Actions for Monitoring the Gonipterus Pest in Eucalyptus on the Cantabrian Coast
Abstract
:1. Introduction
- Galicia: 287,983 ha;
- Asturias: 60,311 ha;
- Cantabria: 39,522 ha;
- Basque Country: 18,750 ha.
2. Materials and Methods
2.1. Study Variables
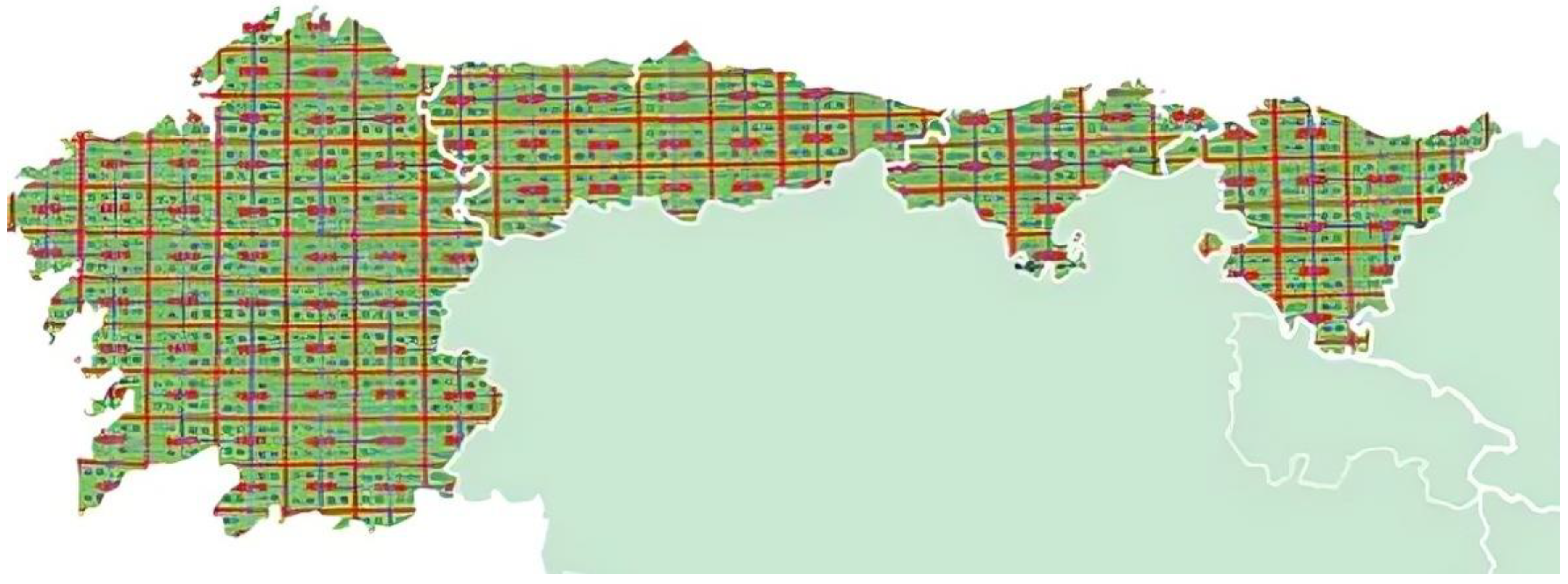
2.2. Study Area
2.3. Information Used
- -
- data provided by the authorities in the respective AR (Xunta de Galicia and Principado de Asturias);
- -
- data supplied by the Spanish Association of Pulp, Paper and Cardboard Manufacturers (ASPAPEL);
- -
- data provided by ADRA Engineering and Environmental Management (SLP) on the two regions;
- -
- data from additional surveys by the Forest Owners Association of Asturias (PROFOAS);
