Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Climate Data
2.2. Experimental Design
2.3. Amino Acid and NaCl Treatments
2.4. Sampling Date
2.4.1. Growth Parameters
2.4.2. Leaf Gas Exchange
2.4.3. Chlorophyll Fluorescence
2.4.4. Leaf Temperature
2.4.5. Leaf Chlorophyll Index
2.4.6. Relative Water Content (RWC)
2.4.7. Determination of Na+ and K+ Accumulation
2.4.8. Malondialdehyde (MDA) Concentration
2.4.9. Proline Concentration
2.5. Statistical Analysis
3. Results
3.1. Growth Character Measurement
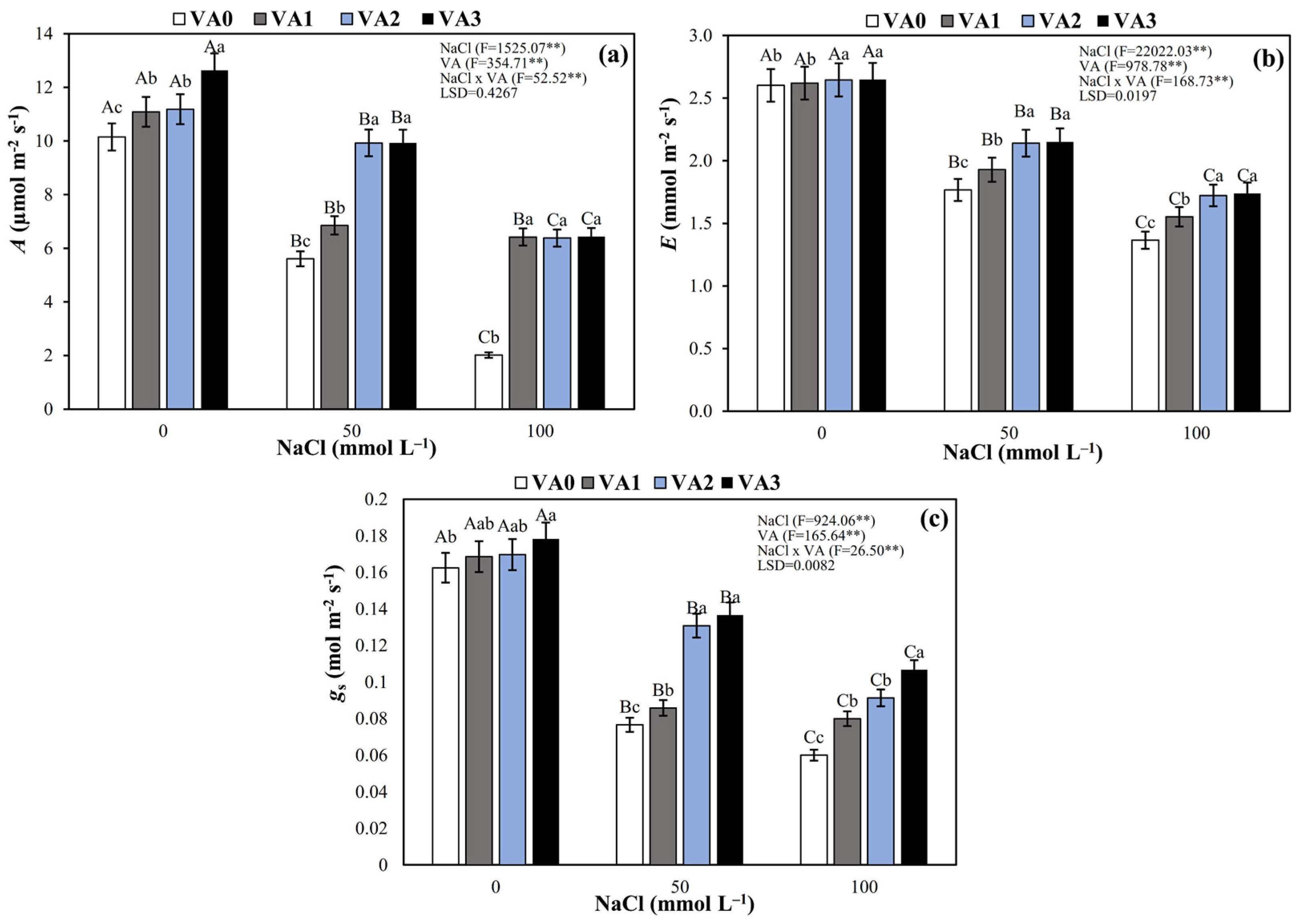
3.2. Leaf Gas Exchange
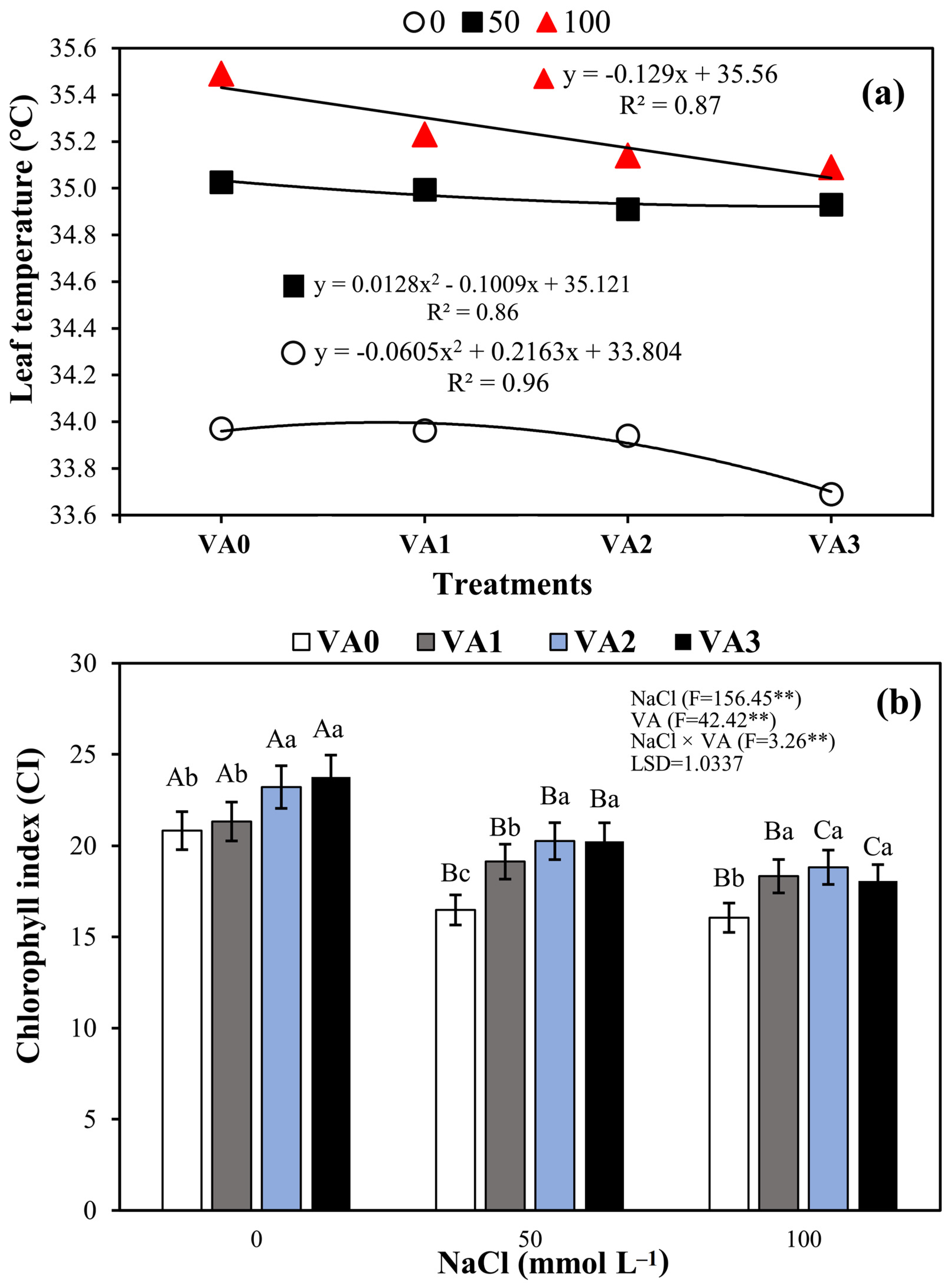
3.3. Leaf Temperature (LT) and Leaf Chlorophyll Index (LCi)
3.4. Maximum Quantum Efficiency of PSII (Fv/Fm) and Photochemistry Efficiency of PSII (Fv/Fo)
3.5. Relative Water Content (RWC) and Water-Use Efficiency (iWUE)
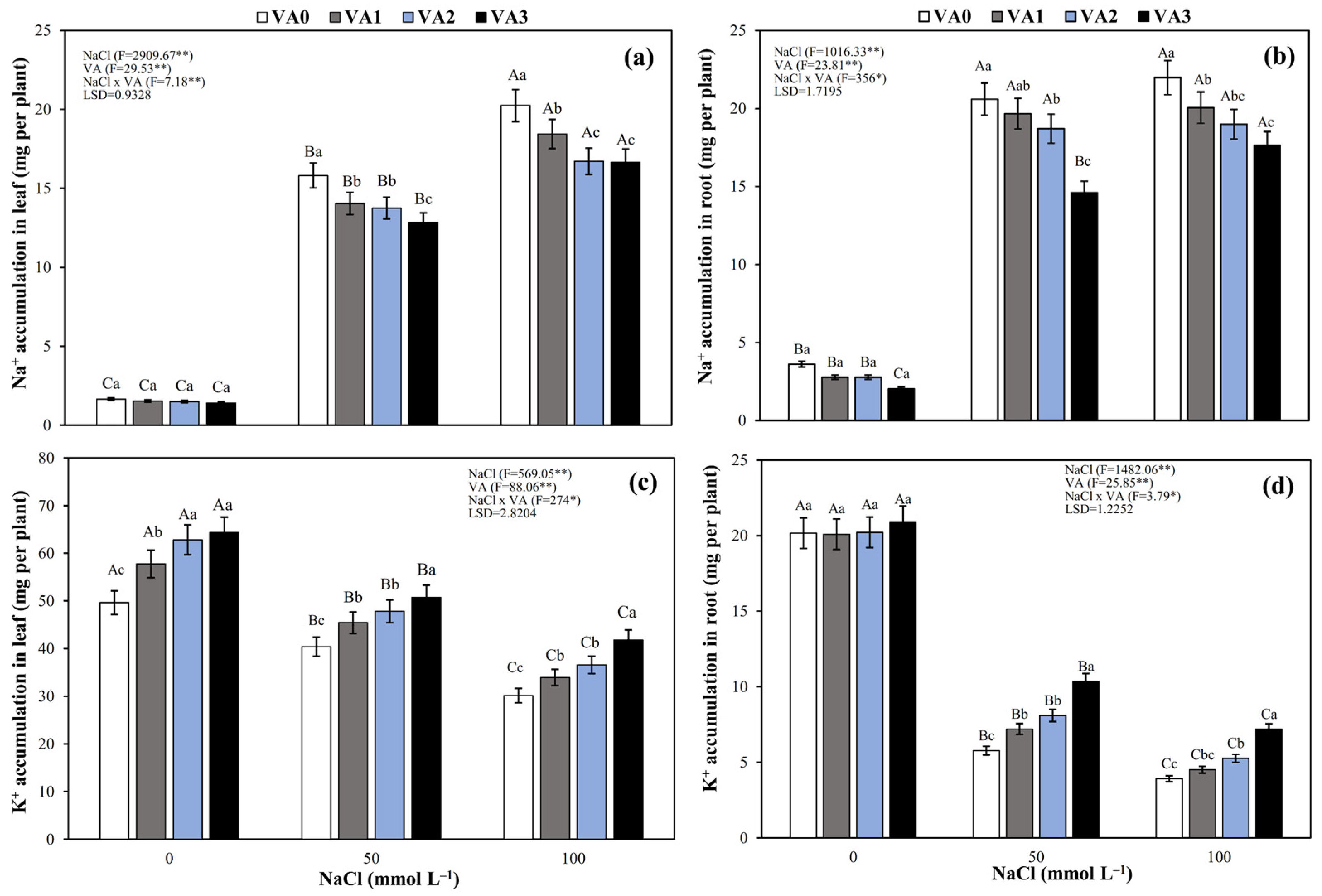
3.6. Na+ and K+ Accumulation
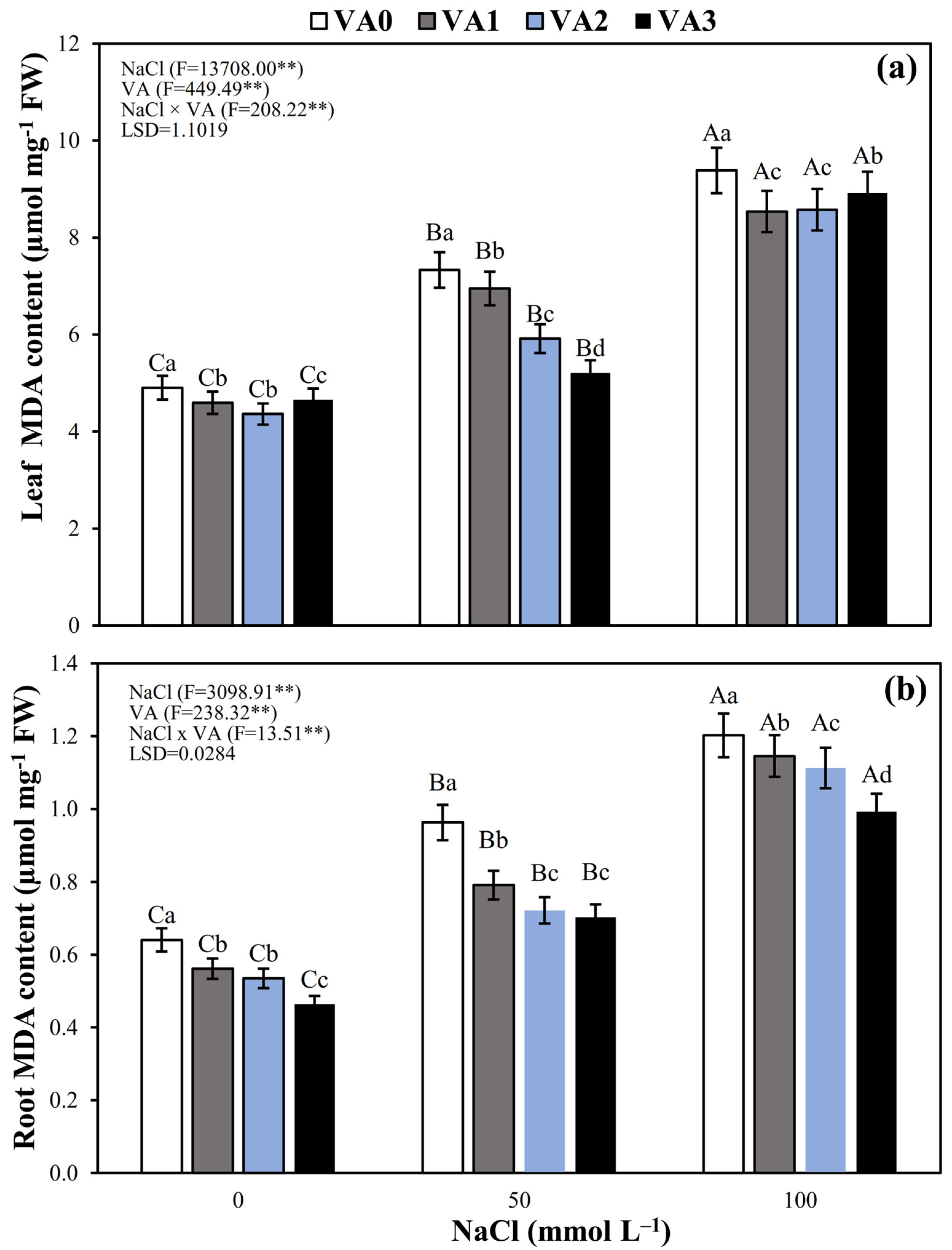
3.7. Leaf and Root MDA Concentration
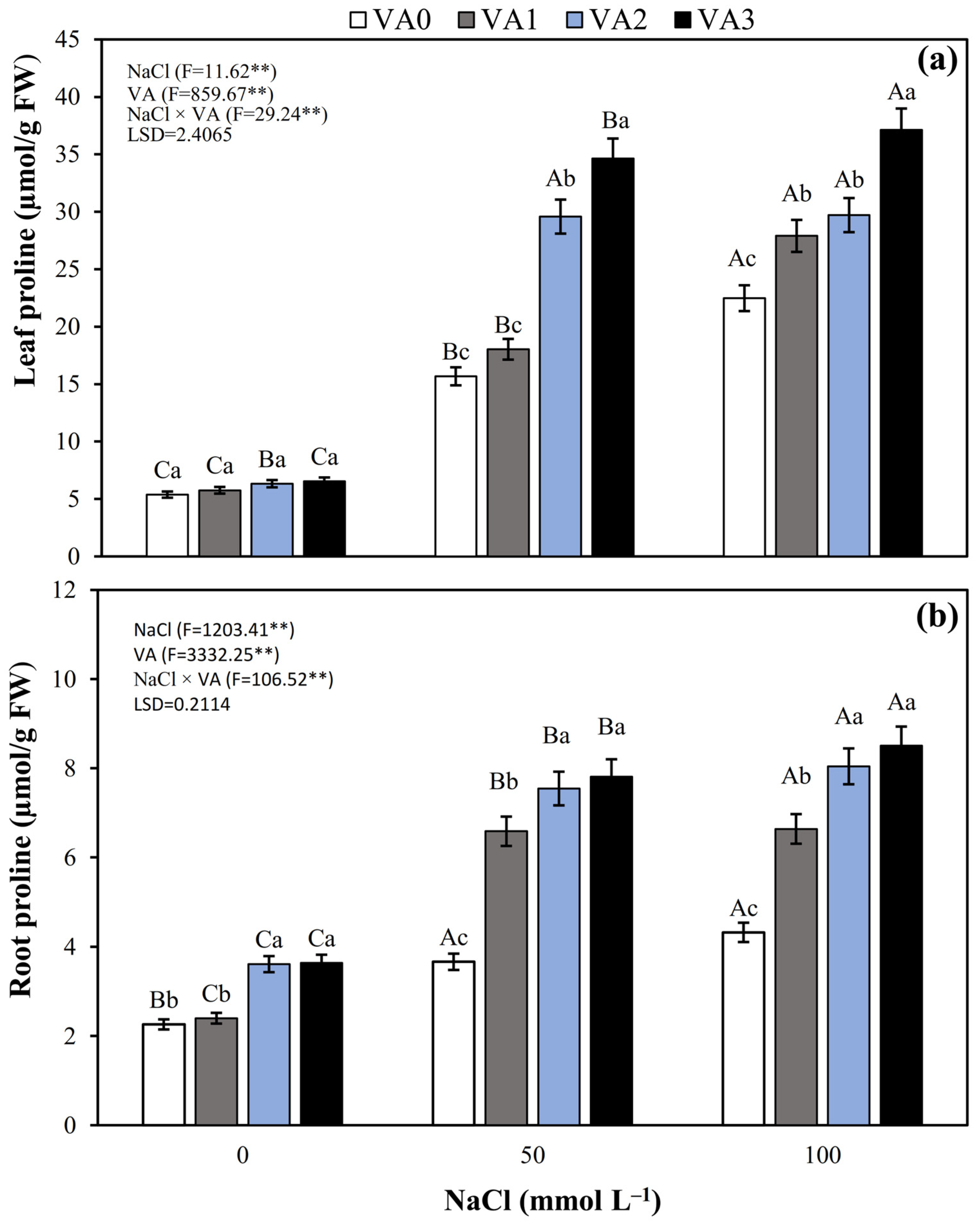
