Suitability Evaluation of Three Tropical Pasture Species (Mulato II, Gatton Panic, and Rhodes Grass) for Cultivation under a Subtropical Climate of Australia
Abstract
:1. Introduction
2. Methodology
2.1. Experimental Site, Plot Establishment, and Management
2.2. Forage Accumulation and Plant Composition
2.3. Sward Structure and Canopy Bulk Density
2.4. Canopy Light Interception, Carbon Assimilation
2.5. Leaf Area Index and Specific Leaf Area
2.6. Nutritive Value
2.7. Calculations and Statistical Analyses
2.7.1. Fitting Light Response Curve
2.7.2. Fitting the CO2 Response Curve (A/Ci Curve)
3. Results
3.1. Carbon Exchange Characteristics and Photosynthesis Biochemistry
3.2. Forage Accumulation and Plant Part Composition
3.3. Sward Structural Parameters
3.4. Nutritive Composition
3.5. Mineral Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooke, R.F.; Daigle, C.L.; Moriel, P.; Smith, S.B.; Tedeschi, L.O.; Vendramini, J.M.B. Cattle adapted to tropical and subtropical environments: Social, nutritional, and carcass quality considerations. J. Anim. Sci. 2020, 98, skaa014. [Google Scholar] [CrossRef]
- Sollenberger, L.E.; Vendramini, J.M.B.; Pedreira, C.G.S.; Rios, E.F. Warm-season grasses for humid areas. In Forages; Wiley: Hoboken, NJ, USA, 2020; pp. 331–345. [Google Scholar]
- Volenec, J.; Nelson, C.; Barnes, R. Physiology of forage plants. In Forages: The Science of Grassland Agriculture; Wiley: Hoboken, NJ, USA, 2007; Volume 2, pp. 37–52. [Google Scholar]
- Nelson, C.; Moser, L.E. Plant factors affecting forage quality. In Forage Quality, Evaluation, and Utilization; Wiley: Hoboken, NJ, USA, 1994; pp. 115–154. [Google Scholar]
- Sage, R.F.; Kubien, D.S. Quo vadis C 4? An ecophysiological perspective on global change and the future of C 4 plants. Photosynth. Res. 2003, 77, 209–225. [Google Scholar] [CrossRef]
- Baptistella, J.L.C.; de Andrade, S.A.L.; Favarin, J.L.; Mazzafera, P. Urochloa in tropical agroecosystems. Front. Sustain. Food Syst. 2020, 4, 119. [Google Scholar] [CrossRef]
- Paul, B.K.; Koge, J.; Maass, B.L.; Notenbaert, A.; Peters, M.; Groot, J.C.J.; Tittonell, P. Tropical forage technologies can deliver multiple benefits in Sub-Saharan Africa. A meta-analysis. Agron. Sustain. Dev. 2020, 40, 22. [Google Scholar] [CrossRef]
- Lowe, K.F.; Hume, D.E.; Fulkerson, W.J. Perennial forage and pasture crops—Species and varieties. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. Ser. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Argel, M.; Pedro, J.; Miles, J.W.; Guiot García, J.D.; Cuadrado Capella, H.; Lascano, C.E. Cultivar mulato II (Brachiaria híbrido CIAT 36087). In Gramínea de Alta Qualidade e Produçao Forrageira, Resistentes as Cigarrinhas e Adaptada a Solos Tropicais Acidos; CIAT: Cali, Colombia, 2007. [Google Scholar]
- Fisher, M.; Kerridge, P. The agronomy and physiology of Brachiaria species. In Brachiaria: Biology, Agronomy, and Improvement; CIAT: Cali, Colombia, 1996; pp. 43–52. [Google Scholar]
- Pontes, L.d.S.; Baldissera, T.C.; Giostri, A.F.; Stafin, G.; dos Santos, B.R.C.; Carvalho, P.C.d.F. Effects of nitrogen fertilization and cutting intensity on the agronomic performance of warm-season grasses. Grass Forage Sci. 2017, 72, 663–675. [Google Scholar] [CrossRef]
- Silva, V.J.; Pedreira, C.G.S.; Sollenberger, L.E.; Silva, L.S.; Yasuoka, J.I.; Almeida, I.C.L. Carbon assimilation, herbage plant-part accumulation, and organic reserves of grazed ‘Mulato II’ brachiariagrass pastures. Crop Sci. 2016, 56, 2853–2860. [Google Scholar] [CrossRef]
