Leaf Functional Traits and Relationships with Soil Properties of Zanthoxylum planispinum ‘dintanensis’ in Plantations of Different Ages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Plot Setting
2.3. Plant Description and Identification
2.4. Sample Collection
2.5. Selection of Leaf Functional Traits and Soil Properties Parameters
2.6. Index Determination Method
2.6.1. Leaf Traits Determination
2.6.2. Determination of Soil Physical and Chemical Properties
2.7. Data Processing
3. Results
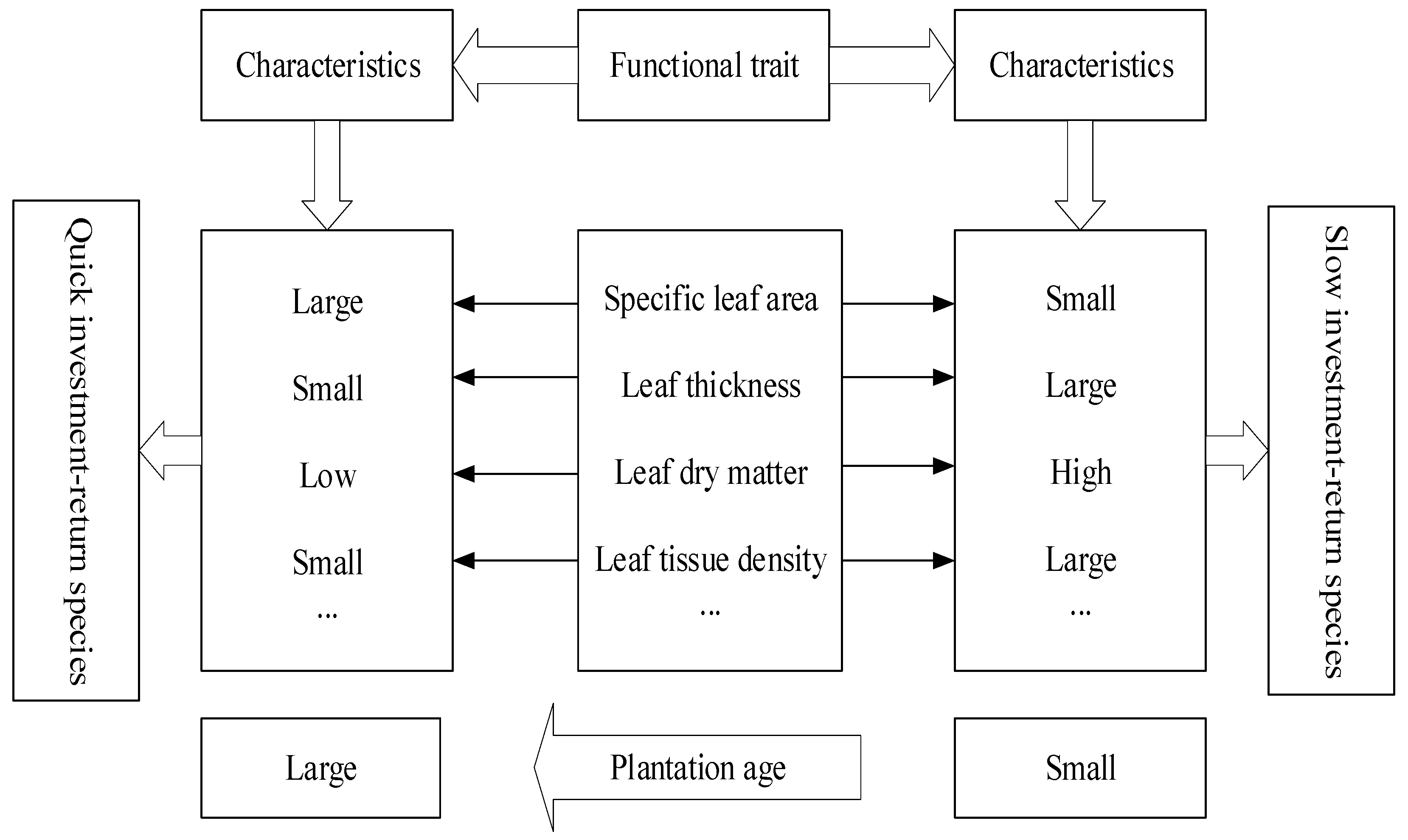
