Effect of a Biostimulant Based on Polyphenols and Glycine Betaine on Tomato Plants’ Responses to Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth, Stress Treatments, and Biostimulant Application in the Greenhouse
2.2. Quantification of Photosynthetic Pigments
2.3. Ion Quantification
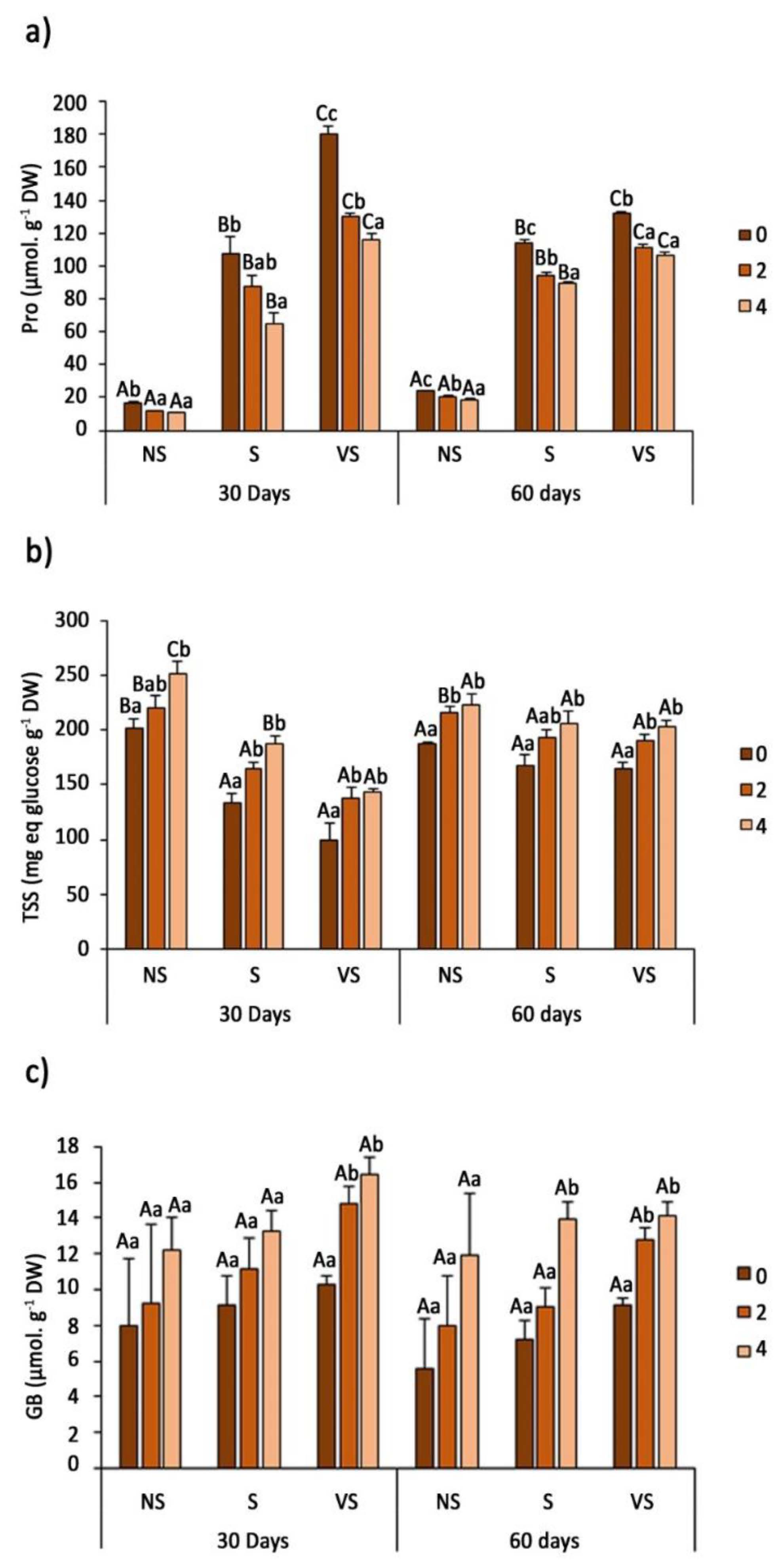
2.4. Osmolyte Determination
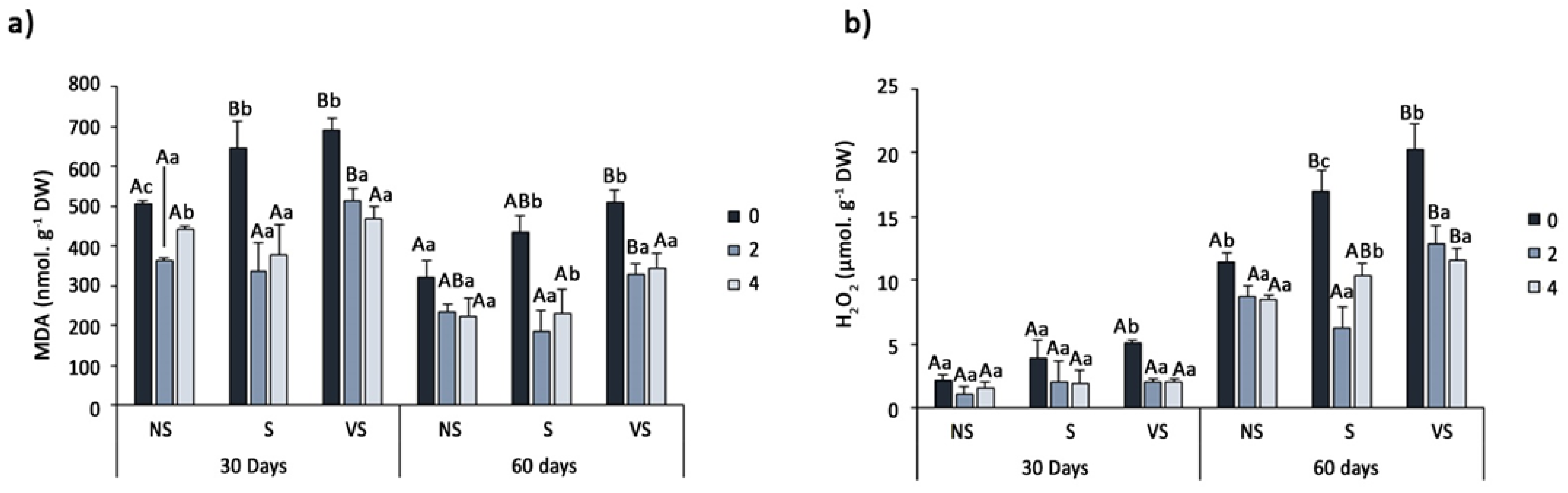
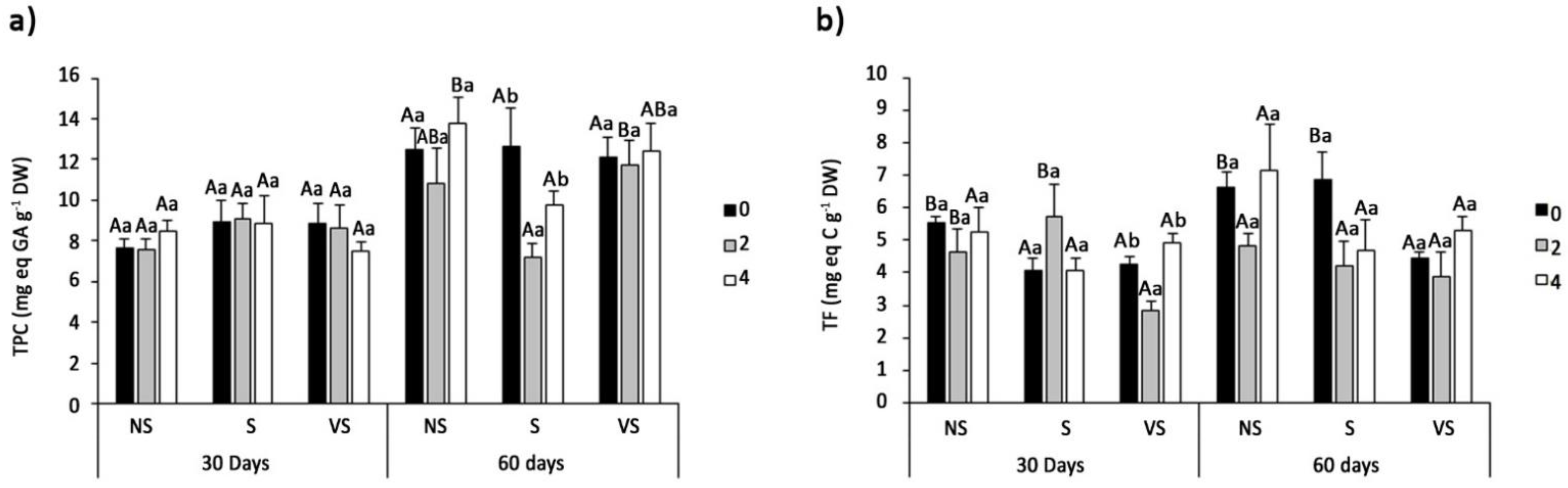
2.5. Determination of Oxidative Stress Markers and Non-Enzymatic Antioxidants
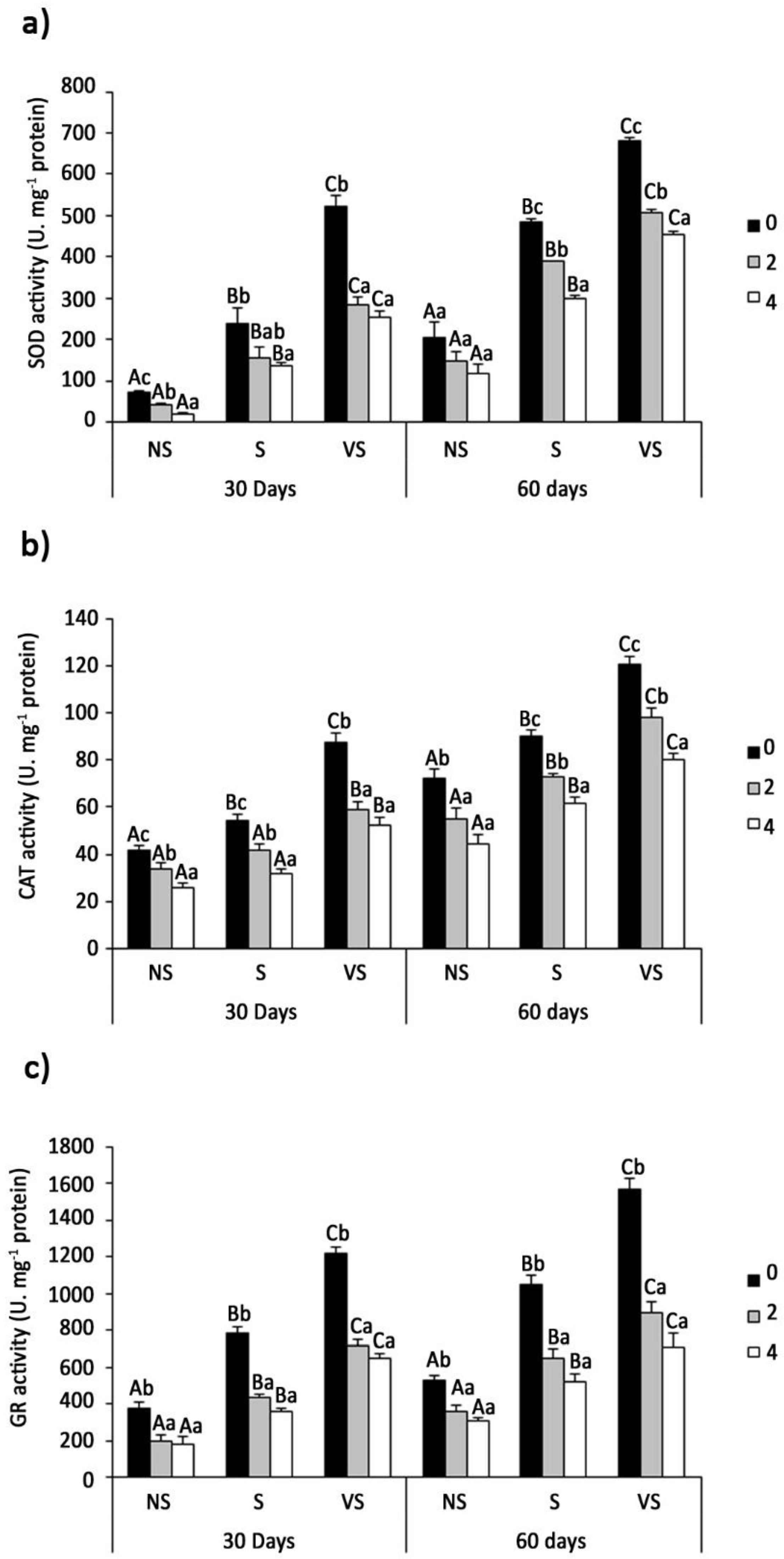
2.6. Antioxidant Enzyme Activities
2.7. Statistical Analyses
3. Results
3.1. Electrical Conductivity of the Substrate
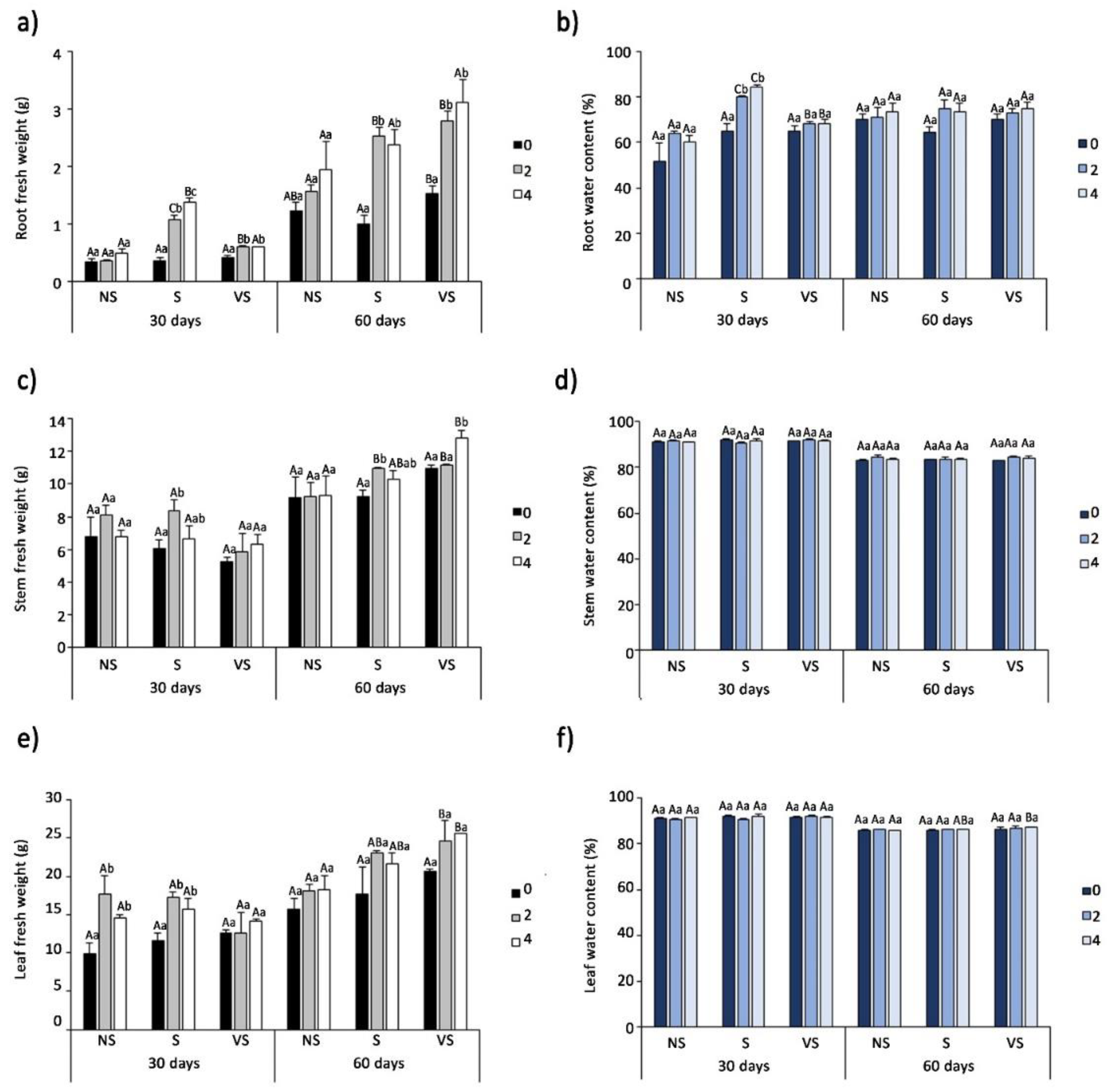
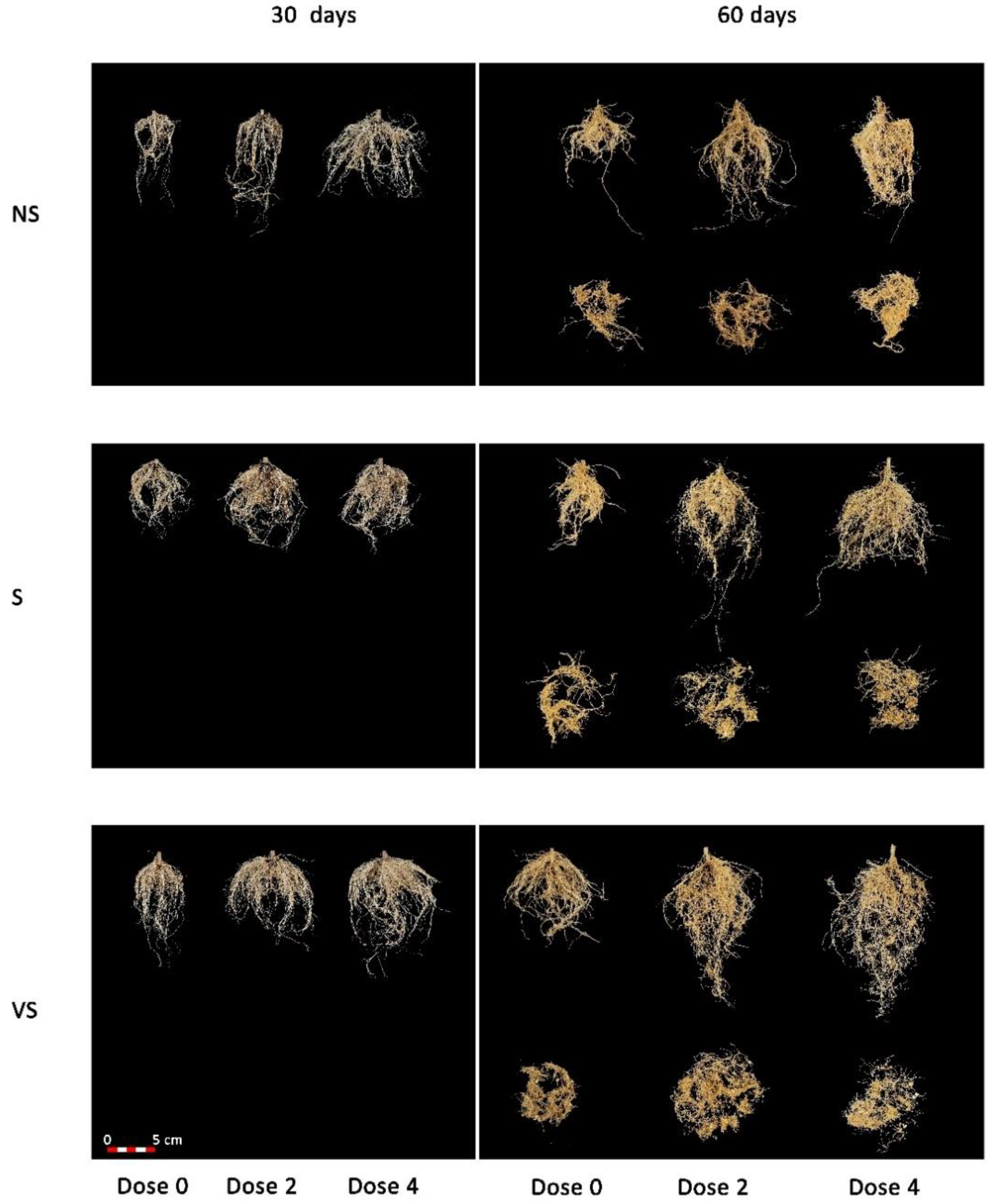
3.2. Effect of Salt Treatments on Plant Growth
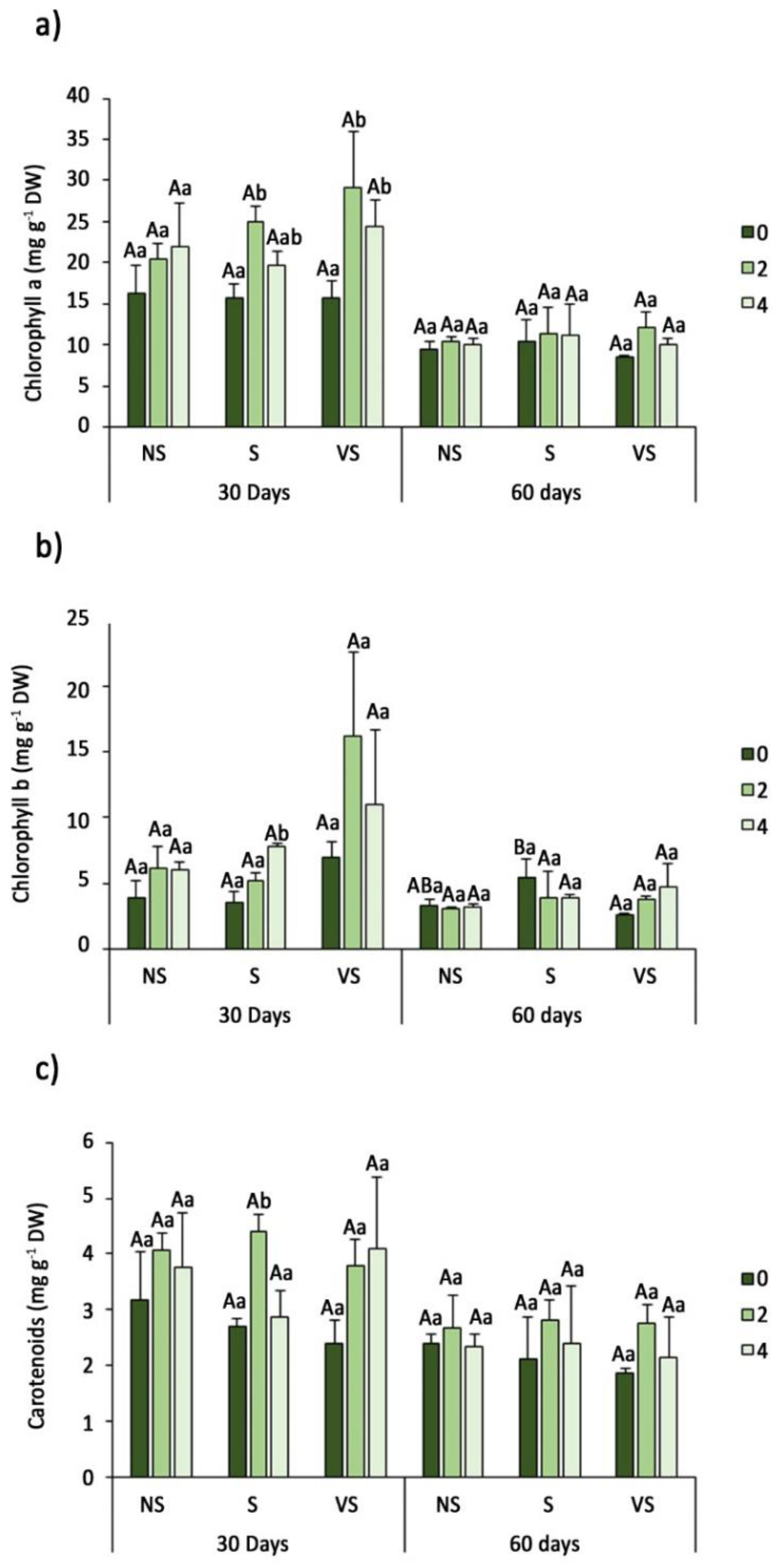
3.3. Photosynthetic Pigments Contents
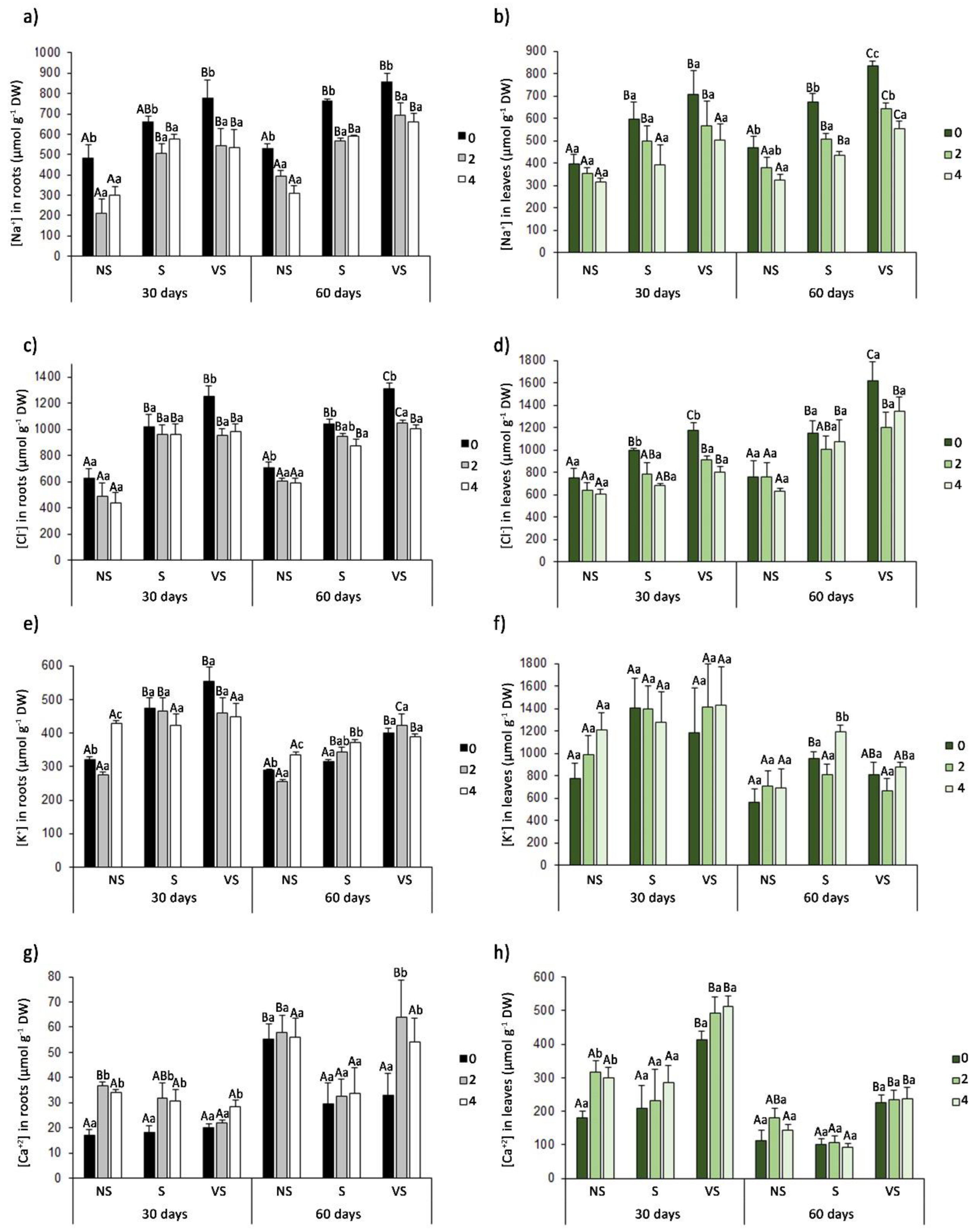