3.8. Leaf and Root Proline Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.D.M.; Junior, G.D.S.S.; Viciedo, D.O.; Díaz, Y.P.; Calzada, K.P.; Gratão, P.L. Silicon Alleviates Sodium Toxicity in Sorghum and Sunflower Plants by Enhancing Ionic Homeostasis in Roots and Shoots and Increasing Dry Matter Accumulation. Silicon 2020, 13, 475–486. [Google Scholar] [CrossRef]
- Viciedo, D.O.; Prado, R.D.M.; Martinez, C.A.; Habermann, E.; Branco, R.B.F.; Piccolo, M.D.C.; Hurtado, A.C.; Calzada, K.P.; Tenesaca, L.F.L. Water stress and warming impact nutrient use efficiency of Mombasa grass (Megathyrsus maximus) in tropical conditions. J. Agron. Crop Sci. 2020, 207, 128–138. [Google Scholar] [CrossRef]
- Viciedo, D.O.; Prado, R.D.M.; Martínez, C.A.; Habermann, E.; Piccolo, M.D.C. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Al Murad, M.; Khan, A.L.; Muneer, S. Silicon in Horticultural Crops: Cross-talk, Signaling, and Tolerance Mechanism under Salinity Stress. Plants 2020, 9, 460. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Kalanaki, M.; Ritzema, H.; Bamshad, R.; Jones, E.; Fazilatnia, M. Application of bio-desalinization for reclamation of salt-affected soil under composted cow manure and deficit irrigation with saline water. Paddy Water Environ. 2020, 18, 469–479. [Google Scholar] [CrossRef]