3.1. The Variation Characteristics of Z. planispinum ‘dintanensis’ Suitability Strategy and Its Economic Spectrum at Different Plantation Ages
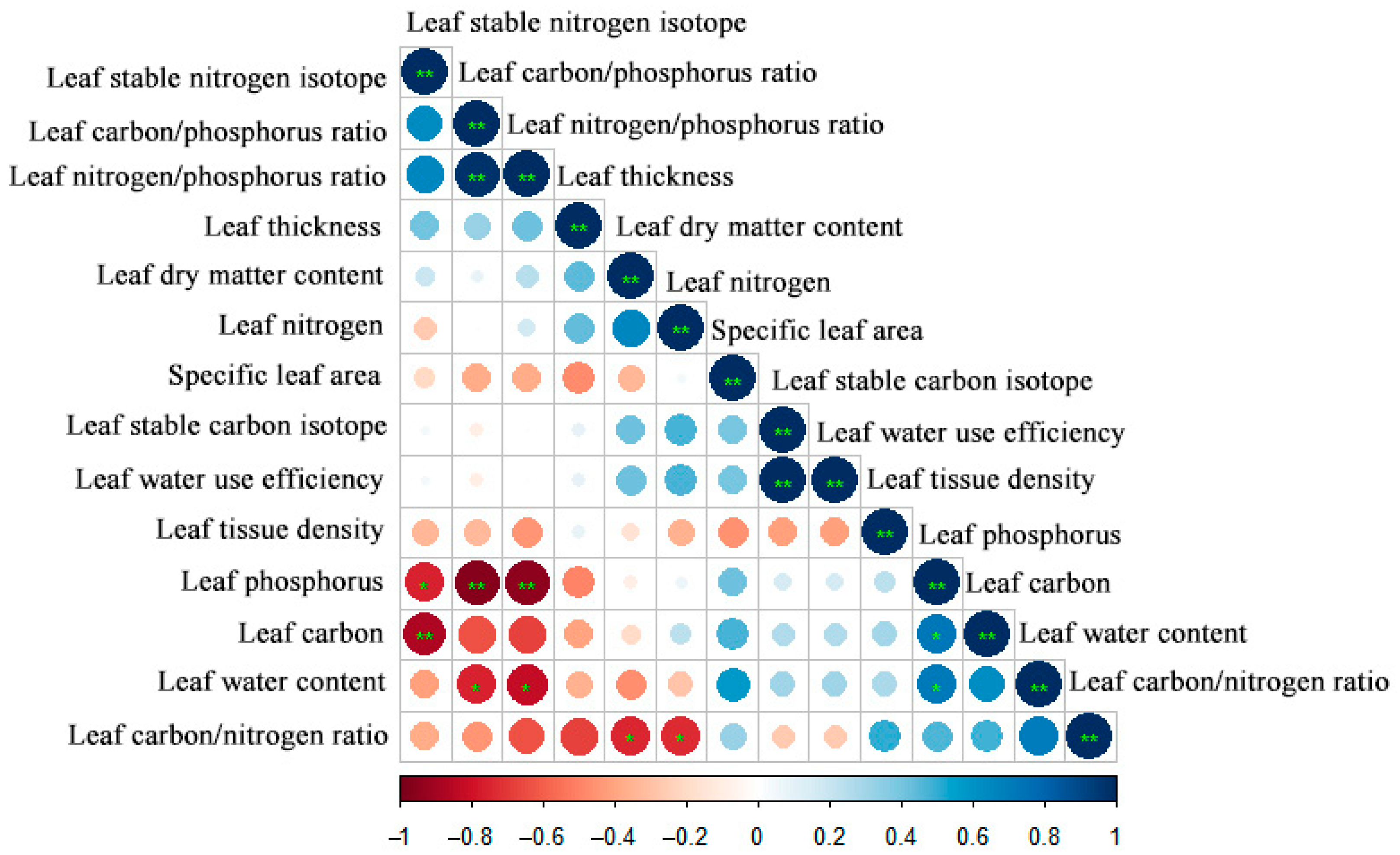
3.2. Trade-Off and Synergistic Relationship between Leaf Functional Traits of Z. planispinum ‘dintanensis’
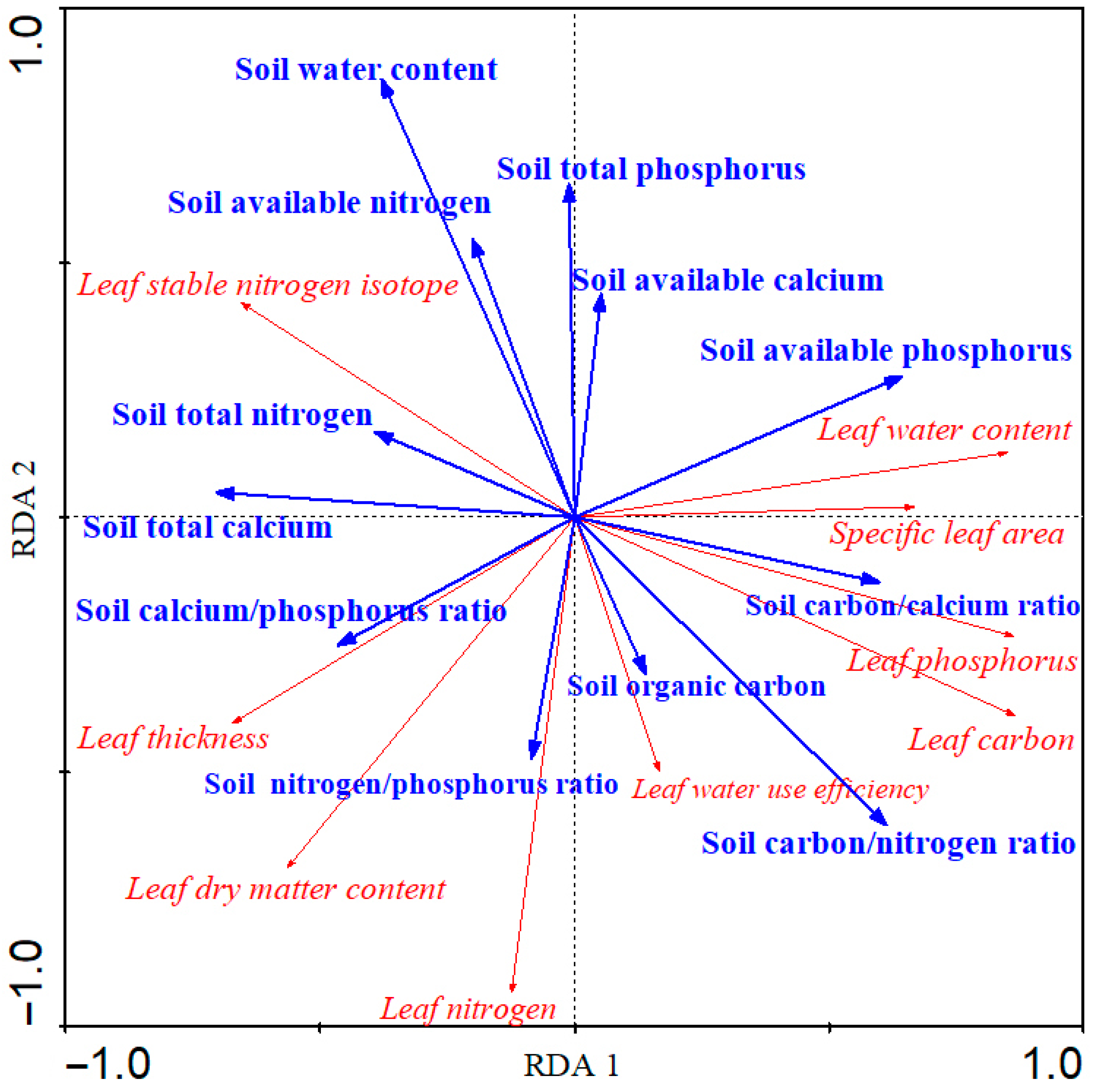
3.3. The Relationships between Soil Factors and Leaf Functional Traits of Z. planispinum ‘dintanensis’
4. Discussion
4.1. The Leaf Economic Spectrum and Suitability Strategy of Z. planispinum ‘dintanensis’ Varies with Plantation Age
4.2. The Trade-Off and Synergic Relationship among Leaf Functional Traits of Z. planispinum ‘dintanensis’
4.3. The Regulation Effect of Soil Factors on the Leaf Functional Traits of Z. planispinum ‘dintanensis’
5. Conclusions
- (1)
- The coefficients of variation of leaf functional traits of Z. planispinum ‘dintanensis’ ranged from 0.41% to 39.51%, mostly with medium and low variation. The 5–7, 10–12, and 20–22-year-old plantations were laid at the “slow investment-return” end of the economic spectrum while 28–32-year plantations were close to the “fast investment-return” end.
