Determining Optimal Levels of Pruning in Hylocereus undatus [(Haw.) Britton and Rose] in Trellis Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Crop Management
2.2. Treatments, Experimental Designs and Measured Parameters
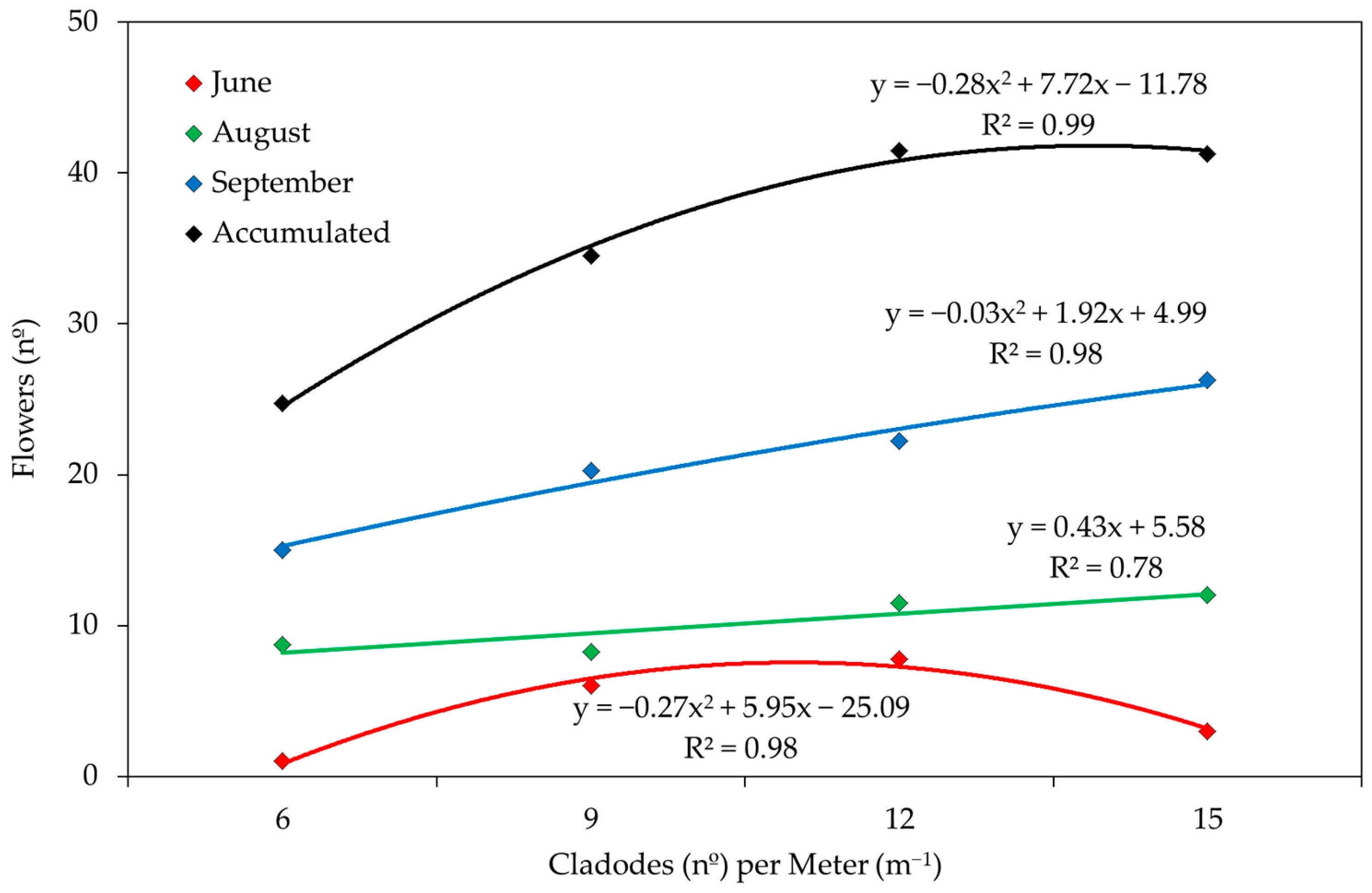
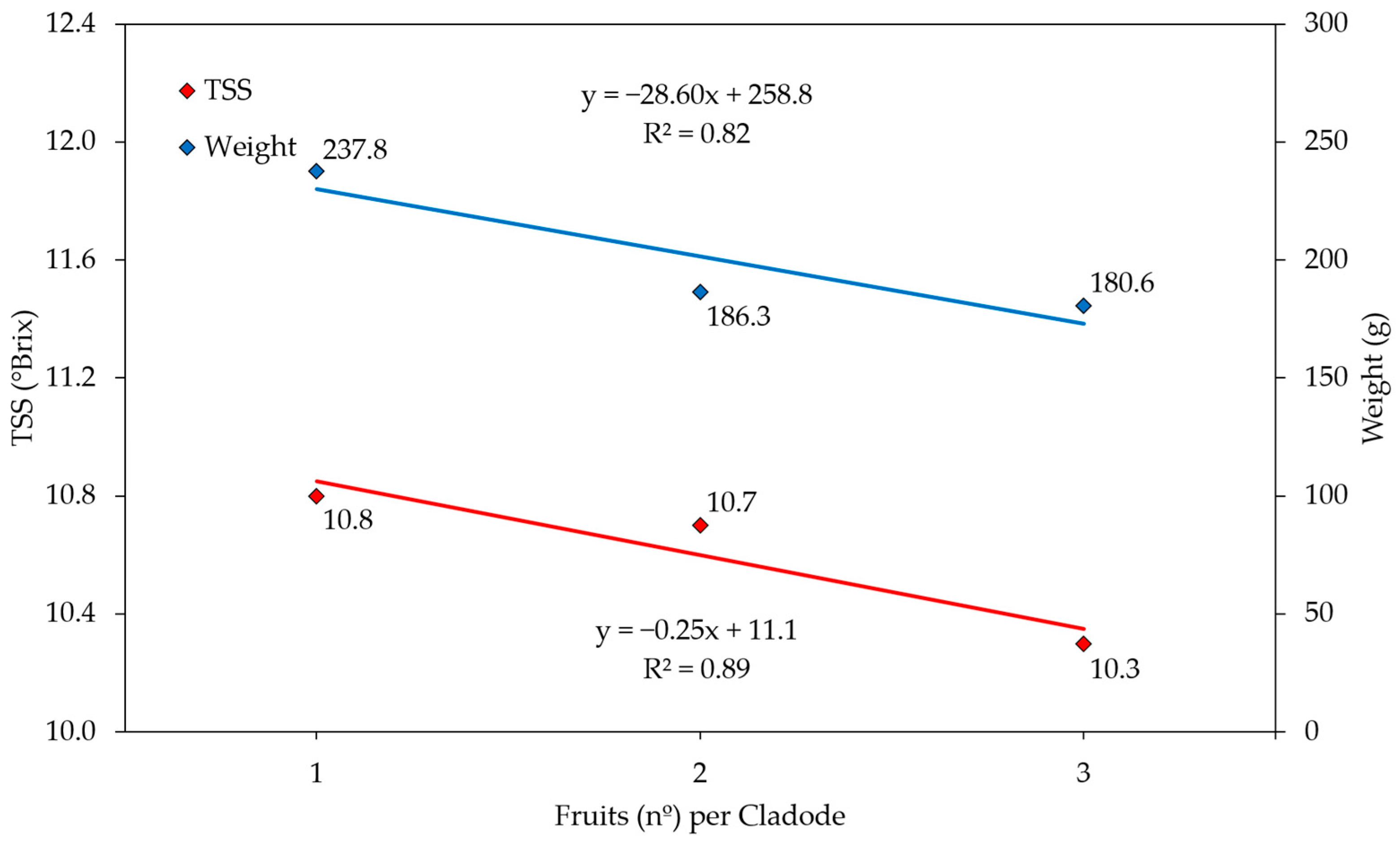
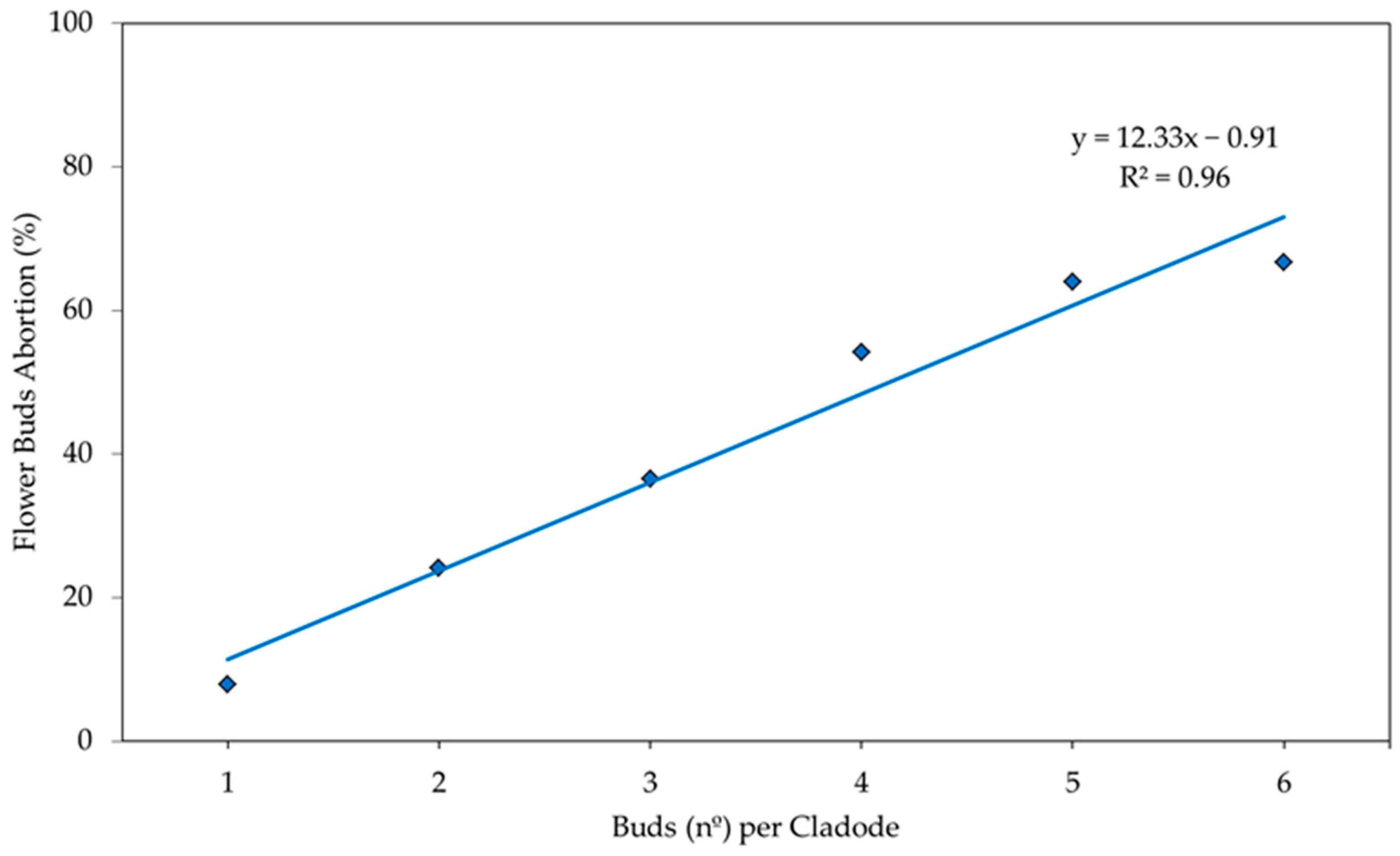
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angonese, M.; Motta, G.E.; Farias, N.S.; Molognoni, L.; Daguer, H.; Brugnerotto, P.; Costa, A.C.O.; Müller, C.M.O. Organic dragon fruits (Hylocereus undatus and Hylocereus polyrhizus) grown at the same edaphoclimatic conditions: Comparison of phenolic and organic acids profiles and antioxidant activities. LWT Food Sci. Technol. 2021, 149, 111924. [Google Scholar] [CrossRef]
- Luu, T.T.H.; Le, T.L.; Huynh, N.; Quintela-Alonso, P. Dragon fruit: A review of health benefits and nutrients and its sustainable development under climate changes in Vietnam. Czech J. Food Sci. 2021, 39, 71–94. [Google Scholar] [CrossRef]
