The Physiological and Biochemical Response of Field Bean (Vicia faba L. (partim)) to Electromagnetic Field Exposure Is Influenced by Seed Age, Light Conditions, and Growth Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Cultivation Conditions
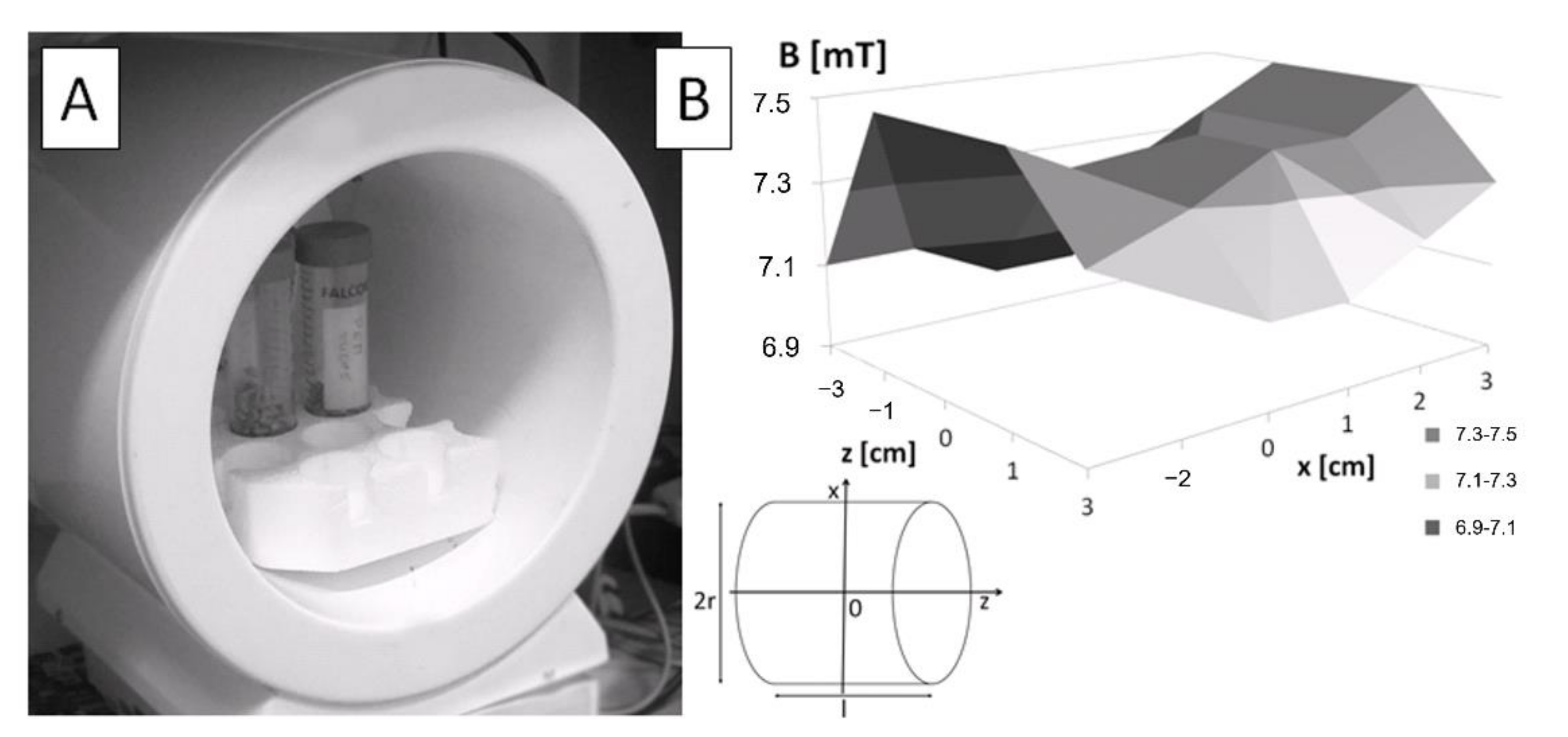
2.2. Exposure to Electromagnetic Field
2.3. Cultivation Conditions
2.4. Seed Germination Analysis
CVG = 100 [(N1 + N2 +…+ Nn)/(N1T1 + N2T2 +…+ NnTn)]
t50 = Ti + [(((N + 1)/2) − Ni) (Tj − Ti)]/(Nj − Ni)
2.5. Measurement of Seedling Morphological Parameters
2.6. Plant Material Collections
2.7. Examination of Seeds Water Uptake and Membrane Integrity
2.8. α-Amylase Assay
2.9. Hydrogen Peroxide Measurement
2.10. Determination of Photosynthetic Pigments
2.11. Quantification of Phytohormones by Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.12. Statistical Analysis
3. Results
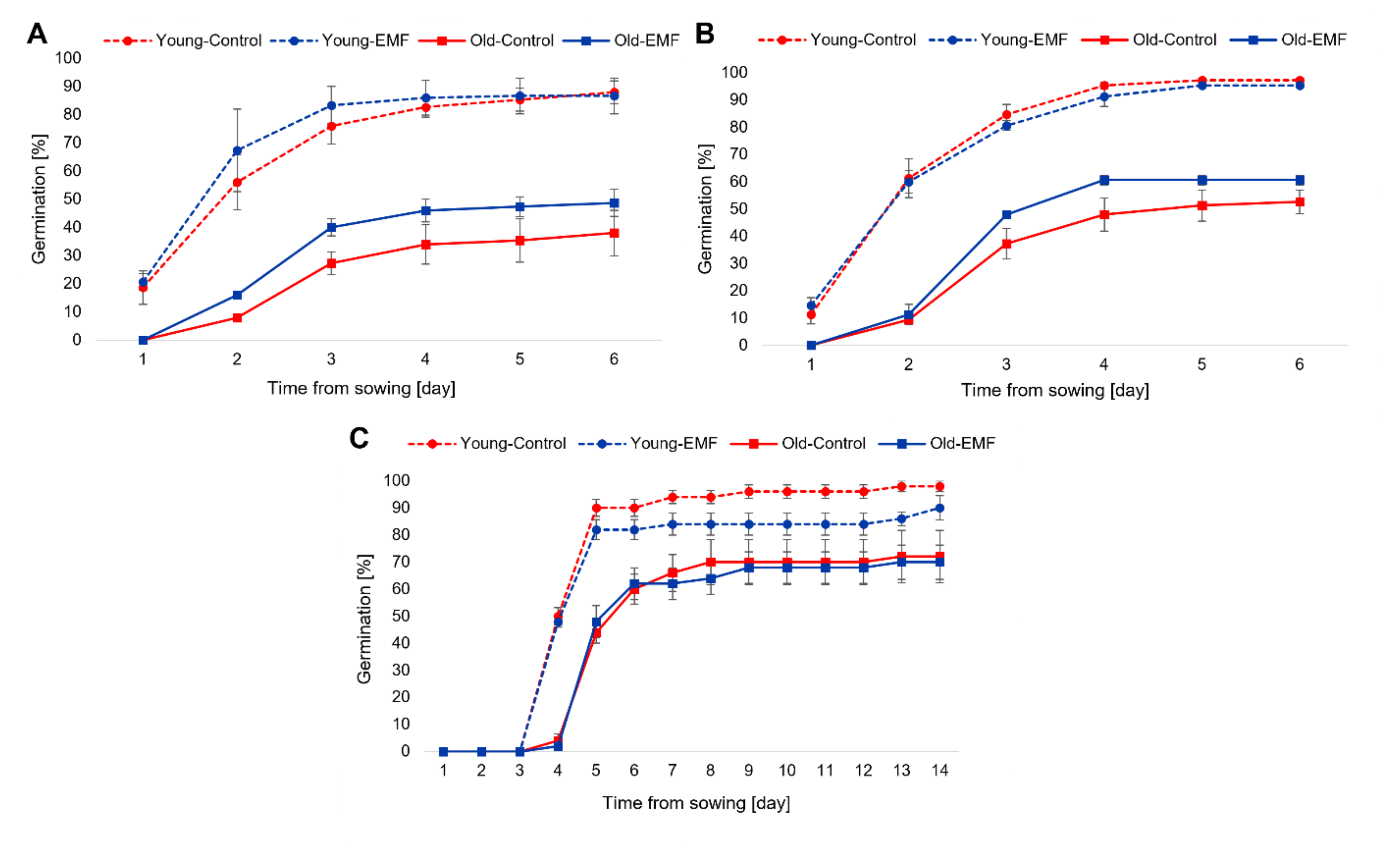
3.1. Effect of Seed Age and EMF Exposure on Germination Kinetics of Petri Dish-Sown and Pot-Sown Field Bean Seeds
3.2. Analysis of Germination Parameters (G, GI, MGT, CVG, t50)
3.3. Morphometric Analysis of Seedling’s Growth in Control Groups
3.4. EMF Treatment Effect on Growth Parameters
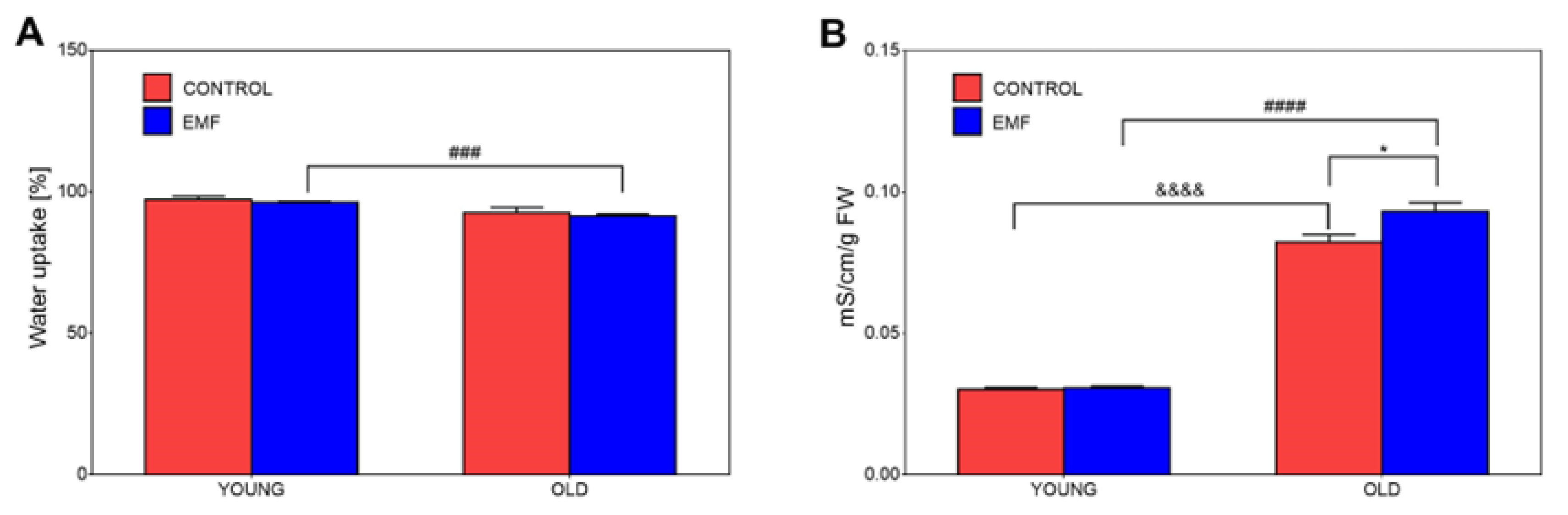
3.5. Assessment of Water Uptake and Membrane Integrity of Field Bean Seeds
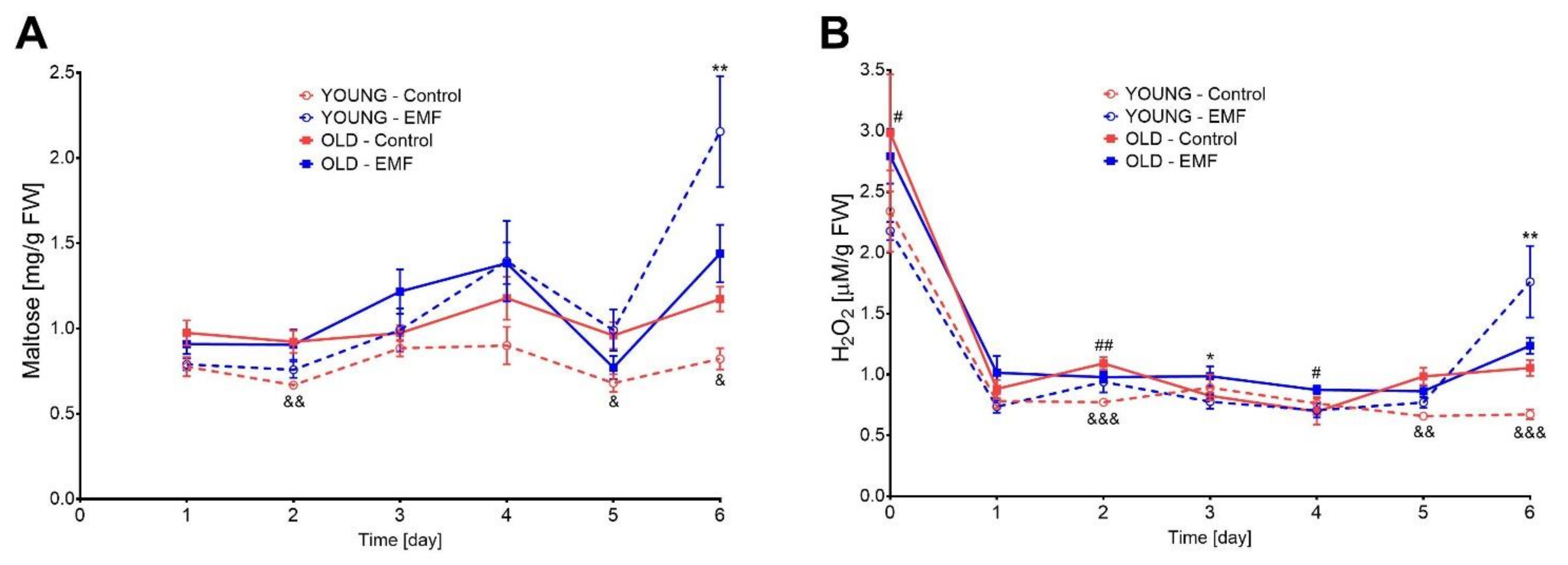
3.6. Amylolytic Activity and H2O2 Content in Seeds of Field Bean
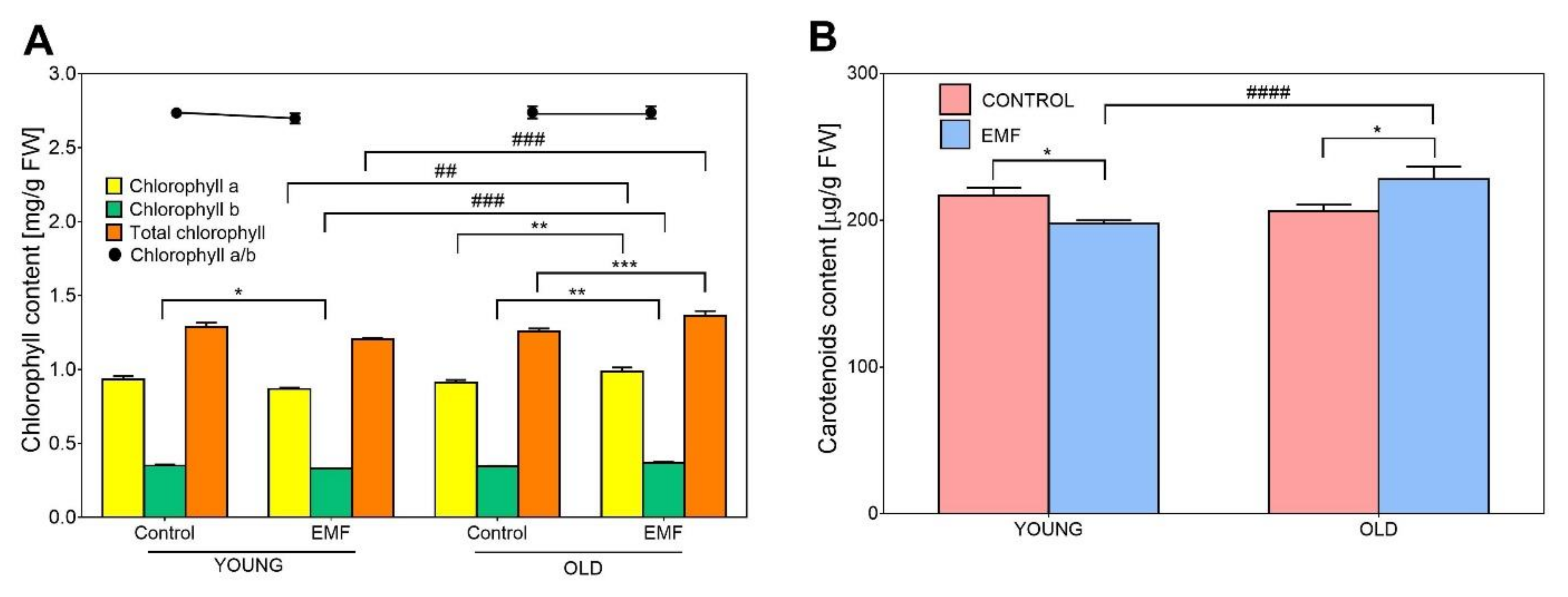
3.7. Effect of Seed Age and EMF Exposure on Photosynthetic Pigments Content
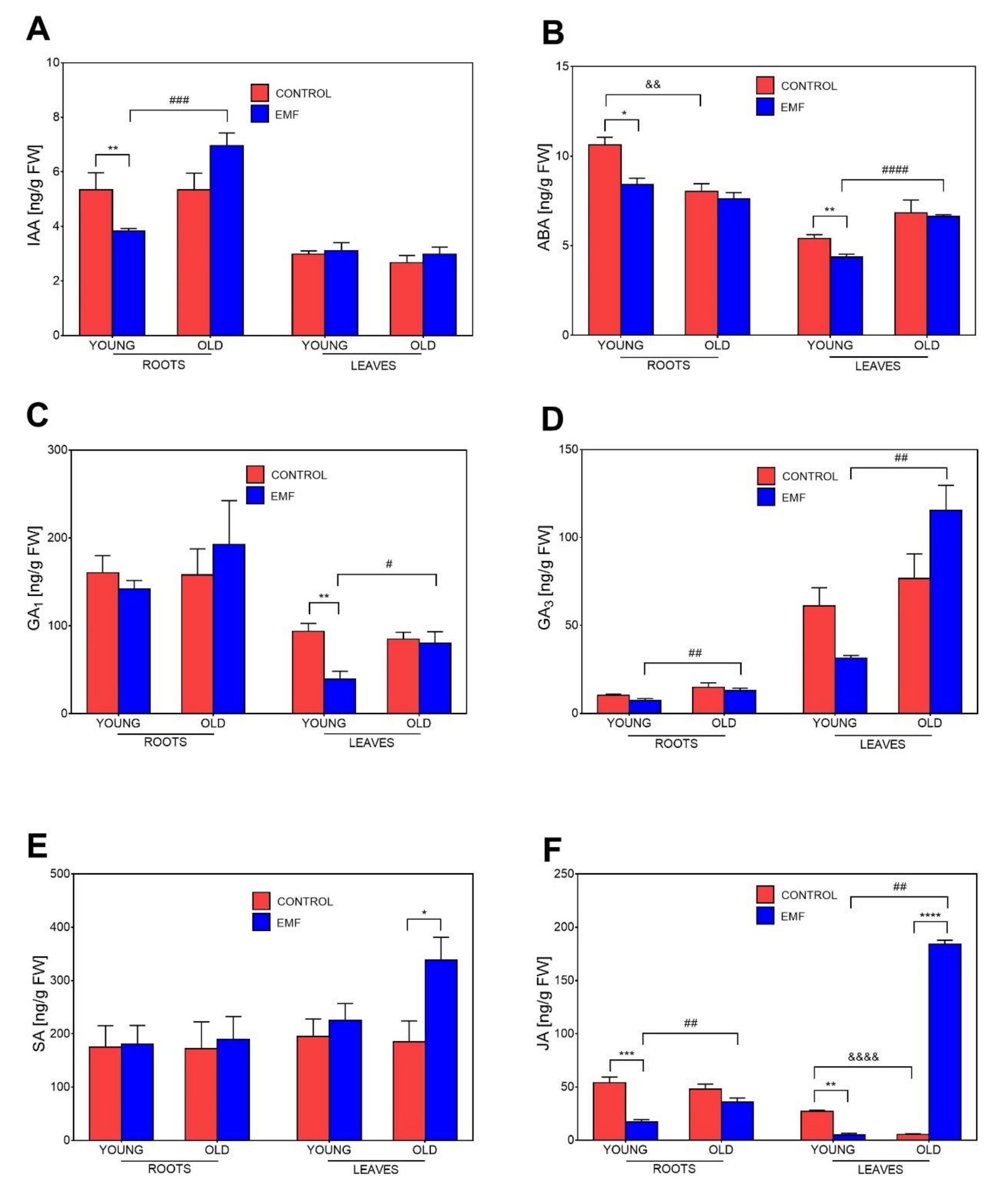
3.8. Influence of Seed Age and EMF Exposure on Phytohormone Levels in Seedlings Growing in Substrate-Filled Pots
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert, M.; Thomas, A.; Bergez, J.-E. Processes of Adaptation in Farm Decision-Making Models. A Review. Agron. Sustain. Dev. 2016, 36, 64. [Google Scholar] [CrossRef]
- Laik, R.; Kumara, B.H.; Pramanick, B.; Singh, S.K.; Nidhi; Alhomrani, M.; Gaber, A.; Hossain, A. Labile Soil Organic Matter Pools Are Influenced by 45 Years of Applied Farmyard Manure and Mineral Nitrogen in the Wheat—Pearl Millet Cropping System in the Sub-Tropical Condition. Agronomy 2021, 11, 2190. [Google Scholar] [CrossRef]
- Hampton, J.G.; Conner, A.J.; Boelt, B.; Chastain, T.G.; Rolston, P. Climate Change: Seed Production and Options for Adaptation. Agriculture 2016, 6, 33. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Sun, J.; Meng, J.; Tao, J. Deterioration of Orthodox Seeds during Ageing: Influencing Factors, Physiological Alterations and the Role of Reactive Oxygen Species. Plant Physiol. Biochem. 2021, 158, 475–485. [Google Scholar] [CrossRef]