- -
- data provided by the Spanish Confederation of Forestry Producer Organisations (COSE) for each region.
2.4. Focus Groups
2.5. Statistical Analysis
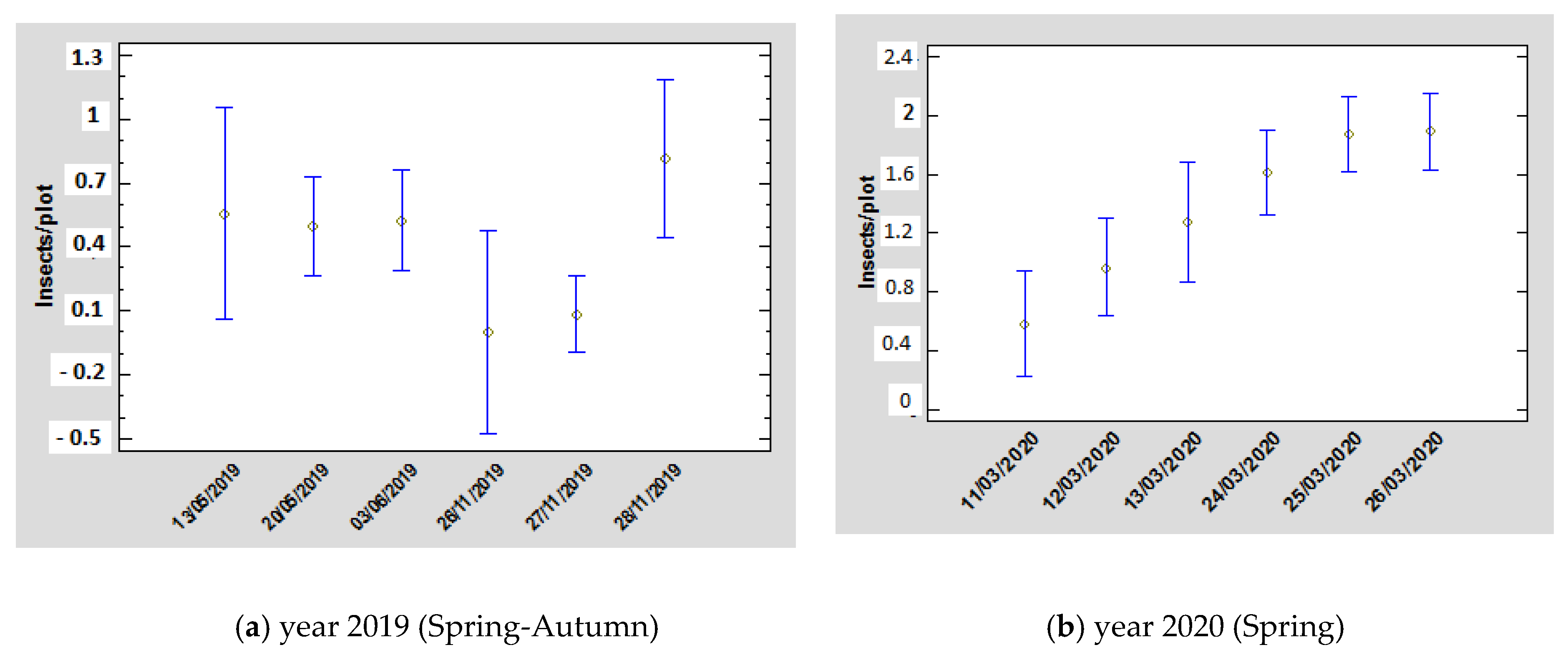
3. Results
3.1. Results of Focus Group
3.1.1. Sample Selection
- Location: eucalyptus stands that cover the spatial variation of the Eucalyptus globulus species in the study region;
- Level of impact: stands with different degrees of defoliation caused by G. platensis;
- Age of the stands: young eucalyptus stands between three and six years old;
- Accessibility: stands with ease of access for monitoring.
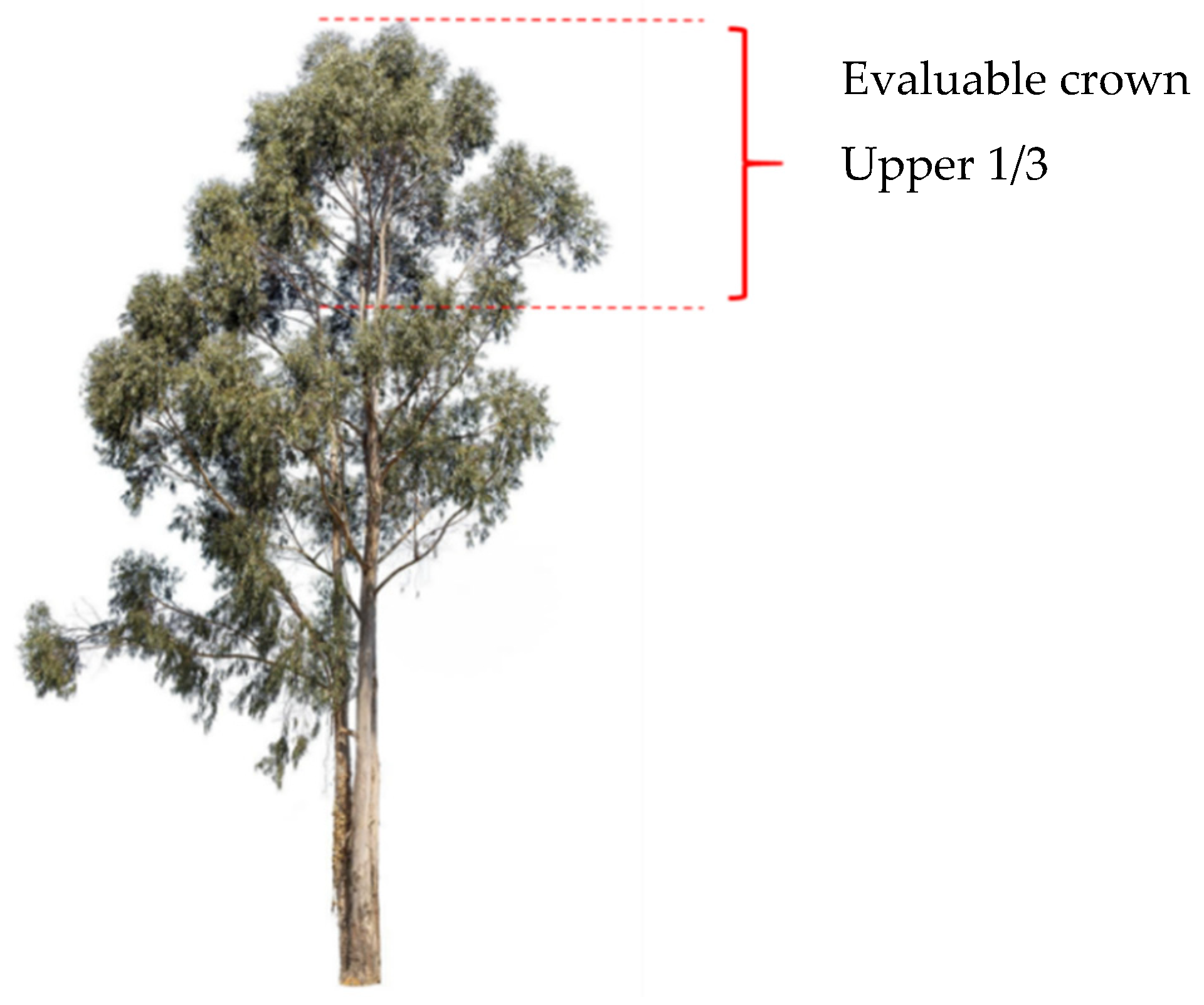