- Etesami, H.; Noori, F. Soil Salinity as a Challenge for Sustainable Agriculture and Bacterial-Mediated Alleviation of Salinity Stress in Crop Plants. In Saline Soil-Based Agriculture by Halotolerant Microorganisms; Springer: Singapore, 2019; pp. 1–22. [Google Scholar] [CrossRef]
- Joshi, S.; Nath, J.; Singh, A.K.; Pareek, A.; Joshi, R. Ion transporters and their regulatory signal transduction mechanisms for salinity tolerance in plants. Physiol. Plant. 2022, 174, e13702. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Hossain, A.; Barutçular, C.; Iqbal, M.A.; Islam, M.S.; Fahad, S.; Sytar, O.; Çiğ, F.; Meena, R.S.; Erman, M. Consequences of Salinity Stress on the Quality of Crops and Its Mitigation Strategies for Sustainable Crop Production: An Outlook of Arid and Semi-arid Regions. In Environment, Climate, Plant and Vegetation Growth; Springer: Cham, Switzerland, 2020; pp. 503–533. [Google Scholar] [CrossRef]
- Kim, Y.; Mun, B.-G.; Khan, A.L.; Waqas, M.; Kim, H.-H.; Shahzad, R.; Imran, M.; Yun, B.-W.; Lee, I.-J. Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS ONE 2018, 13, e0192650. [Google Scholar] [CrossRef]