- (2)
- Z. planispinum ‘dintanensis’ tended to suit the karst environment via making trade-off and coordinating leaf functional traits.
- (3)
- Soil carbon/nitrogen ratio, soil total calcium, soil water content, soil available phosphorus, soil carbon/calcium ratio were highly correlated with leaf functional traits, while soil elemental stoichiometry had a greater reflection on leaf functional traits than their own content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wigley, B.J.; Charles-Dominique, T.; Hempson, G.P.; Stevens, N.; TeBeest, M.; Archibald, S.; Bond, W.J.; Bunney, K.; Coetsee, C.; Donaldson, J.; et al. handbook for the standardised sampling of plant functional traits in disturbance-prone ecosystems, with a focus on open ecosystems. Aust. J. Bot. 2020, 68, 473–531. [Google Scholar] [CrossRef]
- Ameztegui, A.; Paquette, A.; Shipley, B.; Heym, M.; Messier, C.; Grave, D. Shade tolerance and the functional trait: Demography relationship in temperate and boreal forests. Funct. Ecol. 2017, 31, 821–830. [Google Scholar] [CrossRef]
- Pezner, A.K.; Pivovarof, A.L.; Sun, W.; Sharifi, M.R.; Rundel, P.W.; Seibt, U. Plant functional traits predict the drought response of native California plant species. Int. J. Plant Sci. 2020, 181, 256–265. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Anderegg, L.D.L.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; Hillerislambers, J.; Penuelas, J. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef]
- Chen, L.L.; Deng, Q.; Yuan, Z.Y.; Mu, X.M.; Kallenbach, R.L. Age-related C:N:P stoichiometry in two plantation forests in the Loess Plateau of China. Ecol. Eng. 2018, 120, 14–22. [Google Scholar] [CrossRef]
- Wang, J.N.; Wang, J.Y.; Wang, L.; Zhang, H.; Guo, Z.W.; Wang, G.G.; Smith, W.K.; Wu, T.G. Does stoichiometric homeostasis differ among tree organs and with tree age? For. Ecol. Manag. 2019, 453, 117673. [Google Scholar] [CrossRef]
- Chang, Y.J.; Li, N.W.; Wang, W.; Liu, X.J.; Du, F.F.; Yao, D.R. Nutrients resorption and stoichiometry characteristics of different-aged plantations of Larix Kaempferi in the Qingling Mountains, central China. PLoS ONE 2017, 12, e0189424. [Google Scholar] [CrossRef]
- Fan, H.B.; Wu, J.P.; Liu, W.F.; Yuan, Y.H.; Hu, L.; Cai, Q.K. Linkages of plant and soil C:N:P stoichiometry and their relationships to forest growth in subtropical plantations. Plant Soil 2015, 392, 127–138. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, A.M.; Yan, S.W.; Rafay, L.; Du, K.; Wang, D.J.; Ge, Y.G.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soils Sediments 2019, 19, 511–521. [Google Scholar] [CrossRef]
- Nelson, P.R.; McCune, B.; Roland, C.; Stehn, S. Non-parametric methods reveal non-linear functional trait variation of lichens along environmental and fire age gradients. J. Veg. Sci. 2015, 26, 848–865. [Google Scholar] [CrossRef]
- He, B.; Li, Q.; Feng, T.; Xue, X.H.; Li, W.J.; Liu, Y. Variation in leaf functional traits of different-aged Pinus massoniana communities and relationships with soil nutrients. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2020, 44, 181–190. (In Chinese) [Google Scholar] [CrossRef]