- Habermann, E.; Dias de Oliveira, E.A.; Contin, D.R.; Delvecchio, G.; Viciedo, D.O.; de Moraes, M.A.; de Mello Prado, R.; de Pinho Costa, K.A.; Braga, M.R.; Martinez, C.A. Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiol. Plant. 2019, 165, 383–402. [Google Scholar] [CrossRef]
- Pedreira, B.C.; Pedreira, C.G.S.; Lara, M.A.S. Leaf age, leaf blade portion and light intensity as determinants of leaf photosynthesis in Panicum maximum Jacq. Grassl. Sci. 2015, 61, 45–49. [Google Scholar] [CrossRef]
- Dias-Filho, M.B. Photosynthetic light response of the C4 grasses Brachiaria brizantha and B. humidicola under shade. Sci. Agric. 2002, 59, 65. [Google Scholar] [CrossRef] [Green Version]
- Baumont, R.; Prache, S.; Meuret, M.; Morand-Fehr, P. How forage characteristics influence behaviour and intake in small ruminants: A review. Livest. Prod. Sci. 2000, 64, 15–28. [Google Scholar] [CrossRef]
- Lemaire, G.; Da Silva, S.C.; Agnusdei, M.; Wade, M.; Hodgson, J. Interactions between leaf lifespan and defoliation frequency in temperate and tropical pastures: A review. Grass Forage Sci. 2009, 64, 341–353. [Google Scholar] [CrossRef]
- Peyraud, J.L.; Comeron, E.A.; Wade, M.H.; Lemaire, G. The effect of daily herbage allowance, herbage mass and animal factors upon herbage intake by grazing dairy cows. Ann. Zootech. 1996, 45, 201–217. [Google Scholar] [CrossRef]
- Gastal, F.; Lemaire, G. Defoliation, shoot plasticity, sward structure and herbage utilization in pasture: Review of the underlying ecophysiological processes. Agriculture 2015, 5, 1146–1171. [Google Scholar] [CrossRef]
- Hodgson, J. Influence of sward characteristics on diet selection and herbage intake by the grazing animal. In Proceedings of the Nutritional Limits to Animal Production from Pastures, St. Lucia, QLD, Australia, 24–28 August 1981. [Google Scholar]
- Jacobs, A.A.A.; Scheper, J.A.; Benvenutti, M.A.; Gordon, I.J.; Poppi, D.P.; Elgersma, A. Tensile fracture properties of seven tropical grasses at different phenological stages. Grass Forage Sci. 2011, 66, 551–559. [Google Scholar] [CrossRef]
- Ungar, R. Bite horizons and dimensions for cattle grazing herbage to high levels of depletion. Grass Forage Sci. 1999, 54, 357–364. [Google Scholar] [CrossRef]
- Benvenutti, M.A.; Pavetti, D.R.; Poppi, D.P.; Mayer, D.G.; Gordon, I.J. Ingestive behaviour and forage intake responses of young and mature steers to the vertical differentiation of sugarcane in pen and grazing studies. J. Agric. Sci. 2017, 155, 1677–1688. [Google Scholar] [CrossRef]
- Benvenutti, M.A.; Pavetti, D.R.; Poppi, D.P.; Gordon, I.J.; Cangiano, C.A. Defoliation patterns and their implications for the management of vegetative tropical pastures to control intake and diet quality by cattle. Grass Forage Sci. 2016, 71, 424–436. [Google Scholar] [CrossRef]
- Benvenutti, M.A.; Findsen, C.; Savian, J.V.; Mayer, D.G.; Barber, D.G. The effect of stage of regrowth on the physical composition and nutritive value of the various vertical strata of kikuyu (Cenchrus clandestinus) pastures. Trop. Grassl. Forrajes Trop. 2020, 8, 141–146. [Google Scholar] [CrossRef]
- Isbell, R. The Australian Soil Classification, 2nd ed.; CSIRO Publishing: Clayton, VIC, Australia, 2016. [Google Scholar]
- Allen, R.G. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper 56; FAO—Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- DairyOne. Dairy One Forage Lab Analytical Procedures. Available online: https://dairyone.com/download/forage-forage-lab-analytical-procedures/ (accessed on 15 March 2021).
