Abstract
This study aimed to establish a strategy for drought-tolerant selection in Arabica coffee genotypes in Brazil, combining field trials under natural conditions with controlled growth condition experiments. A group of genotypes was evaluated in the greenhouse using 28 morphological, anatomical, and physiological traits. In addition, the productivity of genotypes was evaluated in the field. Under water deficit in the greenhouse, 13 studied traits showed significant genetic variance. For these traits, the heritability coefficients estimated based on the progeny mean ranged from 65 to 92%. A selection index combined with predicted means was applied at these traits to select four progenies for drought tolerance under greenhouse conditions. Under field conditions, the selection applied to genetic values that favored positive genetic gains in four progenies, indicating the potential use of these cultivars for breeding advanced generations. The selection results of coffee plants under greenhouse conditions matched the selection results in the field in a water-deficit region by 50% based on productivity; thus, two genotypes (H419-3-3-7-16-11 and H516-2-1-1-7-2) were identified that have both the productivity and morpho-anatomical traits that confer greater drought tolerance. Finally, we discussed possible strategies for the development of drought-tolerant cultivars.
1. Introduction
The importance of coffee for the Brazilian and global economies is undeniable. Coffee is one of the most traded commodities in the world. However, in most arable areas, coffee plants are often exposed to various types of environmental stress during their life cycle, such as drought periods [1]. Among the environmental factors that negatively impact coffee growth and productivity, drought is particularly important because of its deleterious effects and its frequent occurrence in cultivation areas worldwide [2]. It is estimated that drought is responsible for approximately half of the annual losses in agricultural production, exceeding the damage caused by all other stressors combined [3].
In Brazil, the largest coffee producer in the world, all coffee-producing regions are affected by heat conditions combined with intermittent droughts. The greatest losses are observed in new coffee plantations, where younger plants face problems because their root systems are not fully established, which affects water absorption. This situation drastically reduces coffee production and leads to an increase in the price of coffee in the global market. Thus, coffee agribusiness was identified as one of the most vulnerable sectors to climate change in Brazilian agriculture due to the high sensitivity of coffee production to variations in temperature and rainfall, in addition to the money generated from this activity [4].
The development of more drought-tolerant cultivars, as well as the development of technologies that help plants tolerate prolonged periods of drought, is essential for the maintenance of global agricultural production [5]. In recent years, plants tolerant to water stress have been the subject of intense research [6,7,8,9,10,11]. Many public-sector plant breeding programs in both developed and developing countries have worked to maximize productivity in coffee cultivars that combine drinking quality, drought-tolerance and durable host resistance to cope with the changing climate and demands of consumer markets more efficiently.
In Brazil, many institutions have been working for several years on coffee tree drought adaptation. In collaboration with France, experiments set up in the semi-desert Cerrado region are geared towards controlling the duration of the drought period using central-pivot irrigation [12]. Currently, Arabica coffee cultivars such as Acauã [13], Siriema [14], Catuai SH3 [15] and IPR 103 [16], in addition to clonal cultivars of Coffea canephora [17], minimize the negative effects of drought. Similarly, researchers from Yemen and, later, East Africa produced arabica coffee cultivars (e.g., SL28 in Kenya) with relatively higher drought tolerance when compared to the original Ethiopian germplasm [18]. The Central Coffee Research Institute (CCRI), India, has developed improved arabica, Coffea racemosa and robusta selections for commercial cultivation and are currently under evaluation for drought tolerance [19].
To date, the genetic improvement program of Minas Gerais, the world’s largest coffee producer, works by associating field data with data obtained in a greenhouse to select genotypes with higher productivity coupled with physiological phenotyping to obtain high performance genotypes with multiple characteristics of interest [11]. This complementary approach is very important to assess the performance of genotypes in environments with limited water availability for selection purposes. From this perspective, two components should be considered, the genotypic variability and the heritability of the traits used, with the goal of evaluating the effects of selection on these traits.
Thus, this study aimed to establish a strategy for drought-tolerant selection in Arabica coffee genotypes in Brazil, combining field trials under natural conditions with controlled growth condition experiments. We estimated genetic parameters for 28 traits that provide information on the possibility of genetic gain and help with decision-making in breeding practices. Subsequently, field evaluations were used to validate the selection methodology and determine whether the method was accurate. We also investigated genotypic correlations between these traits as knowledge of these correlations can assist in the design of efficient breeding programs aimed at selecting more drought-tolerant genotypes. The early selection of genotypes in the initial seedling stage can save time and resources that would be lost during the analysis of genotypes with lower performance [20]. The success of this strategy depends on the existence of genetic variation in the relevant traits. Through this strategy, we identified genotypes that have both the ability to produce and morpho-anatomical traits that confer greater drought tolerance. The possibility of generating coffee plants that are better adapted to limited water availability makes this research very relevant.
2. Materials and Methods
2.1. Plant Materials
In the breeding program of the Agricultural Research Corporation of Minas Gerais (EPAMIG), crosses between commercial cultivars of Catuaí and Híbrido de Timor were carried out, aiming to unite the agronomic characteristics of Catuaí and the resistance to rust of the Híbrido de Timor. After the fourth genealogical selection cycle, seedlings (F5 generation) were formed from the best progenies for conducing in Experiment 1. Genealogical selection is a plant breeding method based on the individual selection of plants in the segregating population with the assessment of each progeny separately.
The progenies evaluated in Experiment 1 and Experiment 2 were: H419-3-3-7-16-2 (genotype 1); H419-3-3-7-16-11 (genotype 2); H419-3-4-4-13 (genotype 3), H419-5-2-4-18 (genotype 4), H419-5-4-5-6-1 (genotype 5); H419-6-2-4-2-2 (genotype 6); H419-6-2-7-1-1 (genotype 7); H516-2-1-1-7-1 (genotype 8); H516-2-1-1-12-1 (genotype 9) and H516-2-1-1-14-3 (genotype 10). Genotypes 1, 2, 3, 4, 5, 6 and 7 originated from the cross between Catuaí Amarelo IAC 30 x Timor Hybrid UFV 445-46; genotypes 8, 9 and 10 originated from the cross between Catuaí Amarelo IAC 86 x Timor Hybrid UFV 446-08.