- Appelhans, M.S.; Reichelt, N.; Groppo, M.; Paetzold, C.; Wen, J. Phylogeny and biogeography of the pantropical genus Zanthoxylum and its closest relatives in the proto-Rutaceae group (Rutaceae). Mol. Phylogenetics Evol. 2018, 126, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Zheng, W.; Zhong, X.P.; Yin, B. Stoichiometric characteristics in Zanthoxylum planispinum var. dintanensis plantation of different ages. Agron. J. 2020, 113, 685–695. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, R.; Pei, Y.D. Soil erosion resistance characteristics of Zanthoxylum Bungeanum and Lonicera Japonica forest land in canyon areas of karst plaeau. Acta Pedol. Sin. 2019, 56, 466–474. [Google Scholar] [CrossRef]
- Wei, C.S.; Zuo, Z.L. Analysis and countermeasure research on the cause of the decline of Zanthoxyhum planispiunum var. dingtanensis industry. Guizhou For. Sci. Technol. 2016, 144, 60–64. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.T.; Feng, Y.X.; Guo, S.S.; Pang, X.; Zhang, D.; Geng, Z.F. Insecticidal and repellent efficacy against stored-product insects of oxygenated monoterpenes and 2-dodecanone of the essential oil from Zanthoxylum planispinum var. dintanensis. Environ. Sci. Pollut. Res. 2019, 26, 24988–24997. [Google Scholar] [CrossRef]
- Qu, S.; Wang, R.; Yang, Y.; Pei, Y.D.; Li, K.F.; Hu, J.D. Change characteristics of soil nutrients during Zanthoxylum bungeanum var. dingtanensis grow process in karst plateau. Non-Wood For. Res. 2020, 38, 183–191. (In Chinese) [Google Scholar]
- Li, H.; Yu, Y.H.; Long, J.; Li, J. Responses of leaf functional traits of Zanthoxylum planispinum var. dintanensis to premature senescence. Chin. J. Ecol. 2021, 40, 1695–1704. (In Chinese) [Google Scholar] [CrossRef]
- Tu, Y.L.; Wei, C.S.; Zuo, Z.L.; Lu, Y.M. A new Zanthoxylum Genus—Z. planipinum var.dingtanensis and the research of its species classification. Guizhou Sci. 2001, 19, 77–801. (In Chinese) [Google Scholar]
- Yu, Y.H.; Wu, Y.G.; Song, Y.P.; Li, Y.T. Carbon and nitrogen stable isotope abundance and soil stoichiometry of Zanthoxylum planispinum var. dintanensis plantations of different ages. Agronomy 2022, 12, 1248. [Google Scholar] [CrossRef]
- Cernusak, L.A. Gas exchange and water-use efficiency in plant canopies. Plant Biol. 2020, 22, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil Agro-Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2010. [Google Scholar]
- Huang, X.L.; Wang, J.; Zhu, Q.; Wu, M.L.; Liu, Y. Effect of soil nutrients on leaf functional traits of different life form plants. Acta Bot. Boreali-Occident. Sin. 2018, 38, 2293–2302. (In Chinese) [Google Scholar] [CrossRef]
- Puglielli, G.; Varone, L. Inherent variation of functional traits in winter and summer leaves of Mediterranean seasonal dimorphic species: Evidence of a ’within leaf cohort’ spectrum. AOB Plant 2018, 10, ply027. [Google Scholar] [CrossRef]
- Hecking, M.J.; Zukswert, J.M.; Drake, J.E.; Dovciak, M.; Burton, J.I. Montane temperate-boreal forests retain the leaf economic spectrum despite intraspecific variability. Front. For. Glob. Change 2022, 4, 754063. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature. 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Westoby, Y.M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261268. [Google Scholar] [CrossRef]
- Yu, Y.H.; Song, Y.P.; Zhong, X.P.; Li, Y.T.; Ying, B. Growth decline mechanism of Zanthoxylum planispinum var. dintanensis in the canyon area of Guizhou Karst Plateau. Agron. J. 2021, 113, 852–862. [Google Scholar] [CrossRef]