- Kumar, M.; Mitra, S.; Mazumdar, S.P.; Majumdar, B.; Saha, A.R.; Singh, S.R.; Pramanick, B.; Gaber, A.; Alsanie, W.F.; Hossain, A. Improvement of Soil Health and System Productivity through Crop Diversification and Residue Incorporation under Jute-Based Different Cropping Systems. Agronomy 2021, 11, 1622. [Google Scholar] [CrossRef]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Dutta, A.; Trivedi, A.; Nath, C.P.; Gupta, D.S.; Hazra, K.K. A Comprehensive Review on Grain Legumes as Climate-smart Crops: Challenges and Prospects. Environ. Chall. 2022, 7, 100479. [Google Scholar] [CrossRef]
- Mariotti, M.; Andreuccetti, V.; Arduini, I.; Minieri, S.; Pampana, S. Field Bean for Forage and Grain in Short-Season Rainfed Mediterranean Conditions. Ital. J. Agron. 2018, 13, 208–215. [Google Scholar] [CrossRef]
- Sulas, L.; Roggero, P.P.; Canu, S.; Seddaiu, G. Potential Nitrogen Source from Field Bean for Rainfed Mediterranean Cropping Systems. Agron. J. 2013, 105, 1735–1742. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed Priming: A Potential Supplement in Integrated Resource Management Under Fragile Intensive Ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Araújo, S.S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical Methods for Seed Invigoration: Advantages and Challenges in Seed Technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (MF) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Muszyński, S.; Dziwulska, A. Electromagnetic Fields and Electromagnetic Radiation as Non-Invasive External Stimulants for Seeds (Selected Methods and Responses). Int. Agrophys. 2007, 21, 95–100. [Google Scholar]
- Anand, A.; Kumari, A.; Thakur, M.; Koul, A. Hydrogen Peroxide Signaling Integrates with Phytohormones during the Germination of Magnetoprimed Tomato Seeds. Sci. Rep. 2019, 9, 8814. [Google Scholar] [CrossRef]
- Cecchetti, D.; Pawełek, A.; Wyszkowska, J.; Antoszewski, M.; Szmidt-Jaworska, A. Treatment of Winter Wheat (Triticum aestivum L.) Seeds with Electromagnetic Field Influences Germination and Phytohormone Balance Depending on Seed Size. Agronomy 2022, 12, 1423. [Google Scholar] [CrossRef]
- Podleśny, J.; Podleśna, A.; Gładyszewska, B.; Bojarszczuk, J. Effect of Pre-Sowing Magnetic Field Treatment on Enzymes and Phytohormones in Pea (Pisum sativum L.) Seeds and Seedlings. Agronomy 2021, 11, 494. [Google Scholar] [CrossRef]
- Vian, A.; Davies, E.; Gendraud, M.; Bonnet, P. Plant Responses to High Frequency Electromagnetic Fields. BioMed Res. Int. 2016, 2016, 1830262. [Google Scholar] [CrossRef]
- Ribeiro-Oliveira, J.P. Electromagnetism and Plant Development: A New Unknown in a Known World. Theor. Exp. Plant Physiol. 2019, 31, 423–427. [Google Scholar] [CrossRef]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and Physical Stress Factors Used to Enhance Vegetables Production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Podleśny, J.; Misiak, L.E.; Podleśna, A.; Pietruszewski, S. Concentration of Free Radicals in Pea Seeds after Pre-Sowing Treatment with Magnetic Field. Int. Agrophys. 2005, 19, 243–249. [Google Scholar]
