G.; Yang, G. Effects of Priming Rice Seeds with Decoyinine on Fitness Traits and Virus Transmission Ability of the Small Brown Planthopper, Laodelphax striatellus. Agronomy 2023, 13, 864. https://doi.org/10.3390/agronomy13030864
Shah AZ, Zhang Y, Gui W, Qian M, Yu Y, Xu G, Yang G. Effects of Priming Rice Seeds with Decoyinine on Fitness Traits and Virus Transmission Ability of the Small Brown Planthopper, Laodelphax striatellus. Agronomy. 2023; 13(3):864. https://doi.org/10.3390/agronomy13030864
Chicago/Turabian StyleShah, Amir Zaman, Yuanyuan Zhang, Wei Gui, Mingshi Qian, Youxin Yu, Gang Xu, and Guoqing Yang. 2023. "Effects of Priming Rice Seeds with Decoyinine on Fitness Traits and Virus Transmission Ability of the Small Brown Planthopper, Laodelphax striatellus" Agronomy 13, no. 3: 864. https://doi.org/10.3390/agronomy13030864
APA StyleShah, A. Z., Zhang, Y., Gui, W., Qian, M., Yu, Y., Xu, G., & Yang, G. (2023). Effects of Priming Rice Seeds with Decoyinine on Fitness Traits and Virus Transmission Ability of the Small Brown Planthopper, Laodelphax striatellus. Agronomy, 13(3), 864. https://doi.org/10.3390/agronomy13030864







