Effect of Regulated Deficit Irrigation on the Quality of ‘Arbequina’ Extra Virgin Olive Oil Produced on a Super-High-Intensive Orchard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Processing
- Control (T0): full irrigated conditions [14].
- RDI (T1): trees had no water restriction along phases I and III (the beginning of olive fruit growth and fruit maturation with oil accumulation, respectively, while regulated irrigation was applied at phase II (pit hardening), with 37% and 54% of water reduction in the 2018 and 2019 seasons, respectively.
- Confederation RDI (T2): same conditions established in T1 (deficit irrigation applied at phase II), but with a water restriction set up at donation of Guadalquivir hydrographic confederation (67% of water reduction in 2018 and 72% of water reduction in 2019).
- Confederation SDI (T3): sustained deficit irrigation with the same water restrictions as T2 but during the whole cycle of the olive tree.
2.2. Quality Parameters
2.3. Determination of Antioxidant Activity and Quantification of Total Phenolic Content
2.4. Fatty Acid Profile
2.5. Volatile Compound Profile
2.6. Descriptive Sensory Analysis
2.7. Statistical Treatment
3. Results and Discussion
3.1. Irrigation Stress, Water Used and Production
3.2. Analytical Parameters of Olive Oil Quality
3.3. Determination of Antioxidant Activity and Quantification of Total Phenolic Content
3.4. Fatty Acids Profile
3.5. Volatile Compound Profile
3.6. Descriptive Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IOC. Olive Oil Production in Europe. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 15 July 2022).
- FAO. Food-Based Dietary Guidelines-Spain. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/countries/Spain/en (accessed on 15 July 2022).
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds. Stud. Nat. Prod. Chem. 2018, 57, 41–77. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Moutinho-Pereira, J.; Gonçalves, B.; Ferreira, H.M.F.; Correia, C.M. Changes in growth, gas exchange, xylem hydraulic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2007, 60, 183–192. [Google Scholar] [CrossRef]
- Gomez, J.A.; Guzmán, M.G.; Giraldez, J.V.; Fereres, E. The influence of cover crops and tillage on water and sediment yield, and on nutrient, and organic matter losses in an olive orchard on a sandy loam soil. Soil Tillage Res. 2009, 106, 137–144. [Google Scholar] [CrossRef]
- Torres, J.; Fernández-Ondoño, E.; Garcia-Fuentes, A.; Valenzuela, L.; Siles, G.; Valle-Tendero, F. Las cubiertas vegetales como herramienta en el olivar ecológico. Rev. Agropecu. Agric. 2013, 967, 742–970. [Google Scholar]
- Arbizu-Milagro, J.; Castillo-Ruiz, F.J.; Tascón, A.; Peña, J.M. How could precision irrigation based on daily trunk growth improve super high-density olive orchard irrigation efficiency? Agronomy 2022, 12, 756. [Google Scholar] [CrossRef]
- Ministerio de Agricultura, Pesca y Alimentación (MAPA). Gobierno de España. Encuesta Sobre Superficies y Rendimientos Dultivos (ESYRCE). Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/resultados-de-anos-anteriores/ (accessed on 15 July 2022).
