1. Introduction
Creeping perennial weeds are clonal plants with subterranean reproductive organs. They frequently occur in arable cropping [
1]. While annual weeds exclusively germinate from seeds stored in seed banks and emerge as seedlings, creeping perennials additionally sprout from vegetative sources. The ramets are root or rhizome fragments of creeping perennials. As vegetative offspring, these ramets are genetically identical to the mother plant. Besides seeds, ramets are distributing units of creeping perennials [
2]. The dispersal of clonal plants via ramets is a wide-spread option in various types of ecosystems. Like seeds, these ramets can be further distributed or remain close to the mother plant. In arable cropping, ramets often result from cultivation with cutting tools [
1].
Soil tillage tools such as mouldboard ploughs or chisel ploughs reach depths of 10 up to 30 cm in the soil and can destroy the subterranean organs of creeping perennials. Shallow working cultivators or disc harrows as well as PTO-driven equipment such as rotary hoes or rotary cultivators pull out and cut root or rhizome fragments closer to the surface. Together, these tools produce fragments, which survive as ramets and can establish new plants.
On arable fields carrying combined crops such as cereals or oil seed rape, perennial weeds are cut above ground at harvest. Especially stubble cultivation after harvest, will affect the below-ground parts of perennial weeds and may cut the creeping roots or rhizomes into smaller fragments, hence producing ramets. The ability of these ramets to sprout and develop shoots in their early growth will determine their success in establishing plants. Close to the surface and under dry conditions in full summer, ramet sprouting success seems limited, while under wet conditions, ramet sprouting is very likely to be successful [
3].
Cultivation including ploughing will take place in autumn in Central and Northern Europe to prepare the ground for planting winter crops or summer crops in the cooler Nordic climate. Despite the time in the year, soil cultivation to prepare a seedbed for the next crop can kill the young sprouts but may also transfer unsprouted ramets to places in the soil that are more favorable for sprouting. The sprout production of these ramets in late autumn is the focus of this paper.
Elymus repens (L.) Gould,
Cirsium arvense (L.) Scop. and
Sonchus arvensis L. are important arable creeping perennial species in temperate regions of Europe [
4,
5,
6,
7,
8]. The management of these weeds is challenging in organic farming as well as in conventional farming. While the monocot species
E. repens uses rhizomes the two dicot species produce horizontal adventitious roots as vegetative organs. All three species are known to be able to sprout from ramets [
1,
9,
10,
11].
As ramets are vegetative offspring from a continuously growing plant, the conditions under which the mother plant produced the ramets may influence the sprouting success. In autumn, the mother plants wither, probably due to decreasing day length, lower temperature or frost stopping the above-ground plant growth or other stress factors and plant characteristics [
12].
We carried out a pot experiment with ramets of
E. repens,
C. arvense and
S. arvensis to investigate the effect of temperature on the sprouting and early growth of ramets. Before this ramet experiment, the mother plants either were cultivated under ambient climatic conditions of Southern Norway in two periods [
13] or experienced climate change treatments with elevated temperature and CO
2 in autumn [
14]. The ramets were obtained on two dates in autumn and exposed to three different temperature regimes. We analyzed sprouting and early growth as influenced by temperature and previous conditions. We expected that exposing the ramets to higher temperature would result in more sprouting and better growth of the sprouts.
We hypothesized that (1) the examined species would react differently, (2) nevertheless, species-specific ramet sprouting and sprout growth would be favored by higher temperatures, and (3) the preconditions of the mother plant growth (plant age, climate change, time in autumn) would affect ramet sprouting.
2. Materials and Methods
The sprouting ability of rhizomes of
E. repens and creeping roots of
C. arvense and
S. arvensis was investigated on plant material from two previous experiments. In these previous experiments, all plants were cultivated in containers at Særheim, Norway (58°47′ N, 5°41′ E). Both previous experiments were finished late October 2005. The experiment described here relied on plant material from: (1) the experiment “Plant Age” (PA), which included plant material with different lengths of a pre-growth period [
13] and (2) the experiment ”Climate Change” (CC) that included simulated climate change treatments [
14].
