Abstract
Garden pea (Pisum sativum L.) is an important legume crop, which is widely planted in Yunnan-Guizhou Plateau and Sichuan Basin of southwest China. It has developed rapidly in spring planting agroecological zone in Northwest China in recent years. The major constraints to its cultivation are lodging and infection of powdery mildew. Breeding of high yielding cultivars resistant to powdery mildew is of great significance for the sustainability of pea production, because few local garden pea cultivars are resistant to the disease. Varietal diversification is needed to develop pea cultivars with resistance to lodging and powdery mildew. Breeding work was initiated to develop a high-yielding garden pea cultivar with medium maturity, double podding and resistance to powdery mildew. Longwan 5 (X9002) is high yielding, superior quality, multiple resistance, and climate resilient garden pea cultivar developed by hybridization between Shuanghua 101 and Baofeng 3. It is a semi-leafless pea variety with superiority over existing approved varieties Qizhen 76 and Xucai 1 in terms of green pod yield, medium maturity, and double podding. Longwan 5 gave a significantly higher average green pod yield (12,376 kg/ha) than check varieties Qizhen 76 (11,132 kg/ha) and Xucai 1 (11,649 kg/ha) across five locations and three years, which was 11.2% and 6.3% higher than control varieties, respectively. This variety is tolerant to lodging, powdery mildew disease, and wide climate resilient for spring cultivation as well as for autumn cultivation in irrigated conditions or rain-fed agricultural areas with annual precipitation of 450–650 mm in China. Cultivation of this variety on large scale will surely increase the production of peas in China and will also prove beneficial for farmers increasing their income.
1. Introduction
Peas (Pisum sativum L.) are cultivated worldwide in temperate climates and are the fourth most important legume crop. Meanwhile, garden peas are also one of the main edible bean crops in China, with a perennial planting area of about 1.4 million hectares, accounting for 55.3% of the world’s total area [1]. China is the world’s largest producer of garden peas and plays an important role in the world’s pea production. China has a variety of agricultural ecological types. Garden pea is often cultivated during winter in subtropical and early spring in temperate zones by smallholder farmers. Winter sowing areas are mainly in Yunnan, Jiangsu, Sichuan, Guangdong, Fujian, Zhejiang, and Shandong; spring sowing areas are mainly in Gansu, Ningxia, Inner Mongolia, Hebei, and other places [2]. In recent years, there has been an unprecedented demand for new varieties of garden peas from farmers due to the improvement in people’s diets and the fact that peas have a short growing period and are easy to manage, which can enrich the soil [3]. However, for a long time, when introducing and breeding pea varieties in China, the cultivar improvement of dry pea is concerned. The breeding of pea plant type was mainly vine type, and the improvement of modern pea cultivar resistant to biotic and abiotic stress was mainly focused on resistance to drought in the Northern Arid and Semi-arid region [4], and resistance to frost for winter-hardy pea cultivar [5], and resistance to root rot diseases (Aphanomyes euteiches) [6]. Unfortunately, the program of garden pea cultivar with high resistance to lodging and powdery mildew (Erysiphe pisi DC.) and wide suitability for irrigated cropland were largely neglected. The improvement of semi-leafless peas with resistance to lodging, resistance to powdery mildew, and better suitability for irrigated conditions or rain-fed agricultural areas with annual precipitation of 450–650 mm may be the most effective measure to increase pea production potential and the relative profitability of grain legumes.
Semi-leafless pea is a leaf mutant caused by the afila gene (af) [7], with normal stipules and all leaflets are replaced by tendril complexes which improve canopy structure and ventilation and light transmission conditions of the colony [8]. Moreover, developed tendrils can intertwine each other to form scaffolding structures, thus significantly improving the lodging resistance of pea. Lodging is a major constraint to the production of many crops including pea [9]. The lodging resistance breeding of pea has important application value in agricultural production [10].
Modern field pea cultivars with better lodging resistance are very dependent on the afila gene, as they tend to be bush-type tendrilled architecture with low-leaf biomasses [11]. Over more than the past 20 years, steady progress has been made in improving the agronomic and quality characteristics of semi-leafless field pea as evidenced in the cultivars released in Canada, USA, France and other main pea-producing countries [12]. However, breeding and utilization of semi-leafless pea varieties in China lag far behind outside countries. Over the past decade, few semi-leafless dry pea varieties such as Yunwan 35 [13], Longwan 6 [14], and Longwan 10 [15] were registered and released; but semi-leafless garden pea varieties are even few. Semi-leafless varieties of pea have considerable agronomic importance and they have been suggested that they may have a better standing ability [16], and higher resistance to powdery mildew than conventional varieties [17].
Developing high-yielding, strong lodging resistance and powdery mildew resistant cultivars are important in pea breeding strategy [18], and evaluation across locations and years would form a basis for breeding [19]. In China, dry pea breeding programs has been strengthened with the launch of the China Agriculture Research System of MOF and MARA-Food Legumes (CARS-08) in 2008, but little attention has been paid to the improvement of garden pea varieties. Current commercial garden pea varieties, Qizhen 76, Tiancui 761, Taizhong 11, introduced from the World Vegetable Centre (AVRDC), Tainan, Taiwan [2], which are susceptible to powdery mildew and a long maturity period in spring sowing areas. Moreover, these climbing garden varieties are expensive to trellis with wooden posts and plastic strings used for vine staking, while semi-leafless garden pea varieties could reduce these costs [11]. Therefore, developing new powdery mildew resistant and early maturing cultivars is a paramount solution for the resource-poor farmers growing pea. This work presents the overall performances of the recently developed and released garden cultivar Longwan 5, an edible-podded sweet pea cultivar with high resistance to powdery mildew.