- Gratão, P.; Monteiro, C.C.; Tezotto, T.; Carvalho, R.F.; Alves, L.R.; Peters, L.P.; Azevedo, R.A. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. BioMetals 2015, 28, 803–816. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and Defence Mechanisms of Vegetable Crops against Drought, Heat and Salinity Stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Linh, N.T.; Cham, L.T.T.; Thang, V.N. Effects of Salinity Stress on the Growth, Physiology, and Yield of Soybean (Glycine max (L.) Merrill). Vietnam J. Agric. Sci. 2021, 4, 1043–1055. [Google Scholar] [CrossRef]
- Safwat, G.; Salam, H.S.A. The Effect of Exogenous Proline and Glycine Betaine on Phyto-biochemical Responses of Salt-stressed Basil Plants. Egypt. J. Bot. 2022, 62, 537–547. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous Melatonin Improves Salt Tolerance by Mitigating Osmotic, Ion, and Oxidative Stresses in Maize Seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.D.M.; Junior, G.D.S.S.; Gratão, P.L.; Felisberto, G.; Viciedo, D.O.; dos Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of the plasma membrane H+-ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2014, 115, 481–494. [Google Scholar] [CrossRef]
- Matysiak, K.; Kierzek, R.; Siatkowski, I.; Kowalska, J.; Krawczyk, R.; Miziniak, W. Effect of Exogenous Application of Amino Acids L-Arginine and Glycine on Maize under Temperature Stress. Agronomy 2020, 10, 769. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rohman, M.M.; Anee, T.I.; Huang, Y.; Fujita, M. Exogenous Silicon Protects Brassica napus Plants from Salinity-Induced Oxidative Stress Through the Modulation of AsA-GSH Pathway, Thiol-Dependent Antioxidant Enzymes and Glyoxalase Systems. Gesunde Pflanz. 2018, 70, 185–194. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Elhamid, E.M.A.; Sadak, M.S. Comparative study for the effect of arginine and sodium nitroprusside on sunflower plants grown under salinity stress conditions. Bull. Natl. Res. Cent. 2019, 43, 118. [Google Scholar] [CrossRef]
- Hanc, F.; Tuncer, G. How Do Foliar Application of Melatonin and L-Tryptophan Affect Lettuce Growth Parameters Under Salt Stress? Turk. J. Agric.-Food Sci. Technol. 2020, 8, 960–964. [Google Scholar]