- Cano-Lamadrid, M.; Girón, I.; Pleite, R.; Burló, F.; Corell, M.; Moriana, A.; Carbonell-Barrachina, A. Quality attributes of table olives as affected by regulated deficit irrigation. LWT Food Sci. Technol. 2015, 62, 19–26. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Lipan, L.; Andreu, L.; Martín-Palomo, M.; Carbonell-Barrachina, A.; Hernández, F.; Sendra, E. Effect of regulated deficit irrigation on the quality of raw and table olives. Agric. Water Manag. 2019, 221, 415–421. [Google Scholar] [CrossRef]
- Corell, M.; Martín-Palomo, M.; Sánchez-Bravo, P.; Carrillo, T.; Collado, J.; Hernández-García, F.; Girón, I.; Andreu, L.; Galindo, A.; López-Moreno, Y.; et al. Evaluation of growers’ efforts to improve the sustainability of olive orchards: Development of the hydroSOStainable index. Sci. Hortic. 2019, 257, 108661. [Google Scholar] [CrossRef]
- Sánchez-Bravo, P.; Collado-González, J.; Corell, M.; Noguera-Artiaga, L.; Galindo, A.; Sendra, E.; Hernández, F.; Martín-Palomo, M.J.; Carbonell-Barrachina, A. Criteria for hydroSOS quality index. Application to extra virgin olive oil and processed table olives. Water 2020, 12, 555. [Google Scholar] [CrossRef]
- Barranco Navero, D.; Fernández Escobar, R.; Rallo Romero, L. El Cultivo Del Olivo; Mundi-Prensa: Madrid, Spain, 2017. [Google Scholar]
- Corell, M.; Pérez-López, D.; Andreu, L.; Recena, R.; Centeno, A.; Galindo, A.; Moriana, A.; Martín-Palomo, M. Yield response of a mature hedgerow oil olive orchard to different levels of water stress during pit hardening. Agric. Water Manag. 2021, 261, 107374. [Google Scholar] [CrossRef]
- EEC. Commision Regulation (EEC) No. 2568/91 on the Characteristics of Olive Oil and Olive-Pomace Oil and on the Relevant Methods of Analysis. 2019. Available online: https://leap.unep.org/countries/eu/national-legislation/commission-regulation-eec-no-256891-characteristics-olive-oil-and (accessed on 17 July 2021).
- IOC. Determination of Peroxide Value; DEC-III-12/106-VI/2017; IOC: Madrid, Spain, 2017. [Google Scholar]
- IOC. Determination of Free Fatty Acids, Cold Method; DECISION DEC-III-8/106-VI/2017; IOC: Madrid, Spain, 2017. [Google Scholar]
- IOC. Spectrophotemtric Investigation in the Ultraviolet; DEC-III.4/109-VI/2019; IOC: Madrid, Spain, 2019. [Google Scholar]
- Tuberoso, C.I.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- ISO-12966-2; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017.