2. Materials and Methods
2.1. Cross Information and Breeding Method
Longwan 5 is a garden pea cultivar developed by hybridization between female parent Shuanghua 101 and male parent Baofeng 3. Medium maturity, resistance to powdery mildew, attractive dark green pod with sickle shape, high yielding and lodging resistance were major breeding objectives for the development of the cultivar. The cross between Shuanghua 101 × Baofeng 3 was made in the summer of 2005 at the Qinwangchuan Experimental Station (QES) of Academy of Gansu Agriculture Sciences (AGAS), Lanzhou, China (latitude: 36°43′ N; longitude: 103°38′ E; altitude: 1950 m mean sea level; Annual precipitation: 319 mm; Annual average temperature: 5.9 °C). Shuanghua 101 is a leafy, long vine length and powdery mildew susceptible pure line selected from Qizhen 76. Baofeng 3 is a semi-leafless pea cultivar resistant to powdery mildew, derived from the cross Zhongwan 5 × 90-EP-10, where Zhongwan 5 was developed at Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS—CAAS), Beijing, China, and 90-EP-10 was obtained from the Institute of Crop Breeding and Cultivation, Qinghai Academy of Agriculture and Forestry Sciences (ICBC—QAAFS), Xining, China.
2.2. Selection Process
The breeding method used for Longwan 5 was a pedigree selection in combination with single seed descent. All breeding activities of the F1–F5 generations were conducted in Qinwangchuan Research Station (QRS). In 2006, 20 F1 were planted. A total of 725 F2 seeds were bulked and in 2007, F2 seeds were planted and 120 healthy superior plants were selected (Table 1). All F3 to F5 plant progenies were grown and single plant selection was based on medium maturity, resistance to powdery mildew, lodging resistance, attractive dark green curved pod, and high green pod yield and seed were collected separately. The selected plants from the F5 were grown as F6 progeny rows and bulk-harvested to form breeding lines. Plant progenies at the F7 generation were evaluated in a preliminary screening trial for green pod yield, lodging resistance, resistance to powdery mildew and main characteristics in Yongdeng, Gansu, in 2010. One of the selected lines from the screening trial based on the performance for these traits was X9002 and evaluated in a replicated preliminary yield test at Yongdeng, Gansu province, in 2011. From 2012 to 2014, It was entered into Gansu Pea Coordinated Trials (GPCT) and evaluated in replicated trials at five locations. X9002, named Longwan 5, was registered in the Gansu Crop Cultivar Catalogue (GCCC) in 2015, and subsequently registered in National Pea Varieties Catalogue in China (NPVCC, Registration code: GPD Wandou (2018) 620008) in 2018.

Table 1.
Population size and selection intensity in each segregating generation.
2.3. Testing Sites and Field Evaluation
All F1 to F6 plant progenies selection and preliminary screening trial were performed in irrigated field condition at Qinwangchuan Experimental Station (QES) of Academy of Gansu Agriculture Sciences (AGAS), Lanzhou, China. Then multi-sites advanced yield trials were conducted in different agroecological zones for yield traits performance. From 2012 to 2014, Longwan 5 was evaluated in replicated trials at five sites in northwest China with two sites (Yongdeng and Minle) in Hexi Corridor Irrigated Zone (HCIZ), two sites (Tianzhu and Gannan) in High-Latitude Climates (HLC), and one site (Dingxi) in Northern Arid and Semi-arid region (NASR). For the five sites, long-term average total precipitation varied from 319 mm to 621 mm per year, with 53.2–62.2% of the yearly precipitation occurring during the pea growing season. The long-term average annual temperature of the sites was 5.0 °C, with yearly average temperatures ranging from 4.1 °C to 7.1 °C. The daily minimum temperatures in spring varied from −4 °C to −7 °C, and the daily maximum temperatures in summer varied from 19 °C to 26 °C between sites. Additional information on soil types, biotic or crop management factors and climatic characteristics of experimental sites are presented (Table 2).

Table 2.
Locational and climatic characteristics of multi-sites in different agricultural climatic zoning, soil types, agricultural climatic zone, and growing conditions during the pea growing season.
2.4. Field Evaluation
Garden pea lines together with check cultivars were evaluated for pod yield traits performance during the main cropping season and location-year was treated as a single environment. During each year, the experiment with genotypes was carried out in a randomized complete block design (RCBD) with three replications. The plot size was 2 × 5 m (10 m2) having six rows and spacing of 40 cm between rows and 3–5 cm between plants was maintained. Diammonium phosphate (46% P, 18% N) fertilizer with 150 kg/ha was pre-plant incorporated and appropriate cultural practices (weed removal by hand, disease and pest control) were followed. Appropriate irrigation was performed in Minle and Yongdeng sites at the seedling and flowering stages. Peas are usually sown between mid-March and early April. Green pods are picked from late June to mid-July. The lodging resistance of the pea cultivar was identified according to the method described by Bilgili and Uzun et al. [20]. At the full flowering and pod filling stages, lodging tolerance was rated on a 1–5 scale, where 5 = no lodging and 1 = entire plot lodged.