- Podleśny, J.; Pietruszewski, S.; Podleśna, A. Efficiency of the Magnetic Treatment of Broad Bean Seeds Cultivated under Experimental Plot Conditions. Int. Agrophys. 2004, 18, 65–71. [Google Scholar]
- Radhakrishnan, R.; Ranjitha Kumari, B.D. Pulsed Magnetic Field: A Contemporary Approach Offers to Enhance Plant Growth and Yield of Soybean. Plant Physiol. Biochem. 2012, 51, 139–144. [Google Scholar] [CrossRef]
- Ivankov, A.; Zukiene, R.; Nauciene, Z.; Degutyte-Fomins, L.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. The Effects of Red Clover Seed Treatment with Cold Plasma and Electromagnetic Field on Germination and Seedling Growth Are Dependent on Seed Color. Appl. Sci. 2021, 11, 4676. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Pauzaite, G.; Naucienė, Z.; Zukiene, R.; Degutyte-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V. Seed Treatment with Cold Plasma and Electromagnetic Field Induces Changes in Red Clover Root Growth Dynamics, Flavonoid Exudation, and Activates Nodulation. Plasma Processes Polym. 2021, 18, 2000160. [Google Scholar] [CrossRef]
- Thomas, S.; Anand, A.; Chinnusamy, V.; Dahuja, A.; Basu, S. Magnetopriming Circumvents the Effect of Salinity Stress on Germination in Chickpea Seeds. Acta Physiol. Plant. 2013, 35, 3401–3411. [Google Scholar] [CrossRef]
- Kataria, S.; Baghel, L.; Guruprasad, K.N. Pre-Treatment of Seeds with Static Magnetic Field Improves Germination and Early Growth Characteristics under Salt Stress in Maize and Soybean. Biocatal. Agric. Biotechnol. 2017, 10, 83–90. [Google Scholar] [CrossRef]
- Aguilar, C.H.; Dominguez-Pacheco, A.; Carballo, A.; Cruz-Orea, A.; Ivanov, R.; Luis, J.; Pastor, J. Alternating Magnetic Field Irradiation Effects on Three Genotype Maize Seed Field Performance. Acta Agroph. 2009, 14, 7–17. [Google Scholar]
- Iqbal, M.; Haq, Z.U.; Jamil, Y.; Ahmad, M.R. Effect of Presowing Magnetic Treatment on Properties of Pea. Int. Agrophys. 2012, 26, 25–31. [Google Scholar] [CrossRef]
- Bujak, K.; Frant, M. Influence of pre-sowing seed stimulation with magnetic field on spring wheat yielding. Acta Agroph. 2009, 14, 19–29. [Google Scholar]
- Cakmak, T.; Dumlupinar, R.; Erdal, S. Acceleration of Germination and Early Growth of Wheat and Bean Seedlings Grown under Various Magnetic Field and Osmotic Conditions. Bioelectromagnetics 2010, 31, 120–129. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Magnetic Fields: How Is Plant Growth and Development Impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Trawiński, T.; Szczygieł, M.; Wyszkowska, J.; Kluszczyński, K. Analysis of Magnetic Field Distribution and Mechanical Vibration of Magnetic Field Exciter under Different Voltage Supply. In Information Technologies in Biomedicine; Piȩtka, E., Kawa, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 613–622. [Google Scholar]
