Bacterial Community and Chemical Composition of Mixed Fresh Cactus Forage and Buffel Grass Hay during Aerobic Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Local Conditions
2.2. Treatments and Experimental Design
2.3. Chemical Analysis
2.4. Microbial Populations
2.5. Analysis of the Bacterial Community of Silages by 16s rRNA Marker Gene Sequencing (Metataxonomics) Using High-Throughput Sequencing
2.6. Statistical Analysis
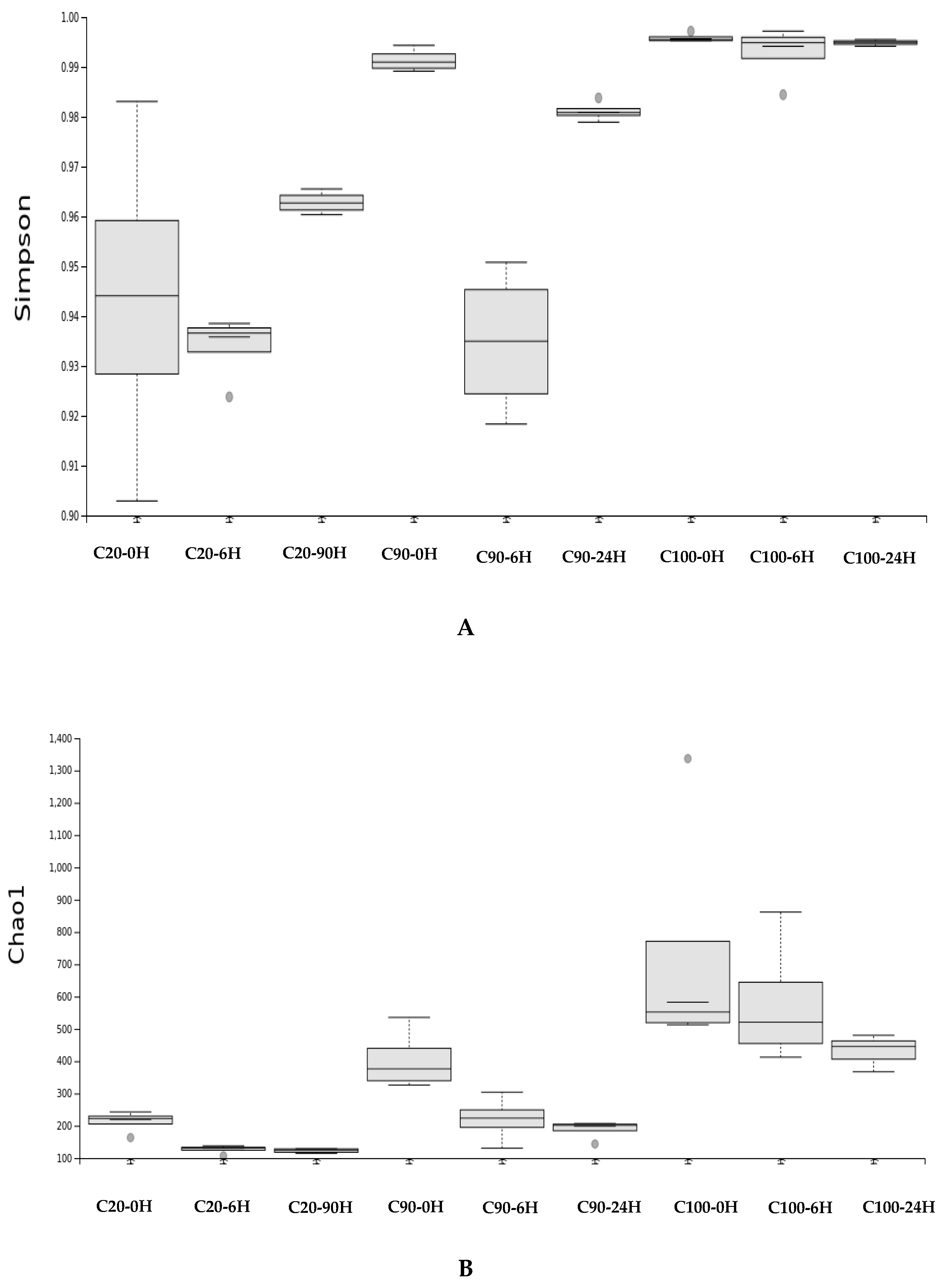
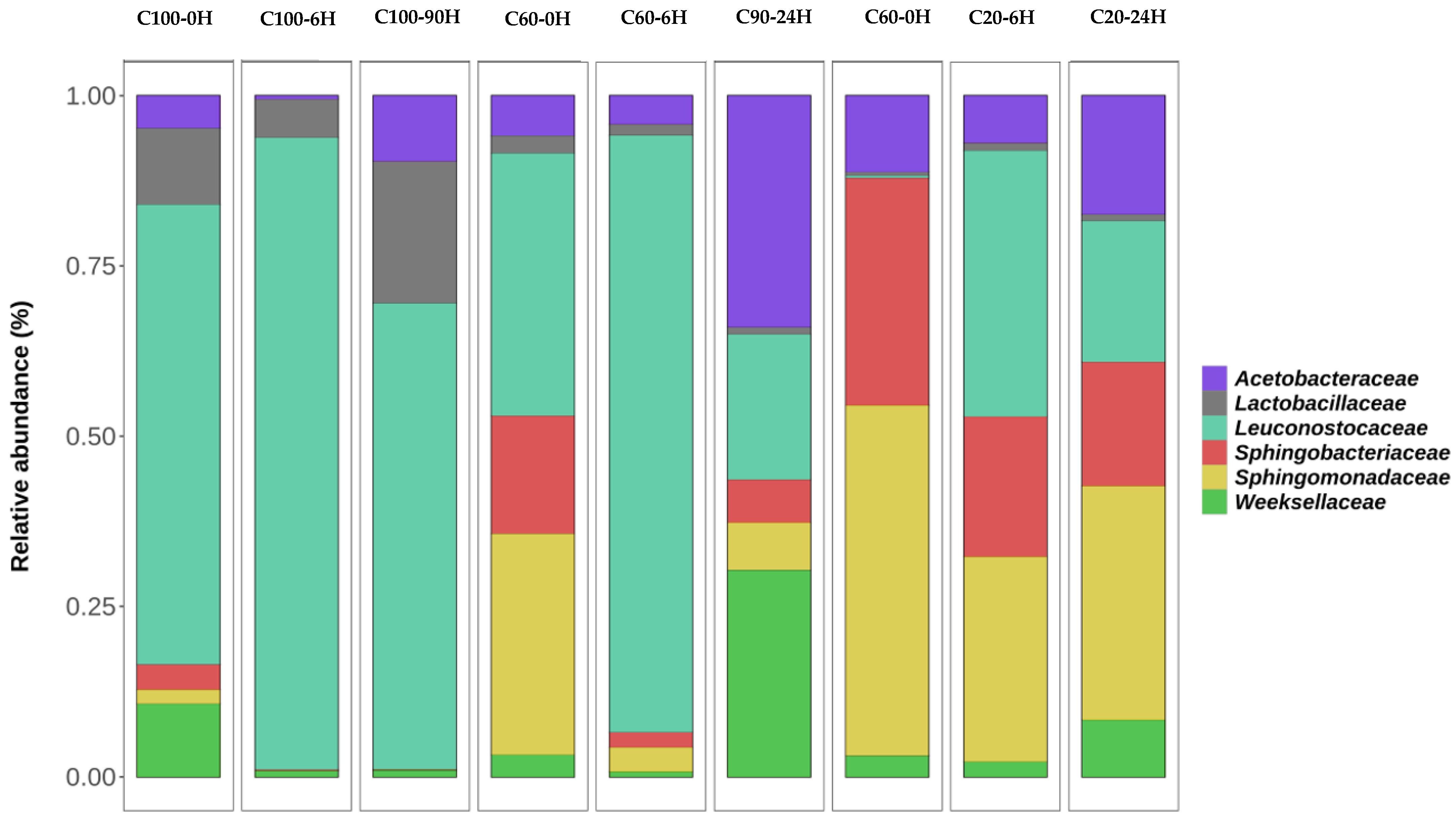
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melo, A.A.S.; Ferreira, M.A.; Véras, A.S.C.; Lira, M.A.; Lima, L.E.; Pessoa, R.A.S.; Bispo, S.V.; Cabral, A.M.D.; Azevedo, M. Desempenho leiteiro de vacas alimentadas com caroço de algodão em dieta à base de palma forrageira. Pesq. Agrop. Bra. 2006, 41, 1165–1171. [Google Scholar] [CrossRef]
- Marques, O.F.C.; Gomes, L.S.P.; Mourthé, M.H.F.; Braz, T.G.S.; Pires Neto, O.S. Palma forrageira: Cultivo e utilização na alimentação de bovinos. Cad. Ciências Agrárias 2017, 9, 75–93. [Google Scholar]
- Costa, R.G.; Treviño, I.H.; Medeiros, G.R.; Medeiros, N.A.; Pinto, T.F.; Oliveira, R.L. Effects of replacing corn with cactus pear (Opuntia fícus indica Mill) on the performance of Santa Inês lambs. Small Rum. Res. 2012, 102, 13–17. [Google Scholar] [CrossRef]
- Pinho, R.M.A.; Santos, E.M.; Oliveira, J.S.; Carvalho, G.G.P.; Silva, T.C.; Macêdo, A.J.S.; Corrêa, Y.R.; Zanine, A.M. Does the level of forage neutral detergent fiber affect the ruminal fermentation, digestibility and feeding behavior of goats fed cactus pear? Anim. Sci. J. 2018, 89, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.J.A.; Ferreira, M.A.; Oliveira, J.C.V.; Santos, D.C.; Chagas, J.C.C.; Alves, A.M.S.V.; Silva, A.E.M.; Freitas, W.R. Replacement of Tifton hay by spineless cactus in Girolando post-weaned heifers’ diets. Trop. Anim. Heath Prod. 2017, 50, 149–154. [Google Scholar] [CrossRef]
- Paulino, R.S.; Oliveira, J.S.; Santos, E.M.; Pereira, G.A.; Ramos, J.P.F.; César Neto, J.M.; Cruz, G.F.L.; Leite, G.M.; Satake, F.; Silva, A.L.; et al. Spineless cactus use management on microbiological quality, performance, and nutritional disorders in sheep. Trop. Anim. Heath Prod. 2021, 53, 1–14. [Google Scholar] [CrossRef]