2.2. Experiment 1: Evaluation of Coffee Progenies in Field Conditions in a Region with a Long and Defined Dry Season
2.2.1. Productivity Analysis
This study was conducted on private property in Tourmaline (lat 20°05′ S, long 50°47′ W and alt 820 m above sea level), located in the Jequitinhonha Valley, MG, Brazil. The region is characterized by a water deficit in terms of coffee production, with a long and defined dry season from March to October. Ten coffee progenies and the Catuaí Vermelho IAC 99 and Catuaí Vermelho IAC 144 cultivars were evaluated for productivity (PROD) in the first five harvests between May and July of each year: first (H1), second (H2), third (H3), fourth (H4) and fifth harvest (H5). The harvest was carried out in individual plots, and the volume of field coffee (coffee fruits of mixed maturity) per plot was converted into the number of 60 kg bags of processed coffee per hectare (bags ha−1). The experimental design was randomized blocks with three replicates and six plants per plot. The spacing between the plants was 4.0 × 1.0 m. Sowing and crop management were performed according to the technical recommendations for the cultivation of the species.
2.2.2. Statistical Analysis
The variances and the prediction of random effects were estimated using the restricted maximum likelihood/best linear unbiased prediction (REML/BLUP) procedure with the aid of the software SELEGEN-REML/BLUP [8]. The equation was used to calculate the PROD data for the five crops. Furthermore, each of the five separate harvests (H1, H2, H3, H4 and H5) was analyzed using the model, where y is the data vector, m is the vector of the effects of evaluation–replicate–environmental combinations (assumed to be fixed) added to the general mean, g is the vector of the genotypic effects (assumed to be random), p is the vector of the plot effects (random), and e is the error or residual vector (random). The uppercase letters represent the incident matrixes for the referenced effects.
Based on estimates of the components of variance, the individual heritabilities and other coefficients of determination associated with the random effects of the models were estimated as described in Resende [21]. The variance components were submitted to the likelihood ratio test at 5% probability.
2.3. Experiment 2: Evaluation of Coffee Progenies under Water Deficit in Greenhouse
Seeds of the ten coffee progenies described were obtained from open pollination in Experiment 1 during the first harvest (H1) and were planted in 26 L pots until the developmental stage, with five pairs of leaves for studying in Experiment 2. The cultivars Catuaí Vermelho IAC 99, Catuaí Vermelho IAC 144 and the Siriema (genotypes 11, 12 and 13, respectively) in the seedling stage were used as checks. The “Siriema” has adaptive responses to water scarcity [14] and the cultivars from the Catuai group are widely adopted by Brazilian producers. A mixture of soil, sand and cattle manure (3:3:1, v/v/v) was used as the substrate. Topdressing fertilization was performed with 75 g of formulated NPK 20-00-20, divided into three plots at 30-day intervals.
The plants were kept in a greenhouse under a low-density polyethylene cover with the average temperature of 28 °C and the average relative humidity of 72%. After 6 months of growth, the seedlings were subjected to two environmental conditions, full irrigation (control) and drought, with water stress imposed by the total suspension of irrigation for 18 days. The experimental design consisted of a randomized complete block design (RCBD) with four replicates for each genotype. For all analyses (Ψpd, gas exchange and anatomy), leaves were harvested at the 3rd and 4th nodes (from the apex) of the plagiotropic branches.
2.3.1. Phenotypic Analyses
The morphological traits were evaluated one day before the onset of drought stress by assessing the stem diameter (SD) at ground level (mm), the plant height (H) (cm) and the total leaf area (LA), according to the leaf size method [22]. In addition, the number of plagiotropic branches (NPB) were counted, and their insertion angle (APL) with the orthotropic branch was determined, as was the length (cm) of the first plagiotropic branch at the bottom of the plants (LPL). At the end of the experiment, the tissues were placed in a forced-air oven at 70 °C for 96 h until a constant weight was reached to determine the shoot dry mass (SM), root dry mass (RM), root length (RL) and total dry mass (TM). In addition, we estimated the ratio of the root to the shoot masses (RMSM) and the ratio of the root mass to the leaf area (RMLA).
2.3.2. Physiological Analyses
The predawn leaf water potential (Ψpd) was determined in the leaves using a pressure pump chamber according to Scholander et al. [23]. Water potential was measured in fully expanded mature leaves at 0, 14 and 18 days after the onset of water stress, always in the morning (between 04:30 and 05:30 a.m.) at an average temperature of 17 °C to avoid the inhibitory effects of light and temperature on water potential.
The gas exchange values were measured using a portable infrared gas analyzer (IRGA, LI-6400XT Portable Photosynthesis System, LI-COR, Lincoln, NE, USA). The following parameters were evaluated: net photosynthetic rate (A), conductance (gs) and transpiration (E). These evaluations were performed on the same days as the water potential assessments, on clear days between 8:00 a.m. and 11:00 a.m.
2.3.3. Anatomical Analyses
Anatomical analyses were performed before water stress was imposed on the leaves. A total of 52 fully expanded leaves (four biological replicates for each genotype) were collected from the plagiotropic branches in the middle third of the plants and stored in 70% ethanol (v/v). The cross sections used for the anatomical analyses were obtained with an LPC table microtome. The sections were cleared with sodium hypochlorite (1.25% active chlorine), triple washed with distilled water, stained with an astra blue-safranin solution (0.1% astra blue and 1% safranin at a ratio of 7:3), and subsequently, mounted on semi-permanent slides with 50% glycerol (v/v). The slides were observed and photographed under an Olympus BX60 optical microscope coupled to a Canon A630 digital camera. The images were analyzed with UTHSCSA ImageTool image analysis software.