- Bieńkowski, P.; Wyszkowska, J. Technical aspects of exposure to magnetic fields of extremely low frequencies (ELF) in biomedical research. Med. Pr. 2015, 66, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Ranal, M.A.; de Santana, D.G. How and Why to Measure the Germination Process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Kader, M. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar]
- Coolbear, P.; Francis, A.; Grierson, D. The Effect of Low Temperature Pre-Sowing Treatment on the Germination Performance and Membrane Integrity of Artificially Aged Tomato Seeds. J. Exp. Bot. 1984, 35, 1609–1617. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: San Diego, CA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Pu, C.-H.; Lin, S.-K.; Chuang, W.-C.; Shyu, T.-H. Modified QuEChERS Method for 24 Plant Growth Regulators in Grapes Using LC-MS/MS. J. Food Drug Anal. 2018, 26, 637–648. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Sherin, G.; Aswathi, K.P.R.; Puthur, J.T. Photosynthetic Functions in Plants Subjected to Stresses Are Positively Influenced by Priming. Plant Stress 2022, 4, 100079. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant Hormone Regulation of Abiotic Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Salvi, P.; Manna, M.; Kaur, H.; Thakur, T.; Gandass, N.; Bhatt, D.; Muthamilarasan, M. Phytohormone Signaling and Crosstalk in Regulating Drought Stress Response in Plants. Plant Cell Rep. 2021, 40, 1305–1329. [Google Scholar] [CrossRef]
- Ruzic, R.; Jerman, I.; Gogala, N. Effects of Weak Low-Frequency Magnetic Fields on Spruce Seed Germination under Acid Conditions. Can. J. For. Res. 1998, 28, 609–616. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common Sunflower (Helianthus annus L.) Seeds with Radio-Frequency Electromagnetic Field and Cold Plasma Induces Changes in Seed Phytohormone Balance, Seedling Development and Leaf Protein Expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Katsenios, N.; Karkanis, A.; Papastylianou, P.; Triantafyllidis, V.; Travlos, I.; Bilalis, D.J. Effects of Presowing Pulsed Electromagnetic Treatment of Tomato Seed on Growth, Yield, and Lycopene Content. Sci. World J. 2014, 2014, 369745. [Google Scholar] [CrossRef]
- Muszyński, S.; Gagoś, M.; Pietruszewski, S. Short-Term Pre-Germination Exposure to ELF Magnetic Field Does Not Influence Seedling Growth in Durum Wheat (Triticum Durum). Pol. J. Environ. Stud. 2009, 18, 1065–1072. [Google Scholar]