- Abidi, S.; Ben Salem, H.; Martín-García, A.I.; Molina-Alcaide, E. Ruminal fermentation of spiny (Opuntia amyclae) and spineless (Opuntia ficus indica f. inermis) cactus cladodes and diets including cactus. Anim. Feed Sci. Technol. 2009, 149, 333–340. [Google Scholar] [CrossRef]
- Andrade-Montemayor, H.M.; Cordova-Torres, A.V.; Casca, T.G.; Kawas, J.R. Alternative foods for small ruminants in semiarid zones, the case of Mesquite (Proposis laevigata spp.) and Nopal (Opuntia spp.). Small Rumi. Res. 2011, 98, 83–92. [Google Scholar] [CrossRef]
- Isaac, A.A. Overview of Cactus (Opuntia Ficus-Indica (L): A Myriad of Alternatives. Stud. Ethno-Med. 2016, 10, 195–205. [Google Scholar] [CrossRef]
- Vimalkumar, S.P.; Purohit, H.J.; Raje, D.V.; Parmar, N.; Patel, A.B.; Jones, O.A.H.J.; Joshi, C.G. The effect of a high-roughage diet on the metabolism of aromatic compounds by rumen microbes: A metagenomic study using Mehsani buffalo (Bubalus bubalis). Appl. Microbiol. Biothechnol. 2016, 100, 1319–1331. [Google Scholar] [CrossRef]
- Zárate-Castrejón, J.L.; Martínez-Martínez, T.O.; Angeles-Núñez, J.G.; Concha-Valdez, F.G.; Verde-Calvo, J.R.; Guzmán, M.E.R. Survival of Salmonella Enteritidis and Escherichia coli in cactus cladodes under domestic marketing conditions in Mexico. Afr. J. Microbiol. Res. 2016, 10, 1999–2006. [Google Scholar] [CrossRef]
- Chaudhry, V.; Runge, P.; Sengupta, P.; Doehlemann, G.; Parker, J.E.; Kemen, E. Shaping the leaf microbiota: Plant–microbe–microbe interactions. J. Exp. Bot. 2020, 72, 36–56. [Google Scholar] [CrossRef]
- Robazza, W.S.; Teleken, J.T.; Gomes, G.A. Modelagem Matemática do Crescimento de Microrganismos em Alimentos. Trends Comput. Appl. Math. 2010, 11, 101–110. [Google Scholar] [CrossRef]
- Gilbert, R.A.; Tomkins, N.; Padmanabha, J.; Gough, J.M.; Krause, D.O.; Mcsweeney, C.S. Effect of finishing diets on Escherichia coli populations and prevalence of enterohaemorrhagic E. coli virulence genes in cattle faeces. J. Appl. Microbiol. 2005, 99, 885–894. [Google Scholar] [CrossRef]