- Sánchez-Rodríguez, L.; Kranjac, M.; Marijanović, Z.; Jerković, I.; Corell, M.; Moriana, A.; Carbonell-Barrachina Ángel, A.; Sendra, E.; Hernández, F. Quality attributes and fatty acid, volatile and sensory profiles of “Arbequina” hydroSOStainable olive oil. Molecules 2019, 24, 2148. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, L.; Kranjac, M.; Marijanović, Z.; Jerković, I.; Pérez-López, D.; Carbonell-Barrachina, A.; Hernández, F.; Sendra, E. Arbequina olive oil composition is affected by the application of regulated deficit irrigation during pit hardening stage. J. Am. Oil Chem. Soc. 2020, 97, 449–462. [Google Scholar] [CrossRef]
- NIST. National Institute of Standars and Technology Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 15 July 2022).
- IOC. International Olive Council: Trade Standard on Olive Oils and Olive-Pomace Oils. Available online: https://www.internationaloliveoil.org/what-we-do/chemistry-standardisation-unit/standards-and-methods/ (accessed on 15 July 2022).
- Ahumada-Orellana, L.E.; Ortega-Farías, S.; Searles, P.S. Olive oil quality response to irrigation cut-off strategies in a super-high density orchard. Agric. Water Manag. 2018, 202, 81–88. [Google Scholar] [CrossRef]
- del Campo, M.G.; García, J.M. Summer deficit-irrigation strategies in a hedgerow olive cv. Arbequina orchard: Effect on oil quality. J. Agric. Food Chem. 2013, 61, 8899–8905. [Google Scholar] [CrossRef] [PubMed]
- Sena-Moreno, E.; Cabrera-Bañegil, M.; Rodríguez, J.M.P.; De Miguel, C.; Prieto, M.H.; Martín-Vertedor, D. Influence of water deficit in bioactive compounds of olive paste and oil content. J. Am. Oil Chem. Soc. 2018, 95, 349–359. [Google Scholar] [CrossRef]
- Shavakhi, F.; Rahmani, A.; Moradi, P. Characterization of Iranian olive oils based on biophenolic minor polar compounds and their contribution to organoleptic properties. Yuzuncu Yıl Univ. J. Agric. Sci. 2021, 31, 365–376. [Google Scholar] [CrossRef]
- Dag, A.; Naor, A.; Ben-Gal, A.; Harlev, G.; Zipori, I.; Schneider, D.; Birger, R.; Peres, M.; Gal, Y.; Kerem, Z. The effect of water stress on super-high- density ‘Koroneiki’ olive oil quality. J. Sci. Food Agric. 2014, 95, 2016–2020. [Google Scholar] [CrossRef]
- Hernández, M.L.; Velázquez-Palmero, D.; Sicardo, M.D.; Fernández, J.E.; Diaz-Espejo, A.; Martínez-Rivas, J.M. Effect of a regulated deficit irrigation strategy in a hedgerow ‘Arbequina’ olive orchard on the mesocarp fatty acid composition and desaturase gene expression with respect to olive oil quality. Agric. Water Manag. 2018, 204, 100–106. [Google Scholar] [CrossRef]