3.4. Ion Accumulation in Roots and Leaves
3.5. Osmolyte Quantification
3.6. Oxidative Stress Markers and Non-Enzymatic Antioxidants
3.7. Antioxidant Enzyme Activities
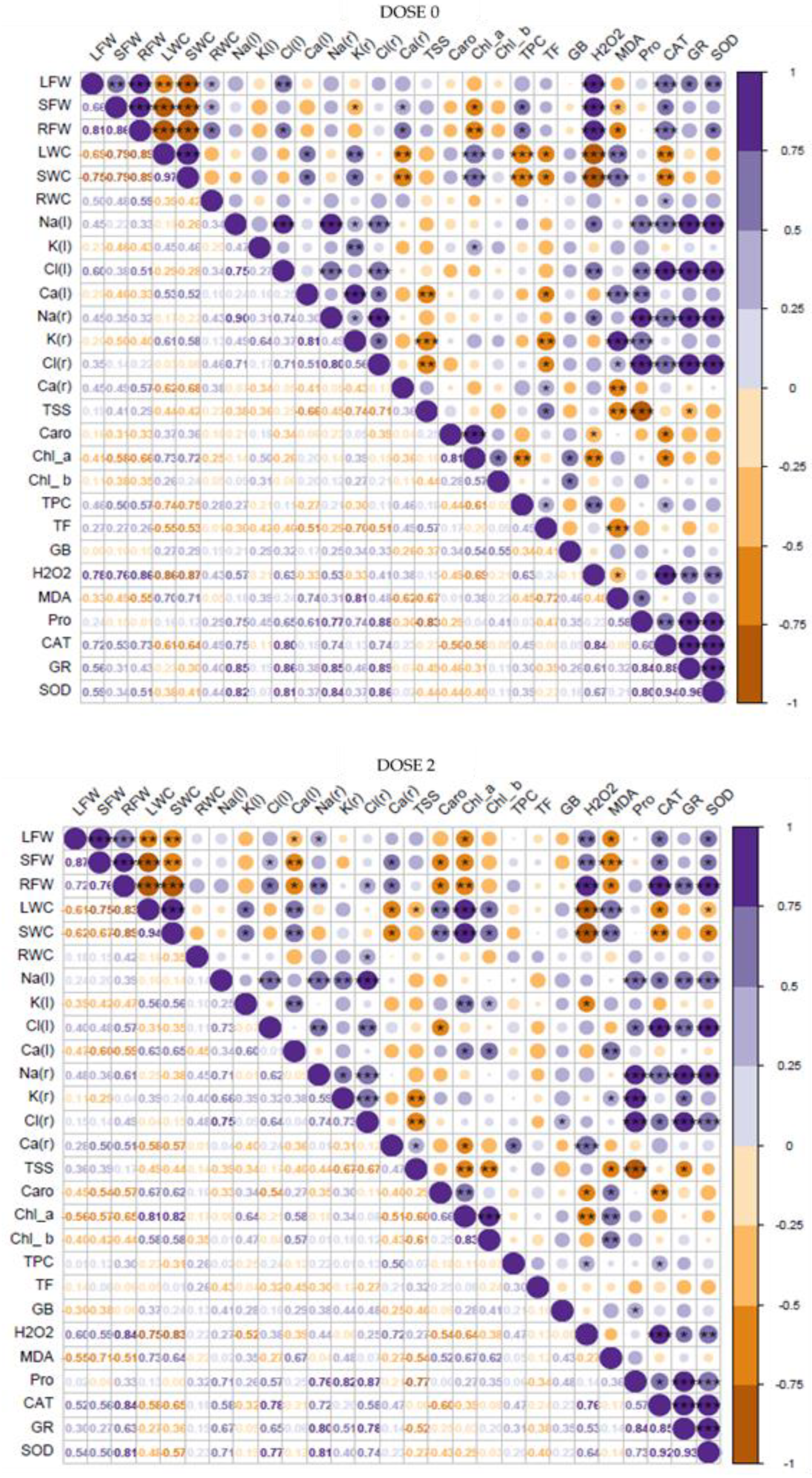
3.8. Correlation and Principal Component Analyses
4. Discussion
4.1. Salinity Effects on Plant Growth and Biochemical Stress Markers
4.2. Biostimulant Effects on Plant Growth and Stress Responses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, P.; Li, Y.; Li, Z.; Sheng, J.; Shen, L. SIMAPK3 enhances tolerance to salt stress in tomato plants by scavenging ROS accumulation and up-regulating the expression of ethylene signaling related genes. Environ. Exp. Bot. 2022, 193, 104698. [Google Scholar] [CrossRef]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity Stress in Arid and Semi-Arid Climates: Effects and Management in Field Crops, Climate Change and Agriculture, Saddam Hussain; Intech Open: London, UK, 2019. