Defense Response to Fusarium Infection in Winter Wheat Varieties, Varying in FHB Susceptibility, Grown under Different Nitrogen Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trial
2.2. Inoculum Production, Inoculation Procedure, and FHB Evaluation
2.3. Sample Preparation and Measurements
2.4. Determination of the Soluble Phenolic Content
2.5. Determination of Total Antioxidant Capacity (TAC)
2.6. Determination of PPO and PAL Activities
2.7. Determination of DON and ZEA
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2019, 139, 429–454. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit. Contam. Part A 2015, 32, 874–910. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals-a review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef]
- Marzec-Schmidt, K.; Börjesson, T.; Suproniene, S.; Jędryczka, M.; Janavičienė, S.; Góral, T.; Karlsson, I.; Kochiieru, Y.; Ochodzki, P.; Mankevičienė, A.; et al. Modelling the Effects of Weather Conditions on Cereal Grain Contamination with Deoxynivalenol in the Baltic Sea Region. Toxins 2021, 13, 737. [Google Scholar] [CrossRef]
- Španić, V.; Dvojković, K.; Babic, J.; Drezner, G.; Zdunić, Z. Fusarium Head Blight Infestation in Relation to Winter Wheat End-Use Quality—A Three-Year Study. Agronomy 2021, 11, 1648. [Google Scholar] [CrossRef]
- Xu, X.M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship between the fungal complex causing fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X2110697. [Google Scholar] [CrossRef]
