Yield Predictive Worth of Pre-Flowering and Post-Flowering Indicators of Nitrogen Economy in High Yielding Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Design
- Three systems of wheat canopy foliar protection in the spring part of the growing season (CFP):
- 1.1.
- N + foliar fertilization with micronutrients (N + Mi, i.e. fungicide control—FC);
- 1.2.
- N + fungicide protection (N + P, FP);
- 1.3.
- N + micronutrients + fungicide protection (N + Mi + FP, MiFP).
- Rates of applied fertilizer N: 0, 40, 80, 120, 160, 200, and 240 kg ha−1.
- (1)
- 80 kg N ha−1: the late winter, before the beginning of winter wheat vegetation in spring;
- (2)
- 160 kg N ha−1: at the end of tillering/beginning of the shoot elongation (BBCH 29/30);
- (3)
- 240 kg N ha−1: at the stage of a flag leaf visible (BBCH 39).
2.3. Plant Sampling
2.4. Calculated Parameters
2.5. Statistical Analysis
3. Results
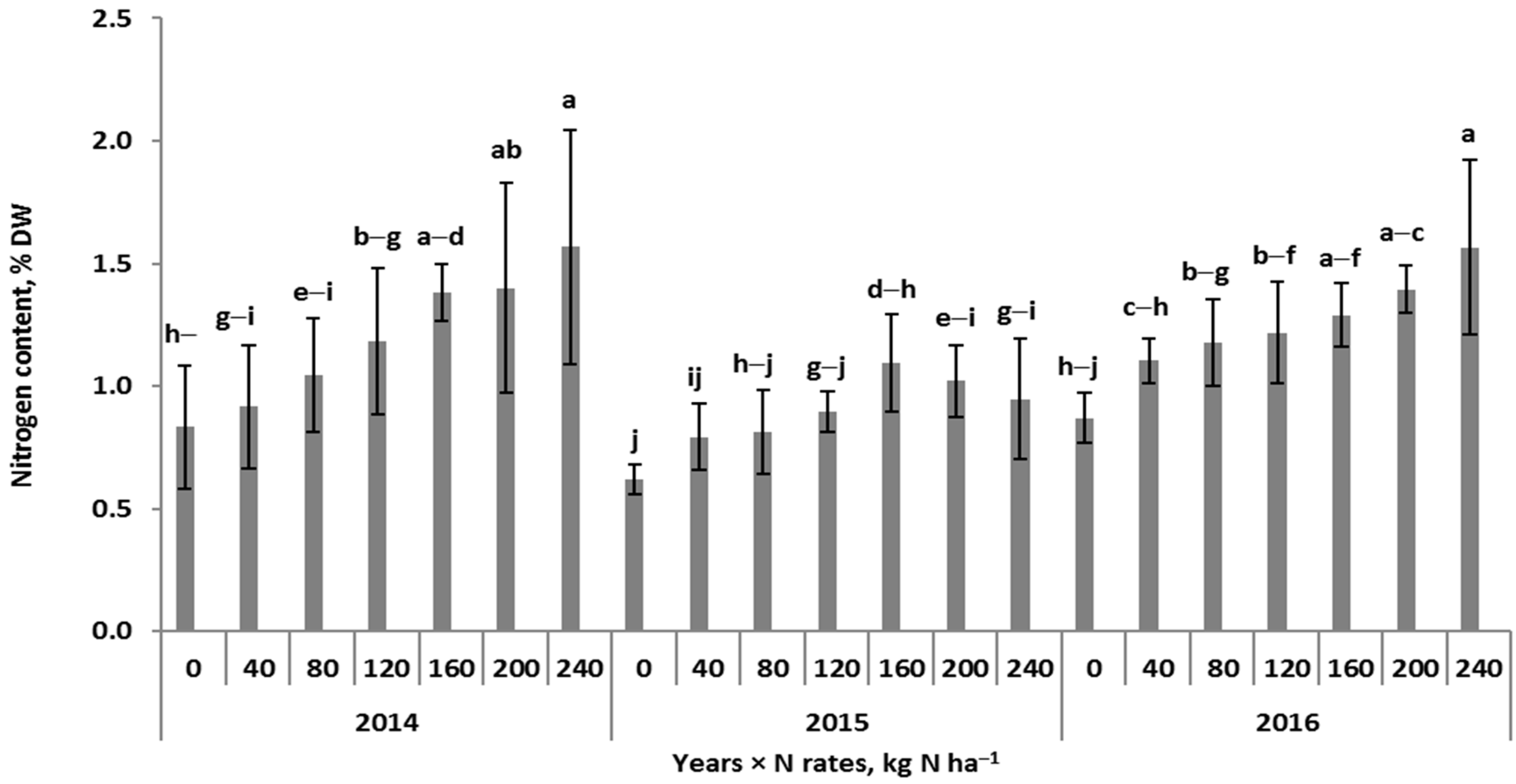
3.1. Patterns of Nitrogen Concentration in Winter Wheat
- 3.
- Yield (GY):
- 4.