2.5. Evaluation Resistance to Powdery Mildew
Reactions to powdery mildew (Erysiphe pisi DC) of Longwan 5 and advanced breeding lines were evaluated during the pod-filling period in field disease nursery on QES in 2014 and 2015 using the method described by Iqbal et al. [21], and greenhouse evaluations using the method described by Sun and Wang [17]. Snap pea cultivar Qizhen 76 harbouring the susceptible gene (Er1), was used as a susceptible check cultivar for evaluating uniformity of inoculation [22], and Xucai 1 carrying resistance gene (er1-2) as a resistant check cultivar [17]. The E. pisi isolate EPBJ (NCBI accession number KR912079) isolated from greenhouse infected pea, was maintained under greenhouse conditions on the susceptible cultivar Longwan 1 as inoculum for the screening trials. The plants were inoculated with EPBJ using conidia shed by shaking heavily infected plants. Longwan 5 and check cultivars were assessed for resistance 10–14 days after inoculation using a 0–4 scale according to the infected foliage area, macroscopic and microscopic density of mycelia, and sporulation on the lower part of the plant [23,24]. Disease severity scales for resistance to powdery mildew are determined according to the infected foliage area in the pea [25]. (A) 0 = no mycelial growth and sporulation on the plant, (B) 1 = sparse mycelial growth and very little sporulation on less than 5% of leaf, (C) 2 = slight to moderate growth mycelium with conidiophores on 5–25% of leaf, (D) 3 = moderate to heavy growth mycelium with sporulation on 25–75% of leaf, and (E) 4 = abundant growth mycelium with heavy sporulation on greater than 75% of leaf. Plants with scores of 0–2 were classified as resistant (R) and those with scores of 3–4 as susceptible (S) [23,24].
2.6. Data Collection and Analysis
Data on green pod yields (GPY) and 11 yield-related traits (YRT) were collected on plot and plant basis from each plot, respectively. Branches per plant (BPP) and date to maturity (DM) were taken when each plot attained 50% flowering and 90% of the pod’s physiological maturity, respectively, and days were calculated beginning from the date of sowing. Data for plant height (PH), pods per plant (PPP), seeds per plant (SPP), hundred green pod weight (HGPW), weight of green pod per plant (WPP), internodes per main stem (IPMS), first blossom node (FBN) and seeds per pod (SPD), double pod rate (DPR) were collected based on five sample plants which were randomly taken from each plot and the average of five sample plants were used for analysis. The measured green pod yield (GPY) value of the plot was converted to kilogram per hectare for analysis. ANOVA was performed using the SPSS statistical program v.26.0 (IBM Corporation, New York, NY, USA) and differences among treatments for all measurements were compared at p = 0.05 and by using Duncan’s multiple-range test.
3. Results
3.1. Morphological Characteristics and Agronomic Traits
Longwan 5 is a semi-leafless sugar snap pea and has white flowers, yellow cotyledons, and wrinkled seeds. It has strong lodging resistance and disease resistance. The data (Table 3) clearly show that Longwan 5 had significant advantages over the check varieties Qizhen 76 (conventional garden pea varieties) and Xucai 1 (semi-leafless garden pea varieties) in plant height, maturity, lodging resistance, pod number per plant, double podding rate, branches per plant, and other agronomic characteristics.

Table 3.
Morphological characteristics and agronomic traits of garden pea lines on-station.
The main morphological and agronomic traits of Longwan 5 (X9002) are presented (Figure 1). Longwan 5 has semi-dwarf stems and dwarf growth habits with PH of 75–90 cm. It produced the average number of BPP (1.7), while Xucai 1 plants had the average number of branches (1.4). The main stem of Longwan 5 has 15–17 nodes with the first blossom at the 8–9th node. Fifty percent flowering of Longwan 5 occurred in 65–70 days with a flowering period of 25–30 days after sowing in spring. The number of flowers per inflorescence is 1 to 2 with white flowers. Longwan 5 has dark green curved pods, mostly double podding per peduncle, and 6–10 pods per plant. DM of Longwan 5 was 3 and 15 days earlier than Xucai 1 and Qizhen 76, respectively. It is an early maturity cultivar having attractive green curved pods with 5–8 wrinkled seeds per pod.
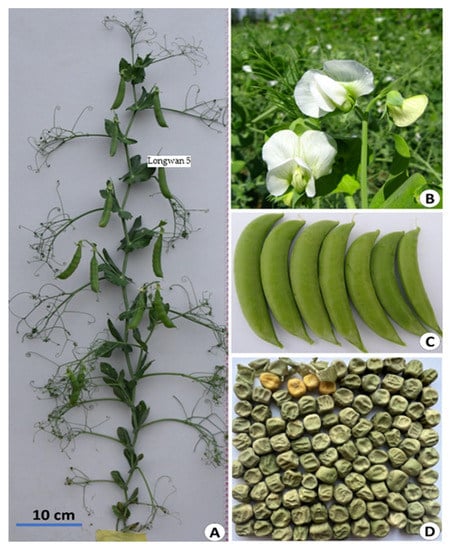
Figure 1.
Morphological characteristics and agronomic traits of semi-leafless sugar snap peas cultivar Longwan 5. (A) Morphological characteristics of individual plants; (B) white flowers; (C) edible sugar snap pods with sickle shape, stripped from plants; (D) normally mature seeds with wrinkled yellow cotyledon.
The data (Table 3) also clearly show that the average PL of Longwan 5 was nearly equal to Xucai 1 but significantly less than Qizhen 76 (8.3 cm). However, the average number of PPP of Longwan 5 (8.0) was higher than that of Xucai 1 (5.3) and Qizhen 76 (6.7). The average HGPW of Longwan 5 (594 g) is nearly equal to Xucai 1 (598 g) but less than Qizhen 76 (627 g). The lodging resistance of Longwan 5 with the best lodging score (1) was better than that of the check varieties Qizhen 76 with the worst lodging score (4). Lodging resistance is related to plant height. The PH of Longwan 5 is about two-thirds of Qizhen 76. Longwan 5 is recommended for sowing from mid-March in Hexi Corridor irrigated zone and northern arid and semi-arid region to early April in the high-latitude climate zone of Northwest China in spring.