- NRC. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition; The National Academies Press: Washington, DC, USA, 2001; p. 405. [Google Scholar]
- RCore Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Marshall, B.; Biscoe, P.V. A Model for C3 leaves describing the dependence of net photosynthesis on irradiance. J. Exp. Bot. 1980, 31, 29–39. [Google Scholar] [CrossRef]
- Leverenz, J.W.; Jarvis, P.G. Photosynthesis in Sitka Spruce. VIII. The effects of light flux density and direction on the rate of net photosynthesis and the stomatal conductance of needles. J. Appl. Ecol. 1979, 16, 919–932. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Duursma, R.A. Plantecophys—An R package for analysing and modelling leaf gas exchange data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Ludlow, M.M.; Wilson, G.L. Photosynthesis of tropical pasture plants, illuminance, carbon dioxide concentration, leaf temperature, and leaf-air vapour pressure difference. Aust. J. Biol. Sci. 1971, 24, 449–470. [Google Scholar] [CrossRef]
- Sonawane, B.V.; Sharwood, R.E.; von Caemmerer, S.; Whitney, S.M.; Ghannoum, O. Short-term thermal photosynthetic responses of C4 grasses are independent of the biochemical subtype. J. Exp. Bot. 2017, 68, 5583–5597. [Google Scholar] [CrossRef]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum. J. Exp. Bot. 2004, 56, 755–763. [Google Scholar] [CrossRef]
- Boschma, S.P.; Murphy, S.R.; Harden, S. Herbage production and persistence of two tropical perennial grasses and forage sorghum under different nitrogen fertilization and defoliation regimes in a summer-dominant rainfall environment, Australia. Grass Forage Sci. 2015, 70, 381–393. [Google Scholar] [CrossRef]
- Lawes, R.; Robertson, M. Seasonal variability of Rhodes grass production in the northern West Australia wheatbelt. In Proceedings of the 14th Australian Society of Agronomy Conference, Adelaide, Australia, 21–25 September 2008; pp. 21–25. [Google Scholar]
- Ivory, D.; Whiteman, P. Effect of temperature on growth of five subtropical grasses. I. Effect of day and night temperature on growth and morphological development. Funct. Plant Biol. 1978, 5, 131–148. [Google Scholar] [CrossRef]
- Pequeno, D.N.L.; Pedreira, C.G.S.; Sollenberger, L.E.; de Faria, A.F.G.; Silva, L.S. Forage accumulation and nutritive value of rachiariagrasses and Tifton 85 Bermudagrass as affected by harvest frequency and irrigation. Agron. J. 2015, 107, 1741–1749. [Google Scholar] [CrossRef]
- Vendramini, J.M.B.; Sollenberger, L.E.; Lamb, G.C.; Foster, J.L.; Liu, K.; Maddox, M.K. Forage accumulation, nutritive value, and persistence of ‘Mulato II’ brachiariagrass in Northern Florida. Crop Sci. 2012, 52, 914–922. [Google Scholar] [CrossRef]
- Hare, M.D. Effect of cutting interval on yield and quality of three brachiaria hybrids in Thailand. Trop. Grassl. Forrajes Trop. 2013, 1, 84–86. [Google Scholar] [CrossRef]
- Simeão, R.M.; Resende, M.D.V.; Alves, R.S.; Pessoa-Filho, M.; Azevedo, A.L.S.; Jones, C.S.; Pereira, J.F.; Machado, J.C. Genomic selection in tropical forage grasses: Current status and future applications. Front. Plant Sci. 2021, 12, 665195. [Google Scholar] [CrossRef]
- Inyang, U.; Vendramini, J.M.B.; Sellers, B.; Silveira, M.L.A.; Lunpha, A.; Sollenberger, L.E.; Adesogan, A.; Paiva, L.M. Harvest frequency and stubble height affect herbage accumulation, nutritive value, and persistence of ‘Mulato II’ Brachiariagrass. Forage Grazinglands 2010, 8, 1–7. [Google Scholar] [CrossRef]
- Moreno, L.S.B.; Boote, K.J.; Sollenberger, L.E.; Dubeux, J.C.B.; Kohmann, M.M.; Pequeno, D.N.L. Shade and nitrogen fertilization affect forage accumulation and nutritive value of C4 grasses differing in growth habit. Crop Sci. 2022, 62, 512–523. [Google Scholar] [CrossRef]
- Nouhoun, Z.; Traoré, T.C.; Sawadogo, E.T.B.P.; Ayantunde, A.; Prasad, K.V.S.V.; Blummel, M.; Balehegn, M.; Rios, E.; Dubeux, J.C.; Boote, K.; et al. Herbage accumulation and nutritive value of Urochloa hybrid cv. ‘Mulato II’, Urochloa ruziziensis and Megathyrsus maximus cv. “C1” in sub-humid zone of West Africa. Agron. J. 2021, 114, 138–147. [Google Scholar] [CrossRef]
- Thomas, D.T.; Lawes, R.A.; Descheemaeker, K.; Moore, A.D. Selection of crop cultivars suited to the location combined with astute management can reduce crop yield penalties in pasture cropping systems. Crop Pasture Sci. 2014, 65, 1022–1032. [Google Scholar] [CrossRef]
- Descheemaeker, K.; Llewellyn, R.; Moore, A.; Whitbread, A. Summer-growing perennial grasses are a potential new feed source in the low rainfall environment of southern Australia. Crop Pasture Sci. 2014, 65, 1033–1043. [Google Scholar] [CrossRef]
- Ward, P.; Ferris, D.; Lawes, R.; Palmer, N.; Micin, S.; Barrett-Lennard, P. Crop yield, pasture yield, and environmental impact of pasture cropping with sub-tropical perennials. In Proceedings of the 16th Australian Society of Agronomy Conference, Armidale, NSW, Australia, 14–18 October 2012; Yunusa, I., Ed.; Australian Society of Agronomy/The Regional Institute: Gosford, NSW, Australia, 2012. Available online: www.regional.org.au/au/asa/2012/pastures/8093_wardpr.Htm (accessed on 20 March 2021).