- Guastalli, E.A.L.; Gama, N.M.S.Q.; Buim, M.R.; Oliveira, R.A.; Ferreira, A.J.P.; Leite, D.S. Índice de patogenicidade, produção de hemolisina e sorogrupo de amostras de Escherichia coli isoladas de aves de postura comercial. Ar. Inst. Biol. 2010, 77, 153–157. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists—AOAC. Official Methods of Analysis, 19th.ed.; AOAC: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Sniffen, C.J.; O'connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agric. 1996, 17, 264–268. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Corsato, C.E.; Scarpare Filho, J.A.; Sales, E.C.J. Teores de carboidratos em órgãos lenhosos do caquizeiro em clima tropical. Rev. Bras. Frut. 2008, 30, 414–418. [Google Scholar] [CrossRef]
- Bolsen, K.K.; Lin, C.; Brent, C.R.; Feyerherm, A.M.; Urban, J.E.; Aimutis, W.R. Effects of silage additives on the microbial succession and fermentation process of alfafa and corn silages. J. Dairy Sci. 1992, 75, 3066–3083. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2018, 37, 852–857. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. Mafft: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Mcmurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. Unifrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Hoffmann, F.L. Fatores limitantes à proliferação de microorganismos em alimentos. Bras. Aliment. 2001, 9, 23–30. [Google Scholar]
- Liu, C.; Hofstra, N.; Franz, E. Impacts of climate change on the microbial safety of pre-harvest leafy green vegetables as indicated by Escherichia coli O157 and Salmonella spp. Int. J. Food Microbiol. 2013, 163, 119–128. [Google Scholar] [CrossRef]
- Solomon, E.B.; Yaron, S.; Matthews, K.R. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 2002, 68, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.L.; Pui, C.F.; Wong, W.C.; Noorlis, A.; Son, R. Biofilm forming ability and time course study of growth of Salmonella Typhi on fresh produce surfaces. Int. Food Res. J. 2012, 19, 71–76. [Google Scholar]