The following traits were evaluated in the cross sections: thickness of the abaxial epidermis (AbE), thickness of the adaxial epidermis (AdE), leaf blade thickness (LBT), thickness of the palisade parenchyma (TPP), thickness of the spongy parenchyma (TSP), thickness of the adaxial cuticle (CT), number of xylem vessels (NXV), xylem vessel thickness (XVT) and phloem thickness (PT). The stomatal density (DEN) (the number of stomata per mm2) and the ratio of the polar to the equatorial diameters of the stomata (PDED) were evaluated for the paradermal sections from the abaxial leaf surfaces.
2.3.4. Statistical Analysis
The variances and the prediction of random effects were estimated via the equation for physiological, morphological and anatomical data before estimating the components of variance, the individual heritabilities and other coefficients of determination. The predicted genotypic values for each genotype were used to determine the selection index based on the sum of ranks from Mulamba and Mock [24].
Genotypic Correlations among Traits
Aiming to determine the associations of the hereditary nature so that they can be used in breeding programs aiming at selecting more drought-tolerant genotypes, the genotypic correlations were estimated by the Genes software [25] among PROD, H1, H2, H3, H4 and H5 (evaluated in Experiment 1), and among the physiological, morphological and anatomical traits which had genetic variability (evaluated in Experiment 2).
3. Results
3.1. Selection under Greenhouse Conditions
To analyze whether the studied variables could be used in the selection process, we estimated the genetic parameters (Table 1). Considering the absence and presence of irrigation and the three evaluation times, the physiological data did not show genetic variability. Therefore, we evaluated the data in the irrigated system and under drought conditions individually using the same model as that used for the anatomical and morphological data. In the irrigated system, heritability did not indicate that selection was possible since under this condition the variations in MPa were low in magnitude. On the other hand, under drought conditions the genetic variation was significant, and the heritability of the genotype average was 79%. Regarding gas exchange, there was no significant genetic variation; thus, these traits were considered inefficient for the early selection of coffee plants under greenhouse conditions for drought tolerance.

Table 1.
Estimates of genetic parameters related to leaf water potential (Ψpd), net photosynthetic rate (A), stomatal conductance (gs), transpiration (E), leaf area (LA), height (H), stem diameter (SD), number of plagiotropic branches (NPB), plagiotropic branch insertion angle (ÂPL), length of the first plagiotropic branch (LPL), root length (RL), root mass (RM), total mass (TM), shoot mass (SM), the ratio of the root to the shoot masses (RMSM), the ratio of the root mass to leaf area (RMLA), thickness of the adaxial epidermis (AdE), cuticle (CT), abaxial epidermis (AbE), leaf blade (LBT), palisade parenchyma (TPP), spongy parenchyma (TSP), ratio of the polar to the equatorial diameters of the stomata (PDED), stomatal density (DEN), number of xylem vessels (NXV), phloem thickness (PT) and xylem vessel thickness (XVT).
Regarding the morphological and anatomical parameters, the likelihood ratio test revealed the existence of genetic variability among the studied genotypes for the traits LA, SD, NPB, RM, TM, SM, CT, LBT, TSP, PDED, NXV and PT. Similarly, the heritability of the mean of the genotypes showed high values for these traits, ranging from 73 to 92%, indicating the possibility of selection. The values observed for the genotypic correlations between environments ranged from 0.79 to 1.0. These parameters indicate that the experiment was conducted properly and that the predicted genetic values were quite accurate, thus demonstrating that selection could be easily applied using these resources. No genetic variability between the studied genotypes was observed for the other traits evaluated, indicating the difficulty of selecting superior plants on the basis of these traits. Therefore, these other traits are not mentioned in the discussion.
Ψpd, LA, SD, NPL, RM, TM, SM, CT, LBT, TSP, PDED, NXV and PT were chosen as the main traits for the greenhouse selection of progenies, as these traits showed satisfactory genetic parameters. According to the Mulamba and Mock index [24], the summed ranks indicated that H516-2-1-1-7-1, H516-2-1-1-12-1, H419-3-3-7-16-2 and H419-3-3-7-16-11 were superior to the other progenies (Figure 1). In the selection of improved progenies, the predicted genotypic values favored genetic gains relative to the genotypic mean, with an increase of 14.35% in LA, of 5.5% in SD, of 5.40% in NPL, of 8.07% in RM, of 9.19% in TM, of 9.56% in SM, of 0.86% in LBT, of 1.26% in TSP, of 1.18% in PDED, of 7.13% in NXV and an increase of 4.11% in PT. The selection of these improved progenies decreased CT by 4.25% and 28.83% in MPa. However, greater gains were obtained for the other traits.
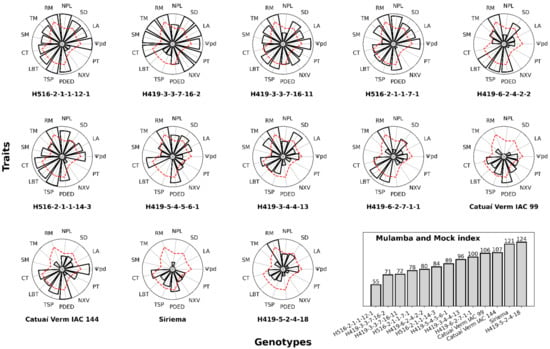
Figure 1.
The predicted additive genetic values regarding the variables of leaf water potential (Ψpd), leaf area (LA), stem diameter (SD), length of the first plagiotropic branch (NPL), root mass (RM), total mass (TM), shoot mass (SM), cuticle (CT), leaf blade (LBT), spongy parenchyma (TSP), ratio of the polar to the equatorial diameters of the stomata (PDED), number of xylem vessels (NXV) and phloem thickness (PT), according to the Mulamba and Mock [12] index. All the data were normalized to have the same scale; the scale for the variables of cuticle (CT) and leaf water potential (Ψpd) were reversed by multiplying by (−1). The dashed red line represents the mean of the traits.