- Grain density (GD):
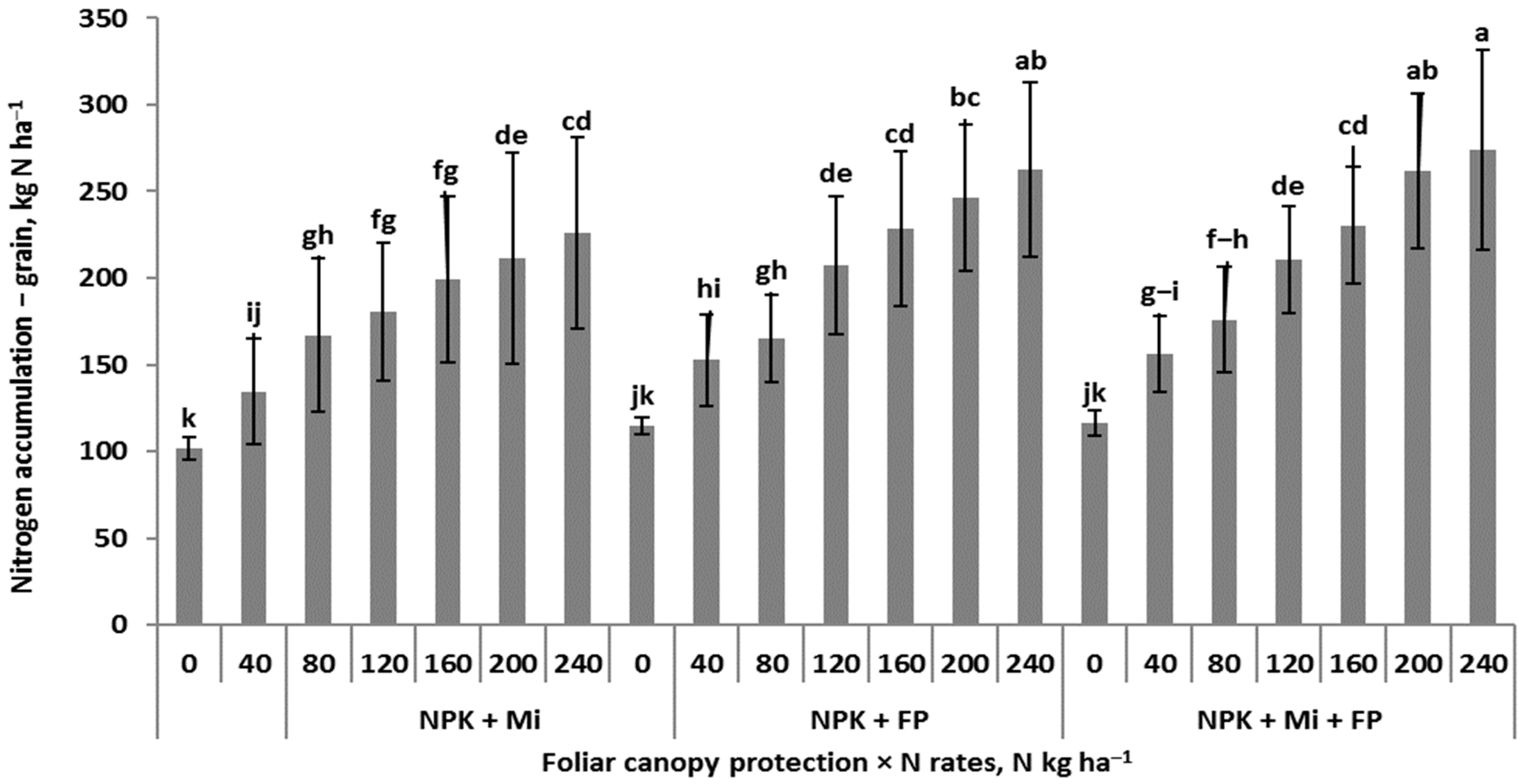
3.2. Patterns of Nitrogen Accumulation in Winter Wheat
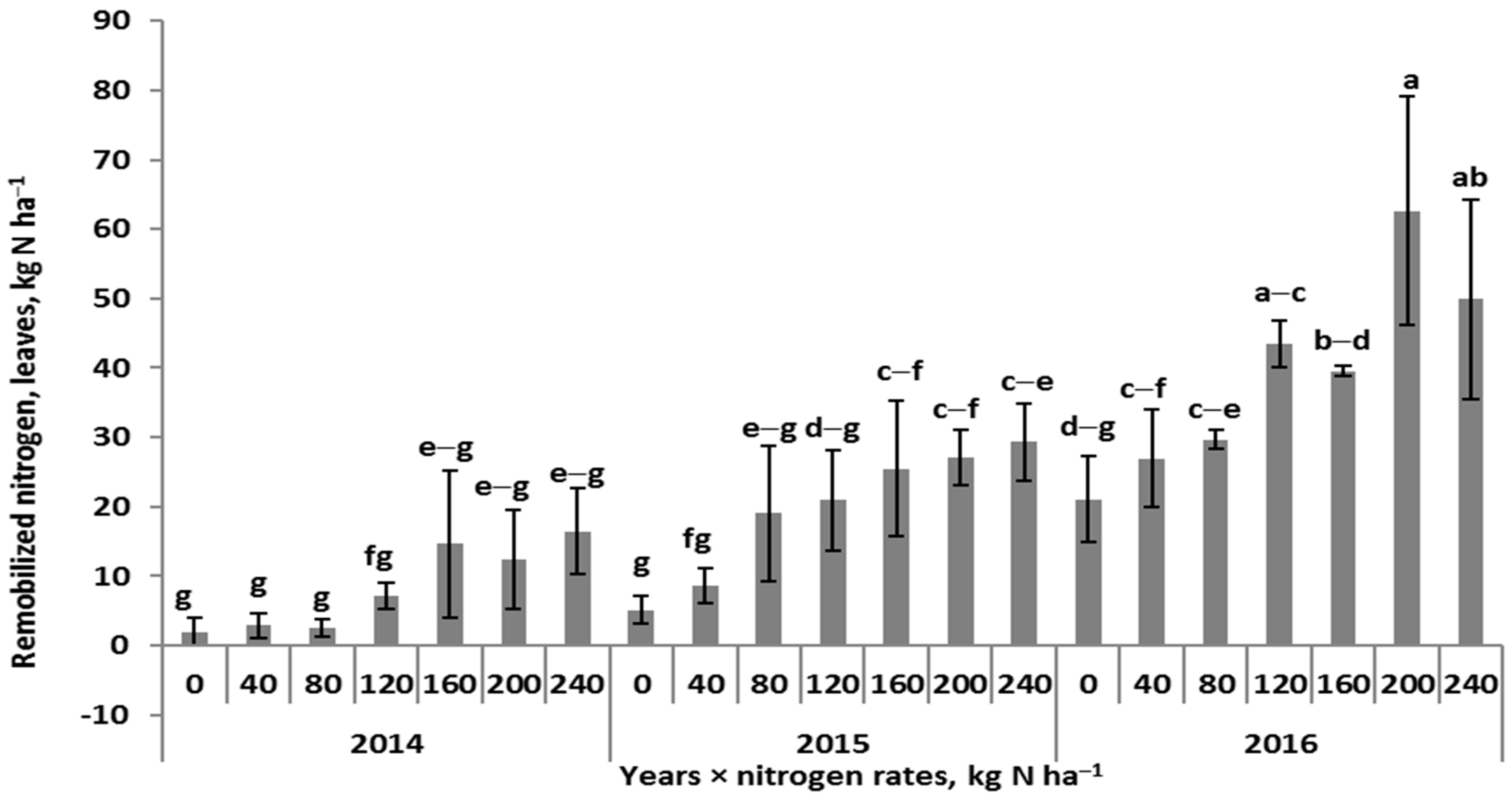
3.3. Indicators of Nitrogen Management by Winter Wheat
4. Discussion
4.1. Nitrogen Content in Wheat Organs as Yield Predictor
4.2. Nitrogen Management by Wheat during the Growing Season
4.3. Indicators of N Management in Winter Wheat during the Spring Vegetation
- (1)
- Leaves are an important, but not dominant, source of N for the growing grains;
- (2)
- Too-low GD, as the physiological sink of winter wheat, had no potential to exploit N resources in leaves during the grain-filling period. This was the case observed in 2016, despite the high remobilized efficiency.