3.2. Assessment of Resistance to Powdery Mildew
In the field trials, severe powdery mildew developed on the susceptible check cultivar Qizhen 76 in 2014 and 2015. Qizhen 76 showed symptoms with a disease score of 4 at the late-flowering to the pod-set stage, while Longwan 5 showed symptoms with a disease score of 1 at the pod-fill to early-maturity stages (Figure 2). The field study results demonstrated that Qizhen 76 is highly susceptible to powdery mildew, whereas Longwan 5 is resistant to powdery mildew. Because of the high degree of infection, whatever the pod yield was recorded in local cultivars, was not saleable. Longwan 5 were resistant and free symptoms were recorded on them.

Figure 2.
Field assessment of resistance to powdery mildew at the pod-fill to early maturity stages in Yongdeng location of spring pea planting agroecological zone in Northwest China. (A) The resistant pea cultivar Longwan 5 with free symptom; (B) the susceptible cultivars Qizhen 76 with white or gray powdery growth on the leaves, pods and stems.
In the greenhouse trials, at 10–14 days post-inoculation, the powdery mildew disease severity of susceptible check Qizhen 76 was rated as score 3 and 4. Whereas, Longwan 5 and Xucai 1 appeared to be high resistance (symptom-free) and resistance with disease scores of 2 to powdery mildew, respectively (Table 4). Based on the symptoms, two classes were observed, one resistant and the other highly susceptible. Local cultivars Qizhen 76 were susceptible with white or gray powdery on the leaves, pods and stems. It is likely that conditions in the field were more conducive to powdery mildew infection and created greater disease pressure and hence a higher disease score on test pea cultivars than was observed in the greenhouse. The greenhouse provided a consistent environment for the screening process, which was able to clearly distinguish between resistant and susceptible cultivars.

Table 4.
Disease reaction to powdery mildew of Longwan 5 together with check cultivars in field and greenhouse conditions from 2014 to 2015.
3.3. Quality Traits
According to the report from the Food Quality Testing Center of the Ministry of Agriculture of China (Table 5), the total protein content of Longwan 5 seed was 28.7%, which was lower than that of Xucai 1 (30.7%) and Qizhen 76 (31.6). However, carbohydrate content of Longwan 5 seed was 47.4%, which was higher than that of Xucai 1 (44.6%) and Qizhen 76 (45.2). Longwan 5 seeds have dietary fiber content of 3.46%, fat content of 1.96%, and lysine content of 1.53%. Green pods of Longwan 5 are crisper in taste than that of Qizhen 76 and Xucai 1, so they do not need to be shelled and are used in recipes in the same way as snow peas.

Table 5.
Evaluation of pea cultivar Longwan 5 together with check varieties for food quality.
3.4. Yield and Adaptability in Different Agroecological Zones
Longwan 5 along with local check varieties Qizhen 76 (conventional garden pea varieties, susceptibility to powdery mildew) and Xucai 1 (semi-leafless garden pea varieties, resistance to powdery mildew) was tested from 2012 to 2014 across five locations in northwest China with Hexi Corridor Irrigated Zone, High-Latitude Climates, and Northern Arid and Semi-arid Region. The grand mean showed that the cultivar Longwan 5 (12,376 kg/ha) gave a significantly higher average green pod yield than check varieties Qizhen 76 (11,132 kg/ha) and Xucai 1 (11,649 kg/ha) across five locations in three years which was 11.2% and 6.3% higher than control varieties, respectively (Table 6).

Table 6.
Mean pod yield (kg/ha) of Longwan 5 along with check varieties Qizhen 76 (conventional garden pea varieties) and Xucai 1 (semi-leafless garden pea varieties) across five locations in three agroecological zones.
In different years and three agroecological zones, the green pod yield of Longwan 5 was significantly different from both control varieties. In Hexi Corridor irrigated zone, the yield of Longwan 5 was 24.2%, 21.9%, and 15.8% higher than that of Qizhen 76, respectively, meanwhile, the yield of Longwan 5 was increased by 8.1%, 8.9%, and 5.9% compared with the control varieties Xucai 1 in 2012, 2013, and 2014, respectively (Table 6). In High-latitude Climates, Longwan 5 had a higher average yield (13,651 kg/ha) across three years, which was 22.6% significantly higher than that of the Qizhen 76, and 6.0% higher than that of the Xucai 1.
In Dingxi site, belonging to the Northern Arid and Semi-arid Region, Longwan 5 had the lowest average yield (7481 kg/ha) across three years, which was 7.1% lower than that of the control cultivar Qizhen 76 (8052 kg/ha); however, it was slightly higher by 7.5% than that of the control cultivar Xucai 1 (6993 kg/ha). The results showed that Longwan 5 was weakly resistant to drought than Qizhen 76.