2.1. Preparing the Plant Material
The experiment described here started by recruiting root/rhizome fragments at Særheim, Norway. The roots/rhizomes were harvested in two occasions: 3–4 October and 31 October–1 November 2005. The roots/rhizomes of the plants from the experimental containers were washed to eliminate the remaining soil and then weighed and measured. A fraction of 1 m of the roots with at least 3 mm diameter was obtained from the mother plants of C. arvense and S. arvensis, while from E. repens a 2 m fraction of the rhizomes was obtained. These roots/rhizomes were wrapped in moist cellulose paper, packed in plastic bags in Styrofoam boxes with a lid and stored at a cool temperature (+4 °C) at Særheim for 0–2 days. On both collecting dates, the roots/rhizomes were taken from their pots in Særheim and transported to Ås, Norway (59°40′ N, 10°46′ E). During transport by plane/car, the ramets were also stored in Styrofoam boxes to ensure cool conditions.
2.2. Experiments
The sprouting experiments were carried out in Ås. The roots/rhizomes were cut into ramets of 5 cm (C. arvense, S. arvensis) or 2 nodes (E. repens), and three ramets were planted in pots with a 12 cm upper diameter at a 1.5–2 cm soil depth. Since the plant material of E. repens was scarce, 1–2 fragments were planted into about half of the pots. The soil was a mixture of sand, clay and peat, amended with balanced nutrients (LOG Gartnerjord, Tjerbo Torvfabrikk, Rakkestad, Norway). The photosynthetic photon flux density was 150–180 µmol m−2 s−1 12 h per day in the growth chambers.
The prepared pots were placed in growth chambers for six weeks. In the sprouting experiments, the factor “Temperature” was tested at three levels. The temperature in “cold” chambers was 4–6 °C, that in “medium” chambers was 8–10 °C and that in “warm” chambers was 12–14 °C. Ranges of temperatures are indicated, as small deviations occurred within them. Growth chamber growth at different temperatures started in all pots at the beginning of either October or November, which represent the two factor levels of the “Test Period”. One growth chamber each carried the pots at the cold or warm temperature level, but, due to a lack of space, four chambers were used for the medium temperature. Within the growth chambers, the pots were rotated twice a week to ensure equal conditions of light and temperature. Water was given as needed during the experimental period.
The root/rhizome fragments harvested on 3–4 October or 31 October–1 November 2005 represent the factor “Test Period” (TP). TP 1 started in early October, and TP 2 in early November. In fact, the factor TP carried two combined influences: the mother plants in TP 2 grew one month longer, and the sprouting test of the ramets started one month later in the year.
Another joint experimental factor, “Origin”, resulted from the previous experiments. For both previous experiments, ecotypes of all three species were obtained from two origins. The mother plants of these origins grew at different latitudes in Norway (59 and 63° N).
The treatments in both previous experiments that provided the plant material were replicated three times, and each replicate grew in a separate pot. The plant material from each pot was used in one replicate of the sprouting test. The three replicates of the sprouting test were each cultivated in a separate pot.
While both previous experiments shared the four factors “Temperature”, “Test Period”, “Origin” and “Replicate”, two factors were specific for one of each experiment.
The previous experiment “Climate Change” investigated the effects of different climate conditions [
14]. The roots/rhizome fragments resulted from plants that had experienced five climate treatments in September and October 2005. The plants grew either in open-top chambers or in the field as a control. Treatments in the open-top chambers consisted in elevating the temperature by 2–2.5 °C (factor level T+), the CO
2–concentration (augmented from 370 ppm to 550 ppm) (C+), or both (C+T+). An ambient control was included in the open-top chambers (AM-O), along with a field control in the outdoor conditions (AM-F). The temperature in the open-top chamber and field control ranged from approx. 15 °C at the start of the experiment to 8–10 °C at the end of the experiment on 1 November. All ramets were obtained from young plants (“Plant Age” = 2 months on 1 September 2005, Tørresen et al. [
14]).
The ramets from the previous experiment “Plant Age” resulted from plants which experienced two pre-growth periods before the main experimental period [
13], but no climate change treatments. This pre-growth period was either 3 months (old plants) or 2 months (young plants) at the start of the previous experiment (1 September 2005). Plants of both “Plant Age” levels previously grew under outdoor field control conditions (AM-F) [
13].