- Xu, T.R.; Wu, X.C.; Tian, Y.H.; Li, Y.; Zhang, W.; Zhang, C.C. Soil property plays a vital role in vegetation drought recovery in karst region of Southwest China. J. Geophys. Res.-Biogeosci. 2021, 126, e2021JG006544. [Google Scholar] [CrossRef]
- Li, Q.; Hou, J.H.; He, N.P.; Xu, L.; Zhang, Z.H. Changes in leaf stomatal traits of different aged temperate forest stands. J. For. Res. 2021, 32, 97–936. [Google Scholar] [CrossRef]
- Grassein, F.; Till-Bottraud, I.; Lavorel, S. Plant resource-use strategies: The importance of phenotypic plasticity in response to a productivity gradient for two subalpine species. Ann. Bot. 2010, 106, 637–645. [Google Scholar] [CrossRef]
- He, Y.; Yao, Y.P.; Jiang, Y.; Liang, S.C.; Li, Y.J.; Liang, H.H.; Zhao, Q.N.; Huang, Y.P.; Lin, C.J. Intraspecific variation of functional traits of woody species in the dominant Cyclobalanopsis glauca community in the karst area of Guilin city, Southwest China. Acta Ecol. 2021, 41, 8237–8245. (In Chinese) [Google Scholar] [CrossRef]
- Windt, C.W.; Nabel, M.; Kochs, J.; Jahnke, S.; Schurr, U. A mobile NMR sensor and relaxometric method to non-destructively monitor water and dry matter content in plants. Front. Plant Sci. 2021, 12, 617768. [Google Scholar] [CrossRef]
- Wei, Y.H.; Liang, W.Z.; Han, L.; Wang, H.Z. Leaf functional traits of Populus euphratica and its response to groundwater depths in Tarim extremely arid area. Acta Ecol. Sin. 2021, 41, 5368–5376. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yang, D.; Zhang, Y.B.; Ellsworth, D.S.; Xu, K.; Zhang, Y.P.; Chen, Y.J.; He, F.L.; Zhang, J.L. Differentiation in stem and leaf traits among sympatric lianas, scandent shrubs and trees in a subalpine cold temperate forest. Tree Physiol. 2021, 41, 1992–2003. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Cao, Y.J.; He, W.J.; Xu, Q.; Xu, C.Y.; Zhang, X.N. Leaf functional traits differentiation in relation to covering materials of urban tree pits. BMC Plant Biol. 2021, 21, 556. [Google Scholar] [CrossRef]
- Chang, Y.N.; Xu, C.B.; Yang, H.; Zhou, J.X.; Hua, W.P.; Zhang, S.H.; Zhong, Q.L.; Li, B.Y. Leaf structural traits vary with plant size in even-aged stands of Sapindus mukorossi. Front. Plant Sci. 2021, 12, 692484. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhu, H.; Cao, Y.J.; Li, J.H.; Zhu, Q.Y.; Yao, J.M.; Xu, C.Y. Effect of simulated warming on leaf functional traits of urban greening plants. BMC Plant Biol. 2020, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The world-wide‘fast-slow’plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Tian, D.; Du, E.; Jiang, L.; Zeng, W.J.; Zou, A.L.; Feng, C.Y.; Xing, A.J.; Wang, W.; Zheng, C.Y.; Ji, C.J.; et al. Responses of forest ecosystems to increasing N deposition in China: A critical review. Environ. Pollut. 2018, 243, 75–86. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zhao, G.X.; Li, M.; Zhang, M.T.; Zhang, L.F.; Zhang, X.F.; An, L.Z.; Xu, S.J. C:N:P Stoichiometry and leaf traits of halophytes in an arid saline environment, northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Zeng, Q.C.; Rattan, L.; Chen, Y.A.; An, S.S. Soil, leaf and root ecological stoichiometry of Caragana Korshinskii on the Loess Plateau of China in relation to plantation age. PLoS ONE 2017, 12, e0168890. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Zhu, S.D.; Jansen, S.; Cao, K.F. Topography strongly affects drought stress and xylem embolism resistance in woody plants from a karst forest in Southwest China. Funct. Ecol. 2021, 35, 566–577. [Google Scholar] [CrossRef]