3.2. Selection under Field Conditions
The total rainfall in the periods corresponding to the five harvests included in the study was 1513, 580, 1115, 1003 and 852 mm. The mean annual temperature was approximately 18 °C (Supplementary Figure S1). The mean PROD ranged between 20.96 and 32.40 bags ha−1. With the selection of superior progenies H419-3-4-4-13, H419-3-3-7-16-11, H516-2-1-1-7-1 and H419-5-4-5-6-1, the predicted genotypic values favored genetic gains of 11.10% relative to the genotypic mean (Figure 2). Heritability indicated that genetic causes accounted for 65% of the phenotypic variance. This value is of significant interest because several genes and environmental factors influence this variable.
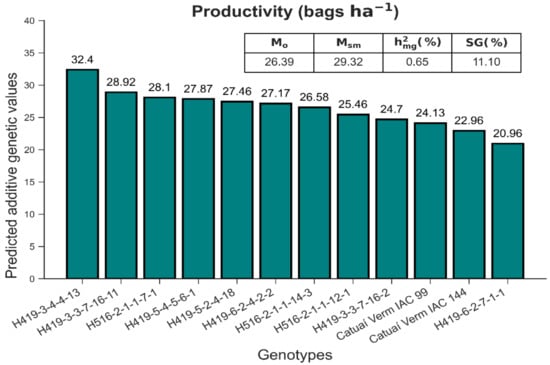
Figure 2.
Predicted additive genetic values for the productivity (bags ha−1) (PROD) in the first five harvests. : overall mean of the experiment; : selected progeny mean; : heritability of genotype average, assuming complete survival; SG (%): estimated gain with the selection of the four best progenies.
3.3. Genotypic Correlations among Traits
Highly significant positive genotypic correlations were observed between the following traits: A × gs, A × E, gs × E, LA × SD, LA × NPB, LA × SM, SD × RM, SD × SM, LBT × TSP, NXV × PT, SD × H3, PROD × H2 and H3 × H5 (Supplementary Table S1). On the other hand, the variables Ψpd and A showed negative correlations with LA and SM, and also with Ψpd × H1. A also showed negative correlations with DPED and NXV. The variables gs and E showed negative correlations with NXV and PT, and gs also showed negative correlations with SM. LA and CT showed negative correlations. PROD did not correlate with any physiological or anatomical variables, indicating that the genetic variation was independent.
4. Discussion
This study aimed to establish a strategy for drought-tolerant selection in Arabica coffee genotypes in Brazil, combining field trials under natural conditions with controlled growth condition experiments. Water deficit is a multifactorial stress. Therefore, several traits were considered in our study as parameters that define drought tolerance. Morphological, anatomical and physiological traits determine water flow in plant tissues, controlling the loss of water vapor on the surface and maintaining water uptake by the roots [26,27], and the selection in the greenhouse was performed on the basis of these traits under water deficit. Notably, drought tolerance is defined as the ability to maintain production under water-deficit conditions, and therefore, a selection was also made based on productivity in the field, where the total annual rainfall was mostly below the 1200 mm required by coffee plants.
The study of statistical genetic parameters aims to know the genetic structure of a population. In this work, of the 28 traits studied in the greenhouse, 13 showed significant genetic variance. The heritability coefficients estimated based on the progeny mean ranged from 65 to 92%. These results indicate that the phenotypic values observed in the progenies are very close to their genetic values and emphasize the importance of parameter estimation in the variables used for selection [28]. In this way, it was possible to recognize that Ψpd, LA, SD, NPL, RM, TM, SM, CT, LBT, TSP, PDED, NXV and PT are heritable. These traits have variability for superior individual selection, aiming for accumulation and perpetuation of genes of interest. Dubberstein et al. [29] used genetic parameters to identify and select coffee plants that show leaf anatomy plasticity under exposure to abiotic factors. However, some researchers have studied genotypes in genetic fixed-effect models (constant between individuals) without estimating the genetic variance and heritability of the phenotypic variation on the studied characters, as in [30,31]. This strategy may not be effective in crop breeding programs that aim to develop drought-tolerant cultivars. Genotypes that presented a heritability lower than 50% have a low genetic influence in the expression of the drought tolerance, causing major responses to environmental conditions [32]. Such genotypes are not expected to maintain their phenotypic behavior of adaptation to drought over time, which makes experimental replication difficult, and may be a false positive source of tolerance. Thus, the genetic variance component must be estimated, as well the heritability of the trait.
It is noteworthy that the population in these studies was the F4:5 generation and that selection gains based on the characteristics that showed variability and heritability could be obtained. Thus, in both experiments, a selection intensity of 40% (four progenies were selected among the ten studied) was considered for the advancement of generations aimed at cultivars more adapted to drought. This percentage ensures a minimum effective number that allows greater efficiency in subsequent selection stages [33]. The application of the selection index combined with predicted means was proposed as a strategy to confirm the feasibility of selection for drought tolerance under greenhouse conditions. Through the Mulamba and Mock index [24], four progenies (H516-2-1-1-7-1, H516-2-1-1-12-1, H419-3-3-7-16-2 and H419-3-3-7-16-11) that favor gains in LA, SD, NPL, RM, TM, SM, LBT, TSP, PDED, NXV and PT were selected.
Findings from a previous study by [34] have indicated that selection for higher bean yield per tree in arabica coffee can be achieved through selection for stem diameter, number and length of plagiotropic branches, and plant height. Our study indicates genetic gain possibility for most of these characteristics and suggests that the selection of plants with the highest LA, SD, NPL, RM, TM and SM could be a useful approach to generating improved productivity in the studied progenies.