2.3. Assessments
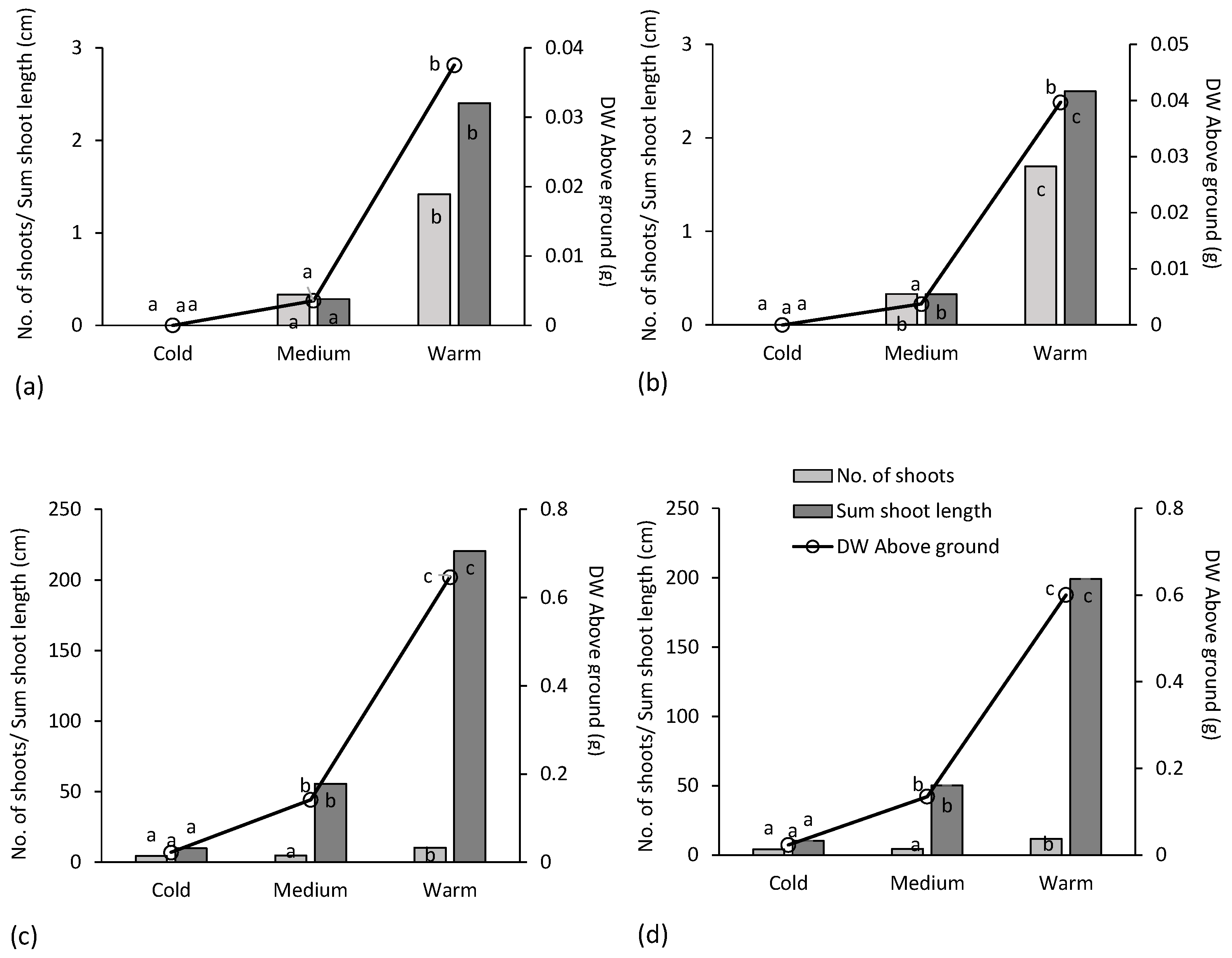
Three and six weeks after the set-up of the experiments, the number of shoots and the total length of all shoots were non-destructively measured. After six weeks, at the end of the experiment, the above-ground dry weight (DW) biomass, was destructively measured. The dry weight was assessed after drying at 60 °C for at least 48 h. Pots with less than 3 planted rhizome or root fragments were converted to values per 3 fragments.