- Cao, J.J.; Wang, X.Y.; Adamowski, J.F.; Biswas, A.; Liu, C.F.; Chang, Z.Q.; Feng, Q. Response of leaf stoichiometry of Oxytropis ochrocephala to elevation and slope aspect. Catena 2020, 194, 104772. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high-and low-rainfall and high-and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef]
- Zhang, S.H.; Zhang, Y.; Xiong, K.N.; Yu, Y.H.; Min, X.Y. Changes of leaf functional traits in karst rocky desertification ecological environment and the driving factors. Glob. Ecol. Conserv. 2020, 24, e01381. [Google Scholar] [CrossRef]
- Fu, P.L.; Zhu, S.D.; Zhang, J.L.; Finnegan, P.M.; Jiang, Y.J.; Lin, H.; Fan, Z.X.; Cao, K.F. The contrasting leaf functional traits between a karst forest and a nearby non-karst forest in south-west China. Funct. Plant Biol. 2019, 46, 907–915. [Google Scholar] [CrossRef]
- Rossatto, D.R.; Carvalho, F.A.; Haridasan, M. Soil and leaf nutrient concentration of tree species support deciduous forests on limestone outcrops as a eutrophic ecosystem. Acta Bot. Brasílica 2015, 29, 231–238. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]

| Age | Tree Height (m) | Density (Plant/ha) | Crown (m) | Coverage (%) | Yield (Plant/kg) |
|---|---|---|---|---|---|
| 5–7 | 3.0 | 1150 | 3 × 3 | 100 | 6–7 |
| 10–12 | 3.0 | 1150 | 3 × 3 | 100 | 7–8 |
| 20–22 | 3.5 | 1000 | 3.5 × 3.5 | 90 | 4–5 |
| 28–32 | 4.2 | 650 | 4 × 5 | 70 | 1–1.5 |
| Age (a) | Soil Water Content (%) | Soil Total Nitrogen (g/kg) | Soil Organic Carbon (g/kg) | Soil Total Phosphorus (g/kg) | Soil Total Calcium (g/kg) | Soil Available Nitrogen (mg/kg) |
|---|---|---|---|---|---|---|
| 5–7 | 22.47 ± 0.85 a | 2.62 ± 0.34 a | 23.65 ± 4.31 a | 0.43 ± 0.11 b | 8.3 ± 2.69 ab | 175 ± 14.14 a |
| 10–12 | 26.23 ± 1.09 a | 2.50 ± 0.30 a | 15.3 ± 0.85 b | 0.80 ± 0.20 ab | 12.75 ± 6.33 a | 162 ± 5.66 a |
| 20–22 | 24.97 ± 4.01 a | 2.00 ± 0.52 a | 15.05 ± 2.47 b | 1.11 ± 0.24 a | 2.5 ± 1.20 b | 222.5 ± 110.71 a |
| 28–32 | 23.23 ± 4.81 a | 2.12 ± 0.43 a | 16.5 ± 2.26 ab | 0.77 ± 0.18 ab | 3.9 ± 1.27 ab | 145 ± 29.70 a |
| Age (a) | Soil Available Phosphorus (mg/kg) | Soil Available Calcium (mg/kg) | Soil Carbon/ Nitrogen Ratio | Soil Nitrogen/Phosphorus Ratio | Soil Carbon/ Calcium Ratio | Soil Calcium/Phosphorus Ratio |
| 5–7 | 32.70 ± 5.80 a | 1109.00 ± 185.26 a | 9.00 ± 0.48 a | 6.37 ± 2.35 a | 2.92 ± 0.42 ab | 20.65 ± 11.31 a |
| 10–12 | 20.2 ± 5.37 a | 989.50 ± 156.27 a | 6.16 ± 0.41 b | 3.27 ± 1.19 ab | 1.36 ± 0.62 b | 17.50 ± 12.38 a |
| 20–22 | 36.65 ± 9.55 a | 1018.50 ± 412.24 a | 7.625 ± 0.76 ab | 1.80 ± 0.08 b | 7.39 ± 4.02 a | 2.14 ± 1.07 a |
| 28–32 | 33.65 ± 7.28 a | 1145.00 ± 35.36 a | 7.86 ± 0.53 a | 2.76 ± 0.10 ab | 4.37 ± 0.85 ab | 5.02 ± 0.47 a |
| Indicator | 5–7 a | 10–12 a | 20–22 a | 28–32 a |
|---|---|---|---|---|
| Leaf thickness (mm) | 0.38 ± 0.01 a (3.17%) | 0.37 ± 0.03 a (7.65%) | 0.36 ± 0.05 a (13.94%) | 0.34 ± 0.09 a (27.43%) |