- (3)
- A strong decrease in the content and amount of N was revealed in leaves, but provided for the high demand of the growing grains. This was the case observed in 2014 and 2015.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Traits | GD | TGW | N31 | N50L | N50S | N65L | N65S | N65E | N65FL | N90L | N90S | N90Ch | N90G | SPAD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GY | 0.99 *** | 0.59 ** | 0.84 *** | 0.90 *** | 0.91 *** | 0.84 *** | 0.80 *** | 0.87 *** | 0.84 *** | 0.46 * | 0.74 *** | 0.60 ** | 0.81 *** | 0.93 *** |
| GD | 1.00 | 0.47 * | 0.84 *** | 0.93 *** | 0.92 *** | 0.86 *** | 0.80 *** | 0.89 *** | 0.85 *** | 0.55 *** | 0.76 *** | 0.68 *** | 0.84 *** | 0.94 *** |
| TGW | 1.00 | 0.44 * | 0.29 | 0.39 * | 0.26 | 0.41 * | 0.32 | 0.34 | −0.31 | 0.23 | −0.13 | 0.25 | 0.42 | |
| N31 | 1.00 | 0.73 *** | 0.67 ** | 0.58 ** | 0.53 * | 0.61 ** | 0.54 * | 0.35 | 0.50 * | 0.45 * | 0.55 * | 0.72 *** | ||
| N50L | 1.00 | 0.94 *** | 0.90 *** | 0.81 *** | 0.83 *** | 0.91 *** | 0.71 *** | 0.87 *** | 0.82 *** | 0.89 *** | 0.92 *** | |||
| N50S | 1.00 | 0.89 *** | 0.79 *** | 0.85 *** | 0.87 *** | 0.59 *** | 0.79 *** | 0.72 *** | 0.85 *** | 0.90 *** | ||||
| N65L | 1.00 | 0.92 *** | 0.86 *** | 0.94 *** | 0.75 *** | 0.91 *** | 0.86 *** | 0.96 *** | 0.93 *** | |||||
| N65S | 1.00 | 0.81 *** | 0.89 *** | 0.60 *** | 0.91 *** | 0.72 *** | 0.93 *** | 0.91 *** | ||||||
| N65E | 1.00 | 0.86 *** | 0.61 *** | 0.74 *** | 0.70 *** | 0.85 *** | 0.90 *** | |||||||
| N65FL | 1.00 | 0.73 *** | 0.91 *** | 0.81 *** | 0.97 *** | 0.94 *** | ||||||||
| N90L | 1.00 | 0.76 *** | 0.89 *** | 0.79 *** | 0.68 *** | |||||||||
| N90S | 1.00 | 0.83 *** | 0.95 *** | 0.87 *** | ||||||||||
| N90Ch | 1.00 | 0.85 *** | 0.78 *** | |||||||||||
| N90G | 1.00 | 0.95 *** |
| Traits | TN31 | NaL50 | NaS50 | TN50 | NaL65 | NaS65 | NaE65 | TN65 | NaL90 | NaS90 | NaCh90 | NaVe90 | NaG90 | TN90 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GD | 0.88 *** | 0.93 ** | 0.95 *** | 0.95 *** | 0.92 *** | 0.92 *** | 0.94 *** | 0.96 *** | 0.71 *** | 0.83 *** | 0.85 *** | 0.82 *** | 0.96 *** | 0.96 *** |
| GY | 0.83 *** | 0.91 *** | 0.94 *** | 0.94 *** | 0.89 *** | 0.91 *** | 0.92 *** | 0.94 *** | 0.64 *** | 0.80 *** | 0.79 *** | 0.77 *** | 0.96 *** | 0.95 *** |
| TN31 | 1.00 | 0.84 *** | 0.81 *** | 0.84 *** | 0.76 *** | 0.75 *** | 0.87 *** | 0.82 *** | 0.77 *** | 0.76 *** | 0.85 *** | 0.81 *** | 0.77 *** | 0.80 *** |
| NaL50 | 1.00 | 0.95 *** | 0.99 *** | 0.92 *** | 0.90 *** | 0.85 *** | 0.92 *** | 0.82 *** | 0.88 *** | 0.88 *** | 0.89 *** | 0.93 *** | 0.95 *** | |
| NaS50 | 1.00 | 0.99 *** | 0.92 *** | 0.92 *** | 0.88 *** | 0.94 *** | 0.72 *** | 0.83 *** | 0.82 *** | 0.82 *** | 0.93 *** | 0.93 *** | ||
| TN50 | 1.00 | 0.94 *** | 0.92 *** | 0.88 *** | 0.94 *** | 0.78 *** | 0.87 *** | 0.86 *** | 0.87 *** | 0.94 *** | 0.95 *** | |||
| NaL65 | 1.00 | 0.97 *** | 0.85 *** | 0.97 *** | 0.80 *** | 0.91 *** | 0.89 *** | 0.90 *** | 0.96 *** | 0.97 *** | ||||
| NaS65 | 1.00 | 0.86 *** | 0.98 *** | 0.72 *** | 0.91 *** | 0.86 *** | 0.86 *** | 0.95 *** | 0.95 *** | |||||
| NaE65 | 1.00 | 0.93 *** | 0.61 ** | 0.73 *** | 0.78 *** | 0.73 *** | 0.86 *** | 0.85 *** | ||||||
| TN65 | 1.00 | 0.74 *** | 0.89 *** | 0.87 *** | 0.86 *** | 0.96 *** | 0.96 *** | |||||||
| NaL90 | 1.00 | 0.86 *** | 0.90 *** | 0.96 *** | 0.73 *** | 0.80 *** | ||||||||
| NaS90 | 1.00 | 0.92 *** | 0.97 *** | 0.88 *** | 0.92 *** | |||||||||
| NaCH90 | 1.00 | 0.97 *** | 0.84 *** | 0.89 *** | ||||||||||
| NaVe90 | 1.00 | 0.85 *** | 0.95 *** | |||||||||||
| NaG90 | 1.00 | 0.99 *** |
| Traits | ΔTN50 | ΔTN65 | NRQ | E- NRQ | C- NRQg | NPFU | E- NPFU | C- NPFUg | NRL | NRS | NRCh | E-NRL | E-NRS | E-NRCh |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GD | 0.86 *** | 0.53 * | 0.94 *** | 0.14 | −0.45 * | 0.75 *** | 0.33 | 0.45 * | 0.89 *** | 0.90 *** | 0.89 *** | 0.76 *** | 0.41 | −0.15 |
| GY | 0.87 *** | 0.52 * | 0.94 *** | 0.21 | −0.46 * | 0.76 *** | 0.34 | 0.46 * | 0.89 *** | 0.90 *** | 0.88 *** | 0.80 *** | 0.45 * | −0.08 |
| ΔTN50 | 1.00 | 0.31 | 0.86 *** | 0.13 | −0.48 * | 0.76 *** | 0.23 | 0.48 * | 0.89 *** | 0.87 *** | 0.70 *** | 0.78 *** | 0.41 | −0.19 |
| ΔTN65 | 1.00 | 0.69 *** | 0.31 | 0.06 | 0.19 | −0.13 | −0.06 | 0.67 ** | −0.69 ** | 0.62 ** | 0.55 * | 0.40 | 0.07 | |
| NRQ | 1.00 | 0.36 | −0.23 | 0.59 ** | 0.07 | 0.23 | 0.97 *** | 0.97 *** | 0.92 *** | 0.88 *** | 0.60 ** | 0.01 | ||
| E-NRQ | 1.00 | 0.50 * | −0.29 | −0.47 * | −0.50 * | 0.29 | 0.28 | 0.45 * | 0.62 ** | 0.86 *** | 0.85 ** | |||
| C-NRQg | 1.00 | −0.89 ** | −0.83 *** | −1.00 ** | −0.31 | −0.28 | 0.07 | −0.10 | 0.32 | 0.56 ** | ||||
| NPFU | 1.00 | 0.72 *** | 0.89 *** | 0.65 ** | 0.61 ** | 0.43 | 0.42 | −0.02 | −0.46 * | |||||
| E-NPFU | 1.00 | 0.83 *** | 0.12 | 0.11 | 0.01 | −0.06 | −0.35 | −0.48 * | ||||||
| C-NPFUg | 1.00 | 0.31 | 0.28 | 0.07 | 0.10 | −0.32 | −0.56 ** | |||||||
| NRL | 1.00 | 0.97 *** | 0.80 *** | 0.88 *** | 0.53 *** | −0.09 | ||||||||
| NRS | 1.00 | 0.82 *** | 0.86 *** | 0.56 ** | −0.11 | |||||||||
| NRCh | 1.00 | 0.77 *** | 0.61 ** | 0.24 | ||||||||||
| E-NRL | 1.00 | 0.75 *** | 0.22 | |||||||||||
| E-NRS | 1.00 | 0.63 ** |
Appendix B

References
- Káš, M.; Mühlbachova, G.; Kusá, H. Winter wheat yields under different soil-climatic conditions in a long-term field trial. Plant Soil Environ. 2019, 65, 27–34. [Google Scholar] [CrossRef]
- Iwańska, M.; Paderewski, J.; Stępień, M.; Rodrigues, P.C. Adaptation of winter wheat cultivars to different environments: A case study in Poland. Agronomy 2020, 10, 632. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://faostat.fao.org/site/567/default.aspx#ancor (accessed on 20 August 2022).