- Rondanini, D.P.; Castro, D.N.; Searles, P.S.; Rousseaux, M.C. Fatty acid profiles of varietal virgin olive oils (Olea europaea L.) from mature orchards in warm arid valleys of Northwestern Argentina (La Rioja). Grasas y Aceites 2011, 62, 399–409. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Perica, S.; Žanetić, M.; Škevin, D. Virgin Olive Oil Phenols, Fatty Acid Composition and Sensory Profile: Can Cultivar Overpower Environmental and Ripening Effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Rumora, A.E.; Lograsso, G.; Hayes, J.M.; Mendelson, F.E.; Tabbey, M.A.; Haidar, J.A.; Lentz, S.I.; Feldman, E.L. The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity. J. Neurosci. 2019, 39, 3770–3781. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- da Silva, M.D.G.; Freitas, A.M.C.; Cabrita, M.J.; Garcia, R. Olive Oil Composition: Volatile Compounds. Olive Oil: Constituents, Quality, Health Properties and Bioconversions; InTech: Rijeka, Croatia, 2012; pp. 17–46. [Google Scholar]
- Servili, M.; Montedoro, G. Contribution of phenolic compounds to virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 602–613. [Google Scholar] [CrossRef]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.F. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A. 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Garrido-Delgado, R.; del Mar Dobao-Prieto, M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC–IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef] [PubMed]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; Harwood, J. Effect of Irrigation on Quality Attributes of Olive Oil. J. Agric. Food Chem. 2009, 57, 7048–7055. [Google Scholar] [CrossRef] [PubMed]

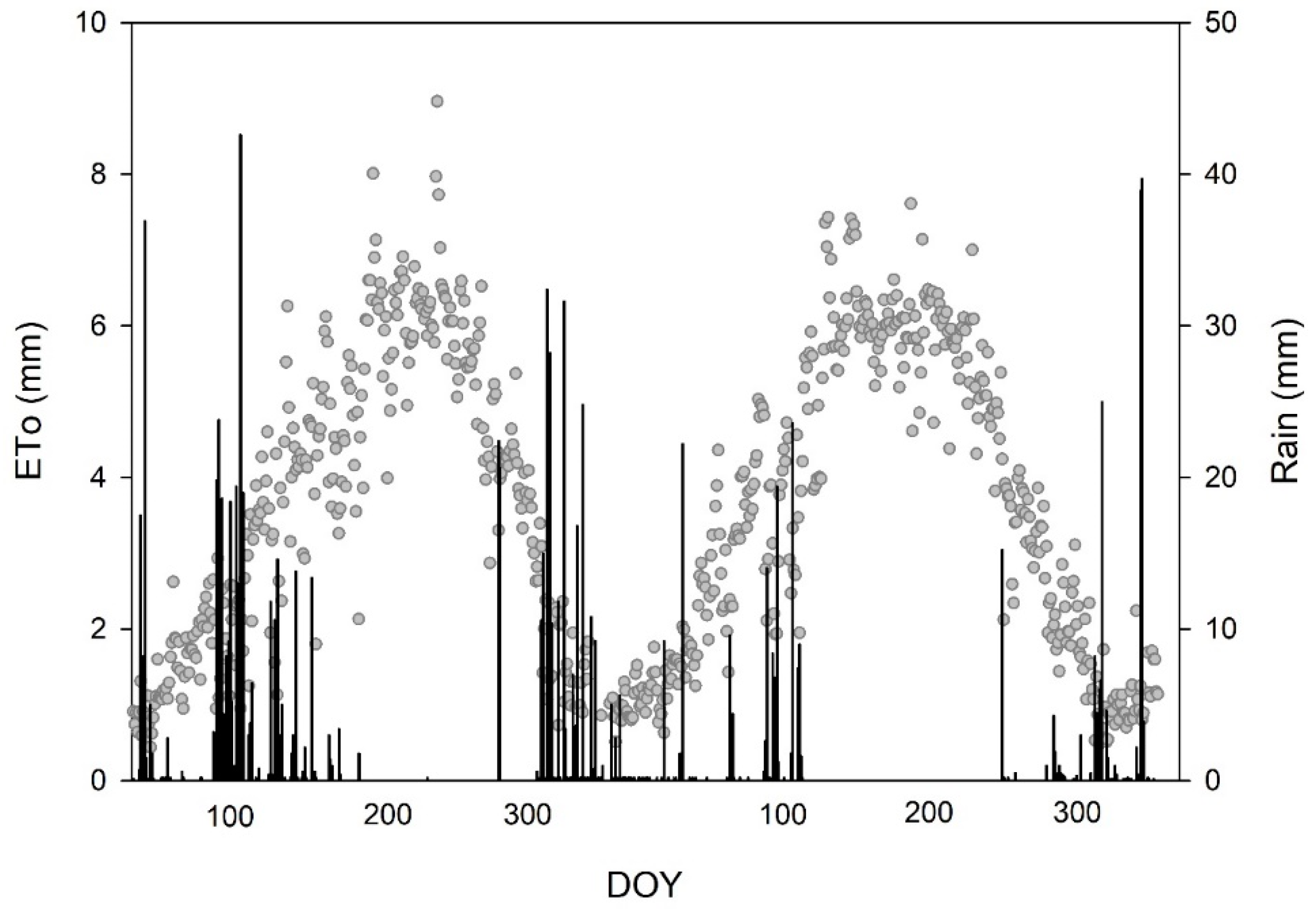
| Factor | Min Ψstem (MPa) | SI Total (MPa Day−1) | SI Phase II (MPa Day−1) | SI Phase III (MPa Day−1) | AW (L m−2) | Olive Oil Production (kg Oil ha−1) |
|---|---|---|---|---|---|---|
| ANOVA † | ||||||
| Season | n.s. | n.s. | n.s. | n.s. | *** | n.s. |
| Irrigation | ** | ** | ** | ** | *** | n.s. |
| Season × Irrigation | ** | ** | ** | ** | *** | n.s. |
| Tukey Multiple Range Test ‡ | ||||||
| Season | ||||||
| 2018 | −3.35 | 76.0 | 54.6 | 16.9 | 304 b | 2198 |
| 2019 | −3.47 | 103 | 71.9 | 30.2 | 484 a | 2025 |
| Irrigation | ||||||
| T0 | −1.88 a | 9.53 c | 9.25 c | 0.17 b | 753 a | 2192 |
| T1 | −2.94 ab | 46.3 bc | 43.24 bc | 2.09 b | 390 b | 2162 |
| T2 | −4.96 c | 179 a | 127 a | 48.8 a | 221 c | 2035 |
| T3 | −3.87 bc | 123 ab | 73.2 ab | 43.9 a | 212 c | 2056 |
| Season × Irrigation | ||||||
| 2018 × T0 | −2.21 ab | 18.4 b | 18.2 b | 0.00 b | 533 b | 2348 |
| 2018 × T1 | −2.74 abc | 40.7 b | 38.7 b | 0.48 b | 334 cd | 2169 |
| 2018 × T2 | −4.71 bc | 140 ab | 104 ab | 30.0 ab | 173 e | 2240 |