- Pratyusha, S. Phenolic Compounds in the Plant Development and Defense: An Overview. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Kiani, R.; Arzani, A.; Maibody, S.A.M.M. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids–Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.; Yu, J.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [Green Version]
- Duba, A.; Goriewa-Duba, K.; Wachowska, U.; Głowacka, K.; Wiwart, M. The Associations between Leaf Morphology, Phenylalanine Ammonia Lyase Activity, Reactive Oxygen Species, and Fusarium Resistance in Selected Species of Wheat with Different Ploidy Levels. Plants 2019, 8, 360. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol Oxidases in Crops: Biochemical, Physiological and Genetic Aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.N.; Sarosh, B.R.; Shetty, H.S. Induction and accumulation of polyphenol oxidase activities as implicated in development of resistance against pearl millet downy mildew disease. Funct. Plant Biol. 2006, 33, 563–571. [Google Scholar] [CrossRef]
- Sorahinobar, M.; Niknam, V.; Ebrahimzadeh, H.; Soltanloo, H. Differential antioxidative responses of susceptible and resistant wheat cultivars against Fusarium head blight. Intl. J. Farm. Allied Sci. 2015, 4, 239–243. [Google Scholar]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Lebeda, A.; Luhova, L.; Sedlarova, M.; Jancova, D. The role of enzymes in plant-fungal pathogens interactions. J. Plant. Dis. Prot. 2001, 108, 89–111. [Google Scholar]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, M.; Mur, L.A.J.; Shen, Q.; Guo, S. Unravelling the Roles of Nitrogen Nutrition in Plant Disease Defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef] [Green Version]