- Medvedev, V.V.; Plisko, I.V.; Bigun, O.N. Comparative characterization of the optimum and actual parameters of Ukrainian Chernozems. Euroasian Soil Sci. 2014, 47, 1044–1057. [Google Scholar] [CrossRef]
- COBORU. Results of Post-Registration Variety Experiments. Winter Cereals. COBORU: Słupia Wielka, Poland, 2019; 68p. Available online: https://www.coboru.gov.pl/ (accessed on 20 September 2022).
- Ceglar, A.; Zampieri, M.; Toreti, A.; Dentner, F. Observed Northward migration of agro-climate zones in Europe will further accelerate under climate change. Eart’s Future 2019, 7, 1088–1101. [Google Scholar] [CrossRef]
- Wójcik-Gront, E.; Iwańska, M.; Wnuk, A.; Oleksiak, T. The analysis of wheat yield variability based on experimental data from 2008−2018 to understand the yield gap. Agriculture 2022, 12, 32. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Sayre, K.D.; Solismoya, E. Identifying factors that determine kernel number in wheat. Field Crops Res. 1998, 58, 223–234. [Google Scholar] [CrossRef]
- Xie, Q.; Mayes, S.; Sparkes, D.L. Preanthesis biomass accumulation and plant organs defines yield components in wheat. Europ. J. Agron. 2016, 81, 15–26. [Google Scholar] [CrossRef]
- Tilley, M.S.; Heiniger, R.W.; Crozier, C.R. Tiller initiation and its effect on yield and yield components in winter wheat. Agron. J. 2019, 111, 1323–1332. [Google Scholar] [CrossRef]
- Pan, W.L.; Kidwell, K.K.; McCracken, V.A.; Bolton, R.P.; Allen, M. Economically optimal wheat yield, protein and nitrogen use component responses to varying N supply and genotype. Front. Plant Sci. 2019, 10, 1790. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Sec. 2015, 493, 178–202. [Google Scholar] [CrossRef]
- Ellmann, T. Effect of intensity of agricultural techniques and grain storage on technological quality of winter wheat, Part I. Quality traits of grain and flour. Acta Sci. Pol. Agric. 2011, 10, 27–36. [Google Scholar]
- Olesen, J.E.; Jorgense, L.N.; Petersen, J.; Mortensen, J.V. Effects of rate and timing of nitrogen fertilizer on disease control by fungicides in winter wheat. 1. Grain yield and foliar disease control. J. Agric. Sci. 2003, 140, 1–13. [Google Scholar] [CrossRef]
- Figueoroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat disease—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Macholdt, J.; Piepho, H.P.; Honermeier, B.; Perryman, S.; MacDonald, A.; Poulton, P. The effects of cropping sequence, fertilization and straw management on the yield stability of winter wheat (1986–2017) in the Broadbalk Wheat Experiment, Rothamsted, UK. J. Agric. Sci. 2010, 158, 65–79. [Google Scholar] [CrossRef]
- Jaczewska-Kalicka, A. Występowanie i szkodliwość najważniejszych chorób pszenicy ozimej w Polsce centralnej. Prog. Plant Prot. 2002, 42, 93–101. [Google Scholar]
- Deliopoulos, T.; Kettlewell, P.S.; Hare, M.C. Fungal disease suppression by inorganic salts: A review. Crop Protect. 2010, 29, 1059–1075. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Functions of nutrient: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Oxford, UK, 2012; pp. 243–248. [Google Scholar]
- Bergmann, W. Nutritional Disorders of Plants; Verlag Gustav Fisher: Jena, Germany, 1992; 741p. [Google Scholar]
- Taiz, L. Agriculture, plant physiology, and human population growth: Past, present, and future. Theor. Exp. Plant Physiol. 2013, 25, 167–181. [Google Scholar] [CrossRef]
- Papakosta, D.K.; Gagianas, A.A. Nitrogen and dry matter accumulation, remobilization, and losses for Mediterranean wheat during grain filling. Agron. J. 1991, 83, 864–870. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Nowicki, B.; Bełka, D.; Kazimierowicz, A.; Kulwicki, M.; Grzebisz, W. Effect of foliar application of micronutrients and fungicides on the nitrogen use efficiency in winter wheat. Agronomy 2022, 12, 257. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Com. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Trávník, K.; Zbíral, J.; Němec, P. Agrochemical Soil Testing—Mehlich III; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 1999. (In Czech) [Google Scholar]
- Zbíral, J. Determination of plant-available micronutrients by the Mehlich 3 soil extractant—A proposal of critical values. Plant Soil Environ. 2016, 62, 527–531. [Google Scholar] [CrossRef]
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971–2010). Fragm Agron. 2008, 31, 74–87. [Google Scholar]
- Meier, U. BBCH Monograph. In Growth Stages of Mono- and Dicotyledonous Plants, 2nd ed.; Federal Biological Research Center for Agriculture and Forestry: Berlin, Germany, 2001; Available online: http://www.jki.bund.de/fileadmin/dam_uploads/_veroeff/bbch/BBCH-Skala_Englisch.pdf (accessed on 14 November 2021).
- PN-EN ISO 20483:2014-02; Cereal Grains and Pulses—Determination of Nitrogen Content and Conversion to Crude Protein—Kjeldah Method. Polski Komitet Normalizacyjny: Warsaw, Poland, 2015; 24p.