- McDonald, P.; Henderson, N.; Heron, S. The Biochemistry of Silage; John Wiley & Sons: Manchester, UK, 1991; pp. 81–151. [Google Scholar]
- Muck, R.E. Microbiologia da silagem e seu controle por meio de aditivos. Rev. Bras. Zoot. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Nascimento, T.V.C.; Carvalho, G.G.P.; Freitas Júnior, J.E.L.; Souza, W.F. Volumosos tratados com asditivos químicos: Valor nutritivo e desempenho de Ruminantes. Arch. Zoot. 2016, 65, 593–604. [Google Scholar]
- Macêdo, A.J.S.; Santos, E.M.; Oliveira, J.S.; Perazzo, A.F. Microbiologia de silagens: Revisão de Literatura. Rev. Elect. Veter. 2017, 18, 1–11. [Google Scholar]
- Carvalho, C.B.M.; Edvan, R.L.; Carvalho, M.L.A.M.; Reis, A.L.A.; Nascimento, R.R. Uso de cactáceas na alimentação animal e seu armazenamento após colheita. Arch. Zoot. 2018, 67, 440–446. [Google Scholar] [CrossRef][Green Version]
- Dinçer, E.; Kıvanç, M. Lipolytic activity of lactic acid bacteria isolated from Turkish pastırma. Anadolu Univ. J. Sci. Technol. C Life Sci. Biotechnol. 2018, 7, 12–19. [Google Scholar] [CrossRef]
- Silva, E.O.O.; Nespolo, C.R.; Sehn, C.P.; Pinheiro, F.C.; Stefani, L.M. Lactic acid bacteria with antimicrobial, proteolytic and lipolytic activities isolated from ovine dairy products. Food Sci. Technol. 2019, 40, 293–299. [Google Scholar] [CrossRef]
- Hui, Y. Handbook of Food Science Tecnhnology and Engineering; CRC Press: Boca Raton, FL, USA, 2006; Volume 149, pp. 8–17. [Google Scholar]
- Trabulsi, L.R.; Alterthum, F.; Martinez, M.B.; Campos, L.C.; Gompertz, O.F.; Rácz, M.L. Microbiologia, 5th ed.; Atheneu: São Paulo, Brazil, 2008. [Google Scholar]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–138. [Google Scholar] [CrossRef]
- Remenant, B.; Jaffres, E.; Dousset, X.; Pilet, M.F.; Zagorec, M. Bacterial spoilers of food: Behavior, fitness and functional properties. Food Microbiol. 