- Lemmens, M.; Haim, K.; Lew, H.; Ruckenbauer, P. The effect of nitrogen fertilization on Fusarium head blight development and deoxynivalenol contamination in wheat. J. Phytopathol. 2004, 152, 1–8. [Google Scholar] [CrossRef]
- Heier, T.; Jain, S.K.; Kogel, K.-H.; Pons-Kühnemann, J. Influence of N-fertilization and fungicide strategies on Fusarium head blight severity and mycotoxin content in winter wheat. J. Phytopathol. 2005, 153, 551–557. [Google Scholar] [CrossRef]
- Chai, X.; Zhou, T.; Liu, Y. Effects of nitrogen deficiency induced by straw decomposition after incorporation into soil on the autophagy and pathogenicity of Fusarium graminearum based on an off-line simulation. J. Phytopathol. 2021, 169, 239–246. [Google Scholar] [CrossRef]
- Huber, D.M.; Watson, R.D. Nitrogen form and plant disease. Annu. Rev. Phytopathol. 1974, 12, 139–165. [Google Scholar] [CrossRef]
- Matić, M.; Vuković, R.; Vrandečić, K.; Štolfa Čamagajevac, I.; Ćosić, J.; Vuković, A.; Sabljić, K.; Sabo, N.; Dvojković, K.; Novoselović, D. Oxidative Status and Antioxidative Response to Fusarium Attack and Different Nitrogen Levels in Winter Wheat Varieties. Plants 2021, 10, 611. [Google Scholar] [CrossRef]
- Matić, M.; Vuković, R.; Vrandečić, K.; Štolfa Čamagajevac, I.; Vuković, A.; Ćosić, J.; Dvojković, K.; Novoselović, D. The Effect of Nitrogen Fertilization and Fusarium culmorum Inoculation on the Biomarkers of Oxidative Stress in Wheat Flag Leaves. Poljoprivreda 2021, 27, 15–24. [Google Scholar] [CrossRef]