- Bavec, F.; Bavec, M. Chlorophyll meter readings of winter wheat cultivars and grain yield prediction. Comm. Plant Soil Anal. 2022, 32, 2709–2719. [Google Scholar] [CrossRef]
- Duan, J.; Wu, Y.; Zhou, Y.; Ren, X.; Shao, Y.; Feng, W.; Zhu, Y.; Wang, Y.; Guo, T. Grain number response to pre-anthesis dry matter and nitrogen in improving wheat yield in the Huang-Huai Plain. Sci. Rep. 2018, 8, 7126. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, J.F.; Kitchen, N.R.; Raun, W.R.; Schepers, J.S. Responsive in-season nitrogen management for cereals. Comput. Electron. Agric. 2008, 61, 51–62. [Google Scholar] [CrossRef]
- Justus, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelier-Huche, L. Determination of a critical dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Potarzycki, J. Impact of increasing nitrogen rates on the course of the nitrogen critical concentration curve during the vegetative growth of winter wheat. J. Elem. 2014, 19, 549–566. [Google Scholar]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef]
- Mizuta, K.; Araki, H.; Takahashi, T. Shifting time of intensive nitrogen topdressing later to the stem-elongation phase reduced lower internodes and lodging risk of wheat. Plant Prod. Sci. 2020, 23, 427–435. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, D.; Schnurbusch, T. Plant and floret growth at distinct developmental stages during the stem elongation phase in wheat. Front. Plant Sci. 2018, 9, 330. [Google Scholar] [CrossRef]
- Triboi, E.; Triboi-Blondel, A.-M. Productivity and grain or seed composition: A new approach to an old problem—Invited paper. Europ. Agron. J. 2002, 16, 163–186. [Google Scholar] [CrossRef]
- Bogard, M.; Allard, V.; Brancourt-Hulmel, M.; Heumez, E.; Machet, J.M.; Jeuffroy, M.H.; Gate, P.; Martre, P.; Le Gouis, J. Deviation from the grain protein concentratione grain yield negative relationship is highly correlated to post-anthesis N uptake in winter wheat. J. Exp. Bot. 2010, 61, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- Slafer, G.A.; Savin, N.; Sadras, V.O. Course and fine regulation of wheat yield components in response to genotype and environment. Field Crops Res. 2014, 157, 71–83. [Google Scholar] [CrossRef]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kichey, T.; Hirel, B.; Heumez, E.; Dubois, F.; Le Gouis, J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlate with agronomic traits and nitrogen physiological markers. Field Crops Res. 2007, 102, 22–32. [Google Scholar] [CrossRef]
- Heitholt, J.J.; Croy, L.I.; Maness, N.O.; Nguyen, H.T. Nitrogen partitioning in genotypes of winter-wheat differing in grain N-concentration. Field Crops Res. 1990, 23, 133–144. [Google Scholar] [CrossRef]
- Kong, L.G.; Xie, Y.; Hu, L.; Feng, B.; Li, S.D. Remobilization of vegetative nitrogen to developing grain in wheat (Triticum aestivum L.). Field Crops Res. 2016, 196, 134–144. [Google Scholar] [CrossRef]
- Bonnett, G.D.; Incoll, L.D. The potential preanthesis and post-anthesis contributions of stem internodes to grain yield in crops of winter barley. Ann. Bot. 1992, 69, 219–225. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol. 2008, 10 (Suppl. 1), 23–36. [Google Scholar] [CrossRef]


| Soil, cm | pH | Corg % | P | K | Mg | Ca | Cu | Mn | Zn | Fe | Nmin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | kg ha−1 | ||||||||||
| 2013/2014 | |||||||||||