| Specific leaf area (cm2/g) | 87.37 ± 1.31 b (1.50%) | 89.70 ± 6.29 b (7.02%) | 91.88 ± 20.65 b (22.47%) | 132.22 ± 2.81 a (2.12%) |
| Leaf dry matter content (%) | 32.50 ± 0.71 a (2.18%) | 33.00 ± 1.41 a (4.29%) | 32.00 ± 1.41 a (4.42%) | 31.00 ± 1.41 a (4.56%) |
| Leaf water content (%) | 64.59 ± 0.35 b (0.55%) | 63.46 ± 0.54 b (0.85%) | 66.00 ± 0.45 a (0.69%) | 65.95 ± 0.33 a (0.51%) |
| Leaf tissue density (g/cm3) | 0.33 ± 0.01 a (2.18%) | 0.33 ± 0.03 a (8.58%) | 0.32 ± 0.02 a (6.73%) | 0.31 ± 0.03 a (9.13%) |
| Leaf nitrogen (g/kg) | 23.70 ± 0.57 a (2.39) | 22.25 ± 0.50 a (2.22%) | 21.00 ± 1.98 a (9.43%) | 22.10 ± 2.97 a (13.44%) |
| Leaf phosphorus (g/kg) | 1.64 ± 0.35 a (21.19%) | 1.02 ± 0.23 a (22.19%) | 1.67 ± 0.40 a (24.21%) | 1.63 ± 0.25 a (15.62%) |
| Leaf carbon (g/kg) | 419.00 ± 18.38 a (4.39%) | 373.00 ± 9.90 a (2.65%) | 399.50 ± 30.41 a (7.61%) | 415.00 ± 12.73 a (3.07%) |
| Leaf carbon/nitrogen ratio | 17.70 ± 1.20 a (6.75%) | 16.76 ± 0.07 a (0.42%) | 19.04 ± 0.35 a (1.86%) | 18.99 ± 3.13 a (16.46%) |
| Leaf carbon/phosphorus ratio | 263.38 ± 67.05 a (25.46%) | 373.81 ± 73.22 a (19.59%) | 244.91 ± 41.02 a (16.75%) | 257.13 ± 32.35 a (12.58%) |
| Leaf nitrogen/phosphorus ratio | 14.79 ± 2.79 a (18.84%) | 22.31 ± 4.47 a (20.03%) | 12.85 ± 1.92 a (14.92%) | 13.87 ± 3.99 a (28.75%) |
| Leaf stable nitrogen isotope (‰) | 0.86 ± 0.02 b (2.17%) | 3.20 ± 0.15 a (4.80%) | 2.17 ± 0.86 ab (39.51%) | 1.80 ± 0.72 ab (39.82%) |
| Leaf stable carbon (‰) | −28.31 ± 0.30 a (1.07%) | −28.10 ± 0.12 a (0.41%) | −27.84 ± 1.12 a (4.03%) | −27.90 ± 0.88 a (3.14%) |
| Leaf water use efficiency (umol/mol) | 81.21 ± 3.18 a (3.92%) | 83.48 ± 1.22 a (1.46%) | 86.15 ± 11.81 a (13.71%) | 85.54 ± 9.20 a (10.76%) |
| Item | Ordination Axes of RDA | ||||
|---|---|---|---|---|---|
| Axis 1 | Axis 2 | Axis 3 | Axis 4 | Sum of All Canonical | |
| Eigenvalues | 0.434 | 0.240 | 0.161 | 0.075 | 1 |
| Cumulative percentage variance of functional traits/% | 43.4 | 67.4 | 83.4 | 90.9 | _ |
| Soil Factor | Explained Variance (%) | p |
|---|---|---|
| Soil carbon/nitrogen ratio | 12.06 | 0.004 |
| Soil total calcium | 11.04 | 0.008 |
| Soil water content | 10.52 | 0.012 |
| Soil available phosphorus | 10.27 | 0.024 |
| Soil carbon/calcium ratio | 9.20 | 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Yu, Y.; Li, Y. Leaf Functional Traits and Relationships with Soil Properties of Zanthoxylum planispinum ‘dintanensis’ in Plantations of Different Ages. Agronomy 2022, 12, 1891. https://doi.org/10.3390/agronomy12081891
Song Y, Yu Y, Li Y. Leaf Functional Traits and Relationships with Soil Properties of Zanthoxylum planispinum ‘dintanensis’ in Plantations of Different Ages. Agronomy. 2022; 12(8):1891. https://doi.org/10.3390/agronomy12081891
Chicago/Turabian StyleSong, Yanping, Yanghua Yu, and Yitong Li. 2022. "Leaf Functional Traits and Relationships with Soil Properties of Zanthoxylum planispinum ‘dintanensis’ in Plantations of Different Ages" Agronomy 12, no. 8: 1891. https://doi.org/10.3390/agronomy12081891
APA StyleSong, Y., Yu, Y., & Li, Y. (2022). Leaf Functional Traits and Relationships with Soil Properties of Zanthoxylum planispinum ‘dintanensis’ in Plantations of Different Ages. Agronomy, 12(8), 1891. https://doi.org/10.3390/agronomy12081891