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; p. 164. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Mapping of Salt-Affected Soils-Technical Manual; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Butcher, K.; Wick, A.F.; DeSutter, T.M.; Chatterjee, A.; Harmon, J. Soil Salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Nishimura, T.; Cha-um, S.; Takagaki, M.; Ohyama, K. Survival percentage, photosynthetic abilities and growth characters of two indica rice (Oryza sativa L. spp. indica) cultivars in response to isosmotic stress. Sp. J. Agric. Res. 2011, 9, 262–270. [Google Scholar] [CrossRef]
- Rabie, G.H. Influence of arbuscular mycorrhizal fungi and kinetin on the response of mungbean plants to irrigation with seawater. Mycorrhiza 2005, 15, 225–230. [Google Scholar] [CrossRef]
- Siringam, K.; Juntawong, N.; Cha-um, S.; Kirdmanee, C. Salt stress induced ion accumulation, ion homeostasis, membrane injury and sugar contents in salt-sensitive rice (Oryza sajtiva L. spp. indica) roots under isoosmotic conditions. Afri. J. Biotechnol. 2011, 10, 1340–1346. [Google Scholar]
- Chawla, S.; Jain, S.; Jain, V. Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J. Plant Biochem. Biotechnol. 2013, 22, 27–34. [Google Scholar] [CrossRef]
- González-Orenga, S.; Grigore, M.-N.; Boscaiu, M.; Vicente, O. Constitutive and induced salt tolerance mechanisms and potential uses of Limonium Mill. species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1;4 and HKT1;5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef]
- Cendrero-Mateo, M.P.; Carmo-Silva, A.E.; Porcar-Castell, A.; Hamerlynck, E.P.; Papuga, S.A.; Moran, M.S. Dynamic response of plant chlorophyll fluorescence to light, water and nutrient availability. Funct. Plant Biol. 2015, 42, 746–757. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ibrahim, M.; Farid, M.; Adrees, M.; Bharwana, S.A.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F. Mechanisms of silicon-mediated alleviation of drought and salt stress in plants: A review. Environ. Sci. Pollut. Res. 2015, 22, 15416–15431. [Google Scholar] [CrossRef]
- Abdallah, M.M.S.; Ramadan, A.A.; El-Bassiouny, H.M.S.; Bakry, B.A. Regulation of antioxidant system in wheat cultivars by using chitosan or salicylic acid to improve growth and yield under salinity stress. Asian J. Plant Sci. 2020, 19, 114–126. [Google Scholar]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, A.P.; Chakraborty, R. Understanding the potential of root microbiome influencing salt-tolerance in plants and mechanisms involved at the transcriptional and translational level. Physiol. Plant. 2021, 173, 1657–1681. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Paes de Melo, B.; Carpinetti, P.d.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M.F.d.S. Abiotic stresses in plants and their markers: A practice view of plant stress responses and programmed cell death mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Singh, J.; Takhur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer Nature Singapore Private Ltd.: Singapore, 2018; pp. 27–46. [Google Scholar]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic Stress—A Review. Agron. J. 2019, 9, 335. [Google Scholar] [CrossRef]
- De Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and their role in improving plant growth under abiotic stresses. In Biostimulants in Plant Science; Mirmajlessi, S.M., Ed.; Intech Open: London, UK, 2019; pp. 3–16. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAO Statistical Databases (FAOSTAT). Available online: http://www.fao.org/faostat/es (accessed on 6 January 2020).