| 0–30 | 6.9 | 1.3 | 234 VH 5 | 231 M | 105 VL | 988 L | 0.4 L | 27.2 L | 3.6 M | 536 H | 86.4 |
| 30–60 | 6.7 | 1.1 | 234 VH | 237 M | 103 VL | 876 L | 0.4 L | 25.7 L | 3.5 M | 541 H | |
| 2014/2015 | |||||||||||
| 0–30 | 7.1 | 2.2 | 185 H | 185 M | 165 M | 2045 M | 3.5 M | 85.5 M | 6.3 H | 268 M | 129.0 |
| 30–60 | 7.2 | 2.1 | 161 H | 157 L | 155 L | 2063 M | 3.5 M | 93.8 M | 5.6 H | 269 M | |
| 2015/2016 | |||||||||||
| 0–30 | 6.6 | 1.6 | 202 VH | 281 M | 165 M | 1480 L | 2.8 M | 61.9 M | 6.1 H | 347 M | 110.0 |
| 30–60 | 6.6 | 1.4 | 139 H | 222 M | 163 L | 1504 L | 2.5 M | 62.0 M | 3.7 M | 231 M | |
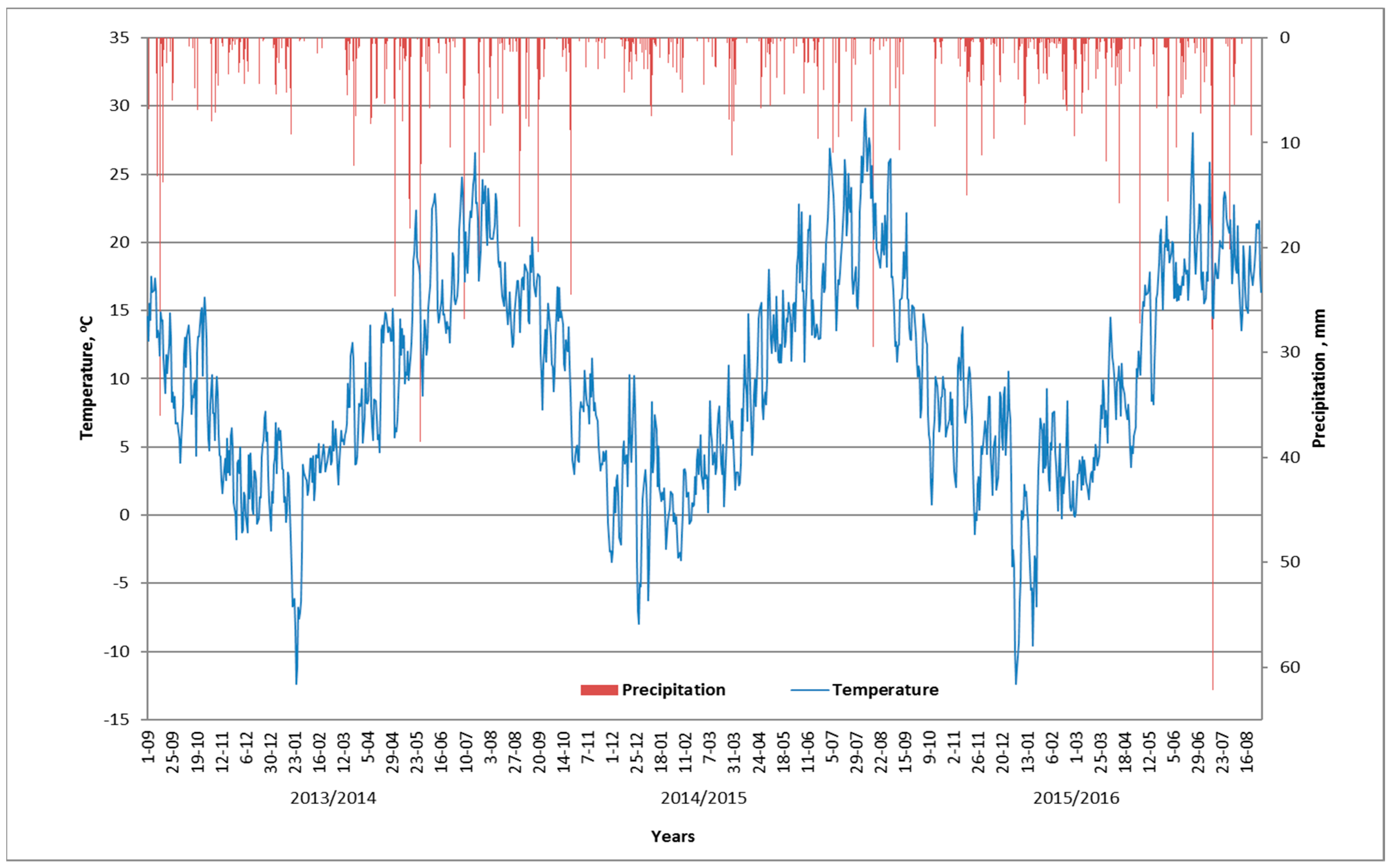
| Growing Season | March | April | May | June | July |
|---|---|---|---|---|---|
| 2013/2014 | 2.0 qw | 1.3 qd | 3.4 ew | 0.6 vd | 1.1 qd |
| 2014/2015 | 2.8 vw | 0.8 d | 0.6 vd | 0.7 vd | 0.7 vd |
| 2015/2016 | 4.3 ew | 1.7 qw | 1.2 qd | 0.7 vd | 2.2 w |
| Stage of Wheat Growth | Microelements | Fungicide |
|---|---|---|
| BBCH 30/31 | Cu + Mn → 60 + 140 g ha−1 | Capalo 337.5 SE → 1.5 dm3 ha−1 |
| BBCH 39 | Cu + Mn + Zn → 15 + 60 + 100 g ha−1 | Adexar Plus → 2 dm3 ha−1 |
| BBCH 65 | - | Osiris 65 EC → 2 dm3 ha−1 |
| Factor | Factor | N31 | N50L | N50S | N65L | N65S | N65E | N65FL | N90L | N90S | N90Ch | N90G | SPAD65 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | |||||||||||||
| Year (Y) | 2014 | 3.2 c | 2.7 b | 1.3 b | 1.5 b | 0.8 b | 1.9 c | 1.9 c | 1.2 a | 0.3 b | 0.5 c | 2.0 c | 552.3 |
| 2015 | 3.8 b | 3.3 a | 1.4 a | 1.4 b | 0.9 a | 3.0 a | 2.5 b | 0.9 b | 0.3 b | 1.0 a | 2.1 b | 553.1 | |
| 2016 | 4.0 a | 3.4 a | 1.4 a | 2.3 a | 0.8 b | 2.1 b | 3.2 a | 1.2 a | 0.5 a | 0.8 b | 2.4 a | 552.4 | |
| p | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | |
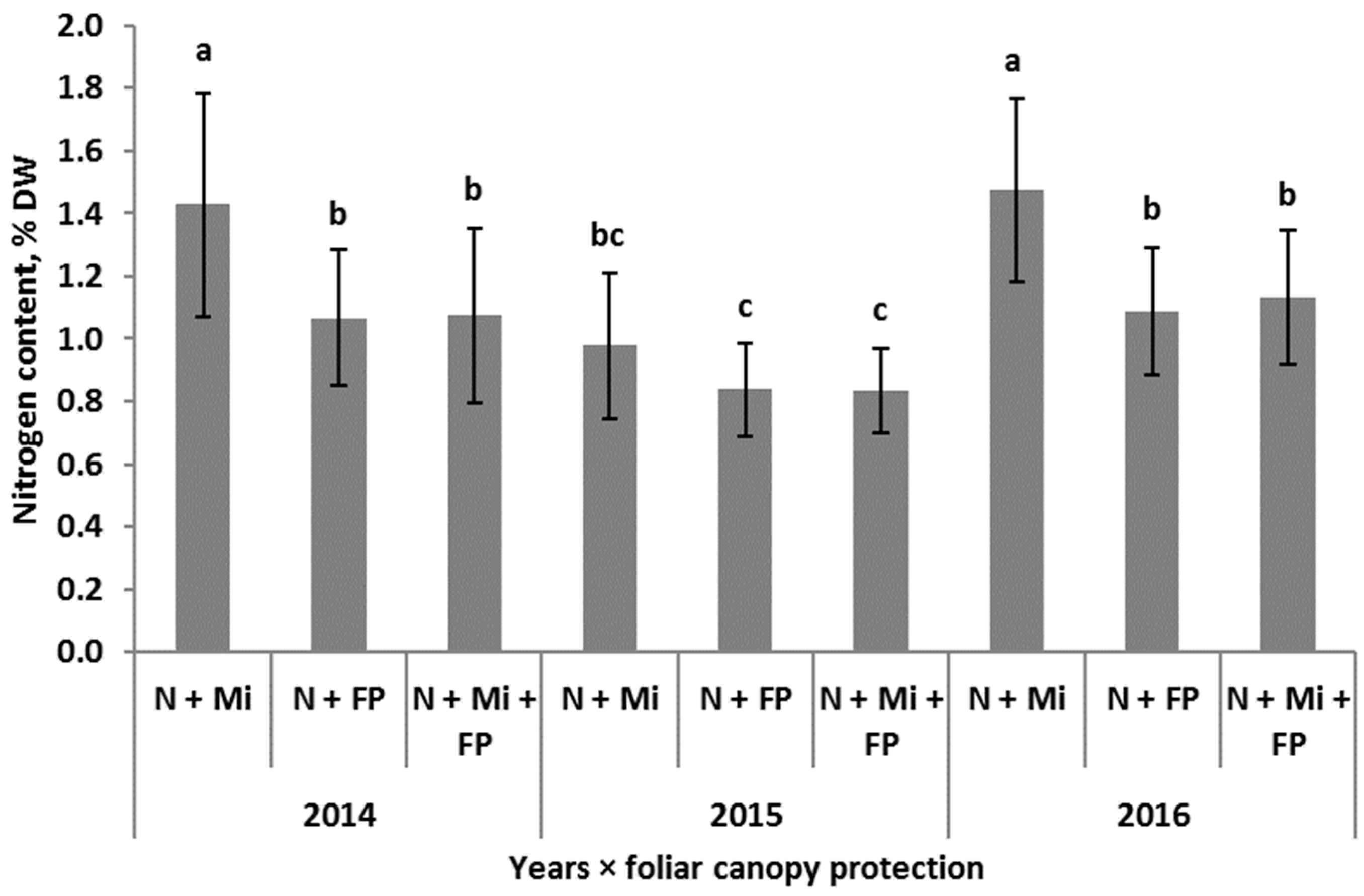
| Foliar canopy protection | N + Mi | 3.5 b | 3.1 | 1.3 | 1.8 | 0.8 b | 2.3 | 2.5 | 1.3 a | 0.4 | 0.8 a | 2.2 | 536.7 c |
| (CFP) | N + P | 3.7 a | 3.2 | 1.4 | 1.8 | 0.9 a | 2.4 | 2.5 | 1.0 b | 0.4 | 0.7 b | 2.1 | 555.6 b |
| N + Mi + P | 3.7 a | 3.1 | 1.4 | 1.7 | 0.8 a | 2.3 | 2.6 | 1.0 b | 0.4 | 0.7 b | 2.1 | 565.6 a | |
| p | *** | ns | ns | ns | *** | ns | ns | *** | ns | ** | ns | *** | |
| Nitrogen rates | 0 | 2.9 b | 2.4 c | 1.1 a | 1.2 d | 0.6 d | 2.1 c | 2.0 d | 0.8 d | 0.3 d | 0.7 d | 1.8 f | 455.2 g |
| (kg N ha−1) | 40 | 3.8 a | 2.8 b | 1.2 ab | 1.4 cd | 0.7 cd | 2.2 c | 2.1 d | 0.9 c | 0.3 cd | 0.7 cd | 1.9 e | 502.9 f |
| 80 | 3.8 a | 3.0 b | 1.3 b | 1.5 c | 0.7 cd | 2.3 bc | 2.2 d | 1.0 bc | 0.4 cd | 0.7 cd | 2.0 f | 526.8 e | |
| 120 | 3.8 ‡,a | 3.3 a | 1.4 a | 1.7 b | 0.8 c | 2.4 ab | 2.6 c | 1.1 b | 0.4 bc | 0.8 bc | 2.1 d | 552.4 d | |
| 160 | 3.8 ‡,a | 3.5 †,a | 1.5 †,a | 2.1 a | 0.9 b | 2.4 ab | 2.8 bc | 1.3 a | 0.4 ab | 0.9 ab | 2.3 c | 594.5 c | |
| 200 | 3.8 ‡,a | 3.5 †,a | 1.5 †,a | 2.1 a | 1.0 a | 2.5 a | 3.0 ab | 1.3 a | 0.4 a | 0.8 a | 2.4 b | 611.6 b | |
| 240 | 3.8 ‡ | 3.5 †,a | 1.5 †,a | 2.2 a | 1.1 a | 2.5 a | 3.2 a | 1.4 a | 0.5 a | 0.9 a | 2.5 a | 625.1 a | |
| p | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Source of variation for interactions | |||||||||||||
| Y × CFP | *** | ns | ns | ns | ns | ns | * | ** | ns | * | ns | ns | |
| Y × N | *** | ns | ns | * | ns | ns | ns | ** | ns | ns | ns | ns | |
| CFP × N | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | *** | |
| Y × CFP × N | ns | ns | ns | ns | ** | ns | ns | ns | ns | ns | ns | ns | |