- Snijders, C.H.A.; Van Eeuwijk, F.A. Genotype x strain interactions for resistance to Fusarium head blight caused by Fusarium culmorum in winter wheat. Theor. Appl. Genet. 1991, 81, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Marumo, K.; Waite, J.H. Optimization of hydroxylation of tyrosine and tyrosine-containing peptides by mushroom tyrosinase. Biochim. Biophys. Acta 1986, 872, 98–103. [Google Scholar] [CrossRef]
- Havir, E.A.; Hanson, K.R. [72a] L-phenylalanine ammonia-lyase (potato tubers). Meth. Enzymol. 1970, 17, 575–581. [Google Scholar] [CrossRef]
- Mesterházy, A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Španić, V.; Ćosić, J.; Zdunić, Z.; Drezner, G. Characterization of agronomical and quality traits of winter wheat (Triticum aestivum L.) for Fusarium head blight pressure in different environments. Agronomy 2021, 11, 213. [Google Scholar] [CrossRef]
- Krnjaja, V.; Mandić, V.; Lević, J.; Stanković, S.; Petrović, T.; Vasić, T.; Obradović, A. Influence of N-fertilization on Fusarium head blight and mycotoxin levels in winter wheat. Crop Prot. 2015, 67, 251–256. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364/5–L364/24. [Google Scholar]
- Gunnaiah, R.; Kushalappa, A.C. Metabolomics Deciphers the Host Resistance Mechanisms in Wheat Cultivar Sumai-3, against Trichothecene Producing and Non-Producing Isolates of Fusarium graminearum. Plant Physiol. Biochem. 2014, 83, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Chrpová, J.; Orsák, M.; Martinek, P.; Lachman, J.; Trávníčková, M. Potential Role and Involvement of Antioxidants and Other Secondary Metabolites of Wheat in the Infection Process and Resistance to Fusarium spp. Agronomy 2021, 11, 2235. [Google