| 2018 × T3 | −3.75 abc | 105 ab | 57.6 ab | 37.1 ab | 175 e | 2034 |
| 2019 × T0 | −1.54 a | 0.681 b | 0.338 b | 0.34 b | 972 a | 2037 |
| 2019 × T1 | −3.15 abc | 51.8 b | 47.8 ab | 3.70 b | 446 bc | 2154 |
| 2019 × T2 | −5.21 c | 219 a | 151 a | 66.0 a | 269 de | 1831 |
| 2019 × T3 | −3.99 abc | 141 ab | 88.9 ab | 50.7 ab | 248 de | 2077 |
| Factor | Peroxide Index (meq O2 kg−1) | Acidity Index (% Oleic Acid) | K232 | K270 | Δk | Commercial Classification |
|---|---|---|---|---|---|---|
| ANOVA † | ||||||
| Season | *** | n.s. | n.s. | *** | n.s. | |
| Irrigation | *** | *** | ** | n.s. | n.s. | |
| Season × Irrigation | *** | *** | n.s. | *** | n.s. | |
| Tukey Multiple Range Test ‡ | ||||||
| Season | ||||||
| 2018 | 8.21 b | 0.240 | 1.80 | 0.086 b | 0.004 | EVOO ¥ |
| 2019 | 12.0 a | 0.214 | 1.76 | 0.127 a | 0.002 | EVOO |
| Irrigation | ||||||
| T0 | 12.6 a | 0.317 a | 2.00 a | 0.101 | 0.002 | EVOO |
| T1 | 9.73 b | 0.207 b | 1.78 ab | 0.101 | 0.004 | EVOO |
| T2 | 8.74 b | 0.192 b | 1.66 b | 0.111 | 0.004 | EVOO |
| T3 | 9.34 b | 0.191 b | 1.69 ab | 0.113 | 0.002 | EVOO |
| Season × Irrigation | ||||||
| 2018 × T0 | 11.8 ab | 0.417 a | 1.96 | 0.082 c | 0.004 | EVOO |
| 2018 × T1 | 8.15 bc | 0.206 b | 1.96 | 0.086 bc | 0.003 | EVOO |
| 2018 × T2 | 5.87 c | 0.187 b | 1.57 | 0.088 bc | 0.004 | EVOO |
| 2018 × T3 | 7.02 bc | 0.150 b | 1.72 | 0.089 bc | 0.004 | EVOO |
| 2019 × T0 | 13.4 a | 0.218 b | 2.04 | 0.120 ab | 0.001 | EVOO |
| 2019 × T1 | 11.3 ab | 0.208 b | 1.61 | 0.116 abc | 0.004 | EVOO |
| 2019 × T2 | 11.6 ab | 0.197 b | 1.75 | 0.134 a | 0.004 | EVOO |
| 2019 × T3 | 11.7 ab | 0.232 b | 1.66 | 0.137 a | 0.000 | EVOO |
| Factor | TPC (mg GAEL L−1) | ABTS (mmol Trolox eq L−1) | DPPH (mmol Trolox eq L−1) |
|---|---|---|---|
| ANOVA † | |||
| Season | ** | ** | * |
| Irrigation | ** | n.s. | n.s. |
| Season × Irrigation | ** | ** | * |
| Tukey Multiple Range Test ‡ | |||
| Season | |||
| 2018 | 86.1 a | 0.262 b | 0.179 b |
| 2019 | 74.9 b | 0.439 a | 0.289 a |
| Irrigation | |||
| T0 | 53.7 b | 0.325 | 0.233 |
| T1 | 77.0 ab | 0.404 | 0.223 |
| T2 | 92.9 a | 0.337 | 0.201 |
| T3 | 98.5 a | 0.337 | 0.279 |
| Season × Irrigation | |||
| 2018 × T0 | 59.0 ab | 0.198 b | 0.089 b |
| 2018 × T1 | 90.4 ab | 0.341 ab | 0.193 ab |
| 2018 × T2 | 91.9 ab | 0.220 b | 0.196 ab |
| 2018 × T3 | 103 a | 0.289 ab | 0.239 ab |
| 2019 × T0 | 48.5 b | 0.452 a | 0.377 a |
| 2019 × T1 | 63.6 ab | 0.467 a | 0.254 ab |
| 2019 × T2 | 93.9 ab | 0.454 a | 0.206 ab |
| 2019 × T3 | 93.7 ab | 0.384 ab | 0.319 ab |