The gains obtained considering these anatomical traits can confer greater drought tolerance because the higher NXV and greater PT indicate a greater flow of water, minerals and carbohydrates in the leaves of these genotypes [35]. Higher NXV values are associated with more efficient hydraulic conductivity because they protect the xylem from cavitation, moderate the movement of water in the shoots and help maintain a moist rhizosphere for the continuous growth of roots and the uptake of water and nutrients [36,37]. In addition to providing these advantages under water deficit, the plasticity of vascular tissues ensures adequate transport of water to the shoots, favoring rapid growth under water availability conditions [38]. The gains in TSP and LBT are also noteworthy, since the plants had thicker leaves, allowing them to maintain a higher water content in the leaves in the drought environment, and also had more spongy parenchyma, which could improve the water conductivity of these plants and may result in better CO2 diffusion to the palisade cells where CO2 is required for photosynthesis [39]. In addition, a greater TSP favors greater reflectance and refraction of light, allowing greater light diffusion, which may contribute to photochemical efficiency, minimizing oxidative stress under water deficit conditions.
In contrast, on the basis of the ranking results considering both the morpho-anatomical variables and the water potential simultaneously, greenhouse selection showed no gains for water potential (the only physiological trait used for selection). This result occurred mainly because there is a negative correlation between LA and water potential: the greater the LA is, the greater the transpiration area, and, consequently, the higher the water consumption. In addition, the coupling of the canopy architecture and the environment is essential in determining the water balance of plants because it alters the absorption and emission of energy, which in turn affects leaf temperature, vapor pressure, transpiration rate and water absorption [40]. Thus, the use of water potential as an early selection criterion for drought tolerance may result in the selection of genetic materials with less vegetative development and vice versa.
Many studies give important insights into the morphological, anatomical, physiological and molecular factors of drought tolerance [11,27,41,42,43,44,45], but the knowledge of the genetic variation in the relevant traits of the studied population is required for use in breeding programs.
In this work, all growth variables were positively correlated with each other and negatively correlated with Ψpd and gas exchange (Supplementary Table S1), but were not correlated with PROD. Therefore, the traits conferring tolerance to water stress under field conditions can be different from those under greenhouse conditions which determine the gas exchange and morphological traits [46]. The positive correlation between Ψpd and gs observed in our study suggests that selection based on water status can also select for genotypes with greater stomatal sensitivity to a water deficit. This trait restricts A under field conditions, with negative effects on production, but allows the maintenance of the water status of the plant for longer periods [47]. It is also noteworthy that the negative correlation of Ψpd with H1 indicates that selection based only on Ψpd under greenhouse conditions can select for less productive young plants under field conditions. Furthermore, SD was positively correlated with H3 in the field, indicating that a larger SD may ensure adequate transport of water and nutrients to the shoots, favoring productivity under conditions of the reestablishment of water availability [38].
In coffee plants, at least four consecutive harvests are required for the successful selection of progeny [48]. Therefore, we chose to consider five harvests instead of using only the years of greatest water deficit (2006, 2007 and 2010). Under field conditions, selection applied to the genetic values that favored positive genetic gains in the progenies H419-3-4-4-13, H419-3-3-7-16-11, H516-2-1-1-7-1 and H419-5-4-5-6-1, indicating the potential use of these cultivars for breeding advanced generations. The progenies derived from the hybrid H419 were among the most promising and constituted three of the four best-ranked progenies. The hybrid H419 refers to the cross between Catuaí Amarelo IAC 30 and the accession Timor Hybrid UFV 445-46. The latter parent originates from the Coffee Leaf Rust Research Center (Oeiras, Portugal) and is registered as CIFC 2570, with genetic resistance to the fungus Hemileia vastatrix, which causes coffee leaf rust. Thus, the selected progenies can combine resistance to leaf rust and drought tolerance.
A genetic breeding program for drought tolerance requires medium- to long-term work because drought tolerance is a complex characteristic that is controlled by several genes with great environmental influence. It requires multidisciplinary knowledge and a reliable system of field experimentation where it is possible to analyze the resilience of the coffee tree; that is, to study the greater capacity of the coffee tree to recover after cycles of water deficit. In the present study, the association between histology, physiology, crop science and genetic studies, among other related areas associated with conventional breeding strategies, allowed us to identify genotypes that simultaneously can produce and have morpho-anatomical characteristics that confer greater tolerance to drought.
The predicted genetic parameters show that the results of the selection of coffee plants in the greenhouse based on anatomical and physiological traits have a 50% match with the results of selection performed in the field based on productivity in a region of water deficit. Although the plants were subjected to water stress in the greenhouse, other factors such as light intensity and quality and wind speed, among others, were greatly modified. However, technologies such as growth cabinets and greenhouses that can be programmed to simulate environmental fluctuations experienced by plants under field conditions are not broadly accessible due to their high costs. In addition, evaluation in a greenhouse in the young plant stage does not consider the impacts of water deficit on productivity in the field, which are quite variable depending on the plant phenological phase in which it occurs, i.e., the vegetative development stage or the flowering and fruiting stage [49]. Thus, two of the four progenies selected under greenhouse conditions match the progenies selected under field conditions: H419-3-3-7-16-11 and H516-2-1-1-7-2. After the advancement of generations, the progeny H419-3-3-7-16-11 originated the MGS Turmalina cultivar, which was registered by the Registro Nacional de Cultivares (National Cultivars Registry, RNC) of the Brazilian Ministry of Agriculture, Livestock and Supply (MAPA), Brazil, under number 49086. The H516-2-1-1-7-2 progeny has been used by the breeding program for drought-tolerant cultivar development.