2.4. Statistical Analyses
The three perennial weed species E. repens, S. arvensis and C. arvense are botanically different, and the observed differences were very strong; thus, we analyzed them separately. Two set of analyses were performed with the following main factors:
- (1)
“Plant Age” analysis: Plant Age (2 levels), Test Period (2 levels), Temperature (3 levels), Origin (2 levels)
- (2)
“Climate Change” analysis: Climate Change (5 levels), Test Period (2 levels), Temperature (3 levels), Origin (2 levels)
Two- and three-factor interactions were included in the analyses. The factor Replicate in both set of analyses and the interaction Replicate x Climate Change in the “Climate Change” analyses were considered as random effects. Other factors were fixed.
The dependent variables in the analyses were the number of shoots, the sum of the shoot lengths and the dry weight of the above-ground parts after 6 weeks in the growth chambers (
Table 1). Temporal repeated measures of the number of shoots and the sum of shoot lengths performed through non-destructive assessments after 3 weeks in the growth chambers were compared to the values measured after 6 weeks. Mixed models were used to analyze the data of the final assessments after 6 weeks.
Diagnostic plots of residuals from each model were used to decide if the dependent variable had to be transformed to achieve a dependent variable being approximately normally distributed with homogeneous variance. The sum of the shoot length data of the species and the DW of the above-ground parts of E. repens from both previous experiments were natural logarithm-transformed. As some values of the dependent variable were equal to zero, the constant 1 was added to each value (ln(x + 1)).
Significant effects or interactions in the mixed model were tested (Tukey–Kramer) to detect significant differences between the factor levels. Effects, interactions and differences were considered significant if p ≤ 0.05.
When a significant influence of the factor Climate Change was indicated by the mixed model, the contrasts were tested with an approximated t-test to determine if they could be claimed to be different from zero [
15]. To test for the main effect of elevated CO
2, the contrast C = [(AM-O) + (T+)] − [(C+) + (C+T+)] was used, whereas for the main effect of elevated temperature, the contrast T = [(AM-O) + (C+)] − [(T+) + (C+T+)] was used, and for the interaction CO
2 x temperature, the contrast CT = [(AM-O) + (C+T+)] − [(C+) + (T+)]) (interpreted as a synergistic effect if positive) was used (
Table 1).
All analyses were performed with the procedure ‘proc mixed’ in SAS [
15].
4. Discussion
The experiments included three different species. We hypothesized that each of them reacted differently in terms of sprouting capacity in autumn. The differences were overwhelming, as one species did not sprout in the test periods.
The ramets of
C. arvense sprouted in both test periods; however, the later in autumn they were tested, the lower their sprouting ability.
Cirsium arvense first produced sprouts (assessment after 3 weeks) and then they grew in length (assessment after 6 weeks) (
Table 2). A higher temperature resulted in more sprouts and better growth of the sprouts.
Cirsium arvense required at least a medium temperature (8–10 °C), as the ramets did not sprout at a cold temperature (4–6 °C).
The conditions under which the mother plants of the ramets were grown hardly influenced the sprouting ability of C. arvense. If the mother plants had more time to grow (factor “Plant Age” in the experiment), the sprouting ability was not better than that after a shorter growth time. In our experiments, we used one size of root fragments as ramets. Hence, we can only conclude that ramets of the same size did not profit as concerns their sprouting ability. Clearly, more roots in the field yield more ramets of a certain size. If a mother plant produces more (longer) creeping roots in a longer growing period, this will probably increase the number and the size of the roots, which could then be fragmented into ramets by soil cultivation treatments.