| Factor | C14:0 | C16:0 | C16:1 | C16:1 | C17:0 | C17:1cis10 | C18:0 | C18:1cis9 | C18:1cis11 | C18:2cis6 | C20:0 | C20:1n9 | C18:3n3 | C22:0 | C20:4n6 | Total | MUFA ¥ | PUFA ¥ | SFA ¥ | TI ¥ | AI ¥ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANOVA † | |||||||||||||||||||||
| Season | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Irrigation | n.s. | n.s. | n.s. | *** | *** | *** | n.s. | *** | n.s. | *** | n.s. | n.s. | n.s. | n.s. | n.s. | *** | *** | *** | *** | n.s. | *** |
| Season × irrigation | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Tukey’s Multiple Range Test ‡ | |||||||||||||||||||||
| Season | |||||||||||||||||||||
| 2018 | 0.031 b | 14.6 b | 0.152 b | 1.76 b | 0.083 b | 0.179 b | 1.82 b | 49.6 a | 10.01 a | 11.6 b | 0.355 b | 0.264 b | 0.598 b | 0.111 b | 0.234 a | 91.4 b | 62.0 a | 12.5 b | 17.0 b | 0.424 b | 0.198 b |
| 2019 | 0.045 a | 18.4 a | 0.430 a | 3.8 a | 0.386 a | 0.703 a | 4.17 a | 44.1 b | 5.74 b | 17.4 a | 1.11 a | 0.764 a | 1.77 a | 0.387 a | 0.005 b | 99.3 a | 55.6 b | 19.2 a | 24.5 a | 0.541 a | 0.249 a |
| Irrigation | |||||||||||||||||||||
| T0 | 0.043 | 16.7 | 0.281 | 2.96 a | 0.191 b | 0.374 c | 2.90 | 45.6 b | 8.04 | 15.5 a | 0.722 | 0.521 | 1.262 | 0.253 | 0.114 | 95.5 ab | 57.8 b | 16.9 a | 20.8 ab | 0.482 | 0.226 a |
| T1 | 0.037 | 16.3 | 0.284 | 2.72 b | 0.222 ab | 0.412 bc | 2.93 | 46.0 b | 7.88 | 14.3 bc | 0.721 | 0.516 | 1.158 | 0.240 | 0.113 | 93.8 b | 57.8 b | 15.6 bc | 20.4 b | 0.482 | 0.224 ab |
| T2 | 0.035 | 16.3 | 0.299 | 2.84 ab | 0.265 a | 0.502 a | 3.07 | 46.9 b | 7.56 | 13.7 c | 0.747 | 0.484 | 1.132 | 0.261 | 0.118 | 94.1 b | 58.6 b | 14.9 c | 20.6 ab | 0.486 | 0.223 ab |
| T3 | 0.037 | 16.8 | 0.300 | 2.68 b | 0.260 a | 0.475 ab | 3.10 | 48.9 a | 8.16 | 14.5 b | 0.749 | 0.534 | 1.185 | 0.242 | 0.133 | 98.0 a | 61.1 a | 15.8 b | 21.2 a | 0.479 | 0.221 b |
| Season × irrigation | |||||||||||||||||||||
| 2018 × T0 | 0.038 abc | 14.6 bc | 0.154 b | 1.58 c | 0.069 c | 0.155 d | 1.93 c | 49.2 b | 10.0 a | 12.1 c | 0.362 b | 0.273 b | 0.640 b | 0.111 b | 0.222 a | 91.6 b | 61.5 b | 13.0 cd | 17.1 bc | 0.426 b | 0.198 b |
| 2018 × T1 | 0.029 bc | 14.1 c | 0.145 b | 1.65 c | 0.083 c | 0.168 d | 1.70 c | 47.7 bc | 10.2 a | 11.3 cd | 0.328 b | 0.248 b | 0.564 b | 0.099 b | 0.221 a | 88.5 b | 60.1 bc | 12.1 cd | 16.3 c | 0.422 b | 0.197 b |
| 2018 × T2 | 0.028 bc | 14.3 c | 0.153 b | 1.92 c | 0.095 c | 0.193 d | 1.81 c | 48.8 b | 9.46 a | 10.8 d | 0.362 b | 0.265 b | 0.570 b | 0.129 b | 0.232 a | 89.1 b | 60.8 b | 11.6 d | 16.7 bc | 0.428 b | 0.199 b |