- El-Azeiz, E.H.A.; El Mantawy, R.F.; Mohamed, E.S. Alleviation the Adverse Effects of Salinity Stress on Soybean Cultivars by Foliar Spraying of Arginine. Menoufia J. Soil Sci. 2021, 6, 343–362. [Google Scholar] [CrossRef]
- Ruas, J.; Salvador, M.; Amazonas, L. Acompanhamento Da Safra Brasileira. Cia. Nac. Abast. 2020, 7, 1–89. [Google Scholar]
- EMBRAPA. Limpeza de Areia Para Experimentos Em Nutrição de Plantas; EMBRAPA: Dourados, Brazil, 2018; pp. 1–5. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of Development Descriptions for Soybeans, Glycine Max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Viciedo, D.O.; Prado, R.D.M.; Toledo, R.L.; Aguilar, D.S.; Dos Santos, L.C.N.; Hurtado, A.C.; Calzada, K.P.; Aguilar, C.B. Physiological role of silicon in radish seedlings under ammonium toxicity. J. Sci. Food Agric. 2020, 100, 5637–5644. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bataglia, O.C.; Teixeira, J.P.F.; Furlani, P.R.; Furlani, A.M.C.; Gallo, J.R. Métodos de Análise Química de Plantas, 1st ed.; Instituto Agronômico de Campinas: Campinas, Brazil, 1983.