5. Conclusions
The strategy for selecting drought-tolerant Arabica coffee genotypes must combine the predicted genetic parameters for character identification of characteristics that are heritable in field trials and controlled growth condition experiments. The method applied to select plants tolerant to drought under greenhouse conditions was adapted from the breeding routine for plant selection, focusing on the development of drought-tolerant cultivars. Through this strategy, we identified genotypes that have both the ability to produce and morpho-anatomical traits that confer greater drought tolerance. The early selection results of coffee plants under greenhouse conditions matched the selection results in the field in a water-deficit region by 50% based on productivity, indicating that selection in the field is still necessary. Therefore, the strategy for the breeding program aimed at developing drought-tolerant cultivars is: (1) screening and selection of genotypes within a large population of plants in the field in a region with a water deficit for at least four harvests; (2) evaluation of the genotypes selected in the field regarding morpho-anatomical traits that confer tolerance under controlled drought conditions; and (3) identification of genotypes selected simultaneously in the field and the greenhouse for the development of drought-tolerant cultivars. Using statistical genetic tools integrating field and greenhouse approaches can accelerate selection cycles, maximize genetic gain and reduce the release time of new cultivars. Such approaches must complement each other appropriately, resulting in efficiency and safety for selecting and developing more promising, productive and drought-tolerant cultivars.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12092167/s1, Figure S1: Variability of total rainfall (mm), and average temperature average (°C), according to the meteorological station (Tourmaline, MG), from first five harvests (first (H1), second (H2), third (H3), fourth (H4) and fifth harvest (H5)); Table S1: Genotypic correlation matrix (r) among leaf water potential (Ψpd), net photosynthetic rate (A), stomatal conductance (gs), transpiration (E), leaf area (LA), stem diameter (SD), number of plagiotropic branches (NPB), root dry mass (RM), total mass (TM), shoot dry mass (SM), cuticle thickness (CT), leaf blade thickness (LBT), thickness of the spongy parenchyma (TSP), the ratio of the polar to the equatorial diameters of the stomata (PDED), number of xylem vessels (NXV), phloem thickness (PT) and productivity in the first five harvests (first (H1), second (H2), third (H3), fourth (H4) and fifth harvest (H5)), and also with five crops (PROD).
Author Contributions
J.C.d.R.A. and V.A.S. conceived and designed the study. A.M.R. performed most of the experiments. A.M.R. and M.d.O.S. performed the physiological analysis. A.M.R. and C.E.B. performed the morphological analysis. A.M.R. and E.M.d.C. performed the anatomical analysis and A.C.B.d.O., A.A.P. and C.E.B. conducted the field condition experiment. J.C.d.R.A. and V.A.S. performed the statistical analysis. V.A.S., G.R.C., A.C.B.d.O., J.P.R.A.D.B., G.P.B. and C.E.B. supervised the research and improved the manuscript. J.C.d.R.A. and V.A.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Instituto Nacional de Ciência e Tecnologia do Café (INCT-Café) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarships and productivity granted, and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), the Instituto Nacional de Ciência e Tecnologia do Café (INCT-Café), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Consórcio Brasileiro de Pesquisa e Desenvolvimento do Café—Consórcio Pesquisa Café for the financial support.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Nakabayashi, R.; Saito, K. Integrated Metabolomics for Abiotic Stress Responses in Plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Barchet, G.L.H.; Dauwe, R.; Guy, R.D.; Schroeder, W.R.; Soolanayakanahally, R.Y.; Campbell, M.M.; Mansfield, S.D. Investigating the Drought-Stress Response of Hybrid Poplar Genotypes by Metabolite Profiling. Tree Physiol. 2014, 34, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, Salt, and Temperature Stress-Induced Metabolic Rearrangements and Regulatory Networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate Change and Coffee Quality: Systematic Review on the Effects of Environmental and Management Variation on Secondary Metabolites and Sensory Attributes of Coffea arabica and Coffea canephora. Front. Plant Sci. 2021, 12, 708013. [Google Scholar] [CrossRef]
- Silva, V.A.; Prado, F.M.; Antunes, W.C.; Paiva, R.M.C.; Ferrão, M.A.G.; Andrade, A.C.; Di Mascio, P.; Loureiro, M.E.; DaMatta, F.M.; Almeida, A.M. Reciprocal Grafting between Clones with Contrasting Drought Tolerance Suggests a Key Role of Abscisic Acid in Coffee Acclimation to Drought Stress. Plant Growth Regul. 2018, 85, 221–229. [Google Scholar] [CrossRef]
- Lima, E.A.; Furlanetto, C.; Nicole, M.; Gomes, A.C.M.M.; Almeida, M.R.A.; Jorge-Júnior, A.; Correa, V.R.; Salgado, S.M.; Ferrão, M.A.G.; Carneiro, R.M.D.G. The Multi-Resistant Reaction of Drought-Tolerant Coffee ‘Conilon Clone 14’ to Meloidogyne Spp. and Late Hypersensitive-Like Response in Coffea canephora. Phytopathology 2015, 105, 805–814. [Google Scholar] [CrossRef]