| 2018 × T3 | 0.027 c | 15.3 b | 0.154 b | 1.88 c | 0.087 c | 0.198 d | 1.86 c | 52.5 a | 10.6 a | 12.3 c | 0.369 b | 0.269 b | 0.617 b | 0.105 b | 0.260 a | 96.6 a | 65.6 a | 13.2 c | 17.8 b | 0.420 b | 0.196 b |
| 2019 × T0 | 0.048 a | 18.8 a | 0.407 a | 4.34 a | 0.314 b | 0.592 c | 3.86 b | 42.0 e | 6.05 b | 18.9 a | 1.08 a | 0.768 a | 1.88 a | 0.395 a | 0.005 b | 99.4 a | 54.1 d | 20.8 a | 24.5 a | 0.538 a | 0.254 a |
| 2019 × T1 | 0.044 ab | 18.5 a | 0.424 a | 3.79 b | 0.362 ab | 0.656 bc | 4.16 ab | 44.3 de | 5.56 b | 17.4 b | 1.12 a | 0.785 a | 1.75 a | 0.382 a | 0.005 b | 99.2 a | 55.5 d | 19.2 b | 24.5 a | 0.543 a | 0.250 a |
| 2019 × T2 | 0.041 abc | 18.2 a | 0.445 a | 3.76 b | 0.436 a | 0.811 a | 4.33 a | 44.9 cde | 5.66 b | 16.6 b | 1.13 a | 0.703 a | 1.70 a | 0.393 a | 0.005 b | 99.1 a | 56.3 d | 18.3 b | 24.5 a | 0.543 a | 0.246 a |
| 2019 × T3 | 0.046 a | 18.2 a | 0.445 a | 3.49 b | 0.434 a | 0.752 ab | 4.34 a | 45.3 cd | 5.70 b | 16.7 b | 1.13 a | 0.798 a | 1.75 a | 0.378 a | 0.006 b | 99.5 a | 56.5 cd | 18.4 b | 24.6 a | 0.539 a | 0.246 a |
| Compound | RT (min) ¥ | RIExp ¥ | RILit ¥ | ANOVA † | Irrigation Treatment | |||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | |||||
| Ethanol | 2.263 | 419 | 427 | *** | 12.85 a ‡ | 7.10 b | 7.16 b | 7.87 b |
| Acetic acid | 3.383 | 680 | 660 | n.s. | 3.14 | 3.65 | 3.83 | 1.96 |
| 3-Pentanone | 4.381 | 737 | 703 | n.s. | 1.35 | 1.35 | 0.77 | 1.05 |
| Propanoic acid | 4.677 | 750 | 745 | n.s. | 0.11 | 0.33 | 0.83 | 1.05 |
| Hexanal | 6.688 | 831 | xxx | n.s. | 4.45 | 3.43 | 3.65 | 3.33 |
| trans-2-Hexenal | 8.903 | 904 | 854 | *** | 91.67 a | 70.69 b | 56.08 c | 96.28 a |
| trans-2-Hexen-1-ol | 9.157 | 910 | 887 | n.s. | 47.22 | 47.46 | 38.06 | 43.98 |
| 4,8-Dimethyl-1,7-nonadiene | 12.998 | 1000 | - | n.s. | 1.67 | 1.72 | 1.70 | 2.54 |
| Benzaldehyde | 13.893 | 1017 | 961 | ** | 2.05 b | 8.14 a | 1.47 b | 1.30 b |
| 3-Hexen-1-ol, acetate | 14.579 | 1031 | 1009 | n.s. | 9.47 | 3.47 | 11.77 | 15.53 |
| Hexyl acetate | 14.806 | 1035 | 997 | ** | 3.54 a | 0.35 c | 2.33 b | 4.77 a |
| Hexanoic acid | 15.571 | 1050 | 1010 | n.s. | 1.03 | 0.43 | 0.63 | 0.38 |
| trans-ß-Ocimene | 16.005 | 1058 | 1050 | *** | 0.56 b | 2.19 a | 0.61 b | 0.64 b |
| Benzyl alcohol | 18.621 | 1109 | 1046 | * | 2.66 a | 0.15 b | 2.15 a | 0.99 b |
| 1-Octanol | 18.967 | 1115 | 1068 | n.s. | 0.47 | 0.28 | 0.26 | 0.06 |
| Acetophenone | 19.628 | 1127 | 1065 | ** | 0.27 b | 4.85 a | 0.43 b | 0.25 b |
| Benzoic acid | 27.670 | 1269 | 1210 | ** | 3.89 a | 1.04 b | 3.67 a | 2.50 b |
| Nonanoic acid | 31.899 | 1354 | 1303 | n.s. | 1.24 | 1.98 | 1.98 | 1.05 |