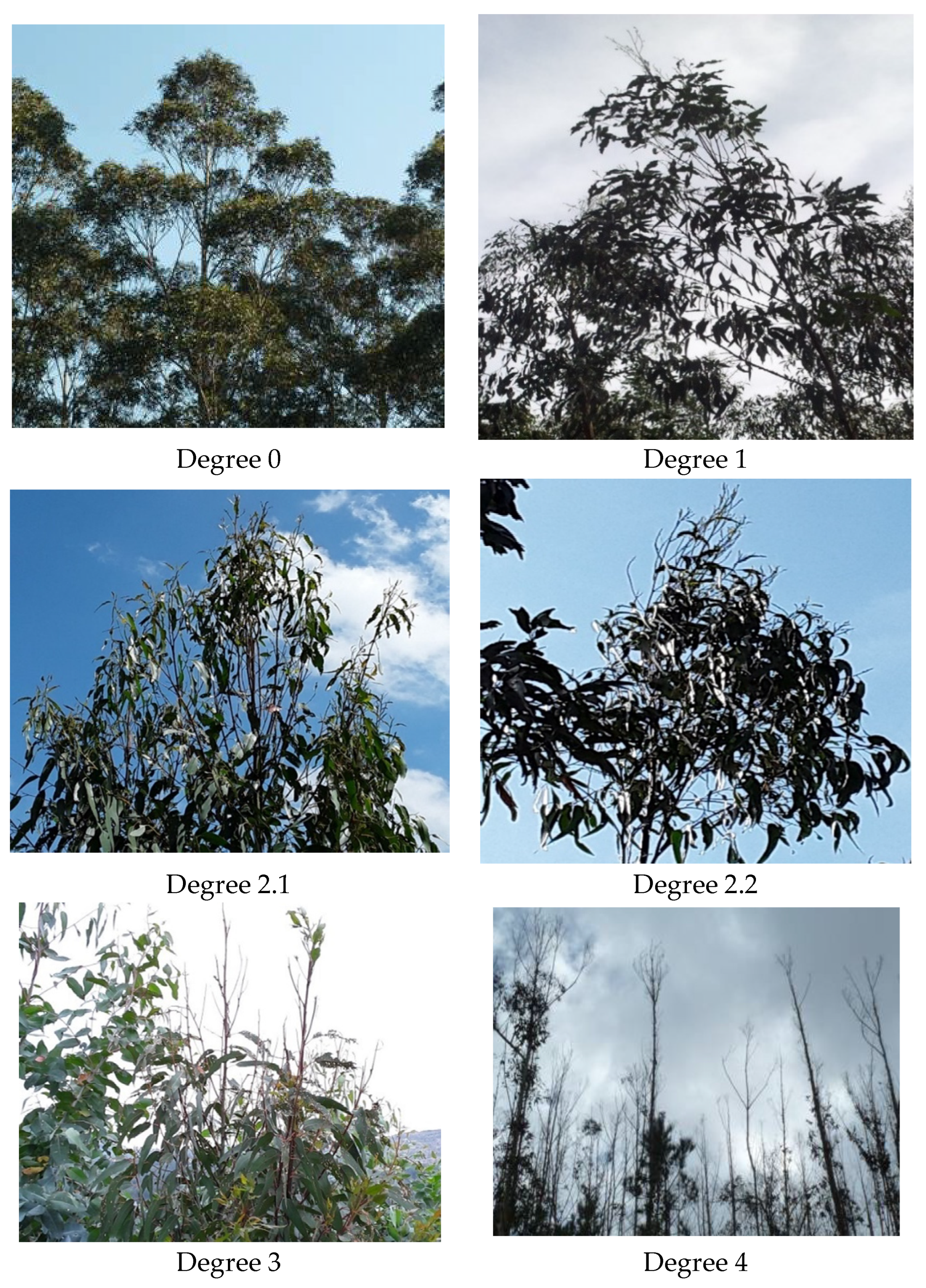
3.1.2. Evaluation of the Degree of Defoliation
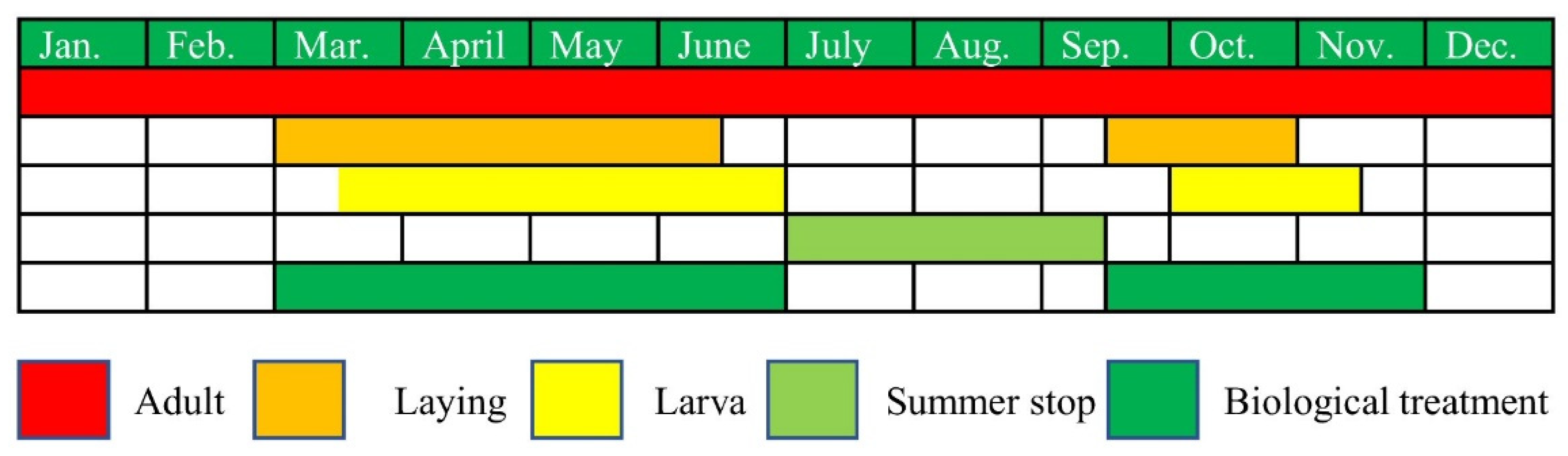
3.1.3. Oothecae Collection
- Date (xx/xx/xxxx);
- Author (first name, surname and company or organisation);
- Plot data;
- Plot no. and/or code;
- Visit no.;
- Area;
- Location (shrubland, town, district, province);
- UTM coordinates (X, Y) and reference system;
- Altitude (m);
- Species;
- Age of the shoot and/or stand (years);
- No. of trees surveyed;
- Degree of defoliation for each individual evaluated (see Figure 4);
- State of the damage;
- No. of fresh oothecae collected from the selected trees;
- Presence of Mycosphaerella spp.;
- Presence of Ctenarytaina spp.