- Mofatto, L.S.; de Carneiro, F.A.; Vieira, N.G.; Duarte, K.E.; Vidal, R.O.; Alekcevetch, J.C.; Cotta, M.G.; Verdeil, J.-L.; Lapeyre-Montes, F.; Lartaud, M.; et al. Identification of Candidate Genes for Drought Tolerance in Coffee by High-Throughput Sequencing in the Shoot Apex of Different Coffea arabica Cultivars. BMC Plant Biol. 2016, 16, 94. [Google Scholar] [CrossRef]
- Alves, G.S.C.; Torres, L.F.; de Aquino, S.O.; Reichel, T.; Freire, L.P.; Vieira, N.G.; Vinecky, F.; This, D.; Pot, D.; Etienne, H.; et al. Nucleotide Diversity of the Coding and Promoter Regions of DREB1D, a Candidate Gene for Drought Tolerance in Coffea Species. Trop. Plant Biol. 2018, 11, 31–48. [Google Scholar] [CrossRef]
- Torres, L.F.; Reichel, T.; Déchamp, E.; de Aquino, S.O.; Duarte, K.E.; Alves, G.S.C.; Silva, A.T.; Cotta, M.G.; Costa, T.S.; Diniz, L.E.C.; et al. Expression of DREB-Like Genes in Coffea canephora and C. arabica Subjected to Various Types of Abiotic Stress. Trop. Plant Biol. 2019, 12, 98–116. [Google Scholar] [CrossRef]
- Kiwuka, C.; Goudsmit, E.; Tournebize, R.; de Aquino, S.O.; Douma, J.C.; Bellanger, L.; Crouzillat, D.; Stoffelen, P.; Sumirat, U.; Legnaté, H.; et al. Genetic Diversity of Native and Cultivated Ugandan Robusta Coffee (Coffea canephora Pierre Ex A. Froehner): Climate Influences, Breeding Potential and Diversity Conservation. PLoS ONE 2021, 16, e0245965. [Google Scholar] [CrossRef]
- De Oliveira Santos, M.; Coelho, L.S.; Carvalho, G.R.; Botelho, C.E.; Torres, L.F.; Vilela, D.J.M.; Andrade, A.C.; Silva, V.A. Photochemical Efficiency Correlated with Candidate Gene Expression Promote Coffee Drought Tolerance. Sci. Rep. 2021, 11, 7436. [Google Scholar] [CrossRef]
- Bertrand, B.; Marraccini, P.; Villain, L.; Breitler, J.-C.; Etienne, H. Healthy Tropical Plants to Mitigate the Impact of Climate Change—As Exemplified in Coffee. In Climate Change and Agriculture Worldwide; Torquebiau, E., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 83–95. ISBN 9789401774604. [Google Scholar]
- De Souza, B.P.; Martinez, H.E.P.; de Carvalho, F.P.; Loureiro, M.E.; Sturião, W.P. Gas Exchanges and Chlorophyll Fluorescence of Young Coffee Plants Submitted to Water and Nitrogen Stresses. J. Plant Nutr. 2020, 43, 2455–2465. [Google Scholar] [CrossRef]
- Dias, P.C.; Araujo, W.L.; Moraes, G.A.B.K.; Barros, R.S.; DaMatta, F.M. Morphological and Physiological Responses of Two Coffee Progenies to Soil Water Availability. J. Plant Physiol. 2007, 164, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Fazuoli, L.C.; Braghini, M.T.; Silvarolla, M.B.; Gonçalves, W.; Mistro, J.C.; Gallo, P.B.; Guerreiro Filho, O. IAC Catuaí SH3—A Dwarf Arabica Coffee Cultivar with Leaf Rust Resistance and Drought Tolerance. Crop Breed. Appl. Biotechnol. 2019, 19, 356–359. [Google Scholar] [CrossRef]
- Sera, T.; Sera, G.H.; Fazuoli, L.C. IPR 103—Rustic Dwarf Arabic Coffee Cultivar More Adapted to Hot Regions and Poor Soils. Crop Breed. Appl. Biotechnol. 2013, 13, 95–98. [Google Scholar] [CrossRef]
- Ferrão, R.G.; Fonseca, A.F.A.; Silveira, J.S.M.; Ferrão, M.A.G.; Bragança, S.M. EMCAPA 8141—Robustão Capixaba, Variedade Clonal De Café Conilon Tolerante À Seca Desenvolvida Para O Estado do Espírito Santo. Rev. Ceres 2000, 273, 555–560. [Google Scholar]
- Van der Vossen, H.; Bertrand, B.; Charrier, A. Next Generation Variety Development for Sustainable Production of Arabica Coffee (Coffea arabica L.): A Review. Euphytica 2015, 204, 243–256. [Google Scholar] [CrossRef]
- Surya Prakash, N.; Devasia, J.; Raghuramulu, Y.; Aggarwal, R.K. Genetic Diversity and Coffee Improvement in India. In Molecular Breeding for Sustainable Crop Improvement; Rajpal, V.R., Rao, S.R., Raina, S.N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 11, pp. 231–268. ISBN 9783319270883. [Google Scholar]
- Silva, P.E.M.; Cavatte, P.C.; Morais, L.E.; Medina, E.F.; DaMatta, F.M. The Functional Divergence of Biomass Partitioning, Carbon Gain and Water Use in Coffea canephora in Response to the Water Supply: Implications for Breeding Aimed at Improving Drought Tolerance. Environ. Exp. Bot. 2013, 87, 49–57. [Google Scholar] [CrossRef]
- De Resende, M.D.V. Software Selegen-REML/BLUP: A Useful Tool for Plant Breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Barros, R.S.; Maestri, M.; Vieira, M.; Braga-Filho, L.J. Determinação De Área De Folhas Do Café (Coffea arabica L. Cv. ‘Bourbon Amarelo’). Rev. Ceres 1973, 20, 44–52. [Google Scholar]
- Scholander, P.F.; Hammel, H.T.; Hemmingsen, E.A.; Bradstreet, E.D. Hydrostatic pressure and osmotic potential in leaves of mangroves and some other plants. Proc. Natl. Acad. Sci. USA 1964, 52, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Mulamba, N.N.; Mock, J.J. Improvement of Yield Potential of the ETO Blanco Maize (Zea mays L.) Population by Breeding for Plant Traits [Mexico]. Egypt. J. Genet. Cytol. 1978, 7, 40–45. [Google Scholar]
- Cruz, C.D. GENES—A Software Package for Analysis in Experimental Statistics and Quantitative Genetics. Acta Sci. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Responses of Different Physiological Parameter Thresholds to Soil Water Availability in Four Plant Species during Prolonged Drought. Agric. For. Meteorol. 2017, 247, 311–319. [Google Scholar] [CrossRef]
- Menezes-Silva, P.E.; Sanglard, L.M.V.P.; Ávila, R.T.; Morais, L.E.; Martins, S.C.V.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araújo, W.L.; Fernie, A.R.; et al. Photosynthetic and Metabolic Acclimation to Repeated Drought Events Play Key Roles in Drought Tolerance in Coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef] [PubMed]