- Lawes, R.A.; Ward, P.R.; Ferris, D. Pasture cropping with C4 grasses in a barley–lupin rotation can increase production. Crop Pasture Sci. 2014, 65, 1002–1015. [Google Scholar] [CrossRef]
- Pembleton, K.G.; Lowe, K.F.; Bahnisch, L.M. Utilising leaf number as an indicator for defoliation to restrict stem growth in rhodes grass (Chloris gayana) cv. Callide. Trop. Grassl. Forrajes Trop. 2009, 43, 79–85. [Google Scholar]
- Pedreira, C.G.S.; Braga, G.J.; Portela, J.N. Herbage accumulation, plant-part composition and nutritive value on grazed signal grass (Brachiaria decumbens) pastures in response to stubble height and rest period based on canopy light interception. Crop Pasture Sci. 2017, 68, 62–73. [Google Scholar] [CrossRef]
- Sollenberger, L.; Burns, J. Canopy characteristics, ingestive behaviour and herbage intake in cultivated tropical grasslands. In Proceedings of the International Grassland Congress, Sao Pedro, Brazil, 11–21 February 2001. [Google Scholar]
- Vendramini, J.M.B.; Sollenberger, L.E.; Soares, A.B.; Da Silva, W.L.; Sanchez, J.M.; Valente, A.L.; Aguiar, A.D.; Mullenix, M.K. Harvest frequency affects herbage accumulation and nutritive value of brachiaria grass hybrids in Florida. Trop. Grassl. Forrajes Trop. 2014, 2, 197–206. [Google Scholar] [CrossRef]
- Wilson, J. Influence of temperature and nitrogen on growth, photosynthesis and accumulation of non-structural carbohydrate in a tropical grass, Panicum maximum var. trichoglum. Neth. J. Agric. Sci. 1975, 23, 48–61. [Google Scholar] [CrossRef]
- Khan, N.A.; Farooq, M.W.; Ali, M.; Suleman, M.; Ahmad, N.; Sulaiman, S.M.; Cone, J.W.; Hendriks, W.H. Effect of species and harvest maturity on the fatty acids profile of tropical forages. JAPS 2015, 25, 739–746. [Google Scholar]
- López, S.; Dijkstra, J.; France, J. 4 Prediction of energy supply in ruminants, with emphasis on forage. In Forage Evaluation in Ruminant Nutrition; CAB International: Wallingford, UK, 2000; pp. 63–94. [Google Scholar]
- Esechie, H.A. Distribution of chemical constituents in the plant parts of six tropical-origin forage grasses at early anthesis. J. Sci. Food Agric. 1992, 58, 435–438. [Google Scholar] [CrossRef]