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of Perennial Woody Plants to Seed Treatment by Electromagnetic Field and Low-Temperature Plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J.; Martínez, B.; Fontcuberta, J. Diamagnetic Susceptibility and Root Growth Responses to Magnetic Fields in Lens Culinaris, Glycine Soja, and Triticum Aestivum. Electromagn. Biol. Med. 2004, 23, 97–112. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, W.; Hu, X.; Liu, M.; Xu, X.; Hu, F.; Lan, Y.; Lv, C.; Fang, Y.; Liu, M.; et al. Static Magnetic Field Regulates Arabidopsis Root Growth via Auxin Signaling. Sci. Rep. 2019, 9, 14384. [Google Scholar] [CrossRef]
- Bilalis, D.J.; Katsenios, N.; Efthimiadou, A.; Karkanis, A.; Efthimiadis, P. Investigation of Pulsed Electromagnetic Field as a Novel Organic Pre-Sowing Method on Germination and Initial Growth Stages of Cotton. Electromagn. Biol. Med. 2012, 31, 143–150. [Google Scholar] [CrossRef]
- Vashisth, A.; Joshi, D.K. Growth Characteristics of Maize Seeds Exposed to Magnetic Field. Bioelectromagnetics 2017, 38, 151–157. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought Stress Condition Increases Root to Shoot Ratio via Alteration of Carbohydrate Partitioning and Enzymatic Activity in Rice Seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Novitskii, Y.I.; Novitskaya, G.V.; Serdyukov, Y.A. Lipid Utilization in Radish Seedlings as Affected by Weak Horizontal Extremely Low Frequency Magnetic Field. Bioelectromagnetics 2014, 35, 91–99. [Google Scholar] [CrossRef]
- Vanderstraeten, J.; Gailly, P.; Malkemper, E.P. Low-Light Dependence of the Magnetic Field Effect on Cryptochromes: Possible Relevance to Plant Ecology. Front. Plant Sci. 2018, 9, 121. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Anand, A.; Pandita, V.K.; Nagarajan, S. Pulsed Magnetic Field Improves Seed Quality of Aged Green Pea Seeds by Homeostasis of Free Radical Content. J. Food Sci. Technol. 2016, 53, 3969–3977. [Google Scholar] [CrossRef]
- Martínez, F.R.; Pacheco, A.D.; Aguilar, C.H.; Pardo, G.P.; Ortiz, E.M. Effects of Magnetic Field Irradiation on Broccoli Seed with Accelerated Aging. Acta Agroph. 2014, 21, 63–73. [Google Scholar]
- Bhardwaj, J.; Anand, A.; Nagarajan, S. Biochemical and Biophysical Changes Associated with Magnetopriming in Germinating Cucumber Seeds. Plant Physiol. Biochem. 2012, 57, 67–73. [Google Scholar] [CrossRef]
- Payez, A.; Ghanati, F.; Behmanesh, M.; Abdolmaleki, P.; Hajnorouzi, A.; Rajabbeigi, E. Increase of Seed Germination, Growth and Membrane Integrity of Wheat Seedlings by Exposure to Static and a 10-KHz Electromagnetic Field. Electromagn. Biol. Med. 2013, 32, 417–429. [Google Scholar] [CrossRef]