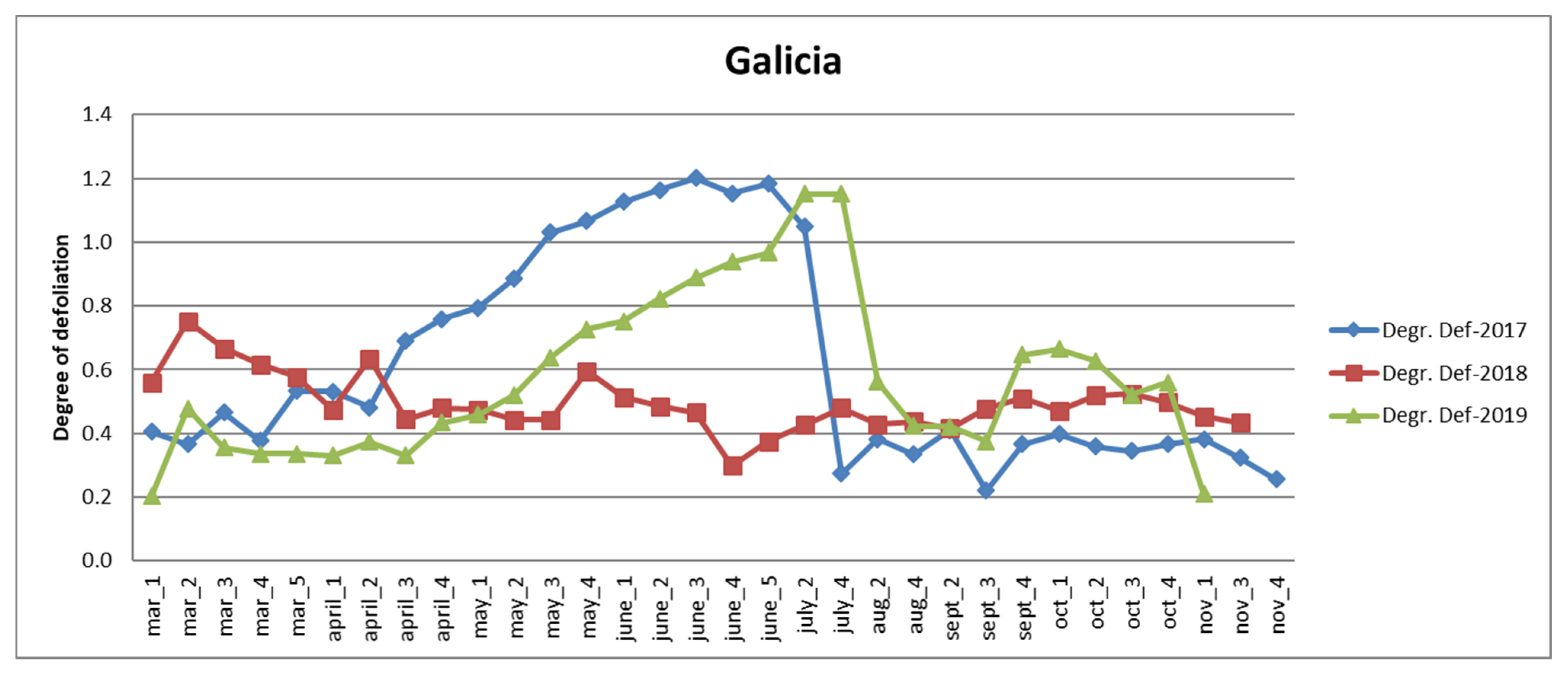
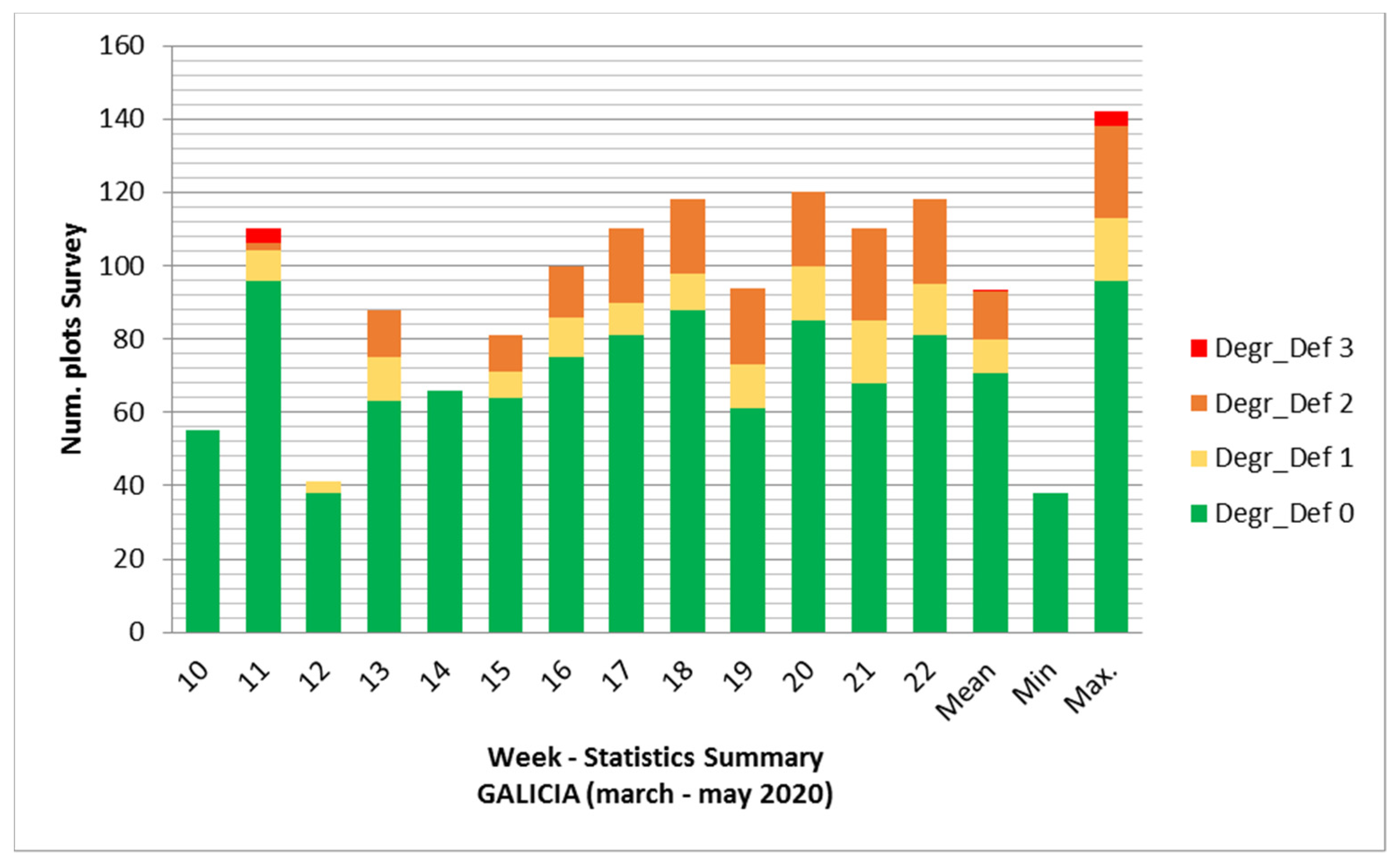
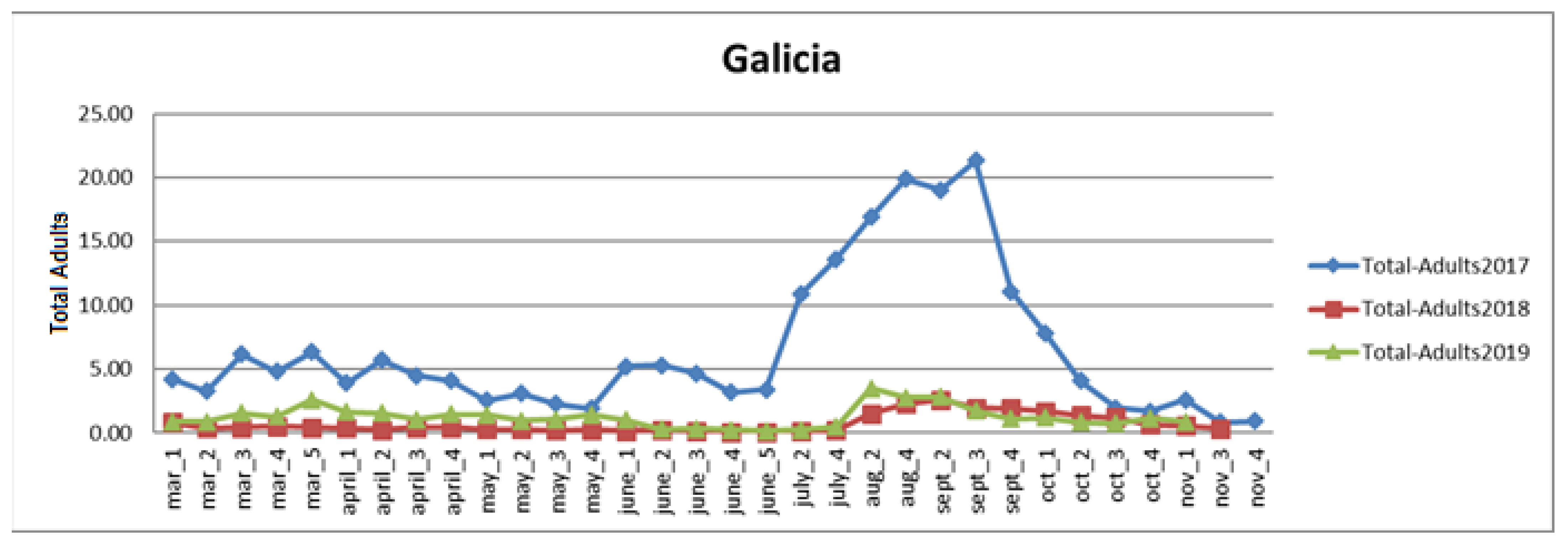
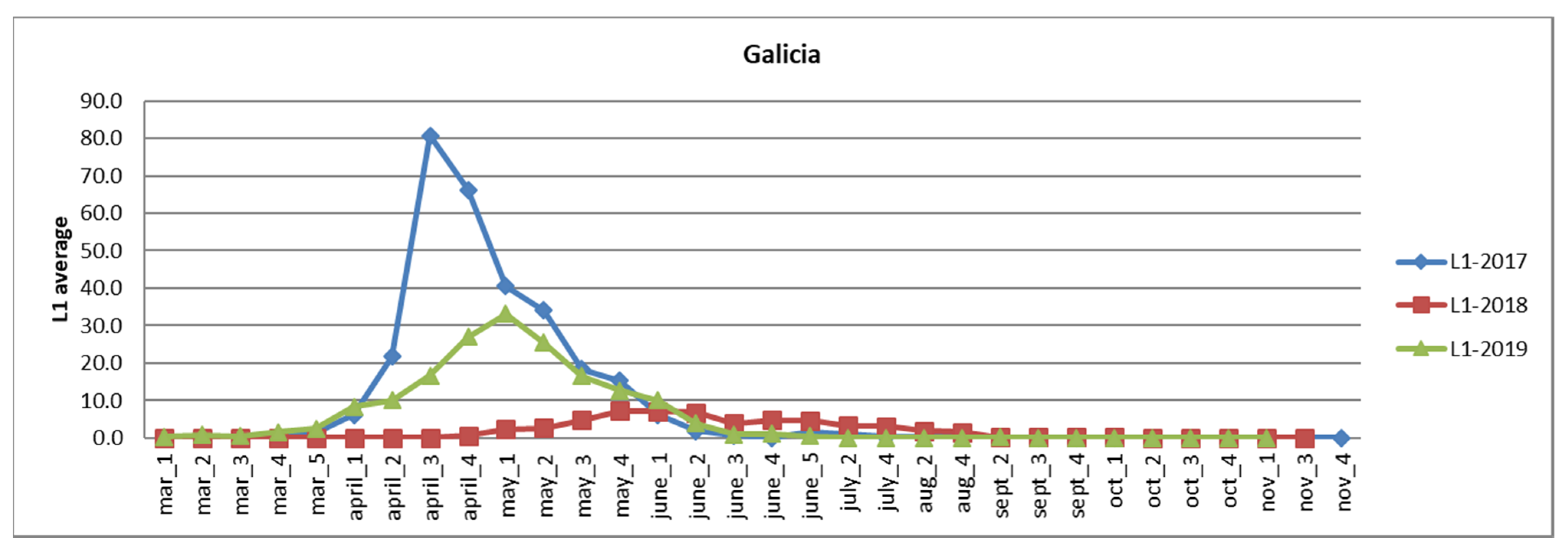
3.2. Analysis of Survey Data in Galicia and Asturias
3.2.1. Galicia
3.2.2. Asturias
3.3. Biological Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ministerio de Agricultura, Pesca y Alimentación. Anuario de Estadística Forestal 2017; Gobierno de España: Madrid, Spain, 2019; Available online: https://www.miteco.gob.es/es/biodiversidad/estadisticas/aef2017_completo_web_tcm30-521729.pdf (accessed on 22 May 2022).
- Ministerio de Agricultura, Pesca y Alimentación. Avance del Anuario de Estadística Forestal 2018; Gobierno de España: Madrid, Spain, 2019; Available online: https://www.mapa.gob.es/estadistica/pags/anuario/2018/anuario/AE18.pdf (accessed on 22 May 2022).