- Attar, Ş.H.; Gündeşli, M.A.; Urün, I.; Kafkas, S.; Kafkas, N.E.; Ercisli, S.; Ge, C.; Mlcek, J.; Adamkova, A. Nutritional analysis of red-purple and white-fleshed pitaya (Hylocereus) species. Molecules 2022, 27, 808. [Google Scholar] [CrossRef]
- Britton, N.L.; Rose, J.N. The Cactaceae: Descriptions and Illustrations of Plants of the Cactus Family; The Carnegie Institution of Washington: Washington, DC, USA, 1920; Volume 2, pp. 183–195. [Google Scholar] [CrossRef]
- Kaiser, R.; Tollsten, L. An introduction to the scent of cacti. Flavour Frag. J. 1995, 10, 153–164. [Google Scholar] [CrossRef]
- Merten, S. A review of Hylocereus production in the United States. J. Prof. Assoc. Cactus Dev. 2003, 5, 98–105. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Santos Gally, R.; Arizmendi, M.C.; Casas, A. Pollination biology of the hemiepiphytic cactus Hylocereus undatus in the Tehuacán Valley, Mexico. J. Arid. Environ. 2007, 68, 1–8. [Google Scholar] [CrossRef]
- Costa, A.C.; Ramos, J.D.; Silva, F.O.R.; Duarte, M.H. Floração e frutificação em diferentes tipos de cladódios de pitaia vermelha em Lavras-MG. Rev. Bras. Frutic. 2014, 36, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.L.; Liao, Y.Y.; Lin, T.S.; Lee, C.L.; Yen, C.R.; Yang, W.J. The photoperiod-regulated bud formation of red pitaya (Hylocereus sp.). HortScience 2012, 47, 1063–1067. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.C.; Chang, J.C. Regulation of floral bud development and emergence by ambient temperature under a long-day photoperiod in white-fleshed pitaya (Hylocereus undatus). Sci. Hortic. 2020, 271, 109479. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Ferrante, A.; Massa, D.; Orlando, M.; Incrocci, L.; Mensuali-Sodi, A. Pitaya, an attractive alternative crop for Mediterranean region. Agronomy 2020, 10, 1065. [Google Scholar] [CrossRef]
- Kakade, V.; Morade, A.; Kadam, D. Dragon Fruit (Hylocereus undatus). In Tropical Fruit Crops: Theory to Practical; Ghosh, S.N., Sharma, R.R., Eds.; Jaya Publishing House: Delhi, India, 2022; pp. 240–257. [Google Scholar]
- Sharma, S.C.; Mittal, R.; Sharma, A.; Verma, V. Dragon fruit: A promising crop with a growing food market that can provide profitable returns to farmers. Int. J. Agric. Sci. Res. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Gunasena, H.P.M.; Pushpakumara, D.K.N.G.; Kariyawasam, M. Dragon fruit: Hylocereus undatus (Haw.) Britton and Rose. In Underutilized Fruit Trees in Sri Lanka; Pushpakumara, D.K.N.G., Gunasena, H.P.M., Singh, V.P., Eds.; World Agroforestry Centre, South Asia Office: New Delhi, India, 2007; Volume 1, pp. 110–141. [Google Scholar]
- Sánchez, F.P. Evaluación de un Cultivo de Pitaya en la Pedanía de La Atalaya en Mazarrón; Universidad Politécnica de Cartagena: Cartagena, Spain, 2020; Available online: http://hdl.handle.net/10317/8525 (accessed on 20 December 2022).