2015, 45, 45–53. [Google Scholar] [CrossRef]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food Spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Pereira, G.A.; Santos, E.M.; Araújo, G.G.L.; Oliveira, J.S.; Pinho, R.M.A.; Zanine, A.M.; Souza, A.F.N.; Macedo, A.J.S.; Neto, J.M.C.; Nascimento, T.V.C. Isolation and identification of lactic acid bacteria in fresh plants and in silage from Opuntia and their effects on the fermentation and aerobic stability of silage. J. Agric. Sci. 2020, 10, 684–692. [Google Scholar] [CrossRef]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 1–22. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef]




| Feeds, g/kg DM | Cactus Forage | Buffel Grass Hay |
|---|---|---|
| Dry matter 1 | 66.41 | 867.44 |
| Organic matter | 918.59 | 929.52 |
| Crude protein | 78.33 | 49.41 |
| Ether extract | 15.68 | 10.76 |
| aNDFom-NDF 2 | 382.82 | 821.23 |
| Lignin (sa) 3 | 18.42 | 48.62 |
| NFC 4 | 441.77 | 48.11 |
| Item | Cactus Forage Levels % | Time, Hours | Overall Average (C) | p-Value 1 | p-Value 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 24 | C | T | C × T | T | ||||
| L | Q | |||||||||||
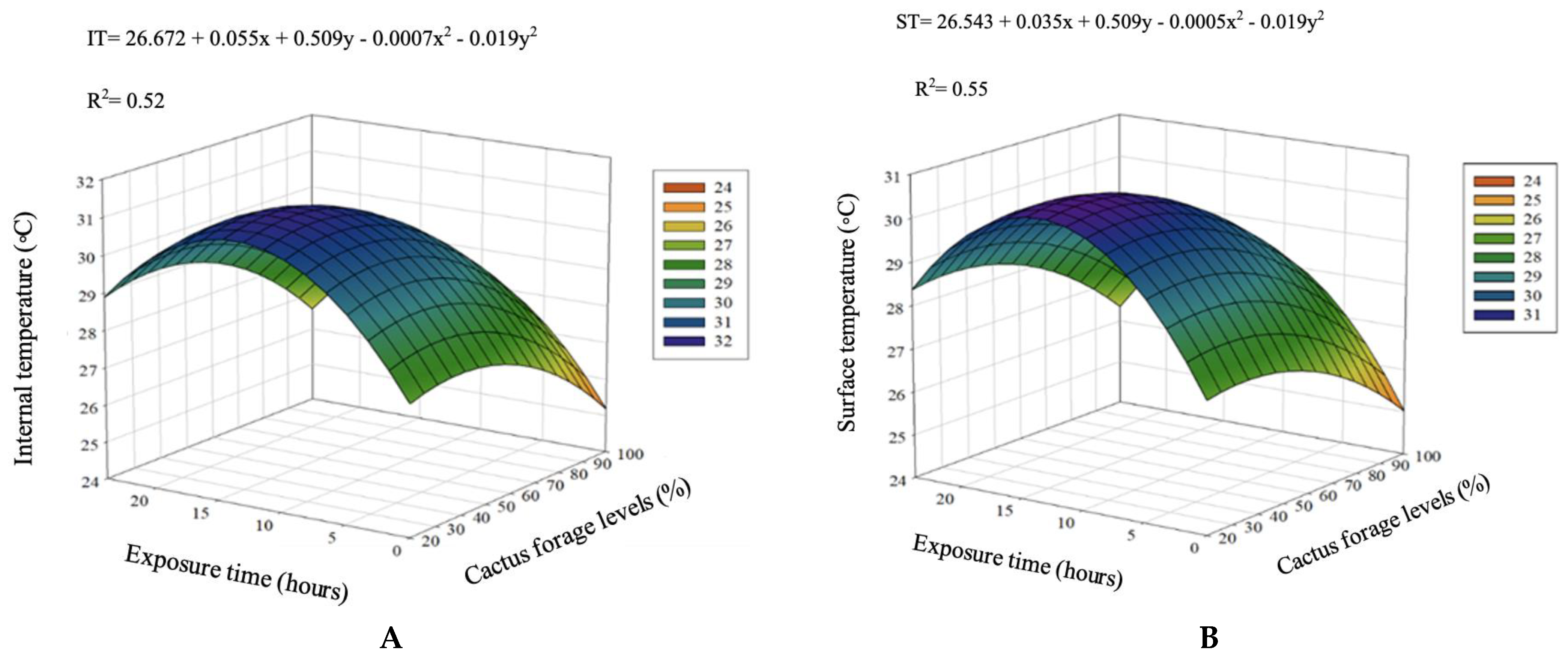
| IT, °C | 20 | 28.60 | 30.98 | 26.68 | 28.88 | 27.22 | 30.47 | <0.001 | <0.001 | <0.001 | <0.001 | 0.397 |
| 60 | 26.58 | 29.28 | 31.82 | 28.64 | 28.84 | 29.03 | <0.001 | <0.001 | <0.001 | <0.001 | 0.044 | |
| 80 | 25.00 | 30.50 | 29.44 | 32.64 | 29.04 | 29.32 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 90 | 25.02 | 27.96 | 27.00 | 29.44 | 28.84 | 27.65 | <0.001 | <0.001 | <0.001 | 0.036 | 0.001 | |
| 100 | 24.76 | 27.54 | 27.40 | 28.74 | 27.22 | 27.13 | <0.001 | <0.001 | <0.001 | 0.679 | 0.163 | |
| Overall Average (T) | 25.99 | 29.25 | 28.47 | 31.67 | 28.23 | |||||||
| ST, °C | 20 | 28.16 | 30.60 | 29.46 | 27.76 | 27.98 | 28.79 | <0.001 | <0.001 | <0.001 | <0.001 | 0.682 |
| 60 | 26.38 | 27.52 | 31.04 | 28.32 | 27.56 | 28.16 | <0.001 | <0.001 | <0.001 | <0.001 | 0.848 | |
| 80 | 24.52 | 29.52 | 28.88 | 31.76 | 27.54 | 28.44 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 90 | 24.58 | 27.28 | 26.88 | 29.12 | 28.08 | 27.19 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | |
| 100 | 24.30 | 26.60 | 27.00 | 28.56 | 26.76 | 26.64 | <0.001 | <0.001 | <0.001 | 0.121 | 0.493 | |
| Overall Average (T) | 25.59 | 28.30 | 28.65 | 29.10 | 27.58 | |||||||
| pH | 20 | 7.17 | 7.38 | 6.94 | 6.31 | 6.39 | 6.84 | <0.001 | <0.001 | <0.001 | <0.001 | 0.923 |