- McNeill, D.M.; Roche, J.R.; Stockdale, C.R.; McLachlan, B.P. Nutritional strategies for the prevention of hypocalcaemia at calving for dairy cows in pasture-based systems. Aust. J. Agric. Res. 2002, 53, 755–770. [Google Scholar] [CrossRef]
- West, J.W.; Mullinix, B.G.; Sandifer, T.G. Changing dietary electrolyte balance for dairy cows in cool and hot environments. J. Dairy Sci. 1991, 74, 1662–1674. [Google Scholar] [CrossRef]
- Chan, P.S.; West, J.W.; Bernard, J.K.; Fernandez, J.M. Effects of Dietary Cation-Anion Difference on Intake, Milk Yield, and Blood Components of the Early Lactation Cow. J. Dairy Sci. 2005, 88, 4384–4392. [Google Scholar] [CrossRef]
- Apper-Bossard, E.; Peyraud, J.L.; Faverdin, P.; Meschy, F. Changing Dietary Cation-Anion Difference for Dairy Cows Fed with Two Contrasting Levels of Concentrate in Diets. J. Dairy Sci. 2006, 89, 749–760. [Google Scholar] [CrossRef] [Green Version]
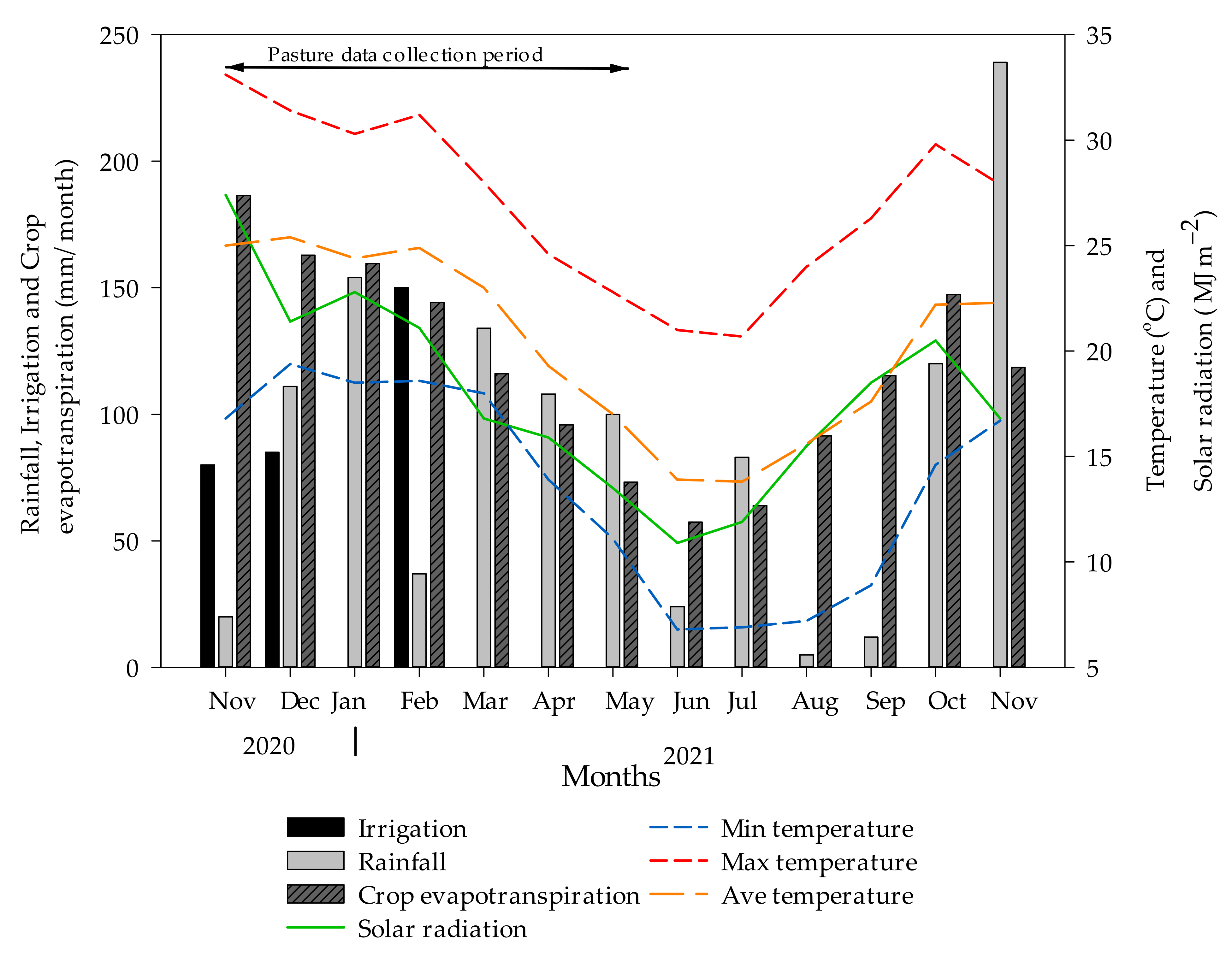
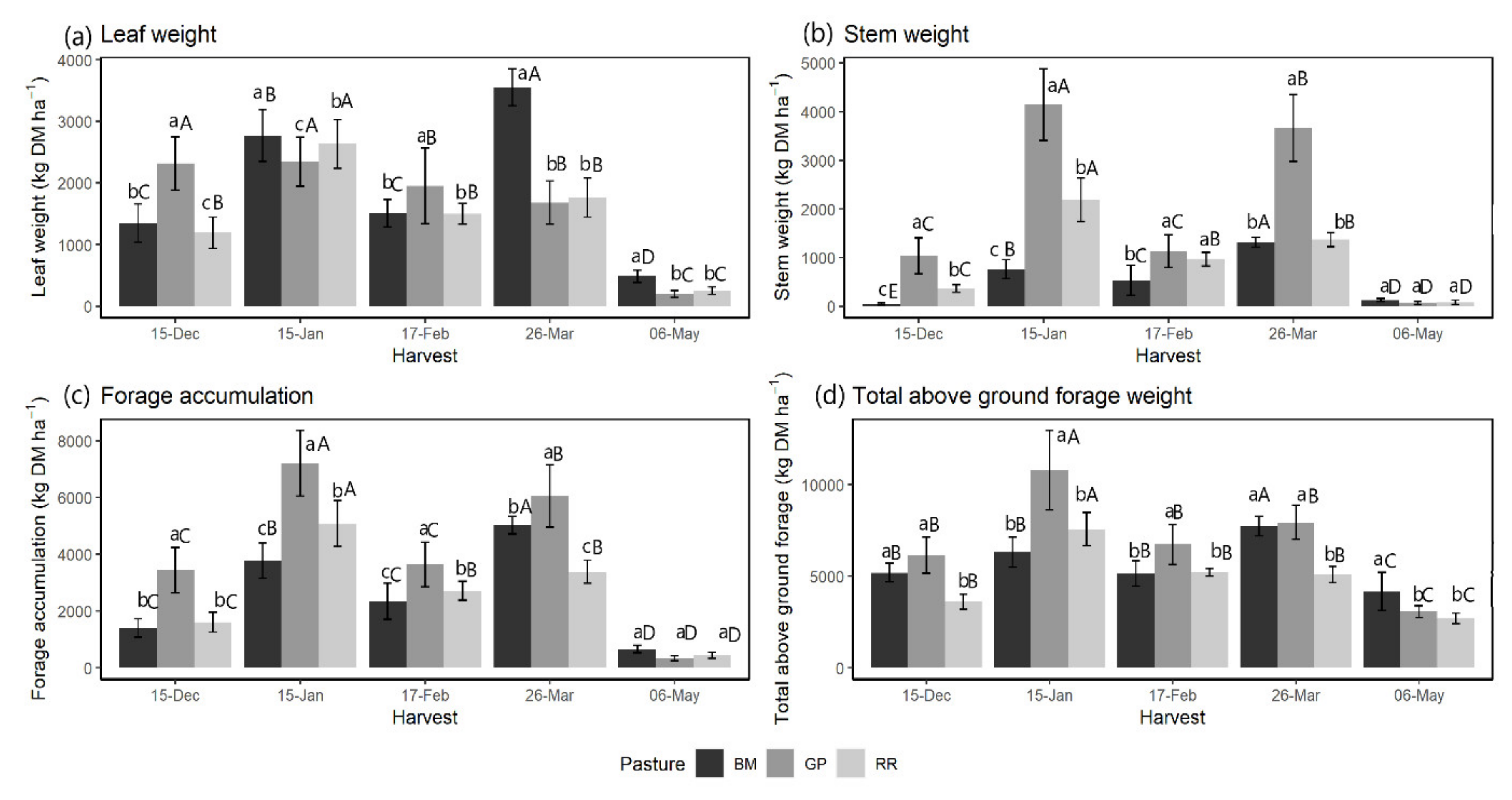
| Photosynthetic Parameters | Pastures | ||
|---|---|---|---|
| RR | BM | GP | |
| Maximum photosynthesis rate (Amax) (µmol CO2 m−2 s−1) | 15.79 ± 0.4 c | 28.95 ± 0.98 a | 25.04 ± 1.34 b |
| Photosynthetic efficiency (µmol CO2 photon−1) | 0.026 ± 0.001 c | 0.056 ± 0.004 a | 0.031 ± 0.002 b |
| Dark respiration (Rd) (µmol CO2 m−2 s−1) | 0.79 ± 0.1 c | 2.41 ± 0.3 a | 0.91 ± 0.3 b |
| Curvature parameter (ϴ) | 0.86 ± 0.04 a | 0.84 ± 0.06 a | 0.87 ± 0.07 a |
| Light compensation point (Ic) (µmol CO2 m−2 s−1) | 30.70 b | 43.70 a | 29.01 c |
| Light saturation point (Is) (µmol CO2 m−2 s−1) | 1242.46 b | 1208.22 b | 1538.66 a |
| Photosynthetic Parameters | Pastures | ||
|---|---|---|---|
| RR | BM | GP | |
| Photosynthetic capacity (Pa) (µmol CO2 m−2 s−1) | 15.79 ± 0.4 b | 33.54 ± 1.7 a | 34.27 ± 4.4 a |
| Photosynthetic efficiency (µmol CO2 photon−1) | 0.02 ± 0.001 c | 0.13 ± 0.02 b | 0.24 ± 0.11 a |
| Respiration rate (Rc) (µmol CO2 m−2 s−1) | 2.04 ± 3.5 b | 4.42 ± 0.93 a | 4.23 ± 4.05 a |
| Curvature parameter (ϴ) | 0.86 ± 0.04 a | 0.83 ± 0.06 a | 0.65 ± 0.18 b |
| Maximum carboxylation rate (Vcmax) (µmol CO2 m−2 s−1) | 83.51± 41.23 b | 71.00 ± 4.33 c | 92.60 ± 17.90 a |
| Maximum electron transfer rate (Jmax) (µmol CO2 m−2 s−1) | 118.24± 16.48 b | 122.56 ± 4.60 a | 106.35± 10.93 c |