- Mihara, M.; Uchiyama, M.; Fukuzawa, K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem. Med. 1980, 23, 302–311. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Hossain, A.; Islam, M.S.; Barutçular, C.; Ratnasekera, D.; Kumar, N.; Meena, R.S.; Gharib, H.S.; Saneoka, H.; da Silva, J.A.T. Sustainable soybean production and abiotic stress management in saline environments: A critical review. Aust. J. Crop Sci. 2019, 13, 228–236. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Alfosea-Simón, M.; Zavala-Gonzalez, E.A.; Camara-Zapata, J.M.; Martínez-Nicolás, J.J.; Simón, I.; Simón-Grao, S.; García-Sánchez, F. Effect of foliar application of amino acids on the salinity tolerance of tomato plants cultivated under hydroponic system. Sci. Hortic. 2020, 272, 109509. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Zhang, B. Transcriptome analysis and functional identification of GmMYB46 in soybean seedlings under salt stress. PeerJ 2021, 9, e12492. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Mostofa, M.G.; Rahman, M.; Abdel-Farid, I.B.; Tran, L.-S.P. Extracts from Yeast and Carrot Roots Enhance Maize Performance under Seawater-Induced Salt Stress by Altering Physio-Biochemical Characteristics of Stressed Plants. J. Plant Growth Regul. 2019, 38, 966–979. [Google Scholar] [CrossRef]
- Bolhàr-Nordenkampf, H.R.; Öquist, G. Chlorophyll Fluorescence as a Tool in Photosynthesis Research. In Photosynthesis and Production in a Changing Environment; Springer: Amsterdam, The Netherlands, 1993; pp. 193–206. [Google Scholar]
- Ali, Q.; Haider, M.Z.; Shahid, S.; Aslam, N.; Shehzad, F.; Naseem, J.; Ashraf, R.; Ali, A.; Hussain, S.M. Role of amino acids in improving abiotic stress tolerance to plants. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 175–204. [Google Scholar]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Malik, S.; Adrees, M.; Qayyum, M.F.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Correction to: Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol. Plant. 2019, 41, 72. [Google Scholar] [CrossRef]
- Mustafa, A.; Imran, M.; Ashraf, M.; Mahmood, K. Perspectives of Using L-Tryptophan for Improving Productivity of Agricultural Crops: A Review. Pedosphere 2018, 28, 16–34. [Google Scholar] [CrossRef]
- Freitas, I.S.; Trennepohl, B.I.; Machado, T.; Acioly, S.; Justi, V.; Mello, S.C.; Neto, D.D.; Kluge, R.A.; Azevedo, R.A. Exogenous Application of L-Arginine Improves Protein Content and Increases Yield of Pereskia Aculeata Mill. Grown in Soilless Media Container. Horticulturae 2022, 8, 142. [Google Scholar] [CrossRef]
- Malhotra, C.; Kapoor, R.T. Silicon: A Sustainable Tool in Abiotic Stress Tolerance in Plants. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K., Nahar, K., Alharby, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 333–356. ISBN 978-3-030-06118-0. [Google Scholar]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef]