| 60 | 6.35 | 6.94 | 7.03 | 7.06 | 6.41 | 6.76 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 80 | 6.28 | 6.92 | 6.96 | 6.43 | 6.16 | 6.55 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 90 | 6.35 | 6.41 | 6.78 | 6.49 | 6.47 | 6.50 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 100 | 5.70 | 5.94 | 5.99 | 5.92 | 5.90 | 5.89 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Overall Average (T) | 6.37 | 6.72 | 6.74 | 6.44 | 6.27 | 6.51 | ||||||
| Escherichia coli (Log CFU 3/g) | ||||||||||||
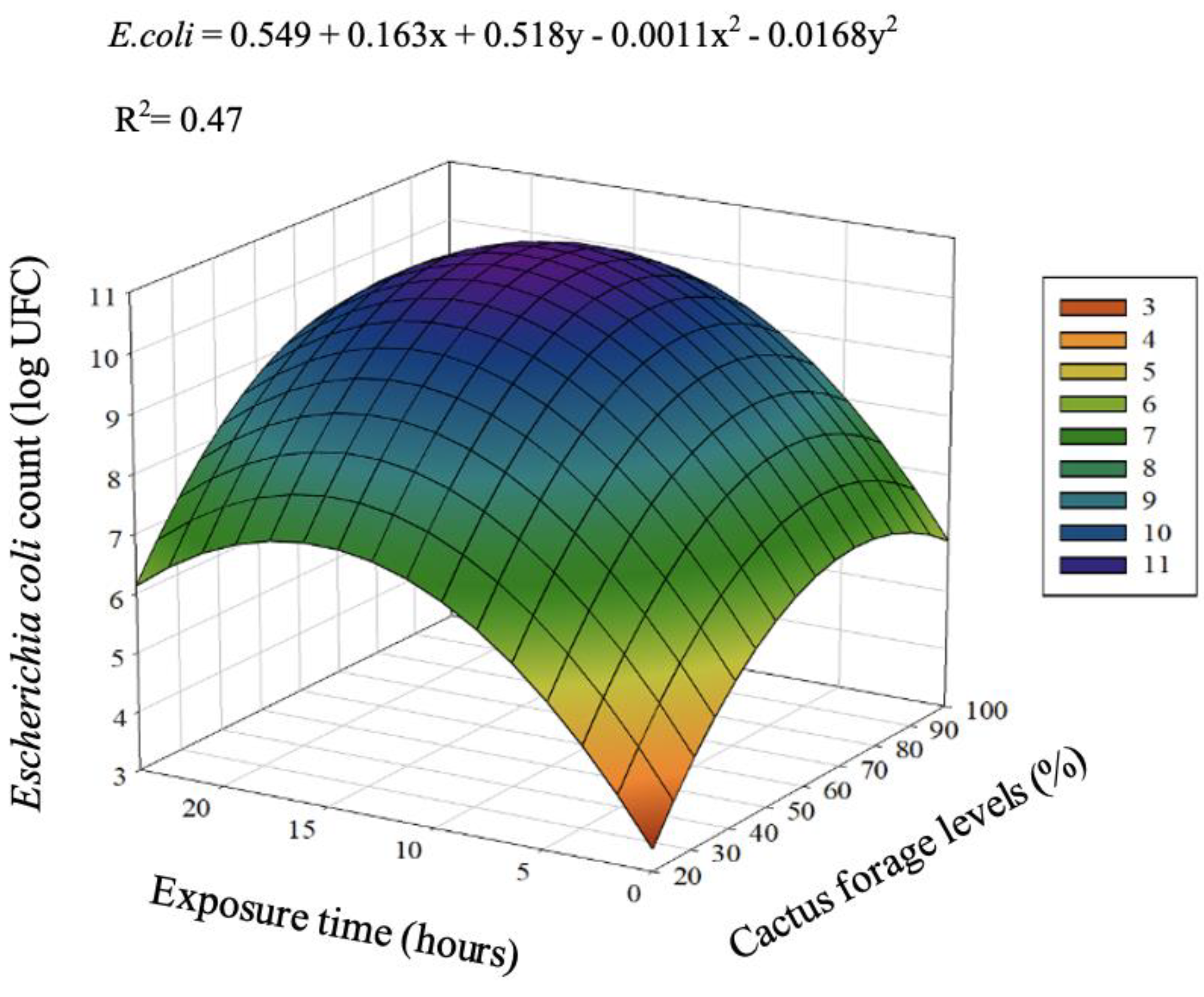
| 20 | 0.00 | 0.00 | 9.72 | 8.77 | 8.64 | 5.43 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 60 | 7.47 | 9.28 | 9.65 | 8.91 | 8.99 | 8.86 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 80 | 7.04 | 9.06 | 9.51 | 8.61 | 8.19 | 8.48 | <0.001 | <0.001 | <0.001 | <0.001 | 0.026 | |
| 90 | 6.79 | 8.69 | 9.30 | 9.13 | 8.83 | 8.55 | <0.001 | <0.001 | <0.001 | 0.029 | 0.170 | |
| 100 | 6.47 | 8.34 | 9.16 | 9.05 | 9.02 | 8.41 | <0.001 | <0.001 | <0.001 | 0.203 | 0.144 | |
| Overall Average (T) | 5.55 | 7.07 | 9.47 | 8.89 | 8.73 | |||||||
| Item | Cactus Forage Levels % | Time, Hours | Overall Average (C) | p-Value 1 | p-Value 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | 24 | C | T | C × T | T | ||||
| L | Q | |||||||||||
| Dry matter, g/kg | 20 | 828.9 | 810.4 | 777.7 | 727.6 | 590.4 | 747.0 | <0.001 | <0.001 | <0.001 | 0.000 | 0. 359 |
| 60 | 432.1 | 431.4 | 431.1 | 397.8 | 388.1 | 416.1 | <0.001 | <0.001 | <0.001 | 0.012 | 0.754 | |
| 80 | 280.7 | 262.2 | 239.1 | 237.4 | 226.9 | 249.3 | <0.001 | <0.001 | <0.001 | 0.019 | 0.202 | |
| 90 | 148.0 | 148.2 | 148.3 | 138.5 | 136.1 | 143.8 | <0.001 | <0.001 | <0.001 | 0.000 | 0.773 | |
| 100 | 66.4 | 65.8 | 64. 6 | 63.4 | 63.2 | 64.7 | <0.001 | <0.001 | <0.001 | 0.000 | 0.002 | |
| Overall Average (T) | 231.8 | 227.0 | 272.8 | 312.9 | 280.9 | |||||||
| Crude protein, g/kg DM | 20 | 50.3 | 49.3 | 48.9 | 49.3 | 57.5 | 51.1 | <0.001 | <0.001 | 0.000 | <0.001 | <0.001 |
| 60 | 57.4 | 55.3 | 53.3 | 53.9 | 54.5 | 54.9 | <0.001 | <0.001 | 0.000 | <0.001 | <0.001 | |