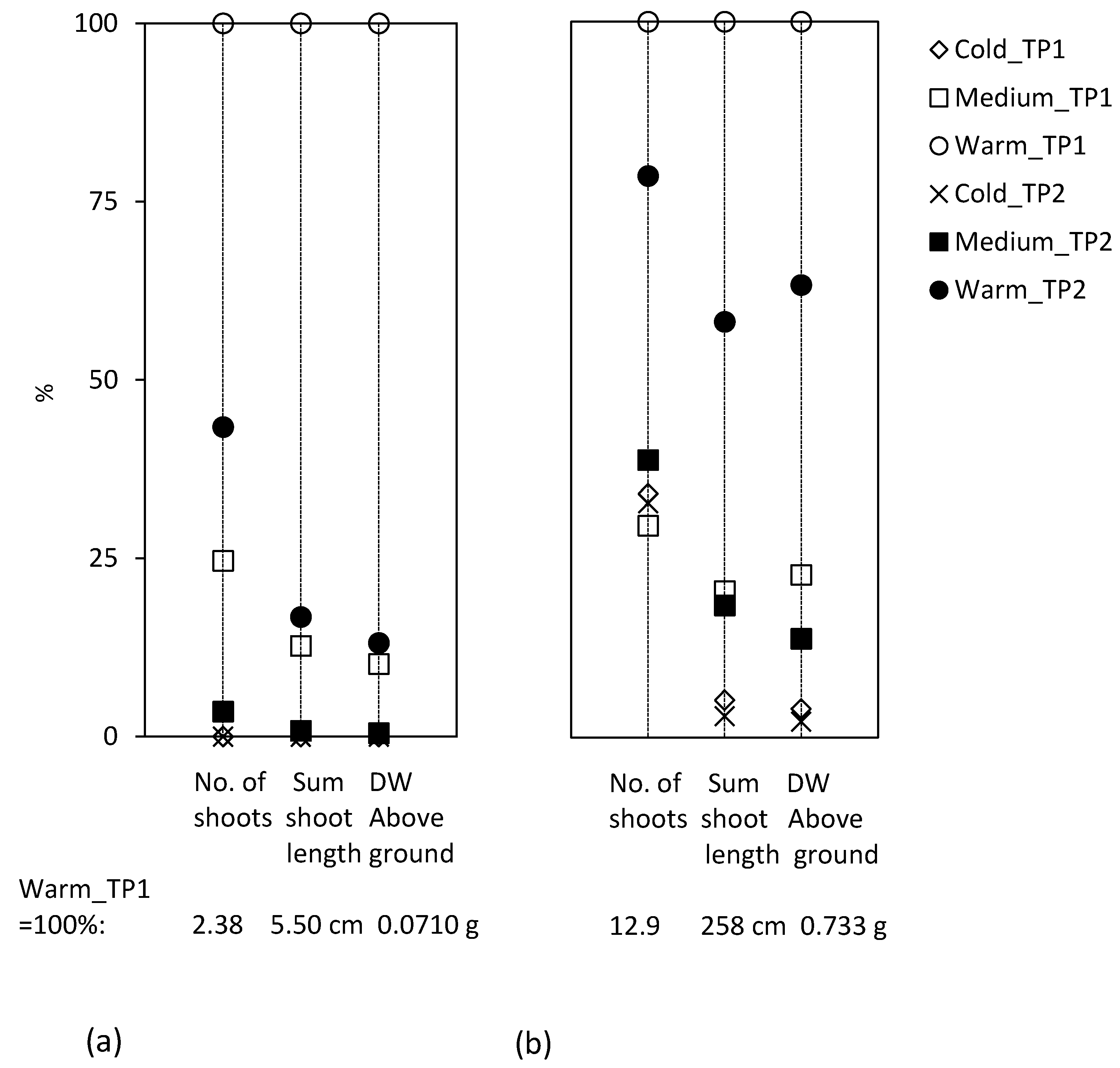
When the mother plants grew under simulated climate change conditions, the sprouting capacity of the ramets was higher in the first test period than in the second period (
Figure 2). In the first test period, only the increase of temperature during the mother plants’ growth resulted in significantly more biomass of the ramet sprouts. For ramets from mother plants grown under increased temperature together with elevated CO
2, this effect was not significant.
A lower sprouting ability in the test period 2 than in the test period 1 could be caused by respiration starving the food resources in the ramets, as
C. arvense withered gradually during autumn with reduced production of photosynthesis products [
13].
With respect to the future climate conditions in autumn, it is evident that the sprouting of
C. arvense ramets will profit if the temperature rises. If the autumn is wet enough, these sprouts will survive with high success. According to Niederstrasser and Gerowitt [
3], only very dry conditions would strongly limit them. Under European Nordic conditions, warmer and wet autumns is a probable scenario.
Cirsium arvense plants also have a deep root system below the plough layer which will not be influenced by soil cultivation and could be difficult to manage [
16].