- Wei, K.; Jin, X.; Chen, X.; Wu, F.; Zhou, W.; Qiu, B.; Qiu, L.; Wang, X.; Li, C.; Zhang, G. The Effect of H2O2 and Abscisic Acid (ABA) Interaction on β-Amylase Activity under Osmotic Stress during Grain Development in Barley. Plant Physiol. Biochem. 2009, 47, 778–784. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Tawaratsumida, T.; Kondo, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Zheng, S.-H.; Yuasa, T.; Iwaya-Inoue, M. Reactive Oxygen Species Are Involved in Gibberellin/Abscisic Acid Signaling in Barley Aleurone Cells. Plant Physiol. 2012, 158, 1705–1714. [Google Scholar] [CrossRef]
- Ramesh, B.; Kavitha, G.; Gokiladevi, S.; Balachandar, R.K.; Kavitha, K.; Gengadharan, A.C.; Puvanakrishnan, R. Effect of Extremely Low Power Time-Varying Electromagnetic Field on Germination and Other Characteristics in Foxtail Millet (Setaria Italica) Seeds. Bioelectromagnetics 2020, 41, 526–539. [Google Scholar] [CrossRef]
- Podleśna, A.; Bojarszczuk, J.; Podleśny, J. Effect of Pre-Sowing Magnetic Field Treatment on Some Biochemical and Physiological Processes in Faba Bean (Vicia faba L. Spp. Minor). J. Plant Growth Regul. 2019, 38, 1153–1160. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different Modes of Hydrogen Peroxide Action During Seed Germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Rochalska, M. Influence of Frequent Magnetic Field on Chlorophyll Content in Leaves of Sugar Beet Plants. Nukleonika 2005, 50, S25–S28. [Google Scholar]
- Pazur, A.; Rassadina, V.; Dandler, J.; Zoller, J. Growth of Etiolated Barley Plants in Weak Static and 50 Hz Electromagnetic Fields Tuned to Calcium Ion Cyclotron Resonance. Biomagn. Res. Technol. 2006, 4, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, M.; Rozhon, W.; Poppenberger, B. The Role of Hormones in the Aging of Plants–A Mini-Review. Gerontology 2014, 60, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins Inhibit Nodule Senescence and Stimulate Nodule Meristem Bifurcation in Pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Huang, R. Auxin Controlled by Ethylene Steers Root Development. Int. J. Mol. Sci. 2018, 19, 3656. [Google Scholar] [CrossRef] [PubMed]
- Humplík, J.F.; Bergougnoux, V.; Van Volkenburgh, E. To Stimulate or Inhibit? That Is the Question for the Function of Abscisic Acid. Trends Plant Sci. 2017, 22, 830–841. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Achard, P.; Cheng, H.; De Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; Van Der Straeten, D.; Peng, J.; Harberd, N.P. Integration of Plant Responses to Environmentally Activated Phytohormonal Signals. Science 2006, 311, 91–94. [Google Scholar] [CrossRef]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Alhaithloul, H.A.S.; Alayafi, A.A.M.; Soliman, M.H. Effect of Gibberellic Acid on Growth, Photosynthesis and Antioxidant Defense System of Wheat under Zinc Oxide Nanoparticle Stress. Environ. Pollut. 2019, 254, 113109. [Google Scholar] [CrossRef]