| 80 | 61.1 | 60.6 | 59.0 | 64.2 | 64.3 | 61.8 | <0.001 | <0.001 | 0.000 | <0.001 | <0.001 | |
| 90 | 65.1 | 64.2 | 63.9 | 67.2 | 72.5 | 66.6 | <0.001 | <0.001 | 0.000 | <0.001 | <0.001 | |
| 100 | 78.3 | 76.4 | 75.8 | 77.7 | 79.0 | 77.3 | <0.001 | <0.001 | 0.000 | <0.001 | <0.001 | |
| Overall Average (T) | 62.4 | 61.2 | 60.2 | 62.3 | 65.6 | |||||||
| WSC 3, g/kg DM | 20 | 1.75 | 1.04 | 1.00 | 0.92 | 0.84 | 1.11 | <0.001 | <0.001 | <0.001 | 0.029 | 0.090 |
| 60 | 1.78 | 1.63 | 1.13 | 0.91 | 0.84 | 1.26 | <0.001 | <0.001 | <0.001 | 0.002 | 0.084 | |
| 80 | 2.54 | 1.76 | 0.75 | 0.73 | 0.66 | 1.29 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 90 | 3.16 | 2.55 | 0.57 | 0.48 | 0.38 | 1.43 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 100 | 6.05 | 3.74 | 0.76 | 0.66 | 0.54 | 2.35 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Overall Average (T) | 3.06 | 2.14 | 0.84 | 0.74 | 0.65 | |||||||
| NH3-N 4, %N total | 20 | 0.010 | 0.010 | 0.010 | 0.016 | 0.010 | 0.011 | <0.001 | <0.001 | <0.001 | 0.381 | 0.267 |
| 60 | 0.010 | 0.010 | 0.029 | 0.020 | 0.013 | 0.016 | <0.001 | <0.001 | <0.001 | 0.725 | 0.000 | |
| 80 | 0.023 | 0.006 | 0.016 | 0.029 | 0.020 | 0.019 | <0.001 | <0.001 | <0.001 | 0.057 | 0.891 | |
| 90 | 0.026 | 0.013 | 0.010 | 0.053 | 0.020 | 0.024 | <0.001 | <0.001 | <0.001 | 0.039 | 0.000 | |
| 100 | 0.066 | 0.020 | 0.026 | 0.013 | 0.060 | 0.037 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Overall Average (T) | 0.027 | 0.012 | 0.018 | 0.026 | 0.025 | |||||||
| BC 5, mg/100 g DM | 20 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | <0.001 | <0.001 | <0.001 | 0.997 | 0.997 |
| 60 | 0.010 | 0.020 | 0.010 | 0.013 | 0.016 | 0.014 | <0.001 | <0.001 | <0.001 | 0.997 | 0.825 | |
| 80 | 0.020 | 0.030 | 0.023 | 0.020 | 0.020 | 0.023 | <0.001 | <0.001 | <0.001 | 0.086 | 0.719 | |
| 90 | 0.036 | 0.046 | 0.043 | 0.066 | 0.060 | 0.050 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 100 | 0.090 | 0.110 | 0.116 | 0.146 | 0.176 | 0.128 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Overall Average (T) | 0.033 | 0.043 | 0.040 | 0.051 | 0.056 | |||||||