4. Discussion
4.1. Breeding Metheds for Peas
Pea is a self-pollinated diploid (2n = 14, x = 7) annual crop and has been the subject of controlled hybridization and selection for decades [26]. However, the progress in pea genomics has lagged behind that of legumes with smaller genomes such as mungbean, cowpea, common bean, adzuki bean, and soybean, largely as a consequence of its genome size (4.45 Gbp) [27,28]. Modern breeding programs in peas typically use either a bulk population, pedigree selection, or a combination of these two procedures. The bulk population method has the advantage of easy population maintenance and delayed selection until the F4 or F5 when selections are near homozygosity and breed true. A disadvantage of the bulk procedure is that certain genotypes may be lost reducing the range of variation present for selection. Marker-assisted selection (MAS) and, more recently, genome-wide association studies (GWAS) and genomic selection (GS), both performed by using genome-wide markers, are becoming important and effective tools for plant breeding, especially in resistant genotype selection [29]. The wide applicability of modern molecular breeding methods such as MAS and GS have already been demonstrated in three major crops, rice, wheat, and maize [30], while in the case of pulses (pea, faba bean, cowpea, mungbean, common bean, and other grain legumes), it is in infancy stage in China. Conventional breeding methods have been successful in improving pea germplasm toward the development of superior cultivars [12].
In this study, we were successful to develop a garden pea cultivar Longwan 5 using a pedigree selection procedure in combination with single seed descent; however, it took 13 years and even longer from hybridization in 2005 to registration and release in 2018. The pedigree method maintains a wider range of the variation initially present and allows specific undesirable genotypes to be removed from the population. However, it is much more time consuming and labor intensive and requires that individual plants be separated for close observation and data collection. Therefore, to increase the efficiency of crop breeding, plant breeders and researchers around the world are using modern molecular breeding methods such as MAS and GS, in addition, the recently developed NGS-based methods such as whole-genome re-sequencing (WGRS) and restriction site-associated DNA sequencing (RADseq) in major pulse crops breeding programmes [31]. These advanced molecular breeding approaches thus represent the next generation of MAS that would greatly assist breeders to strengthen as well as reorient the pulse breeding programmes.
4.2. Genetic Analysis of Resistance to Powdery Mildew
Powdery mildew is an important pea disease, which often occurs in the late stage of pea grain filling, resulting in substantial decreases in pea yield and quality [17]. In this study, powdery mildew began to develop in early July and peaked at the end of July in spring planting agroecological zone, when all the plant parts of the local cultivars were severely infected. The appearance of powdery mildew symptoms during July and its peak incidence by the end of July have also been reported [32]. Qizhen 76 were highly susceptible with pronounced symptoms on the leaves, stems, and pods. Because of the high degree of infection, whatever the pod yield was recorded in local cultivars, was not saleable. Longwan 5 were resistant to powdery mildew with no or very few symptoms on them. Previous several studies had focused on the identification of Chinese pea germplasms resistant to powdery mildew. In the Chinese pea cultivars Longwan 5 (X9002) and Xucai 1, powdery mildew resistance is conferred by alleles er1-2, while in some Chinese pea landraces from Yunnan Province, resistance is conferred by the er1-6 allele, and in the Indian pea cultivar DDR11 is conferred by the er1-7 allele [33].
Powdery mildew is caused by an obligate parasite Erysiphe pisi and is considered one of the most important constraints causing yield reductions in pea, particularly important in climates with warm dry days and cool nights. To date, there have been some encouraging results in the practical biocontrol of several powdery mildew diseases, but they are mostly still limited to the laboratories and await large-scale agricultural trials [34]. Development and utilization of genetic resistance are acknowledged as the most effective, economic and environmentally friendly methods of control [35]. Therefore, the development of cultivars with improved resistance to biotic stresses is a primary goal of plant breeding programs throughout the world. Although varying levels of resistance to powdery mildew have been observed in pea [36,37], only three genes for resistance named er1, er2, and Er3 have been described so far [38]. Among the three genes, gene er1 was harbored by many accessions and has now been characterized with 11 distinct alleles, of which er1-1 and er1-2 are currently used by the breeders [39]. Gene er1 has been extensively used to develop resistant varieties globally [38]. However, er2 and Er3 genes were reported in a few accessions only.
Gene er2 is not used commercially. The gene confers a high level of resistance in some locations but is ineffective in others. The expression of recessive er2 is influenced by temperature and leaf age [40,41]. Dominant gene Er3 was identified from a wild relative of pea (P. fulvum) and has successfully been introgressed in the cultivated genotypes through hybridization [42,43]. Several markers have been reported to be linked to three resistant genes at varying distances in different mapping populations [44,45,46], and are currently being used in MAS strategy to aid in obtaining a complete powdery mildew resistance in pea [47].
4.3. Stability and Adaptability of Garden Pea
An understanding of the stability and adaptability of the crop is basic to its effective improvement by plant breeding. Accurate estimates of stability and adaptability of the genotype through the evaluation of given multi-environment trials are key issues in plant breeding [48]. Pea is grown mostly in cool season climates but also sub-tropical regions over winter or spring. Compared with soybean, faba bean, and other legumes, dry field pea and garden pea have wider adaptability to agroecological environments. Garden peas are grown mostly in temperate and sub-tropics zones. China is the world’s largest producer of garden peas and has a variety of agricultural ecological types [2]. Dry peas are mostly cultivated in Gansu, Qinghai, Inner Mongolia and other provinces, in Northwest of China. Whereas, garden peas are widely grown in the fall or winter, specifically in the highlands of Yunnan, Sichuan, and Guizhou provinces and near the coast [49].
In this study, advanced garden pea lines together with check cultivars were evaluated for pod yield traits performance during the main cropping season in Hexi Corridor irrigated zone (HCIZ), high-latitude climates (HLC), and northern arid and semi-arid region (NASR) of Northwest China. Compared with normal-leafed pea varieties, semi-leafless peas have higher yield potential and adaptability as they have better resistance to lodging under irrigated and rainfed environments, which is consistent with previous studies [15]. In irrigated farming, the traditional cultivation of normal-leafed peas was limited by their high susceptibility to lodging [50]. Lodging is a quantitative trait and is highly affected by environmental conditions. The semi-leafless plant type significantly increased the lodging resistance or standing ability of pea cultivars which reduced green pod yield losses and canopy disease severity [51].