- Ogunbayo, S.A.; Sie, M.; Ojo, D.K.; Sanni, K.A.; Akinwale, M.G.; Toulou, B.; Shittu, A.; Idehen, E.O.; Popoola, A.R.; Daniel, I.O.; et al. Genetic Variation and Heritability of Yield and Related Traits in Promising Rice Genotypes (Oryza sativa L.). J. Plant Breed. Crop Sci. 2014, 6, 153–159. [Google Scholar] [CrossRef]
- Dubberstein, D.; Oliveira, M.G.; Aoyama, E.M.; Guilhen, J.H.; Ferreira, A.; Marques, I.; Ramalho, J.C.; Partelli, F.L. Diversity of Leaf Stomatal Traits among Coffea canephora Pierre Ex A. Froehner Genotypes. Agronomy 2021, 11, 1126. [Google Scholar] [CrossRef]
- Anim-Kwapong, E.; Anim-Kwapong, G.J.; Adomako, B. Indirect Versus Direct Selection of Adaptable Genotypes of Coffea Canephora for Drought Stress and Non-Stress Conditions. J. Crop Improv. 2011, 25, 356–370. [Google Scholar] [CrossRef]
- King’oro, M. Effect of Different Watering Regimes on Agro-Morphology of Selected Coffee Genotypes. AJEA 2014, 4, 1016–1026. [Google Scholar] [CrossRef]
- De Resende, M.D.V.; Duarte, J.B. Precision and Quality Control in Variety Trials. Pesq. Agropec. Trop. 2007, 37, 182–194. [Google Scholar]
- Rocha, R.B.; Vieira, A.H.; Gama, M.M.B.; Rossi, L.M.B. Avaliação Genética De Procedências De Bandarra (Schizolobium amazonicum) Utilizando REML/BLUP (Máxima Verossimilhança Restrita/Melhor Predição Linear Não Viciada). Sci. For. 2009, 37, 351–358. [Google Scholar]
- De Carvalho, A.M.; Mendes, A.N.G.; Carvalho, G.R.; Botelho, C.E.; Gonçalves, F.M.A.; Ferreira, A.D. Correlação Entre Crescimento e Produtividade de Cultivares de Café Em Diferentes Regiões de Minas Gerais, Brasil. Pesq. Agropec. Bras. 2010, 45, 269–275. [Google Scholar] [CrossRef]
- Castro, E.D.; Pereira, F.J.; Paiva, R. Histologia Vegetal: Estrutura E Função De Órgãos Vegetativos, 9th ed.; Editora UFLA: Lavras, Brazil, 2009; 244p. [Google Scholar]
- Prince, S.J.; Murphy, M.; Mutava, R.N.; Durnell, L.A.; Valliyodan, B.; Grover Shannon, J.; Nguyen, H.T. Root Xylem Plasticity to Improve Water Use And Yield In Water-Stressed Soybean. J. Exp. Bot. 2017, 68, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Wang, G.; Augstein, F.; de Vries, J.; Carlsbecker, A. Continuous Root Xylem Formation and Vascular Acclimation To Water Deficit Involves Endodermal ABA Signalling Via miR165. Development 2018, 145, dev.159202. [Google Scholar] [CrossRef]
- Hazman, M.; Brown, K.M. Progressive Drought Alters Architectural and Anatomical Traits of Rice Roots. Rice 2018, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Arena, C.; Izzo, L.G.; Tsonev, T.; Koleva, D.; Tattini, M.; Roeva, O.; De Maio, A.; Loreto, F. Functional and Structural Leaf Plasticity Determine Photosynthetic Performances during Drought Stress and Recovery in Two Platanus Orientalis Populations from Contrasting Habitats. Int. J. Mol. Sci. 2020, 21, 3912. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and Agronomic Performance of the Coffee Crop in the Context of Climate Change and Global Warming: A Review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef]
- Thioune, E.-H.; Strickler, S.; Gallagher, T.; Charpagne, A.; Decombes, P.; Osborne, B.; McCarthy, J. Temperature Impacts the Response of Coffea canephora to Decreasing Soil Water Availability. Trop. Plant Biol. 2020, 13, 236–250. [Google Scholar] [CrossRef]
- Tounekti, T.; Mahdhi, M.; Al-Turki, T.A.; Khemira, H. Water Relations and Photo-Protection Mechanisms during Drought Stress in Four Coffee (Coffea arabica) Cultivars from Southwestern Saudi Arabia. S. Afr. J. Bot. 2018, 117, 17–25. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the Impact of Drought in Coffea Genotypes: Transcriptomic Analysis Supports a Common High Resilience to Moderate Water Deficit but a Genotype Dependent Sensitivity to Severe Water Deficit. Agronomy 2021, 11, 2255. [Google Scholar] [CrossRef]
- De Guedes, F.A.F.; Nobres, P.; Rodrigues Ferreira, D.C.; Menezes-Silva, P.E.; Ribeiro-Alves, M.; Correa, R.L.; DaMatta, F.M.; Alves-Ferreira, M. Transcriptional Memory Contributes to Drought Tolerance in Coffee (Coffea canephora) Plants. Environ. Exp. Bot. 2018, 147, 220–233. [Google Scholar] [CrossRef]
- Marques, I.; Gouveia, D.; Gaillard, J.-C.; Martins, S.; Semedo, M.C.; Lidon, F.C.; DaMatta, F.M.; Ribeiro-Barros, A.I.; Armengaud, J.; Ramalho, J.C. Next-Generation Proteomics Reveals a Greater Antioxidative Response to Drought in Coffea arabica Than in Coffea Canephora. Agronomy 2022, 12, 148. [Google Scholar] [CrossRef]
- Sales, C.R.G.; Molero, G.; Evans, J.R.; Taylor, S.H.; Joynson, R.; Furbank, R.T.; Hall, A.; Carmo-Silva, E. Phenotypic Variation in Photosynthetic Traits in Wheat Grown under Field versus Glasshouse Conditions. J. Exp. Bot. 2022, 73, 3221–3237. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Ramalho, J.D.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- De Oliveira, A.C.B.; Pereira, A.A.; da Silva, F.L.; de Rezende, J.C.; Botelho, C.E.; Carvalho, G.R. Prediction of Genetic Gains from Selection in Arabica Coffee Progenies. Crop Breed. Appl. Biotechnol. 2011, 11, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.M.; Mendes, A.N.G.; Abrahão, J.C.R.; de Santos, M.O.; Silva, V.A. Early Selection of Drought-Tolerant Coffea Arabica Genotypes At The Seedling Stage Using Functional Divergence. Pesq. Agropec. Trop. 2022, 52, e72412. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).