Tørresen et al. [
14] investigated the growing success of the mother plants and found that
C. arvense profited mainly in above-ground biomass production from climate change conditions, while the below-ground variables were hardly changed. Hence, the climate change scenarios induced no quantitative effect in the roots. Our results showed a small quality effect observed in the sprouting performance, but only at increased temperature during the growth of the mother plant.
This study investigated the sprouting of vegetatively produced
C. arvensis ramets. Experiments in growth chambers allowed focusing on the temperature under controlled environment conditions. To what extent vegetative spread adds to the distribution under arable conditions in general is still under discussion. In general, research and review papers attribute great importance to the vegetative spread when considering
C. arvensis [
11,
17,
18]. However, Hettwer and Gerowitt [
19] and Bommarco et al. [
20] investigated the genetic diversity in arable fields and found it surprisingly high. This result does not contradict the importance of dispersal via ramets, as genetic variation in perennial species occurs even if the spread by seedlings is low compared to vegetative propagation [
2]. Competition for light is well known from the literature to limit the success of
C. arvense [
11,
21]. Therefore, carefully treating the soil for a seedbed as early as possible in the summer and establishing a crop with a competitive autumn canopy—either a fast-closing cash crop or a cover crop—seems to be a good strategy to suppress the late-autumn sprouting of
C. arvense. Our results support the conclusion that the ramets of
C. arvense will sprout as long as the temperature is high enough in autumn. Avoiding bare soil as far as possible seems a better option to reduce their sprouting than destroying the sprouted ramets late in autumn, because the plants of
C. arvense quickly establish their creeping root system.
The ramets of E. repens produced many sprouts, more after 6 than after 3 weeks. These sprouts developed a high total length the longer they could grow (two times the shoot length at 6 weeks compared to 3 weeks). This indicated that E. repens had much more biomass after 6 weeks than after 3 weeks in the growth chambers. This was similar to the sprouts of C. arvense.
Elymus repens produced more sprouts at higher temperatures, but unlike
C. arvense, the ramets also sprouted at low temperatures (4–6 °C). Moreover,
E. repens produced rather longer sprouts than more sprouts with higher temperatures. The time in the autumn (factor Test Period) hardly affected
E. repens sprouting behavior. The reason for the difference in temperature requirement for sprouting between
C. arvense and
E. repens is unknown. We speculate that it could be related to differences in how their major metabolic processes respond to temperature in autumn [
11] and to differences in how they prepare for winter (e.g., accumulation of non-structural carbohydrates [
11,
22,
23]).
A species such as E. repens that has leaves during the whole autumn will probably contain constantly more energy in the rhizomes than a species that withers in autumn, such as C. arvense. If the leaves and the above-ground parts wither, the process of respiration will use resources in the roots and thus decrease the sprouting ability and the early growth. Consequently, there would be less photosynthetic active leaves to fill up the resources in the creeping organs again. For both C. arvense and E. repens, less sprouting was seen in the test period 2 than in 1 (number of shoots for C. arvense)/early growth as shoot length and DW Above ground for C. arvense and E. repens).
For
E. repens, no effect of the climate change factors C+ and T+ during the mother plants’ growth was evident (
Figure 3b). A reason could be that in this species, increased growth was apparent in relation to the length of the rhizomes rather than to the thickness, energy level or carbohydrate content in the rhizomes. Our study detected hardly any effect of plant age on the sprouting of
E. repens ramets. The reason for no or very little effect could be that the rhizomes were already well developed. However, more and longer rhizomes were found for older mother plants (factor plant age [
13]) and climate change [
14].
These results showed that the ramets of
E. repens were active the whole autumn and sprouting continued over time. The ramets can sprout and grow as soon as the temperature is above 5–6 °C [
24]. This means that
E. repens is easy to control with stubble cultivation, as it sprouts readily, and it is possible to starve the rhizomes with repeated stubble cultivations followed by ploughing. This is shown by numerous studies [
1,
25]. In conventional farming, this species can also be treated relatively late with glyphosate due to its active growth late in the autumn.
The ramets of S. arvensis did not sprout at all. We consider two possible reasons for this result: (1) unfavorable test conditions and (2) internal characteristics of the ramets.