Scholar] [CrossRef]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. Physiological Function of Phenolic Compounds in Plant Defense System. In Phenolic Compounds-Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Riaz, A.; Riaz, A.; Rattu, A.U.R.; Tahir, M.I.; Azeem, M. Phenylalanine ammonia-lyase (PAL) and peroxidase activity in brown rust infected tissues of Pakistani wheat cultivars. Pak. J. Bot. 2014, 46, 1101–1107. [Google Scholar]
- Chen, C.; Belanger, R.R.; Benhamou, N.; Paulitz, T.C. Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol. Mol. Plant Pathol. 2000, 56, 13–23. [Google Scholar] [CrossRef]
- Deborah, S.D.; Palaniswami, A.; Vidhyasekaran, P.; Velazhahan, R. Time-course study of the induction of defense enzymes, phenolics and lignin in rice in response to infection by pathogen and non-pathogen. J. Plant Dis. Prot. 2001, 108, 204–216. [Google Scholar]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 22, 566. [Google Scholar] [CrossRef] [Green Version]
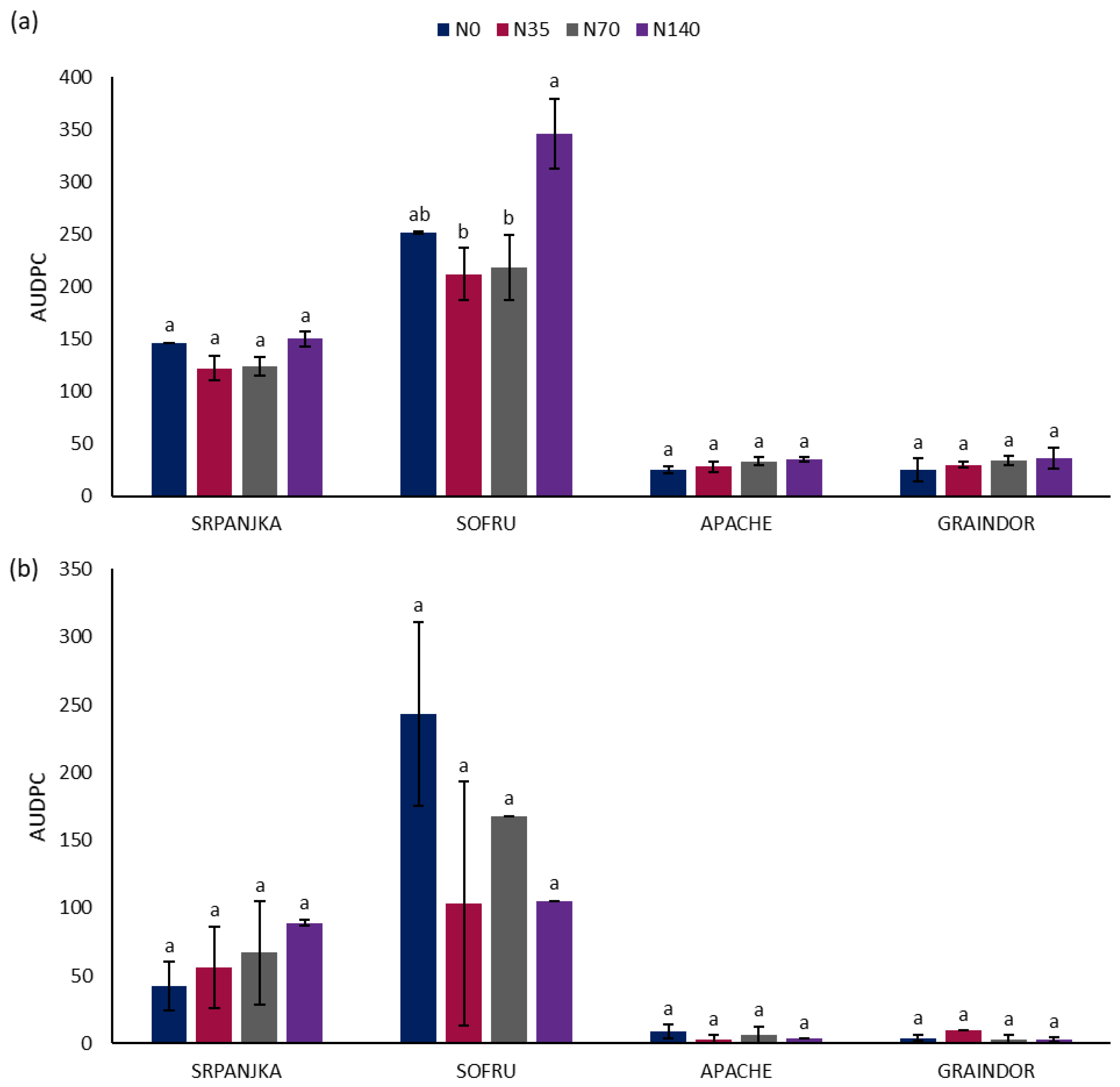
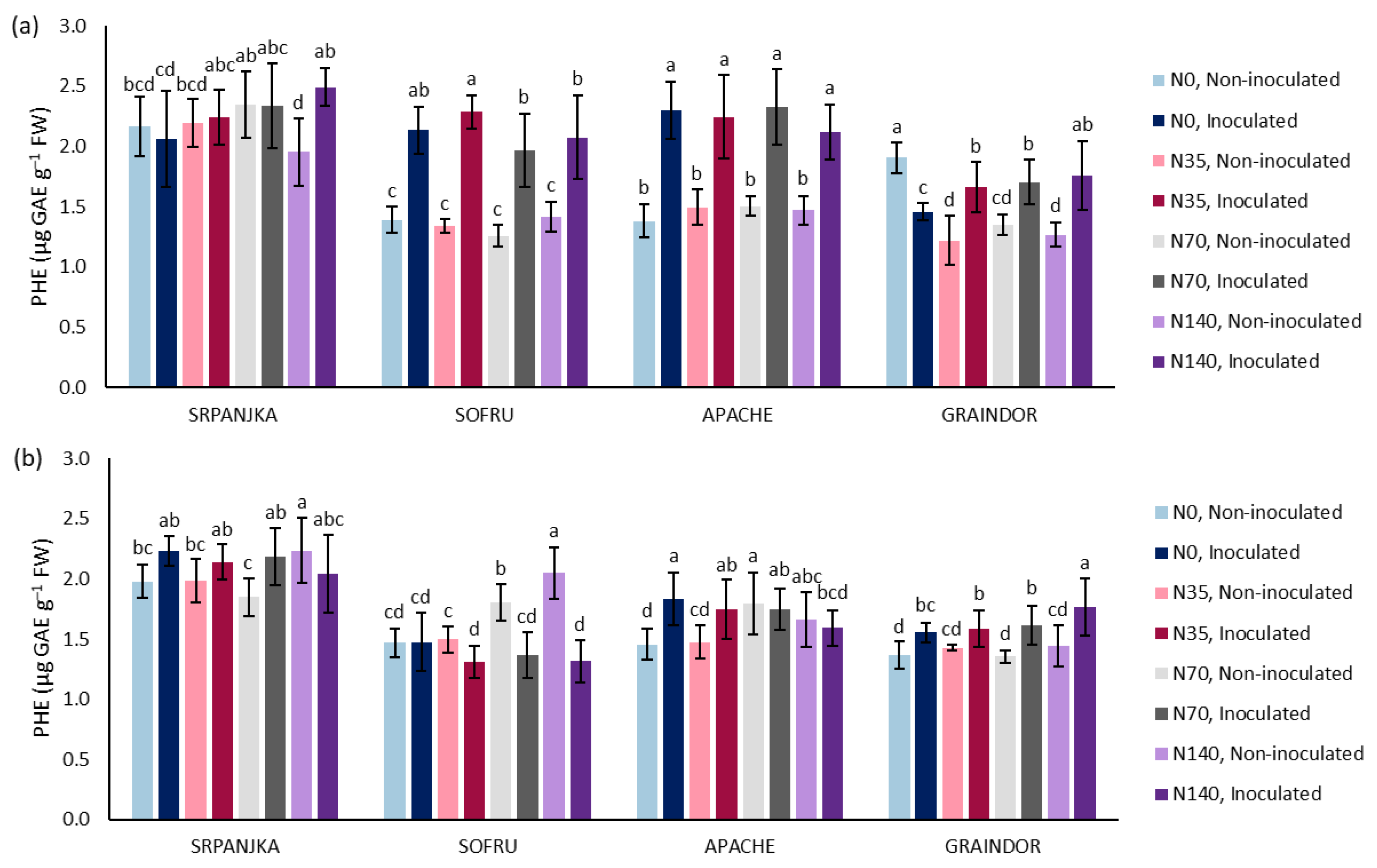

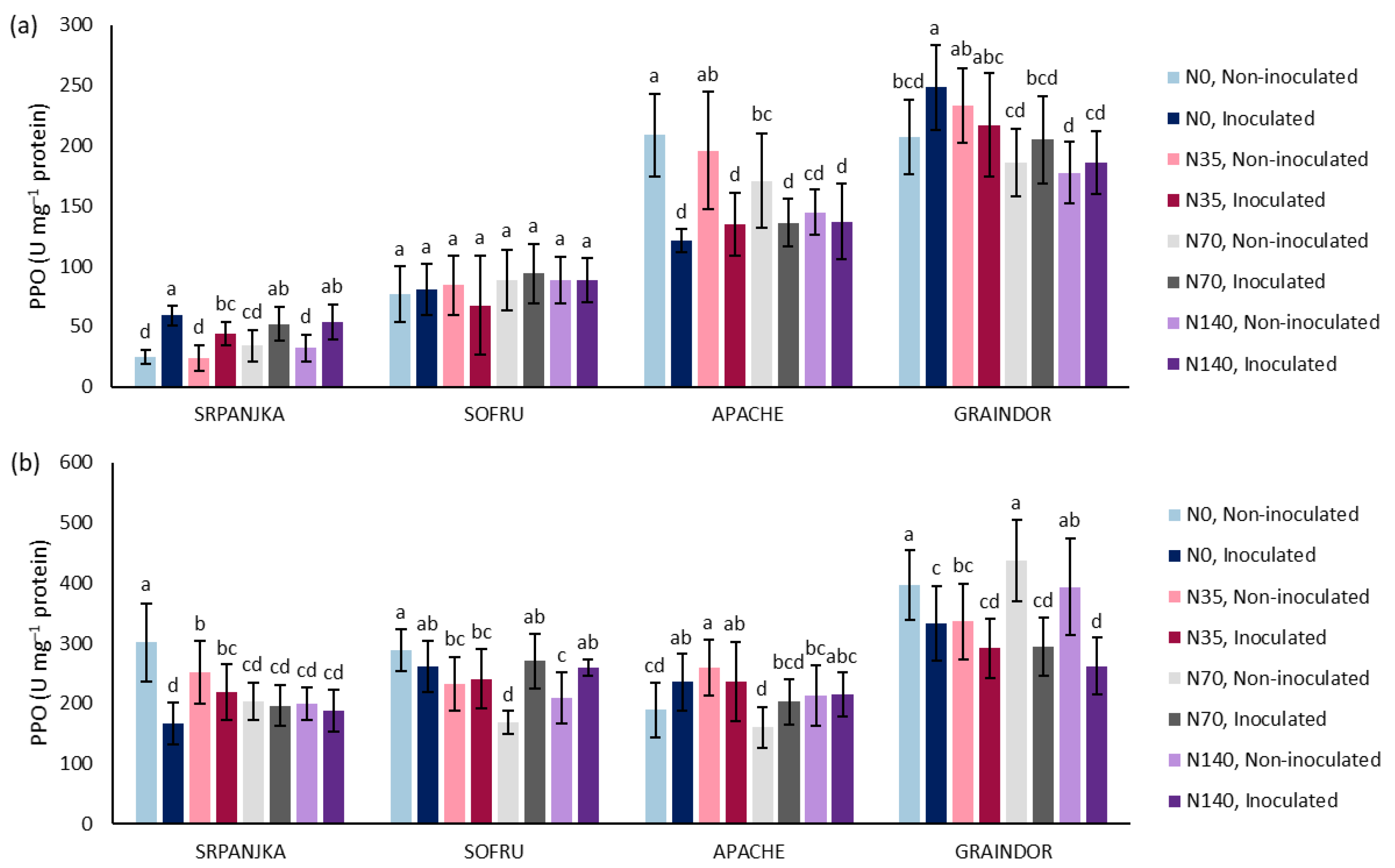
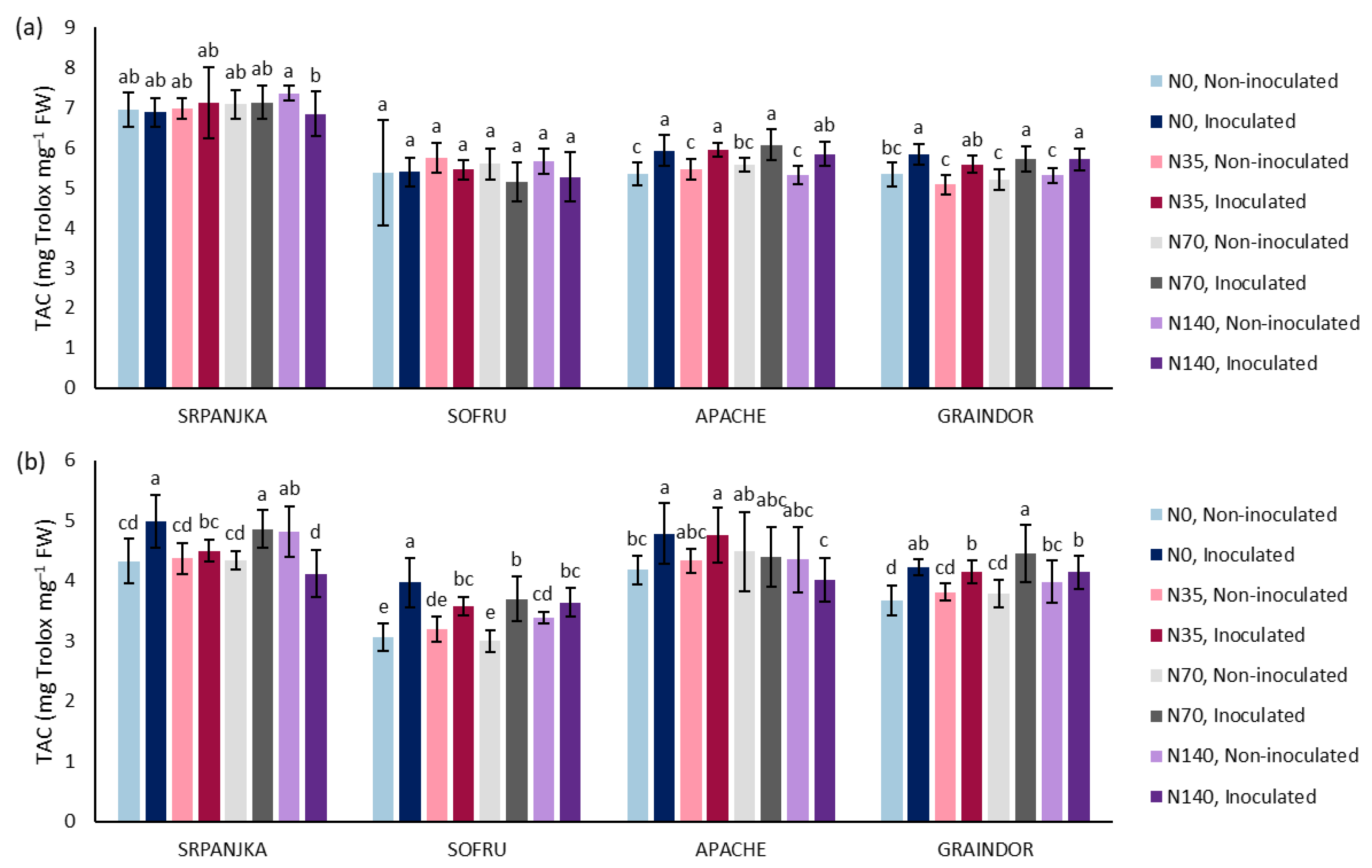
| Location | Soil Type | Season | Residual Soil N (kg N ha−1) | Basic N Fertilization (kg N ha−1) | N Top-Dressing (kg N ha−1) | Total N (kg N ha−1) |
|---|---|---|---|---|---|---|
| Osijek | Eutric Cambisol | 2018/2019 and 2019/2020 | 20 | 74 | 0 | 94 |
| 35 | 129 | |||||
| 70 | 164 | |||||
| 140 | 234 |
| Fusarium Inoculated Wheat Varieties | Nitrogen Treatment | DON Concentration (µg kg−1) | ZEA Concentration (µg kg−1) |
|---|---|---|---|
| Srpanjka | N0 | 1691.30 | ˂8 |