- Yang, F.; Fan, Y.; Wu, X.; Cheng, Y.; Liu, Q.; Feng, L.; Chen, J.; Wang, Z.; Wang, X.; Yong, T.; et al. Auxin-to-Gibberellin Ratio as a Signal for Light Intensity and Quality in Regulating Soybean Growth and Matter Partitioning. Front. Plant Sci. 2018, 9, 56. [Google Scholar] [CrossRef]
- Adams, E.; Turner, J. COI1, a Jasmonate Receptor, Is Involved in Ethylene-Induced Inhibition of Arabidopsis Root Growth in the Light. J. Exp. Bot. 2010, 61, 4373–4386. [Google Scholar] [CrossRef]
- Kaya, A.; Doganlar, Z.B. Exogenous Jasmonic Acid Induces Stress Tolerance in Tobacco (Nicotiana tabacum) Exposed to Imazapic. Ecotoxicol. Environ. Saf. 2016, 124, 470–479. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Abd_Allah, E.F.; Ahmad, P. Jasmonic Acid and Methyl Jasmonate Modulate Growth, Photosynthetic Activity and Expression of Photosystem II Subunit Genes in Brassica oleracea L. Sci. Rep. 2020, 10, 9322. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
| Long-Day Conditions | Continuous Darkness | |||||||
|---|---|---|---|---|---|---|---|---|
| Young— Control | Young— EMF | Old— Control | Old—EMF | Young— Control | Young—EMF | Old— Control | Old—EMF | |
| G | 88 ± 4 | 86.66 ± 6.36 | 38 ± 8.08 & | 48.66 ± 4.81 ## | 97.33 ± 0.66 | 95.33 ± 0.66 | 52.66 ± 4.37 & | 60.66 ± 1.76 #### |
| GI | 203.33 ± 0.82 | 215.33 ± 1.27 | 71.33 ± 1.51 & | 99 ± 0.81 ## | 223.67 ± 0.37 | 218.67 ± 0.27 | 99.33 ± 1.12 & | 120.67 ± 0.17 #### |
| MGT | 2.38 ± 0.1 | 2.04 ± 0.05 | 3.17 ± 0.1 & | 2.92 ± 0.05 ## | 2.41 ± 0.06 | 2.41 ± 0.07 | 3.25 ± 0.08 & | 3.02 ± 0.04 ## |
| CVG | 42.41 ± 0.43 | 49.12 ± 0.26 | 31.77 ± 0.34 & | 34.33 ± 1.68 ## | 41.68 ± 0.23 | 41.63 ± 0.28 | 30.9 ± 0.23 & | 33.17 ± 0.13 # |
| t50 | 1.8 ± 0.15 | 1.64 ± 0.18 | 2.53 ± 0.16 & | 2.39 ± 0.03 # | 1.8 ± 0.12 | 1.77 ± 0.07 | 2.67 ± 0.09 & | 2.53 ± 0.05 ### |
| Young—Control | Young—EMF | Old—Control | Old—EMF | |
|---|---|---|---|---|
| G | 98 ± 2 | 90 ± 4.47 | 72 ± 9.69 & | 70 ± 6.32 # |
| GI | 99.8 ± 2.4 | 89.2 ± 3.84 * | 66.8 ± 7.78 & | 65 ± 5.67 ## |
| MGT | 4.81 ± 0.14 | 5.06 ± 0.27 | 5.74 ± 0.43 | 5.69 ± 0.19 |
| CVG | 20.83 ± 0.58 | 19.98 ± 1.11 | 17.76 ± 1.11 & | 17.65 ± 0.6 |
| t50 | 4.07 ± 0.08 | 4.03 ± 0.09 | 4.73 ± 0.2 & | 4.51 ± 0.24 |
| Long-Day Conditions | |||||
|---|---|---|---|---|---|
| Seedling Growth Parameters | Young—Control | Young—EMF | Old— Control | Old— EMF | |
| Fully emerged roots and epicotyls | Epicotyl length (mm) | 7.48 ± 0.37 | 8.27 ± 0.66 | - | 6.5 ± 0.5 |
| Root length (mm) | 17.45 ± 1.22 | 14.85 ± 1.05 | - | 11.5 ± 0.5 | |
| Seedling fresh weight (mg) | 157.70 ± 8.15 | 142.77 ± 7.97 | - | 145.5 ± 7.5 | |
| Seedling dry weight (mg) | 30.33 ± 4.81 | 47 ± 22.55 | - | 26 ± 0.1 | |
| Only roots protruded | Root length (mm) | 8.38 ± 0.53 | 8.87 ± 0.58 | 6.56 ± 0.38 & | 7.13 ± 0.3 # |
| Emerged embryo fresh weight (mg) | 76.42 ± 3.4 | 83.04 ± 3.18 | 67.21 ± 4.14 | 75.97± 3.55 | |
| Continuous Darkness | |||||
|---|---|---|---|---|---|