The tests were carried out under typical Nordic autumn environmental conditions, with day length of 12 h and highest temperatures of 12–14 °C. It could be the day length was too short, and/or the temperature was too low for
S. arvensis to sprout. Other sprouting studies conducted under test conditions more typical for spring/early summer with longer days (16–18 h) and warmer temperatures (18–25 °C during daytime) confirmed its sprouting ability [
17,
26]. For the other two species investigated, we trust that we tracked their sprouting capacity, as our experimental method resulted in quantities of sprouting comparable to those of other studies under controlled conditions in autumn (
C. arvense: [
17,
27,
28],
E. repens: [
17,
29]).
The internal characteristics can be related to the physiological status of the ramets. As seeds of annual weed species, the ramets may not always sprout. Besides that non-sprouting can result from unfavorable environmental conditions, it can result from internal restrictions. Similar to seeds of annuals, this phenomenon is often called dormancy (innate dormancy according to Håkansson [
1], endodormancy (within the bud) and paradormancy (outside the bud, but in the plant) according to Lang et al. [
30] and Ott et al. [
2]). For the two dicot species investigated here, dormancy in the ramets has been reported to varying degrees [
17,
26,
27,
28,
31].
Under Nordic conditions Håkansson [
32], Håkansson and Wallgren [
26], Fykse [
27], Brandsæter et al. [
17], Andersson et al. [
33] and Liew et al. [
31] reported innate dormancy/endodormancy in the roots for
S. arvensis to occur at the end of the growing season, from August to October. Based on these previous studies, we expected that
S. arvensis might show sprouting restrictions in late September and beginning of October, but not in late October. It is suggested that innate dormancy is enhanced by a short daylength, especially in combination with high temperatures, and is released or broken during periods of cold [
26,
27,
34,
35]. Thus, the temperatures during the growth of the mother plants were, in our study, too high to release dormancy (the mean air temperature in the last two weeks of October was 9.5 °C). The maritime climate conditions in South-West Norway (Særheim) were probably more similar to the UK conditions under which Henson [
36] (referred by Håkansson and Wallgren [
26]) reported reduced sprouting ability as late as November. We cannot finally decide whether a methodological experimental reason or the physiological status of the ramets in relation to the environmental conditions caused no sprouting in
S. arvensis. However, it is most probable that the temperature prior to the test had been too warm to break dormancy. We only used fresh, unshrivelled ramets in the test, which we claim were viable. However, in parallel with the germination tests of seeds from annuals, any final statement regarding dormancy requires distinguishing between viable and non-viable ramets.
We have deduced a methodological and a practical recommendation from the non-successful sprouting of
S. arvensis. Methodologically, we recommend widening the tested temperatures, ensuring a cold growth period of the mother plants prior to ramet collection and performing this type of experiments even later than November. For practical control in arable farming, it seems difficult to stimulate the autumn sprouting of
S. arvensis and use this as a strategy to starve the creeping roots. Dormancy in the ramets increases the risk of reaching the opposite than expected with any intervention. Dormancy is a protecting mechanism for the species, preventing the plants from sprouting under favorable conditions at the wrong time of the year (in autumn) thus avoiding the risk of frost damage later in winter. However, the ramets will sprout in spring as soon at the temperature allows it [
37] when innate dormancy is broken by a cold temperature during late autumn/winter [
35]. An advantage could be that short ramets in autumn may have less energy to sprout/start growing in spring, produce less biomass per plant and thus be more affected by crop competition than longer ramets [
38,
39,
40]. However, there is also a risk that cutting the creeping roots in autumn may increase the abundance of
S. arvensis in spring the next year.
Summing up, the species’ reactions were strongly different, with S. arvensis not sprouting at all, and both C. arvensis and E. repens sprouting. Cirsium arvense required a higher temperature to sprout than E. repens, but both species will profit, as concerns ramet sprouting, from warmer autumn conditions. E. repens is easier to control with stubble cultivation than C. arvensis. For S. arvensis, control strategies in spring and summer will probably be more effective than in autumn, as its sprouting ability might be higher in spring/summer.
We tested the sprouting ability and early growth in conditions typical for a Nordic autumn climate. We recommend testing the three species at a wider range of temperatures to reveal their full temperature requirements in autumn. If researchers repeat our experiments, we also recommend performing a second check of all not sprouted ramets looking viable at additional temperature and daylength well-known to favor sprouting.