| N35 | 1504.80 | ˂8 | |
| N70 | 1822.50 | 16.30 | |
| N140 | 1653.50 | 19.90 | |
| Sofru | N0 | 1769.90 | ˂8 |
| N35 | 1623.10 | 49.80 | |
| N70 | 1487.90 | 17.30 | |
| N140 | 1940.70 | ˂8 | |
| Apache | N0 | 1739.90 | ˂8 |
| N35 | 1761.60 | ˂8 | |
| N70 | 1590.90 | ˂8 | |
| N140 | 1575.70 | ˂8 | |
| Graindor | N0 | 1696.80 | ˂8 |
| N35 | 1702.00 | ˂8 | |
| N70 | 1206.70 | ˂8 | |
| N140 | 1522.70 | ˂8 |
| MS | |||||
|---|---|---|---|---|---|
| Source of Variation | df | PHE | TAC | PAL | PPO |
| VARIETY (V) | 3 | 5.31 *** | 36.95 *** | 75.43 *** | 354,684.34 *** |
| N LEVEL (N) | 3 | 0.01 ns | 0.04 ns | 0.64 ns | 2586.77 * |
| FUSARIUM (F) | 1 | 14.08 *** | 1.66 ** | 0.27 ns | 688.89 ns |
| V × N | 9 | 0.15 ** | 0.21 ns | 2.67 *** | 2957.68 *** |
| V × F | 3 | 2.01 *** | 2.62 *** | 3.05 ** | 15,940.55 *** |
| N × F | 3 | 0.30 *** | 0.19 ns | 0.02 ns | 1789.18 ns |
| V × N × F | 9 | 0.31 *** | 0.11 ns | 1.56 ns | 1941.68 * |
| MS | |||||
|---|---|---|---|---|---|
| Source of Variation | df | PHE | TAC | PAL | PPO |
| VARIETY (V) | 3 | 4.46 *** | 15.53 *** | 26.35 *** | 238,480.99 *** |
| N LEVEL (N) | 3 | 0.17 ** | 0.12 ns | 7.88 *** | 13,153.02 *** |
| FUSARIUM (F) | 1 | 0.11 ns | 6.70 *** | 13.50 ** | 34,039.71 *** |
| V × N | 9 | 0.09 ** | 0.23 * | 1.89 ns | 8876.24 *** |
| V × F | 3 | 1.05 *** | 0.66 *** | 237.92 *** | 56,574.48 *** |
| N × F | 3 | 0.40 *** | 1.99 *** | 4.86 * | 4996.04 ns |
| V × N × F | 9 | 0.15 *** | 0.27 * | 14.94 *** | 11,892.92 *** |
| PHE | TAC | PAL | PPO | |
|---|---|---|---|---|
| PHE | 1 | |||
| TAC | 0.53 *** | 1 | ||
| PAL | −0.21 *** | −0.44 *** | 1 | |