- Hieu, N.T.; Thu, N.N.A.; Linh, D.T.; Thanh, N.T.K.; Van Hoa, N.; Rangaswamy, M. Effect of various degree of canopy pruning on plant growth, yield, and control of canker disease of dragon fruit crop. In Proceedings of the International Conference on Tropical Fruit Pests and Diseases, Kota Kinabalu, Sabah, Malaysia, 25–27 September 2018. [Google Scholar]
- Alam, M.; Hasan, M.D.A.; Mondal, T.; Rathod, K.H.; Chhetri, S. Studies on the intensity of cladode pruning on vegetative and reproductive behaviour of dragon fruit (Hylocereus costaricensis). J. Crop Weed 2022, 18, 104–110. [Google Scholar] [CrossRef]
- Arredondo, E.; Chiamolera, F.M.; Casas, M.; Cuevas, J. Comparing different methods for pruning pitaya (Hylocereus undatus). Horticulturae 2022, 8, 661. [Google Scholar] [CrossRef]
- Papadakis, J. El Clima: Con Especial Referencia a los Climas de América Latina, Península Ibérica, Ex-Colonias Ibéricas y sus Potencialidades Agropecuarias; Albatros: Buenos Aires, Argentina, 1980. [Google Scholar]
- Nerd, A.; Sitrit, Y.; Kaushik, R.A.; Mizrahi, Y. High summer temperatures inhibit flowering in vine pitaya crops (Hylocereus spp.). Sci. Hortic. 2002, 96, 343–350. [Google Scholar] [CrossRef]
- Le Bellec, F.; Judith, R.C. La Pitahaya à la Réunion: Bilan et Perspectives; Cirad-Flhor: Saint-Pierre, France, 1999. [Google Scholar]
- Raveh, E.; Nerd, A.; Mizrahi, Y. Responses of two hemiepiphytic fruit crop cacti to different degrees of shade. Sci. Hortic. 1998, 73, 151–164. [Google Scholar] [CrossRef]
- Oliveira, M.M.T.; Albano-Machado, F.G.; Penha, D.M.; Pinho, M.M.; Natale, W.; Miranda, M.R.A.; Moura, C.F.H.; Alves, R.E.; Corrêa, M.C.M. Shade improves growth, photosynthetic performance, production and postharvest quality in red pitahaya (Hylocereus costaricensis). Sci. Hortic. 2021, 286, 110217. [Google Scholar] [CrossRef]
- Faust, M. Physiology of Temperate Zone Fruit Trees, 1st ed.; Wiley Intersciences: New York, NY, USA, 1989. [Google Scholar]
- Chen, K.; Hu, G.Q.; Lenz, F. Training and shading effects on vegetative and reproductive growth and fruit quality of apple. Gartenbauwissenschaft 1997, 62, 207–213. [Google Scholar]
- Acebedo, M.M.; Cañete, M.L.; Cuevas, J. Processes affecting fruit distribution and its quality in the canopy of olive trees. Adv. Hort. Sci. 2000, 14, 169–175. Available online: https://www.jstor.org/stable/42883271 (accessed on 25 December 2022).