| Seedling Growth Parameters | Young—Control | Young—EMF | Old— Control | Old— EMF | |
| Fully emerged roots and epicotyls | Epicotyl length (mm) | 9.61 ± 0.42 | 11 ± 0.54 * | 9.22 ± 0.74 | 10 ± 0.62 |
| Root length (mm) | 16.83 ± 1.06 | 15.81 ± 0.97 | 12.22 ± 0.85 & | 11.35 ± 0.79 ## | |
| Seedling fresh weight (mg) | 172.55 ± 5.25 | 179.35 ± 7.53 | 147.83 ± 7.31 & | 142.96 ± 6.88 ## | |
| Seedling dry weight (mg) | 32.66 ± 6.12 | 29.33 ± 7.13 | 17.33 ± 2.85 | 20.33 ± 0.88 | |
| Only roots protruded | Root length (mm) | 9.11 ± 0.46 | 9.72 ± 0.46 | 6.44 ± 0.31 &&&& | 7.84 ± 0.25 ***,## |
| Emerged embryo fresh weight (mg) | 104.6 ± 3.47 | 108.62 ± 3.22 | 78.67 ± 3.98 &&&& | 95.72 ± 3.25 **,## | |
| Seedling Growth Parameters | Young—Control | Young— EMF | Old— Control | Old— EMF |
|---|---|---|---|---|
| Stem length (cm) | 31.02 ± 0.83 | 27.95 ± 1.23 * | 29.24 ± 1.06 | 26.79 ± 1.39 |
| Root length (cm) | 20.69 ± 0.54 | 23.43 ± 0.93 ** | 22.28 ± 0.62 | 21.5 ± 1.08 |
| Stem fresh weight (g) | 3.68 ± 0.14 | 3.33 ± 0.17 | 3.6 ± 0.25 | 3.05 ± 0.20 |
| Root fresh weight (g) | 1.87 ± 0.09 | 1.66 ± 0.09 | 1.61 ± 0.10 | 1.61 ± 0.13 |
| Stem dry weight (mg) | 232.90 ± 9.19 | 205.19 ± 14.53 | 216.06 ± 60.79 | 190.78 ± 16.46 |
| Root dry weight (mg) | 115.41 ± 8.85 | 102.64 ± 5.96 | 112.70 ± 27.04 | 99.88 ± 7.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawełek, A.; Wyszkowska, J.; Cecchetti, D.; Dinka, M.D.; Przybylski, K.; Szmidt-Jaworska, A. The Physiological and Biochemical Response of Field Bean (Vicia faba L. (partim)) to Electromagnetic Field Exposure Is Influenced by Seed Age, Light Conditions, and Growth Media. Agronomy 2022, 12, 2161. https://doi.org/10.3390/agronomy12092161
Pawełek A, Wyszkowska J, Cecchetti D, Dinka MD, Przybylski K, Szmidt-Jaworska A. The Physiological and Biochemical Response of Field Bean (Vicia faba L. (partim)) to Electromagnetic Field Exposure Is Influenced by Seed Age, Light Conditions, and Growth Media. Agronomy. 2022; 12(9):2161. https://doi.org/10.3390/agronomy12092161
Chicago/Turabian StylePawełek, Agnieszka, Joanna Wyszkowska, Daniele Cecchetti, Mergi Daba Dinka, Krzysztof Przybylski, and Adriana Szmidt-Jaworska. 2022. "The Physiological and Biochemical Response of Field Bean (Vicia faba L. (partim)) to Electromagnetic Field Exposure Is Influenced by Seed Age, Light Conditions, and Growth Media" Agronomy 12, no. 9: 2161. https://doi.org/10.3390/agronomy12092161
APA StylePawełek, A., Wyszkowska, J., Cecchetti, D., Dinka, M. D., Przybylski, K., & Szmidt-Jaworska, A. (2022). The Physiological and Biochemical Response of Field Bean (Vicia faba L. (partim)) to Electromagnetic Field Exposure Is Influenced by Seed Age, Light Conditions, and Growth Media. Agronomy, 12(9), 2161. https://doi.org/10.3390/agronomy12092161