| PPO | −0.48 *** | −0.53 *** | 0.17 ** | 1 |
| PHE | TAC | PAL | PPO | |
|---|---|---|---|---|
| PHE | 1 | |||
| TAC | 0.57 *** | 1 | ||
| PAL | 0.33 *** | 0.16 * | 1 | |
| PPO | −0.44 *** | −0.20 ** | −0.30 *** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matić, M.; Vuković, R.; Vrandečić, K.; Štolfa Čamagajevac, I.; Ćosić, J.; Vuković, A.; Dvojković, K.; Novoselović, D. Defense Response to Fusarium Infection in Winter Wheat Varieties, Varying in FHB Susceptibility, Grown under Different Nitrogen Levels. Agronomy 2022, 12, 1746. https://doi.org/10.3390/agronomy12081746
Matić M, Vuković R, Vrandečić K, Štolfa Čamagajevac I, Ćosić J, Vuković A, Dvojković K, Novoselović D. Defense Response to Fusarium Infection in Winter Wheat Varieties, Varying in FHB Susceptibility, Grown under Different Nitrogen Levels. Agronomy. 2022; 12(8):1746. https://doi.org/10.3390/agronomy12081746
Chicago/Turabian StyleMatić, Magdalena, Rosemary Vuković, Karolina Vrandečić, Ivna Štolfa Čamagajevac, Jasenka Ćosić, Ana Vuković, Krešimir Dvojković, and Dario Novoselović. 2022. "Defense Response to Fusarium Infection in Winter Wheat Varieties, Varying in FHB Susceptibility, Grown under Different Nitrogen Levels" Agronomy 12, no. 8: 1746. https://doi.org/10.3390/agronomy12081746
APA StyleMatić, M., Vuković, R., Vrandečić, K., Štolfa Čamagajevac, I., Ćosić, J., Vuković, A., Dvojković, K., & Novoselović, D. (2022). Defense Response to Fusarium Infection in Winter Wheat Varieties, Varying in FHB Susceptibility, Grown under Different Nitrogen Levels. Agronomy, 12(8), 1746. https://doi.org/10.3390/agronomy12081746