| Pastures | Total above Ground | Total Forage Accumulation | Total Leaf Mass | Total Stem Mass | Total Dead Mass |
|---|---|---|---|---|---|
| (kg ha−1) DM | |||||
| BM | 28,590 b | 13,200 b | 9660 a | 2775 c | 732 |
| GP | 34,725 a | 20,655 a | 8495 b | 10,040 a | 1200 |
| RR | 24,210 b | 13,220 b | 7335 b | 4955 b | 690 |
| SEM | 1971.8 | 2008.6 | 794.5 | 956.9 | 222.7 |
| Sward Structural Parameters | Leaf Appearance Rate (Leaf Day−1) | Canopy Height (cm) | Stem Height (cm) | Stem: Canopy Height | Leaf: Stem Ratio | LAI (m2 m−2) | SLA (m2 kg−1) | Canopy Light Interception (%) | Canopy Bulk Density (kg ha−1 cm−1) | Stem Bulk Density (kg ha−1 cm−1) | Leaf Bulk Density (kg ha−1 cm−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Harvest | BM | ||||||||||
| 1 | 0.08 aB | 29.8 bC | 11.8 bB | 0.4 aA | 23.5 aA | 2.9 bB | 21.6 aA | 95.7 aA | 175 bA | * | * |
| 2 | 0.10 aB | 43.1 aC | 17.8 bC | 0.4 aB | 3.9 cA | 7.9 aA | 25.0 aA | 94.9 aA | 145 cA | 74 bA | 124 cA |
| 3 | 0.08 aA | 25.6 bB | 10.3 bB | 0.4 aA | 6.1 bA | 3.0 bA | 16.9 bA | 78.7 cB | 203 aA | 128 aA | 159 bA |
| 4 | 0.10 aB | 46.9 aB | 24.9 aB | 0.5 aB | 2.7 cA | 9.1 aA | 22.9 aA | 97.5 aA | 165 bA | 91 bA | 182 aA |
| 5 | 0.08 aB | 23.8 bA | 12.5 bA | 0.5 aA | 4.2 cB | 9.1 aA | 22.4 aB | 81.3 bA | 169 bA | 80 bA | 90 dA |
| Harvest | GP | ||||||||||
| 1 | 0.11 bA | 60.5 cA | 27.3 cA | 0.4 bA | 2.8 bB | 4.6 bA | 19.9 bA | 96.3 aA | 102 bB | * | * |
| 2 | 0.17 aA | 98.9 aA | 69.5 aA | 0.7 aA | 0.5 dC | 5.7 aB | 23.3 bA | 94.4 aA | 109 bB | 80 aA | 86 aB |
| 3 | 0.09 cA | 50.0 dA | 21.6 cA | 0.4 bA | 1.7 cB | 3.5 cA | 15.2 cA | 91.6 aA | 134 aB | 88 aB | 78 aB |
| 4 | 0.14 bA | 75.9 bA | 51.5 bA | 0.7 aA | 0.4 dC | 4.1 bB | 22.4 bA | 95.7 aA | 104 bB | 80 aA | 75 aB |
| 5 | 0.09 cB | 24.6 eA | 12.9 dA | 0.5 aA | 4.9 aB | 4.1 bB | 34.0 aA | 75.0 bB | 125 aB | 90 aA | 41 bB |
| Harvest | RR | ||||||||||
| 1 | 0.09 cB | 41.7 bB | 21.4 bA | 0.3 bA | 3.3 bB | 1.8 cC | 16.9 bB | 91.0 aA | 86 bC | * | * |
| 2 | 0.16 aA | 84.2 aB | 54.0 aB | 0.6 aA | 1.2 cB | 5.0 aB | 17.0 bB | 95.0 aA | 88 bC | 63 bA | 96 aB |
| 3 | 0.09 cA | 46.1 bA | 21.3 bA | 0.5 aA | 1.6 cB | 2.8 bA | 14.7 bA | 89.7 aA | 114 aB | 82 aB | 78 aB |
| 4 | 0.15 aA | 79.6 aA | 57.0 aA | 0.7 aA | 1.3 cB | 2.8 bC | 15.3 bB | 93.8 aA | 64 cC | 34 cB | 86 aB |