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant. 1987, 70, 381–388. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.T. Rapid determination of free proline for water—Stress Studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Valadez-Bustos, M.G.; Aguado-Santacruz, G.A.; Tiessen-Favier, A.; Robledo-Paz, A.; Muñoz-Orozco, A.; Rascón-Cruz, Q.; Santacruz-Varela, A. A reliable method for spectrophotometric determination of glycine betaine in cell suspension and other systems. Anal. Biochem. 2016, 498, 47–52. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 2002, 52, 2375–2380. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; Mello, J. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beyer, W.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Connell, J.P.; Mullet, J.E. Pea chloroplast glutathione reductase: Purification and characterization. Plant Physiol. 1986, 82, 351–356. [Google Scholar] [CrossRef]
- Fieller, E.C.; Pearson, E.S. Tests for rank correlation coefficients. I. Biometrika 1957, 44, 470–481. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Available online: https://ggplot2.tidyverse.org/reference/ (accessed on 15 May 2022).
- Kuhn, M.; Jackson, S.; Cimentada, J. Corrr: Correlations in R. R Package Version 0.4. Available online: https://cran.r-project.org/web/packages/corrr/corrr.pdf (accessed on 15 May 2022).
- Chhabra, R. Classification of salt-affected soils. Arid Land Res. Manag. 2004, 19, 61–79. [Google Scholar] [CrossRef]
- Raza, M.A.; Saeed, A.; Munir, H.; Ziaf, K.; Shakeel, A.; Saeed, N.; Munawar, A.; Rehman, F. Screening of tomato genotypes for salinity tolerance based on early growth attributes and leaf inorganic osmolytes. Arch. Agron. Soil Sci. 2017, 63, 501–512. [Google Scholar] [CrossRef]
- Hajer, A.S.; Malibari, A.A.; Al-Zahrani, H.S.; Almaghrabi, O.A. Responses of three tomato cultivars to sea water salinity 1. Effect of salinity on the seedling growth. Afr. J. Biotechnol. 2006, 5, 855–861. [Google Scholar]
- Bolarín, M.C.; Perez-Alfocea, F.; Estan, M.T.; Caro, M. Response of tomato cultivars to salinity. Plant Soil. 1993, 150, 203–211. [Google Scholar]
- Cuartero, J.; Fernández-Muñoz, R. Tomato and salinity. Sci. Hortic. 1999, 78, 83–152. [Google Scholar] [CrossRef]
- Zengin, F.K.; Munzuroglu, O. Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol. Cracov. Bot. 2005, 47, 157–164. [Google Scholar]
- Al Hassan, M.; Fuertes, M.M.; Sánchez, F.J.R.; Vicente, O.; Boscaiu, M. Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 1–11. [Google Scholar] [CrossRef]
- Plazas, M.; Nguyen, H.T.; González-Orenga, S.; Fita, A.; Vicente, O.; Prohens, J.; Boscaiu, M. Comparative analysis of the responses to water stress in eggplant (Solanum melongena) cultivars. Plant Physiol. Biochem. 2019, 143, 72–82. [Google Scholar] [CrossRef]
- Landa-Faz, A.; González-Orenga, S.; Boscaiu, M.; Rodríguez-Vázquez, R.; Vicente, O. Effect of the pesticide endosulfan and two different biostimulants on the stress responses of Phaseolus leptostachyus plants grown in a saline soil. Agronomy 2021, 11, 1208. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.P.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-induced variation in biochemical markers provides insight into the mechanisms of salt tolerance in common (Phaseolus vulgaris) and runner (P. coccineus) beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef] [Green Version]
- Gong, B.; Wen, D.; Vanden Langenberg, K.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci. Hortic. 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Geng, S.; Ri, Y.J.; Cao, D.; Liu, J.; Shi, D.; Yang, C. Physiological responses and adaptive strategies of tomato plants to salt and alkali stresses. Sci. Hortic. 2011, 130, 248–255. [Google Scholar] [CrossRef]
- De la Torre-González, A.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the proline metabolism and glycine betaine on tolerance to salt stress in tomato (Solanum lycopersicum L.) commercial genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Flynn, R. Interpreting Soil Tests: Unlock the Secrets of Your Soil; Cooperative Extension Service, College of Agricultural, Consumer and Environmental Sciences, New Mexico State University: Las Cruces, NM, USA, 2015; pp. 1–12. [Google Scholar]
- Ahmad, F.; Singh, A.; Kamal, A. Osmoprotective role of sugar in mitigating abiotic stress in plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 53–70. [Google Scholar]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Aziz, A.; Martin-Tanguy, J.; Larher, F. Salt stress-induced proline accumulation and changes in tyramine and polyamine levels are linked to ionic adjustment in tomato leaf discs. Plant Sci. 1999, 145, 83–91. [Google Scholar] [CrossRef]
- Watanabe, S.; Kojima, K.; Ide, Y.; Sasaki, S. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult. 2000, 63, 199–206. [Google Scholar] [CrossRef]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic differences in physiological and biochemical responses tosalinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef]
- Derkaoui, K.M.; Sahnoune, M.; Belkohodja, M. The effect of salinity on accumulation proline, glycine betaine and mineral elements in four varieties of tomatoes. Ukr. J. Ecol. 2022, 12, 58–63. [Google Scholar]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Anjum, N.A.; Aref, I.M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Iqbal, M. Glutathione and proline can coordinately make plants withstand the joint attack of metal (loid) and salinity stresses. Front Plant Sci. 2014, 5, 662. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Gil, R.; Boscaiu, M.; Lull, C.; Bautista, I.; Lid, A.N.; Vicente, Ó. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805–818. [Google Scholar] [CrossRef]