- ASPAPEL (Asociación Española de Fabricantes de Pasta, Papel y Cartón). Innovación en prospección y control fundamentalmente biológico de la plaga Gonipterus. Nota de Prensa 02/11/2017. Available online: http://www.aspapel.es/content/innovacion-en-prospeccion-y-control-fundamentalmente-biologico-de-la-plaga-gonipterus (accessed on 22 May 2022).
- Ministerio de Medio Ambiente y Medio Rural y Marino. Cuarto Inventario Forestal Nacional; Gobierno de España: Galicia, Spain, 2011; Available online: https://www.miteco.gob.es/es/biodiversidad/temas/inventarios-nacionales/galicia_tcm30-531786.pdf (accessed on 22 May 2022).
- Ministerio de Agricultura, Pesca y Alimentación. Cuarto Inventario Forestal Nacional (Asturias, 2012, Cantabria, 2012, País Vasco, 2013); Gobierno de España: Madrid, Spain; Available online: https://www.miteco.gob.es/es/biodiversidad/temas/inventarios-nacionales/inventario-forestal-nacional/publicaciones.aspx (accessed on 22 May 2022).
- Mansilla, J.P. Presencia sobre Eucalyptus globulus Labill de Gonipterus scutellatus Gyll. (Col., Curculionidaé) en Galicia. Boletín de Sanidad Vegetal Plagas 1992, 18, 547–554. [Google Scholar]
- Proyecto de innovación del Grupo Operativo Supra-Autonómico de sanidad sobre Gonipterus en Eucalipto. Documento final GOSSGE. Documento interno. 2020. Available online: https://ec.europa.eu/eip/agriculture/en/find-connect/projects/gossge-grupo-operativo-sobre-la-sanidad-del (accessed on 22 May 2022).