Among the tested environments, the most representative and discriminating regions for green pod yield of garden pea were Hexi Corridor irrigated zone (Minle and Yongdeng locations) and high-latitude climates (Tianzhu and Gannan locations). In the northern arid and semi-arid region (Dingxi location), Longwan 5 had the lowest average yield across three years. Semi-leafless peas were lower in grain seed yield than conventional leaf-type peas in drought conditions [15]. Average pod yields of individual locations were calculated for each test pea variety across three years. There was no significant difference in pea pod yield of Longwan 5 between four locations (Yongdeng, Minle, Tianzhu, Gannan) except Dingxi location, which indicated Longwan 5 has greater stability and adaptability under irrigated conditions or rain-fed agricultural areas with the annual precipitation of 450–650 mm. These findings represent a comprehensive analysis of stability and adaptability of garden pea variety, which may be useful for national and international pea improvement programs.
5. Conclusions
It could be concluded that semi-leafless garden pea cultivar Longwan 5 is superior to existing approved varieties Qizhen 76 and Xucai 1 in terms of green pod yield, medium maturity and double podding. This cultivar is tolerant to lodging, powdery mildew disease, and wide climate resilient for spring cultivation as well as for autumn cultivation under irrigated and rainfed environments in China. Longwan 5 gave a significantly higher average green pod yield (12,376 kg/ha) than check varieties Qizhen 76 (11,132 kg/ha) and Xucai 1 (11,649 kg/ha) across five locations and three years, which was 11.2% and 6.3% higher than control varieties, respectively. Cultivation of this cultivar on large scale will surely increase the production of peas in China and will also prove beneficial for farmers increasing their income. This cultivar may be adapted to other temperate regions of the world. The breeder seeds of Longwan 5, derived from a single line at the F10 generation, are being maintained at Qinwangchuan Research Station, Institute of Crop Sciences, Academy of Gansu Agriculture Sciences (ICS-AGAS), Lanzhou, China, and conserved in the National Crop Genebank of China (NCGC, https://www.cgris.net/, (accessed on: 1 May 2022), GenBank accessions: G0007660). Exclusive rights for the sale and production of the pedigreed seed have been awarded to Golden Silk Road Seeds Industry Co., Ltd., Wuwei, China.
Author Contributions
Conceptualization, X.Y. and X.C.; methodology, J.Y. and X.Y.; software, J.Y.; validation, X.Y. and R.L.; formal analysis, J.Y. and X.Y.; investigation, G.M., R.L., Z.Z. and L.Z.; writing—original draft preparation, X.Y.; writing—review and editing, X.Y. and X.C.; supervision, X.Y. and G.M.; project administration, X.Y. and X.C.; funding acquisition, X.Y. and X.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the China Agriculture Research System of MOF and MARA—Food Legumes (CARS-08-G14) from the Ministry of Agriculture and Rural Affairs of the People’s Republic of China, and the National Natural Science Foundation of China (Grant No: 32260483).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. Value of Agricultural Production, Food and Agricultural Organization Statistical Database. Statistics Division. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QV (accessed on 1 May 2022).
- Li, L.; Yang, T.; Liu, R.; Redden, B.; Maalouf, F.; Zong, X. Food legume production in China. Crop J. 2017, 5, 115–126. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, X.; Hao, J.; Qiu, S.; Lyu, X.; Song, F. Genetic Diversity Analysis of Agronomic Traits in Vegetable Pea Germplasm Resources. J. Sichuan Agric. Univ. 2022, 40, 489–497+590. [Google Scholar]
- Sihui, W. High-yield comprehensive cultivation technologies for dryland pea Dingwan No. 2. Agric. Res. Arid Areas 2006, 24, 108–112. [Google Scholar]
- Liu, R.; Fang, L.; Yang, T.; Zhang, X.; Hu, J.; Zhang, H.; Han, W.; Hua, Z.; Hao, J.; Zong, X. Marker-trait association analysis of frost tolerance of 672 worldwide pea (Pisum sativum L.) collections. Sci. Rep. 2017, 7, 5919. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Han, X.; Chen, A.; Liu, X.; Wei, Z. Investigation on factors affecting pea root rot in Dingxi. Gansu Agric. Sci. Technol. 2020, 05, 14–17. [Google Scholar]
- Snoad, B. A preliminary assessment of ‘leafless peas’. Euphytica 1974, 23, 257–265. [Google Scholar] [CrossRef]