- Calone, R.; Mircea, D.-M.; González-Orenga, S.; Boscaiu, M.; Lambertini, C.; Barbanti, L.; Vicente, O. Recovery from salinity and drought stress in the perennial Sarcocornia fruticosa vs. the annual Salicornia europaea and S. veneta. Plants 2022, 11, 1058. [Google Scholar] [CrossRef]
- Kaya, C.; Sönmez, O.; Aydemir, S.; Dikilitas, M. Mitigation effects of glycinebetaine on oxidative stress and some key growth parameters of maize exposed to salt stress. Turk. J. Agric. For. 2013, 37, 188–194. [Google Scholar] [CrossRef]
- Paradisone, V.; Barrameda-Medina, Y.; Montesinos-Pereira, D.; Romero, L.; Esposito, S.; Ruiz, J.M. Roles of some nitrogenous compounds protectors in the resistance to zinc toxicity in Lactuca sativa cv. Phillipus and Brassica oleracea cv. Bronco. Acta Physiol. Plant 2015, 37, 137. [Google Scholar] [CrossRef]
- Mahboob, W.; Khan, M.A.; Shirazi, M.U. Characterization of salt tolerant wheat (Triticum aestivum) genotypes on the basis of physiological attributes. Int. J. Agric. Biol. 2017, 19, 726–734. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, B. Oxidative stress in five wheat varieties (Triticum aestivum L.) exposed to water stress and study of their antioxidant enzyme defense system, water stress responsive metabolites and H2O2 accumulation. Braz. J. Plant Phys. 2012, 24, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Moussouraki, M.A.; Eleni, T.; Velliou, A.; Goufa, M.; Psychogiou, M.; Papadakis, I.E.; Abraham, E.M. Growth, physiological and biochemical responses of two greek cotton cultivars to salt stress and their impact as selection indices for salt tolerance. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 706–715. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, X.; Nawaz, G.; Yin, J.; Yang, J. Physiological and biochemical responses of four cassava cultivars to drought stress. Sci. Rep. 2020, 10, 6968. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant compounds for plant defence … and for a healthy human diet. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 14–21. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.P.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef] [PubMed]
- Rabie, G.H.; Almadini, A.M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afri. J. Biotechnol. 2005, 4, 210–222. [Google Scholar] [CrossRef]
- Sannazzaro, A.I.; Echeverría, M.; Albertó, E.O.; Ruiz, O.A.; Menéndez, A.B. Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol. Biochem. 2007, 45, 39–46. [Google Scholar] [CrossRef]
- Agboma, P.C.; Jones, M.G.K.; Peltonen-Sainio, P.; Rita, H.; Pehu, E. Exogenous glycine betaine enhances grain yield of maize, sorghum and wheat grown under two supplementary watering regimes. J. Agron. Crop Sci. 1997, 178, 29–37. [Google Scholar] [CrossRef]
- Rajashekar, C.B.; Zhou, H.; Marcum, K.B.; Prakash, O. Glycine betaine accumulation and induction of cold tolerance in strawberry (Fragaria X ananassa Duch.) plants. Plant Sci. 1999, 148, 175–183. [Google Scholar] [CrossRef]
- Heuer, B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003, 165, 693–699. [Google Scholar] [CrossRef]


| Soil Characteristics | Non-Saline (NS) | Saline (S) | Very Saline (VS) |
|---|---|---|---|
| pH | 7.67 | 7.71 | 7.88 |
| ECe (dS m−1) | 2.77 | 7.99 | 12.44 |
| EC1:5 (dS m−1) | 0.43 | 1.32 | 1.74 |
| Na+ (meq L−1) | 1.20 | 2.63 | 5.20 |
| K+ (meq L−1) | 7.83 | 12.67 | 18.22 |
| Ca2+ (meq L−1) | 0.92 | 1.57 | 1.75 |
| Mg2+ (meq L−1) | 15.30 | 38.53 | 42.31 |
| Sand (%) | 27.00 | 22.50 | 28.10 |
| Clay (%) | 35.20 | 36.50 | 35.90 |
| Silt (%) | 37.8 | 41.0 | 36.0 |
| EC1:5 (dS m−1) | NS | S | VS |
|---|---|---|---|
| 0 days (start of treatment) | 0.51 ± 0.00 | 1.50 ± 0.01 | 1.71 ± 0.01 |
| 30 days (end of period 1 *) | 0.46 ± 0.02 | 1.49 ± 0.08 | 1.59 ± 0.07 |
| 60 days (end of period 2 **) | 0.56 ± 0.06 | 1.52 ± 0.01 | 1.98 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuzunaga-Rosas, J.; González-Orenga, S.; Tofei, A.M.; Boscaiu, M.; Moreno-Ramón, H.; Ibáñez-Asensio, S.; Vicente, O. Effect of a Biostimulant Based on Polyphenols and Glycine Betaine on Tomato Plants’ Responses to Salt Stress. Agronomy 2022, 12, 2142. https://doi.org/10.3390/agronomy12092142
Zuzunaga-Rosas J, González-Orenga S, Tofei AM, Boscaiu M, Moreno-Ramón H, Ibáñez-Asensio S, Vicente O. Effect of a Biostimulant Based on Polyphenols and Glycine Betaine on Tomato Plants’ Responses to Salt Stress. Agronomy. 2022; 12(9):2142. https://doi.org/10.3390/agronomy12092142
Chicago/Turabian StyleZuzunaga-Rosas, Javier, Sara González-Orenga, Ana Maria Tofei, Monica Boscaiu, Héctor Moreno-Ramón, Sara Ibáñez-Asensio, and Oscar Vicente. 2022. "Effect of a Biostimulant Based on Polyphenols and Glycine Betaine on Tomato Plants’ Responses to Salt Stress" Agronomy 12, no. 9: 2142. https://doi.org/10.3390/agronomy12092142
APA StyleZuzunaga-Rosas, J., González-Orenga, S., Tofei, A. M., Boscaiu, M., Moreno-Ramón, H., Ibáñez-Asensio, S., & Vicente, O. (2022). Effect of a Biostimulant Based on Polyphenols and Glycine Betaine on Tomato Plants’ Responses to Salt Stress. Agronomy, 12(9), 2142. https://doi.org/10.3390/agronomy12092142