- Mapondera, T.S.; Burgess, T.; Matsuki, M.; Oberprieler, R.G. Identification and molecular phylogenetics of the cryptic species of theGonipterus scutellatus complex (Coleoptera: Curculionidae: Gonipterini). Aust. J. Entomol. 2012, 51, 175–188. [Google Scholar] [CrossRef]
- Mansilla, P.; Pérez, R. El defoliador del eucalipto Gonipterus scutellatus. Phytoma 1996, 81, 36–42. [Google Scholar]
- Romanyk, N.; Cadahia, D. Plagas de Insectos en las Masas Forestales; Sociedad Española de Ciencias Forestales, Ed.; Mundi-Prensa: Madrid, España, 2002; ISBN 978-8484760269. [Google Scholar]
- Mansilla, J.P.; Pérez, R.; Salinero, C. Gonipterus scutellatus Gyll. Defoliador del eucalipto; Estación Fitopatolóxica do Areeiro, Servicio Agrario, Diputación Provincial de Pontevedra: Pontevedra, Spain, 1997; EFA 04/97. [Google Scholar]
- Arzone, A.; Meotto, F. Reperti biologici su Gonipterus scutellatus Gyll. (Col. Curculionidae) inféstate gli eucalipti delia Riviera Ligure. REDIA 1978, 61, 205–222. [Google Scholar]
- Rabasse, J.M.; Perrin, H. Introduction en France du charançon de l’Eucalyptus, Gonipterus scutellatus Gyll. (Col. Curculionidae). Ann. Zool. Ecol. Amm. 1979, 11, 337–347. [Google Scholar]
- Vaccaro, N. Los gorgojos del eucalipto. In Boletín Informativo, Estación Experimental Agropecuaria de Concordia; INTA: New York, NY, USA, 1983. [Google Scholar]
- Sanches, M.A. Influência da Temperatura no Desenvolvimento de Gonipterus Scutellatus Gyllenhal 1833 (Coleóptera, Curculionidae) em Eucalyptus Viminalis Labill, Aspectos Bionômicos e Parasitismo na Região de Curitiba (PR). Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 1993. 65p. [Google Scholar]
- Santolamazza-Carbone, S.; Pestaña-Nieto, M.; Pérez-Otero, R.; Mansilla-Vázquez, P.; Adolfo Cordero-Rivera, A. Winter and spring ecology of Anaphes nitens, a solitary egg-parasitoid of the Eucalyptus snout-beetle Gonipterus scutellatus. BioControl 2009, 54, 195–209. [Google Scholar] [CrossRef]
- Reis, A.R.; Ferreira, L.; Tomé, M.; Araujo, C.; Branco, M. Efficiency of biological control of Gonipterus platensis (Coleoptera: Curculionidae) by Anaphes nitens (Hymenoptera: Mymaridae) in cold areas of the Iberian Peninsula: Implications for defoliation and wood production in Eucalyptus globulus. For. Ecol. Manag. 2012, 270, 216–222. [Google Scholar] [CrossRef]
- Valente, C.; Gonçalves, C.I.; Monteiro, F.; Gaspar, J.; Silva, M.; Sottomayor, M.; Paiva, M.R.; Branco, M. Economic Outcome of Classical Biological Control: A Case Study on the Eucalyptus Snout Beetle, Gonipterus platensis, and the Parasitoid Anaphes nitens. Ecol. Econ. 2018, 149, 40–47. [Google Scholar] [CrossRef]
- Santolamazza-Carbone, S.; Pérez-Rodríguez, A.; García-Fojo, R.; Cordero-Rivera, A. Local augmentation efficiency of Anaphes nitens (Hymenoptera, Mymaridae), the egg parasitoid of Gonipterus platensis (Coleoptera, Curculionidae). J. Appl. Entomol. 2019, 143, 574–583. [Google Scholar] [CrossRef]
- Gumovsky, A.; Little, D.; Rothmann, S.; Jaques, L.; Mayorga, S.E.I. Redescription and first host and biology records of Entedon magnificus (Girault & Dodd) (Hymenoptera, Eulophidae), a natural enemy of Gonipterus weevils (Coleoptera, Curculionidae), a pest of Eucalyptus trees. Zootaxa 2015, 3957, 577–584. [Google Scholar] [CrossRef]
- Loch, A.D. Parasitism of the Eucalyptus weevil, Gonipterus scutellatus Gyllenhal, by the egg parasitoid, Anaphes nitens Girault, in Eucalyptus globulus plantations in southwestern Australia. Biol. Control. 2008, 47, 1–7. [Google Scholar] [CrossRef]
- Cordero-Rivera, A.; Santolamazza-Carbone, S.; Andrés, J.A. Life cycle and biological control of the Eucalyptus snout beetle (Coleoptera, Curculionidae) by Anaphes nitens (Hymenoptera, Mymaridae) in north-west Spain. Agric. For. Entomol. 1999, 1, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Valente, C.; Vaz, A.; Pina, J.; Manta, A.; Sequeira, A. Control strategy against the Eucalyptus Snout Beetle, Gonipterus scutellatus Gyllenhal (Coleoptera, Curculionidae), by the Portuguese cellulose industry. In Eucalyptus in a Changing World, Proceedings of the IUFRO Conference Eucalyptus in a Changing World, Aveiro, Portugal, 11–15 October 2004; Borralho, N., Ed.; Environmental Science: Beijing, China, 2004; pp. 37–51. [Google Scholar]
- Tribe, G.D. Biological control of defoliating, and phloem- or wood-feeding insects in commercial forestry in southern Africa. In Biological Control in IPM Systems in Africa; Neuenschwander, P., Borgemeister, C., Langewald, J., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 113–129. ISBN 9780851996394. [Google Scholar] [CrossRef]
- Tooke, F.G.C. The Eucalyptus Snout-beetle, Gonipterus scutellatus Gyll. A Study of its Ecology and Control by Biological Means. In Entomology Memoirs; Department of Agriculture and Forestry: Pretoria, South Africa, 1955. [Google Scholar]
- Valente, C. Biological Control of Gonipterus Platensis: Current Status and New Possibilities. Ph.D. Thesis, Instituto Superior de Agronomia, Universidade de Lisboa, Lisboa, Portugal, 2018. [Google Scholar]