| 5 | 0.12 bA | 23.6 cA | 10.1 cA | 0.4 bA | 7.6 aA | 2.8 bC | 22.9 aB | 71.5 bC | 115 aB | 83 aB | 42 bB |
| SEM | 0.1 | 3.0 | 2.3 | 0.04 | 1.3 | 0.8 | 1.4 | 3.3 | 16.5 | 10.7 | 14.6 |
| Pastures | Nutritive Value Parameters | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TDN | CP | ADF | NDF | NDICP | Starch | WSC | ESC | NFC | Lignin | CF | Ash | IVTD | NDFD | ME | |
| Leaf | |||||||||||||||
| BM | 688 a | 163 | 282 b | 504 b | 26 b | 15 a | 72 | 63 | 167 a | 35 | 51 | 115 | 776 | 560 b | 10.4 a |
| GP | 678 ab | 172 | 327 a | 553 a | 67 a | 11 b | 64 | 48 | 105 b | 40 | 52 | 118 | 814 | 666 a | 10.2 a |
| RR | 646 bc | 145 | 334 a | 607 a | 58 a | 07 b | 63 | 50 | 80 b | 37 | 43 | 124 | 764 | 618 a | 9.5 b |
| SEM | 10 | 16 | 13 | 25 | 05 | 01 | 05 | 05 | 07 | 04 | 02 | 08 | 21 | 27 | 0.2 |
| Stem | |||||||||||||||
| BM | 605 | 96 | 380 | 641 b | 18 | 10 a | 53 | 51 | 121 a | 49 | 29 a | 113 | 722 | 562 | 8.4 |
| GP | 598 | 68 | 432 | 677 ab | 19 | 07 ab | 51 | 37 | 127 a | 48 | 19 b | 109 | 546 | 434 | 7.9 |
| RR | 554 | 83 | 407 | 709 a | 25 | 05 bc | 47 | 31 | 72 b | 48 | 20 bc | 114 | 648 | 512 | 7.8 |
| SEM | 20 | 10 | 20 | 19 | 03 | 0.8 | 07 | 06 | 7.2 | 05 | 01 | 07 | 90 | 78 | 0.1 |
| Whole plant | |||||||||||||||
| BM | 674 a | 151 a | 300 b | 527 b | 25 b | 14 a | 69 | 61 | 158 a | 37 | 47 | 115 | 766 | 560 | 10.0 a |
| GP | 629 ab | 127 b | 375 a | 608 ab | 45 a | 09 ab | 58 | 45 | 113 b | 44 | 37 | 113 | 710 | 570 | 9.2 ab |
| RR | 616 b | 124 b | 360 a | 641 a | 46 a | 07 b | 59 | 45 | 77 c | 42 | 35 | 121 | 729 | 589 | 8.9 b |
| SEM | 14 | 17 | 19 | 07 | 06 | 01 | 05 | 05 | 06 | 04 | 03 | 07 | 24 | 25 | 0.2 |
| Pastures | Minerals | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | P | Mg | K | Na | Fe | Zn | Cu | Mn | Mo | S | Cl | DCAD | |
| Leaf | |||||||||||||
| BM | 0.51 | 0.46 | 0.81 a | 2.47 a | 0.08 b | 223 | 45.2 a | 7.4 b | 77.6 | 0.36 b | 0.25 b | 0.94 b | 24.4 |
| GP | 0.60 | 0.46 | 0.51 b | 1.55 b | 1.12 a | 185 | 38.2 ab | 10.4 a | 70.2 | 0.60 b | 0.22 b | 1.30 ab | 30.2 |
| RR | 0.52 | 0.45 | 0.25 c | 1.33 b | 1.01 a | 216 | 27.4 b | 10.2 a | 86.2 | 1.04 a | 0.41 a | 1.88 a | 13.1 |
| SEM | 0.05 | 0.05 | 0.04 | 0.08 | 0.09 | 30 | 3.7 | 0.7 | 10.1 | 0.15 | 0.02 | 0.19 | 6.6 |