- Mikic, A.; Mihailovic, V.; Cupina, B.; Kosev, V.; Warkentin, T.; Mcphee, K.; Ambrose, M.; Hofer, J.; Ellis, N. Genetic background and agronomic value of leaf types in pea (Pisum sativum). Ratar. i Povrt. 2011, 48, 275–284. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, C.P. Effect of plant types on grain yield and lodging resistance in pea (Pisum sativum L.). Indian J. Genet. Plant Breed. 2015, 75, 69. [Google Scholar] [CrossRef]
- Tar’An, B.; Warkentin, T.; Somers, D.J.; Miranda, D.; Vandenberg, A.; Blade, S.; Woods, S.; Bing, D.; Xue, A.; DeKoeyer, D.; et al. Quantitative trait loci for lodging resistance, plant height and partial resistance to mycosphaerella blight in field pea (Pisum sativum L.). Theor. Appl. Genet. 2003, 107, 1482–1491. [Google Scholar] [CrossRef]
- Checa, O.; Rodriguez, M.; Wu, X.; Blair, M. Introgression of the Afila Gene into Climbing Garden Pea (Pisum sativum L.). Agronomy 2020, 10, 1537. [Google Scholar] [CrossRef]
- Warkentin, T.D.; Smýkal, P.; Coyne, C.J.; Weeden, N.; Domoney, C.; Bing, D.; Leonforte, A.; Xuxiao, Z.; Dixit, G.P.; Boros, L.; et al. Pea; De Ron, A.M., Ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Hu, C.; Lv, M.; Yang, F.; Yu, H.; Yang, X.; Wang, Y.; Wang, L.; Zhen, A.; Dai, Z.; Tang, Y.; et al. Breeding of a new semi-leafless pea variety Yunwan 35. China Seed Ind. 2022, 06, 87–88. [Google Scholar]
- Yang, X.; Gou, Z.; Zhu, Z.; Wang, C.; Zhang, L.; Min, G. Breeding and Evaluation of a New-Bred Semi-Leafless Pea (Pisum sativum L.) Cultivar Longwan No. 6. Agronomy 2022, 12, 850. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; He, Y.; Zong, X.; Min, G.; Lian, R.; Liu, Z.; Xiang, C.; Li, L.; Xing, B.; et al. Performance of Different Varieties of Spring Field Pea (Pisum sativum L.) under Irrigated and Rainfed Environments in North China. Agronomy 2022, 12, 1498. [Google Scholar] [CrossRef]
- Uzun, A.; Bilgili, U.; Sincik, M.; Filya, I.; Acikgoz, E. Yield and quality of forage type pea lines of contrasting leaf types. Eur. J. Agron. 2005, 22, 85–94. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Z.; Fu, H.; Duan, C.; Wang, X.; Zhu, Z. Resistance to powdery mildew in the pea cultivar Xucai 1 is conferred by the gene er1. Crop J. 2015, 3, 489–499. [Google Scholar] [CrossRef]
- Ondřej, M.; Dostálová, R.; Hýbl, M.; Odstrčilová, L.; Tyller, R.; Trojan, R. Utilization of afila types of pea (Pisum sativum L.) resistant to powdery mildew (Erysiphe pisi DC.) in the breeding programs. Plant Soil Environ. 2003, 49, 481–485. [Google Scholar] [CrossRef]
- Egea-Gilabert, C.; Pagnotta, M.A.; Tripodi, P. Genotype × Environment Interactions in Crop Breeding. Agronomy 2021, 11, 1644. [Google Scholar] [CrossRef]
- Bilgili, U.; Uzun, A.; Sincik, M.; Yavuz, M.; Aydinoǧlu, B.; Çakmakci, S.; Geren, H.; Avcioǧlu, R.; Nizam, I.; Tekeli, A.S.; et al. Forage yield and lodging traits in peas (Pisum sativum L.) with different leaf types. Turk. J. Field Crops 2010, 15, 50–53. [Google Scholar]
- Iqbal, S.M.; Zulkiffal, M.; Rauf, C.A.; Mahmood, T. Screening of pea for resistance against powdery mildew under rainfed conditions. Pak. J. Arid Agric. 1998, 1, 33–35. [Google Scholar]
- Sun, S.; Fu, H.; Wang, Z.; Duan, C.; Zong, X.; Zhu, Z. Discovery of a Novel er1 Allele Conferring Powdery Mildew Resistance in Chinese Pea (Pisum sativum L.) Landraces. PLoS ONE 2016, 11, e147624. [Google Scholar] [CrossRef]
- Vaid, A.; Tyagi, P.D. Genetics of powdery mildew resistance in pea. Euphytica 1997, 96, 203–206. [Google Scholar] [CrossRef]
- Rana, J.C.; Banyal, D.K.; Sharma, K.D.; Sharma, M.K.; Gupta, S.K.; Yadav, S.K. Screening of pea germplasm for resistance to powdery mildew. Euphytica 2013, 189, 271–282. [Google Scholar] [CrossRef]
- Davidson, J.A.; Krysinska-Kaczmarek, M.; Kimber, R.B.E.; Ramsey, M.D. Screening field pea germplasm for resistance to downy mildew (Peronospora viciae) and powdery mildew (Erysiphe pisi). Australas. Plant Path. 2004, 33, 413–417. [Google Scholar] [CrossRef]
- Mcphee, K. Dry pea production and breeding—A mini-review. Food Agric. Environ. 2003, 1, 64–69. [Google Scholar]
- Kreplak, J.; Madoui, M.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Doležel, J.; Greilhuber, J.; Lucretti, S.; Meister, A.; Lysák, M.A.; Nardi, L.; Obermayer, R. Plant Genome Size Estimation by Flow Cytometry: Inter-laboratory Comparison. Ann. Bot. 1998, 82, 17–26. [Google Scholar] [CrossRef]
- Minamikawa, M.F.; Nonaka, K.; Kaminuma, E.; Kajiya-Kanegae, H.; Onogi, A.; Goto, S.; Yoshioka, T.; Imai, A.; Hamada, H.; Hayashi, T.; et al. Genome-wide association study and genomic prediction in citrus: Potential of genomics-assisted breeding for fruit quality traits. Sci. Rep. 2017, 7, 4721. [Google Scholar] [CrossRef]