| Factor | Factor | NaT31 | NaL50 | NaS50 | NaT50 | NaL65 | NaS65 | NaE65 | NaT65 |
|---|---|---|---|---|---|---|---|---|---|
| Level | |||||||||
| Year (Y) | 2014 | 59.1 c | 56.7 c | 79.4 c | 136.1 c | 23.1 c | 51.4 c | 62.7 c | 137.1 c |
| 2015 | 78.7 b | 75.4 b | 102.9 b | 178.3 b | 34.4 b | 94.8 b | 92.6 b | 221.8 b | |
| 2016 | 106.7 a | 92.3 a | 122.2 a | 214.5 a | 58.6 a | 74.4 a | 119.2 a | 252.3 a | |
| p | *** | *** | *** | *** | *** | *** | *** | *** | |
| Foliar protection | N + Mi | 81.5 | 73.2 | 96.4 b | 169.6 | 37.3 | 68.1 b | 86.9 b | 192.4 b |
| (CFP) | N + P | 81.5 | 74.1 | 103.2 ab | 177.3 | 41.2 | 79.4 a | 94.9 a | 215.4 a |
| N + Mi + P | 81.5 | 77.1 | 104.9 a | 182.0 | 37.6 | 73.1 b | 92.7 ab | 203.4 ab | |
| p | ns | ns | * | ns | ns | *** | * | ** | |
| Nitrogen rates | 0 | 49.9 c | 43.8 d | 68.8 d | 112.6 d | 18.0 e | 46.2 e | 64.7 c | 128.8 e |
| (kg N ha−1) | 40 | 77.6 b | 61.7 c | 89.9 c | 151.6 c | 25.6 de | 57.5 de | 82.4 b | 165.5 d |
| 80 | 88.6 ‡,a | 69.7 bc | 94.5 bc | 164.3 bc | 32.4 cd | 66.9 cd | 94.6 ab | 194.0 cd | |
| 120 | 88.6 ‡,a | 78.5 b | 106.7 ab | 185.1 ab | 40.5 bc | 75.3 bc | 100.0 a | 215.8 bc | |
| 160 | 88.6 ‡,a | 90.0 †,a | 116.9 †,a | 206.8 †,a | 47.5 ab | 84.2 ab | 96.7 a | 228.4 ab | |
| 200 | 88.6 ‡,a | 90.0 †,a | 116.9 †,a | 206.8 †,a | 53.8 a | 93.4 a | 102.1 a | 249.3 ab | |
| 240 | 88.6 ‡,a | 90.0 †,a | 116.9 †,a | 206.8 †,a | 53.2 a | 91.1 a | 100.0 a | 244.3 a | |
| p | *** | *** | *** | *** | *** | *** | *** | *** | |
| Source of variation for interactions | |||||||||
| Y × CFP | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y × N | ** | ns | ns | ns | ** | ns | ns | ns | |
| CFP × N | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y × CFP × N | ns | ns | ns | ns | ns | ns | ns | ns | |
| Factor | Factor | NaL90 | NaS90 | NaCH90 | NaVe90 | NaG90 | NaT90 |
|---|---|---|---|---|---|---|---|
| Level | |||||||
| Year (Y) | 2014 | 14.8 b | 20.3 c | 10.1 c | 45.1 b | 179.5 c | 224.6 b |
| 2015 | 15.1 b | 27.6 b | 24.9 b | 67.5 a | 231.0 b | 298.6 a | |
| 2016 | 19.6 a | 32.5 a | 15.5 a | 67.6 a | 164.4 a | 232.0 b | |
| p | *** | *** | *** | *** | *** | *** | |
| Foliar protection | N + Mi | 18.8 a | 26.6 | 17.2 | 62.6 a | 174.4 c | 237.0 b |
| (CFP) | N + P | 15.3 b | 27.9 | 17.2 | 60.4 ab | 196.9 b | 257.3 a |
| N + Mi + P | 15.3 b | 25.9 | 16.0 | 57.2 b | 203.6 a | 260.9 a | |
| p | *** | ns | ns | * | *** | *** | |
| Nitrogen rates | 0 | 8.6 e | 17.7 d | 10.8 c | 37.1 e | 111.1 g | 148.2 g |
| (kg N ha−1) | 40 | 12.8 d | 22.2 cd | 14.5 b | 49.5 d | 147.7 f | 197.2 f |
| 80 | 15.3 cd | 25.0 bc | 16.2 b | 56.6 cd | 169.4 e | 226.0 e | |
| 120 | 16.7 ab | 26.5 bc | 17.3 ab | 60.5 bc | 199.6 d | 260.1 d | |
| 160 | 20.9 a | 29.6 ab | 19.3 a | 69.8 ab | 219.5 c | 289.4 c | |
| 200 | 19.7 a | 32.2 a | 19.4 a | 71.4 a | 239.8 b | 311.2 b | |
| 240 | 21.3 a | 34.5 a | 20.1 a | 75.9 a | 254.3 a | 330.1 a | |
| p | *** | *** | *** | *** | *** | *** | |
| Source of variation for interactions | |||||||
| Y × CFP | ns | ns | ns | ns | ** | ns | |
| Y × N | ns | ns | *** | ns | *** | ** | |
| CFP × N | ns | ns | ns | ns | ** | ns | |
| Y × CFP × N | ns | ns | ns | ns | ns | ns | |
| Factor | Factor | ΔTN50 | ΔTN65 | NRQ | E- NRQ | C- NRQg | NPFU | E- NPFU | C- NPFUg | NRL | NRS | NRCh | E-NRL | E-NRS | E-NRCh |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | kg N ha−1 | % | kg N ha−1 | % | kg N ha−1 | % | |||||||||