| Total | *** | 188 a | 159 b | 137 b | 186 a | |||
| Factor | Fruity | Bitter | Pungent | Overall Defects ¥ | Commercial Classification |
|---|---|---|---|---|---|
| ANOVA † | |||||
| Season | *** | n.s. | *** | n.s. | |
| Irrigation | *** | ** | *** | n.s. | |
| Season × Irrigation | *** | ** | *** | n.s. | |
| Tukey’s Multiple Range Test ‡ | |||||
| Season | |||||
| 2018 | 4.1 b | 1.9 | 1.1 b | 0.0 | EVOO ξ |
| 2019 | 5.8 a | 2.2 | 3.1 a | 0.0 | EVOO |
| Irrigation | |||||
| T0 | 4.0 b | 1.4 b | 1.2 b | 0.0 | EVOO |
| T1 | 4.6 b | 2.1 a | 2.0 ab | 0.0 | EVOO |
| T2 | 5.5 a | 2.3 a | 2.7 a | 0.0 | EVOO |
| T3 | 5.7 a | 2.5 a | 2.5 a | 0.0 | EVOO |
| Season × irrigation | |||||
| 2018 × T0 | 3.2 d | 1.2 b | 0.4 d | 0.0 | EVOO |
| 2018 × T1 | 4.2 cd | 2.0 ab | 1.4 cd | 0.0 | EVOO |
| 2018 × T2 | 4.1 cd | 1.9 ab | 1.3 cd | 0.0 | EVOO |
| 2018 × T3 | 5.1 bc | 2.7 a | 1.4 cd | 0.0 | EVOO |
| 2019 × T0 | 4.9 c | 1.5 ab | 2.0 c | 0.0 | EVOO |
| 2019 × T1 | 5.1 bc | 2.3 ab | 2.5 bc | 0.0 | EVOO |
| 2019 × T2 | 7.0 a | 2.6 a | 4.2 a | 0.0 | EVOO |
| 2019 × T3 | 6.3 ab | 2.2 ab | 3.5 ab | 0.0 | EVOO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Garví, J.M.; Sánchez-Bravo, P.; Hernández, F.; Sendra, E.; Corell, M.; Moriana, A.; Burgos-Hernández, A.; Carbonell-Barrachina, Á.A. Effect of Regulated Deficit Irrigation on the Quality of ‘Arbequina’ Extra Virgin Olive Oil Produced on a Super-High-Intensive Orchard. Agronomy 2022, 12, 1892. https://doi.org/10.3390/agronomy12081892
García-Garví JM, Sánchez-Bravo P, Hernández F, Sendra E, Corell M, Moriana A, Burgos-Hernández A, Carbonell-Barrachina ÁA. Effect of Regulated Deficit Irrigation on the Quality of ‘Arbequina’ Extra Virgin Olive Oil Produced on a Super-High-Intensive Orchard. Agronomy. 2022; 12(8):1892. https://doi.org/10.3390/agronomy12081892
Chicago/Turabian StyleGarcía-Garví, José Miguel, Paola Sánchez-Bravo, Francisca Hernández, Esther Sendra, Mireia Corell, Alfonso Moriana, Armando Burgos-Hernández, and Ángel A. Carbonell-Barrachina. 2022. "Effect of Regulated Deficit Irrigation on the Quality of ‘Arbequina’ Extra Virgin Olive Oil Produced on a Super-High-Intensive Orchard" Agronomy 12, no. 8: 1892. https://doi.org/10.3390/agronomy12081892
APA StyleGarcía-Garví, J. M., Sánchez-Bravo, P., Hernández, F., Sendra, E., Corell, M., Moriana, A., Burgos-Hernández, A., & Carbonell-Barrachina, Á. A. (2022). Effect of Regulated Deficit Irrigation on the Quality of ‘Arbequina’ Extra Virgin Olive Oil Produced on a Super-High-Intensive Orchard. Agronomy, 12(8), 1892. https://doi.org/10.3390/agronomy12081892