| Stem | |||||||||||||
| BM | 0.20 a | 0.39 | 0.53 a | 3.06 a | 0.24 b | 129.8 b | 46.8 a | 5.5 | 83.2 | 0.10 a | 0.19 a | 1.82 | 25.8 |
| GP | 0.21 a | 0.35 | 0.38 b | 1.75 b | 1.43 a | 83.6 b | 33.0 ab | 5.6 | 60.4 | 0.50 a | 0.14 a | 2.26 | 34.0 |
| RR | 0.33 b | 0.37 | 0.20 c | 1.76 bc | 1.56 a | 162.4 a | 25.6 b | 5.4 | 100.0 | 1.44 b | 0.40 b | 1.98 | 21.0 |
| SEM | 0.03 | 0.02 | 0.03 | 0.26 | 0.14 | 19.8 | 5.0 | 0.4 | 15.3 | 0.27 | 0.02 | 0.51 | 8.9 |
| Whole plant | |||||||||||||
| BM | 0.45 | 0.44 | 0.76 a | 2.58 a | 0.11 b | 202 | 44.7 a | 7.1 | 78.2 | 0.29 b | 0.24 b | 1.13 b | 24.1 a |
| GP | 0.42 | 0.42 | 0.45 b | 1.65 b | 1.26 a | 138 | 36.2 ab | 8.2 | 65.9 | 0.49 b | 0.18 b | 1.71 a | 37.0 a |
| RR | 0.46 | 0.42 | 0.23 c | 1.49 b | 1.26 a | 197 | 26.5 b | 8.5 | 91.6 | 1.21 a | 0.41 a | 2.04 a | 6.7 b |
| SEM | 0.05 | 0.05 | 0.05 | 0.11 | 0.09 | 28.5 | 2.9 | 0.8 | 12 | 0.16 | 0.02 | 0.24 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasinghe, P.; Donaghy, D.J.; Barber, D.G.; Pembleton, K.G.; Ramilan, T. Suitability Evaluation of Three Tropical Pasture Species (Mulato II, Gatton Panic, and Rhodes Grass) for Cultivation under a Subtropical Climate of Australia. Agronomy 2022, 12, 2032. https://doi.org/10.3390/agronomy12092032
Jayasinghe P, Donaghy DJ, Barber DG, Pembleton KG, Ramilan T. Suitability Evaluation of Three Tropical Pasture Species (Mulato II, Gatton Panic, and Rhodes Grass) for Cultivation under a Subtropical Climate of Australia. Agronomy. 2022; 12(9):2032. https://doi.org/10.3390/agronomy12092032
Chicago/Turabian StyleJayasinghe, Priyanath, Daniel J. Donaghy, David G. Barber, Keith G. Pembleton, and Thiagarajah Ramilan. 2022. "Suitability Evaluation of Three Tropical Pasture Species (Mulato II, Gatton Panic, and Rhodes Grass) for Cultivation under a Subtropical Climate of Australia" Agronomy 12, no. 9: 2032. https://doi.org/10.3390/agronomy12092032
APA StyleJayasinghe, P., Donaghy, D. J., Barber, D. G., Pembleton, K. G., & Ramilan, T. (2022). Suitability Evaluation of Three Tropical Pasture Species (Mulato II, Gatton Panic, and Rhodes Grass) for Cultivation under a Subtropical Climate of Australia. Agronomy, 12(9), 2032. https://doi.org/10.3390/agronomy12092032