| Year (Y) | 2014 | 77.0 b | 1.1 b | 92.0 c | 66.3 b | 51.8 c | 87.5 a | 73.8 a | 48.2 a | 8.3 a | 31.1 a | 52.6 a | 37.8 c | 58.1 b | 83.1 b |
| 2015 | 99.5 a | 43.5 a | 154.2 b | 68.5 b | 69.1 b | 76.8 a | 37.4 b | 30.9 b | 19.4 b | 67.1 c | 68.0 b | 49.1 b | 69.5 a | 72.2 c | |
| 2016 | 107.9 a | 37.7 a | 184.7 a | 72.3 a | 115.4 a | −20.3 b | −5.8 c | −15.4 c | 39.0 c | 41.0 b | 103.8 c | 61.9 a | 54.2 c | 86.6 a | |
| p | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Foliar protection | N+Mi | 88.1 | 22.7 | 129.7 b | 66.3 b | 79.4 | 44.7 | 32.4 | 20.6 | 18.5 a | 41.5 a | 67.7 a | 39.3 | 58.8 | 79.5 |
| (CFP) | N+P | 95.8 | 38.2 | 155.0 a | 70.3 a | 82.1 | 41.9 | 31.3 | 17.9 | 25.9 b | 51.5 b | 77.6 b | 50.6 | 61.3 | 80.8 |
| N+Mi+P | 100.5 | 21.4 | 146.2 a | 70.4 a | 74.6 | 57.5 | 41.6 | 25.4 | 22.4 ab | 47.1 ab | 76.7 b | 48.9 | 61.7 | 81.7 | |
| p | ns | ns | *** | ** | ns | ns | ns | ns | *** | *** | *** | ** | ns | ns | |
| Nitrogen rates | 0 | 62.7 c | 16.1 | 91.7 d | 69.8 | 83.5 | 19.4 c | 28.3 | 16.5 | 9.4 a | 28.4 a | 53.9 a | 35.4 d | 58.9 | 82.1 |
| (kg N ha−1) | 40 | 74.0 bc | 13.9 | 116.0 cd | 68.1 | 82.9 | 31.7 bc | 37.8 | 17.1 | 12.8 a | 35.3 ab | 67.8 b | 39.1 cd | 58.2 | 80.6 |
| 80 | 75.7 bc | 29.7 | 137.4 bc | 69.7 | 84.6 | 32.0 bc | 29.1 | 15.4 | 17.1 ab | 41.9 bc | 78.4 bc | 40.5 c | 60.2 | 82.1 | |
| 120 | 96.6 ab | 30.7 | 155.3 ab | 70.8 | 80.9 | 44.2 bc | 31.5 | 19.1 | 23.8 bc | 41.8 cd | 82.7 bc | 50.8 ab | 62.9 | 82.0 | |
| 160 | 118.2 ‡,a | 21.6 | 158.5 ab | 67.6 | 74.3 | 61.0 ab | 38.5 | 25.7 | 26.5 b–d | 54.6 de | 77.4 bc | 51.0 ab | 61.5 | 78.7 | |
| 200 | 118.2 ‡,a | 42.5 | 178.0 a | 69.9 | 76.5 | 61.9 ab | 35.9 | 23.5 | 34.0 cd | 61.2 de | 82.7 c | 55.7 a | 63.3 | 80.1 | |
| 240 | 118.2 ‡,a | 37.5 | 168.4 a | 67.3 | 68.5 | 85.8 a | 44.8 | 31.5 | 31.9 d | 79.9 e | 79.9 c | 51.3 b | 59.1 | 79.0 | |
| p | *** | ns | *** | ns | ns | *** | ns | ns | *** | *** | *** | *** | ns | ns | |
| Source of variation for interactions | |||||||||||||||
| Y × CFP | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y × N | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | ns | ns | ns | ns | |
| CFP × N | ns | ns | ns | ** | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| Y × CFP × N | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepaniak, W.; Grzebisz, W.; Potarzycki, J. Yield Predictive Worth of Pre-Flowering and Post-Flowering Indicators of Nitrogen Economy in High Yielding Winter Wheat. Agronomy 2023, 13, 122. https://doi.org/10.3390/agronomy13010122
Szczepaniak W, Grzebisz W, Potarzycki J. Yield Predictive Worth of Pre-Flowering and Post-Flowering Indicators of Nitrogen Economy in High Yielding Winter Wheat. Agronomy. 2023; 13(1):122. https://doi.org/10.3390/agronomy13010122
Chicago/Turabian StyleSzczepaniak, Witold, Witold Grzebisz, and Jarosław Potarzycki. 2023. "Yield Predictive Worth of Pre-Flowering and Post-Flowering Indicators of Nitrogen Economy in High Yielding Winter Wheat" Agronomy 13, no. 1: 122. https://doi.org/10.3390/agronomy13010122
APA StyleSzczepaniak, W., Grzebisz, W., & Potarzycki, J. (2023). Yield Predictive Worth of Pre-Flowering and Post-Flowering Indicators of Nitrogen Economy in High Yielding Winter Wheat. Agronomy, 13(1), 122. https://doi.org/10.3390/agronomy13010122