- Arabzai, M.G.; Gul, H. Application Techniques of Molecular Marker and Achievement of Marker Assisted Selection (MAS) in Three Major Crops Rice, Wheat and Maize. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 82–93. [Google Scholar] [CrossRef]
- Bohra, A.; Pandey, M.K.; Jha, U.C.; Singh, B.; Singh, I.P.; Datta, D.; Chaturvedi, S.K.; Nadarajan, N.; Varshney, R.K. Genomics-assisted breeding in four major pulse crops of developing countries: Present status and prospects. Theor. Appl. Genet. 2014, 127, 1263–1291. [Google Scholar] [CrossRef]
- Zeng, L.; Li, M.Q.; Yang, X.M. Identification of resistance of peas resources to powdery mildew. Grassl. Turf 2012, 32, 35–38. [Google Scholar]
- Sun, S.; Deng, D.; Duan, C.; Zong, X.; Xu, D.; He, Y.; Zhu, Z. Two Novel er1 Alleles Conferring Powdery Mildew (Erysiphe pisi) Resistance Identified in a Worldwide Collection of Pea (Pisum sativum L.) Germplasms. Int. J. Mol. Sci. 2019, 20, 5071. [Google Scholar] [CrossRef] [PubMed]
- Sulima, A.S.; Zhukov, V.A. War and Peas: Molecular Bases of Resistance to Powdery Mildew in Pea (Pisum sativum L.) and Other Legumes. Plants 2022, 11, 339. [Google Scholar] [CrossRef]
- Pheirim, R.; Konjengbam, N.S.; Mahanta, M. Molecular Markers for Powdery Mildew in Pea (Pisum sativum L.): A Review. Legume Res. Int. J. 2022, 45, 399–409. [Google Scholar] [CrossRef]
- Fondevilla, S.; Carver, T.L.W.; Moreno, M.T.; Rubiales, D. Identification and characterization of sources of resistance to Erysiphe pisi Syd. in Pisum spp. Plant Breed. 2007, 126, 113–119. [Google Scholar] [CrossRef]
- Heringa, R.J.; van Norel, A.; Tazelaar, M.F. Resistance to powdery mildew (Erisyphe polygoni D.C.) in peas (Pisum sativum L.). Euphytica 1969, 18, 163–169. [Google Scholar] [CrossRef]
- Fondevilla, S.; Rubiales, D. Powdery mildew control in pea. A review. Agron. Sustain. Dev. 2012, 32, 401–409. [Google Scholar] [CrossRef]
- Devi, J.; Mishra, G.P.; Sagar, V.; Kaswan, V.; Dubey, R.K.; Singh, P.M.; Sharma, S.K.; Behera, T.K. Gene-Based Resistance to Erysiphe Species Causing Powdery Mildew Disease in Peas (Pisum sativum L.). Genes 2022, 13, 316. [Google Scholar] [CrossRef]
- Tiwari, K.; Penner, G.; Warkentin, T. Inheritance of powdery mildew resistance in pea. Can. J. Plant Sci. 1997, 77, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Fondevilla, S.; Carver, T.L.W.; Moreno, M.T.; Rubiales, D. Macroscopic and Histological Characterisation of Genes er1 and er2 for Powdery Mildew Resistance in Pea. Eur. J. Plant Pathol. 2006, 115, 309–321. [Google Scholar] [CrossRef]
- Fondevilla, S.; Torres, A.; Moreno, M.T.; Rubiales, D. Identification of a New Gene for Resistance to Powdery Mildew in Pisum fulvum, a Wild Relative of Pea. Breed. Sci. 2007, 57, 181–184. [Google Scholar] [CrossRef]
- Fondevilla, S.; Cubero, J.I.; Rubiales, D. Confirmation that the Er3 gene, conferring resistance to Erysiphe pisi in pea, is a different gene from er1 and er2 genes. Plant Breed. 2011, 130, 281–282. [Google Scholar] [CrossRef]
- Katoch, V.; Sharma, S.; Pathania, S.; Banayal, D.K.; Sharma, S.K.; Rathour, R. Molecular mapping of pea powdery mildew resistance gene er2 to pea linkage group III. Mol. Breed. 2010, 25, 229–237. [Google Scholar] [CrossRef]
- Nisar, M.; Ghafoor, A. Linkage of a RAPD marker with powdery mildew resistance er-1 gene in Pisum sativum L. Russ. J. Genet 2011, 47, 300–304. [Google Scholar] [CrossRef]
- Srivastava, R.; Mishra, S.; Singh, A.; Mohapatra, T. Development of a coupling-phase SCAR marker linked to the powdery mildew resistance gene ‘er1’ in pea (Pisum sativum L.). Euphytica 2012, 186, 855–866. [Google Scholar] [CrossRef]
- Ghafoor, A.; Mcphee, K. Marker assisted selection (MAS) for developing powdery mildew resistant pea cultivars. Euphytica 2012, 186, 593–607. [Google Scholar] [CrossRef]
- Yan, W.; Frégeau-Reid, J. Genotype by Yield*Trait (GYT) Biplot: A Novel Approach for Genotype Selection based on Multiple Traits. Sci. Rep. 2018, 8, 8242. [Google Scholar] [CrossRef]
- Wu, X.; Li, N.; Hao, J.; Hu, J.; Zhang, X.; Blair, M.W. Genetic Diversity of Chinese and Global Pea (Pisum sativum L.) Collections. Crop Sci. 2017, 57, 1574–1584. [Google Scholar] [CrossRef]
- Tran, C.T.; Becker, H.C.; Horneburg, B. Agronomic performance of normal-leafed and semi-leafless pea (Pisum sativum L.) genotypes. Crop Sci. 2022, 62, 1430–1442. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, V.P.; Srivastava, S.; Singh, A.K.; Chaubey, B.K.; Srivastava, R.K. Estimation of correlation coefficient among yield and attributing traits of field pea (Pisum sativum L.). Legume Res. 2018, 41, 20–26. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).