- Kahveci, H.; Bilginer, N.; Diraz-Yildirim, E.; Kulak, M.; Yazar, E.; Kocacinar, F.; Karaman, S. Priming with salicylic acid, β-carotene and tryptophan modulates growth, phenolics and essential oil components of Ocimum basilicum L. grown under salinity. Sci. Hortic. 2021, 281, 109964. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Ashraf, M. Mitigation of sodium chloride toxicity in Solanum lycopersicum L. by supplementation of jasmonic acid and nitric oxide. J. Plant Interact. 2018, 13, 64–72. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.A.; Babar, A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- De Morais, M.B.; Azevedo, R.A.; Camara, T.R.; Ulisses, C.; De Albuquerque, C.C.; Willadino, L. Antioxidative metabolism in sugarcane (Poaceae) varieties subjected to water and saline stress. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 776–782. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis, and Ascorbate-Glutathione Cycle in Salt Stressed Vigna angularis. Biomolecules 2020, 10, 42. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Liu, B.; Peng, X.; Han, L.; Hou, L.; Li, B. Effects of Exogenous Spermidine on Root Metabolism of Cucumber Seedlings under Salt Stress by GC-MS. Agronomy 2020, 10, 459. [Google Scholar] [CrossRef]
- Chung, Y.S.; Kim, K.-S.; Hamayun, M.; Kim, Y. Silicon Confers Soybean Resistance to Salinity Stress Through Regulation of Reactive Oxygen and Reactive Nitrogen Species. Front. Plant Sci. 2020, 10, 1725. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Z.; Liu, Q.; Pu, G.; Zhang, Y.; Li, J. Research on the adaptive mechanism of photosynthetic apparatus under salt stress: New directions to increase crop yield in saline soils. Ann. Appl. Biol. 2019, 175, 1–17. [Google Scholar] [CrossRef]
- Hessini, K.; Issaoui, K.; Ferchichi, S.; Saif, T.; Abdelly, C.; Siddique, K.H.; Cruz, C. Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Karpets, Y.V.; Kolupaev, Y.E.; Lugovaya, A.A.; Shvidenko, N.V.; Yastreb, T.O. Effects of Nitrate and L-Arginine on Content of Nitric Oxide and Activities of Antioxidant Enzymes in Roots of Wheat Seedlings and Their Heat Resistance. Russ. J. Plant Physiol. 2018, 65, 908–915. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.C.; Otálora, G.; López-Marín, J.; Del Amor, F.M. Merging Heat Stress Tolerance and Health-Promoting Properties: The Effects of Exogenous Arginine in Cauliflower (Brassica oleracea var. botrytis L.). Foods 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña Calzada, K.; Olivera Viciedo, D.; Habermann, E.; Calero Hurtado, A.; Lupino Gratão, P.; De Mello Prado, R.; Lata-Tenesaca, L.F.; Martinez, C.A.; Ajila Celi, G.E.; Rodríguez, J.C. Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants. Agronomy 2022, 12, 2014. https://doi.org/10.3390/agronomy12092014
Peña Calzada K, Olivera Viciedo D, Habermann E, Calero Hurtado A, Lupino Gratão P, De Mello Prado R, Lata-Tenesaca LF, Martinez CA, Ajila Celi GE, Rodríguez JC. Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants. Agronomy. 2022; 12(9):2014. https://doi.org/10.3390/agronomy12092014
Chicago/Turabian StylePeña Calzada, Kolima, Dilier Olivera Viciedo, Eduardo Habermann, Alexander Calero Hurtado, Priscila Lupino Gratão, Renato De Mello Prado, Luis Felipe Lata-Tenesaca, Carlos Alberto Martinez, Gabriela Eugenia Ajila Celi, and Juan Carlos Rodríguez. 2022. "Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants" Agronomy 12, no. 9: 2014. https://doi.org/10.3390/agronomy12092014
APA StylePeña Calzada, K., Olivera Viciedo, D., Habermann, E., Calero Hurtado, A., Lupino Gratão, P., De Mello Prado, R., Lata-Tenesaca, L. F., Martinez, C. A., Ajila Celi, G. E., & Rodríguez, J. C. (2022). Exogenous Application of Amino Acids Mitigates the Deleterious Effects of Salt Stress on Soybean Plants. Agronomy, 12(9), 2014. https://doi.org/10.3390/agronomy12092014