| Item 1, g/kg DM | Cactus Forage Levels % | Time, Hours | SEM 2 | p-Value 3 | p-Value 4 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | ||||||||||||||||
| 20 | 60 | 80 | 90 | 100 | 0 | 3 | 6 | 12 | 24 | C | T | C × T | L | Q | ||
| EE | 16.6 | 21.8 | 16.5 | 11.5 | 15.7 | 20.6 | 19.9 | 17.4 | 14.1 | 10.1 | 0.11 | <0.001 | <0.001 | 0.762 | <0.001 | 0.372 |
| FDN | 788.5 | 768.5 | 713.5 | 647.0 | 382.8 | 667.6 | 686.0 | 655.8 | 635.6 | 651.9 | 1.95 | <0.001 | 0.458 | 0.427 | 0.278 | 0.335 |
| NFC | 54.6 | 44.3 | 74.3 | 106.5 | 273.7 | 102.8 | 92.3 | 113.3 | 132.6 | 112.3 | 19.13 | <0.001 | 0.659 | 0.5695 | 0.473 | 0.314 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.d.; Araújo, G.d.; Santos, E.M.; Oliveira, J.d.; Lambais, É.; Lambais, G.; Nagahama, H.; Zanine, A.; Santos, F.N.; Soares, R.; et al. Bacterial Community and Chemical Composition of Mixed Fresh Cactus Forage and Buffel Grass Hay during Aerobic Exposure. Agronomy 2022, 12, 1927. https://doi.org/10.3390/agronomy12081927
Santos Dd, Araújo Gd, Santos EM, Oliveira Jd, Lambais É, Lambais G, Nagahama H, Zanine A, Santos FN, Soares R, et al. Bacterial Community and Chemical Composition of Mixed Fresh Cactus Forage and Buffel Grass Hay during Aerobic Exposure. Agronomy. 2022; 12(8):1927. https://doi.org/10.3390/agronomy12081927
Chicago/Turabian StyleSantos, Daiane dos, Gherman de Araújo, Edson Mauro Santos, Juliana de Oliveira, Érica Lambais, George Lambais, Hideo Nagahama, Anderson Zanine, Francisco Naysson Santos, Rafael Soares, and et al. 2022. "Bacterial Community and Chemical Composition of Mixed Fresh Cactus Forage and Buffel Grass Hay during Aerobic Exposure" Agronomy 12, no. 8: 1927. https://doi.org/10.3390/agronomy12081927
APA StyleSantos, D. d., Araújo, G. d., Santos, E. M., Oliveira, J. d., Lambais, É., Lambais, G., Nagahama, H., Zanine, A., Santos, F. N., Soares, R., Sobral, G., Justino, E., Lemos, M., & de Oliveira, C. J. (2022). Bacterial Community and Chemical Composition of Mixed Fresh Cactus Forage and Buffel Grass Hay during Aerobic Exposure. Agronomy, 12(8), 1927. https://doi.org/10.3390/agronomy12081927