- Sousa Ferraz, L.E.C.; Cavalcante, Í.H.L.; Teixeira Lobo, J.; Gomes da Cunha, J. Terminal branch density and fruit production in the canopy of high-yield mango orchards. Eur. J. Hortic. Sci. 2020, 85, 118–122. [Google Scholar] [CrossRef]
- Cronje, R.; Human, C.; Ratlapane, I. Pruning strategies for young ‘Nadorcott’ mandarin trees planted in high density orchards in South Africa. Int. J. Fruit Sci. 2021, 21, 921–931. [Google Scholar] [CrossRef]
- Truc, L.T.H.; Son, N.V.; Long, N.N.; Hoa, N.V.; Campbell, J.M.; Fullerton, R.A. Novel trellising and plant management techniques for dragon fruits in Vietnam. In Dragon Fruit—From Production to Market, 1st ed.; Fullerton, R.A., Lee, W.-L., Eds.; Food and Fertilizer Technology Center for the Asian and Pacific Region: Taipei, Taiwan, 2021; pp. 183–194. [Google Scholar]
- Weiss, J.; Nerd, A.; Mizrahi, Y. Flowering behavior and pollination requirements in climbing cacti with fruit crop potential. HortScience 1994, 29, 1487–1492. [Google Scholar] [CrossRef] [Green Version]
- Nerd, A.; Mizrahi, Y. Reproductive biology of cactus fruit crops. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1996; Volume 18, pp. 321–346. [Google Scholar] [CrossRef]
- Dag, A.; Mizrahi, Y. Effect of pollination method on fruit set and fruit characteristics in the vine cactus Selenicereus megalanthus (“yellow pitaya”). J. Horticult. Sci. Biotechnol. 2005, 80, 618–622. [Google Scholar] [CrossRef]
- Cho, J.L.Y.; Ding, P.; Abd. Razak, A.R.; Wahab, Z. Pollen load affects quality of red-fleshed dragon fruit (Hylocereus polyrhizus). Acta Hortic. 2013, 1012, 253–258. [Google Scholar] [CrossRef]


| Pruning Treatments | First Flowering | Second Flowering | ||||
|---|---|---|---|---|---|---|
| Flower Buds Number | Open Flower Number | Flower Buds Abortion (%) | Flower Buds Number | Open Flower Number | Flower Buds Abortion (%) | |
| T6 | 1.25 b | 1.00 b | 20.0 a | 9.50 a | 8.75 a | 7.9 a |
| T9 | 6.25 ab | 6.00 ab | 4.0 a | 9.25 a | 8.25 a | 10.8 a |
| T12 | 9.75 a | 7.75 a | 20.5 a | 12.00 a | 11.50 a | 4.2 a |
| T15 | 3.75 ab | 3.00 ab | 20.0 a | 14.75 a | 12.00 a | 18.6 a |
| p | 0.0208 | 0.0416 | 0.8864 | 0.5679 | 0.6802 | 0.8406 |
| Pruning Treatments | Weight (g) | Diameter (mm) | Length (mm) | TSS (°Brix) |
|---|---|---|---|---|
| T6 | 331.9 a | 70.2 a | 107.5 a | 9.5 ab |
| T9 | 344.6 a | 73.2 a | 111.7 a | 9.3 ab |
| T12 | 262.4 a | 66.9 a | 98.0 a | 10.5 a |
| T15 | 300.1 a | 68.5 a | 109.1 a | 9.2 b |
| p | 0.4088 | 0.2740 | 0.3469 | 0.0293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiamolera, F.M.; Parra, L.; Sánchez, E.; Casas, M.; Hueso, J.J.; Cuevas, J. Determining Optimal Levels of Pruning in Hylocereus undatus [(Haw.) Britton and Rose] in Trellis Systems. Agronomy 2023, 13, 238. https://doi.org/10.3390/agronomy13010238
Chiamolera FM, Parra L, Sánchez E, Casas M, Hueso JJ, Cuevas J. Determining Optimal Levels of Pruning in Hylocereus undatus [(Haw.) Britton and Rose] in Trellis Systems. Agronomy. 2023; 13(1):238. https://doi.org/10.3390/agronomy13010238
Chicago/Turabian StyleChiamolera, Fernando M., Laura Parra, Elisabet Sánchez, Marina Casas, Juan J. Hueso, and Julián Cuevas. 2023. "Determining Optimal Levels of Pruning in Hylocereus undatus [(Haw.) Britton and Rose] in Trellis Systems" Agronomy 13, no. 1: 238. https://doi.org/10.3390/agronomy13010238
APA StyleChiamolera, F. M., Parra, L., Sánchez, E., Casas, M., Hueso, J. J., & Cuevas, J. (2023). Determining Optimal Levels of Pruning in Hylocereus undatus [(Haw.) Britton and Rose] in Trellis Systems. Agronomy, 13(1), 238. https://doi.org/10.3390/agronomy13010238