- Huber, J.; Prinsloo, G. Redescription of Anaphes nitens (Girault) and description of two new species of Anaphes Haliday (Hymenoptera: Mymaridae), parasites of Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae) in Tasmania. Aust. J. Entomol. 1990, 29, 333–341. [Google Scholar] [CrossRef]
- Ward, S.E.; Valente, C.; Gonçalves, C.; Polaszek, A. Rediscovery and redescription of Centrodora damoni (Girault) (Hymenoptera: Aphelinidae) from Australia, an egg parasitoid of Gonipterus spp (Coleoptera: Curculionidae), after nearly a century. Biodivers. Data J. 2016, 4, e7766. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, L.I.; Soliman, E.P.; Valverde-Zauza, E.A.; Stape, J.L.; Wilcken, C.F. First Global Record of Podisus nigrispinus (Hemiptera: Pentatomidae) as Predator of Gonipterus platensis (Coleoptera: Curculionidae) Larvae and Adults. Fla. Entomol. 2017, 100, 675–677. [Google Scholar] [CrossRef] [Green Version]
- Branco, S.; Mateus, E.P.; Gomes da Silva, M.D.R.; Mendes, D.; Rocha, S.; Mendel, Z.; Schütz, S.; Paiva, M.R. Electrophysiological and behavioural responses of the Eucalyptus weevil, Gonipterus platensis, to host plant volatiles. J. Pest Sci. 2019, 92, 221–235, ISSN 1612-4758. [Google Scholar] [CrossRef]
- Damascena, A.P.; De Carvalho, V.R.; Ribeiro, M.F.; Horta, A.B.; Monteiro de Castro e Castro, B.; Zanuncio, A.J.V.; Wilcken, C.F.; Zanuncio, J.C.; Wilcken, S.R.S. Steinernema diaprepesi (Rhabditida: Steinernematidae) parasitizing Gonipterus platensis (Coleoptera: Curculionidae). R. Soc. Open Sci. 2020, 7, 200282. [Google Scholar] [CrossRef]
- Jordan, C.; dos Santos, P.L.; Oliveira, L.R.d.S.; Magalhães Domingues, M.; Costa Gêa, B.C.; Fonseca Ribeiro, M.; Moura Mascarin, G.; Wilcken, C.F. Entomopathogenic fungi as the microbial frontline against the alien Eucalyptus pest Gonipterus platensis in Brazil. Sci. Rep. 2021, 11, 7233. [Google Scholar] [CrossRef]
- Valente, C.; Afonso, C.; Gonçalves, C.I.; Alonso-Zarazaga, M.A.; Reis, A.; Branco, M. Environmental risk assessment of the egg parasitoid Anaphes inexpectatus for classical biological control of the Eucalyptus snout beetle, Gonipterus platensis. BioControl 2017, 62, 457–468. [Google Scholar] [CrossRef]
- Valente, C.; Gonçalves, C.I.; Reis, A.; Branco, M. Pre-selection and biological potential of the egg parasitoid Anaphes inexpectatus for the control of the Eucalyptus snout beetle, Gonipterus platensis. J. Pest Sci. 2017, 90, 911–923. [Google Scholar] [CrossRef]
- Valente, C.; Afonso, C.; Gonçalves, C.I.; Branco, M. Assessing the competitive interactions between two egg parasitoids of the Eucalyptus snout beetle, Gonipterus platensis, and their implications for biological control. Biol. Control 2019, 130, 80–87. [Google Scholar] [CrossRef]
- ICNF (Instituto da Conservação da Natureza e das Florestas, Portugal). Plano de controlo para o inseto Gonipterus Platensis gorgulho-do-eucalipto 2.ªfase FASE 2014–2015, Fitossanidade Florestal, Eucalipto. Portugal. Fevereiro 2015. Available online: https://www.icnf.pt/api/file/doc/c4e7f70b7041669b (accessed on 22 May 2022).
- PLURIFOR Project 2018. (Transnational Plans for the Management of Forests Risks). Plan de Riesgo para el gorgojo del eucalipto, Gonipterus platensis. UE: Portugal, Spain and France, 2018. Available online: https://plurifor.efi.int/es/sobre-nosotros/; https://plurifor.efi.int/wp-content/uploads/WP2/plans/Gonipterus-platensis-risk-plan_ES.pdf (accessed on 22 May 2022).
- Plan Estratégico de Lucha Integrada contra Gonipterus scutellatus. Protocolo de actuaciones para o inventario permanente de danos e presencia da praga. Xunta de Galicia: Santiago, Spain, 2011. Available online: https://www.xunta.gal/hemeroteca/-/nova/008799/medio-rural-apuesta-por-lucha-biologica-para-erradicar-plaga-del-gorgullo-del?langId=es_ES (accessed on 22 May 2022).
- Gobierno de Cantabria. Plan de Lucha Integrada contra los Patógenos Causantes de Daños en las Masas de Eucalipto de Cantabria. Consejería de Medio Rural, Pesca y Alimentación Dirección General del Medio Natural Servicio de Montes; Gobierno de Cantabria: Santander, Spain, 2017; p. 43. Available online: https://dgmontes.org/documents/16835/0/PLI+Eucalipto/8d063c78-03ed-27a0-f071-e3c2f8a1c9fe (accessed on 22 May 2022).
- ASPAPEL (Asociación Española de Fabricantes de Pasta, Papel y Cartón). Protocolo de Prospección y Estimación de Daños ocasionados por el Gontipterus scutellatus Gyll en las Masas de Eucalipto de la Cornisa Cantábrica; ASPAPEL: Madrid, Spain, 2012. [Google Scholar]
- Duarte, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent Advances in Forest Insect Pests and Diseases Monitoring Using UAV Based Data: A Systematic Review. Forests 2022, 13, 911. [Google Scholar] [CrossRef]
- Adame, P.; Alberdi, I.; Cañellas, I.; Hernández, L.; Aguirre, A.; Ruano, A.; Moreno-Fernández, D.; González, A.I.; Torres, M.B.; Fernando Montes, F. Drivers and spread of non-native pests in forests: The case of Gonipterus platensis in Spanish Eucalyptus plantations. For. Ecol. Manag. 2022, 510, 120104. [Google Scholar] [CrossRef]
- Mansilla, P.; Pérez-Otero, R.; Salinero, C. Lucha biológica contra plagas forestales en Galicia. MOL (Sociedad de Ciencias de Galicia) 2020, 20, 2. Available online: http://mol-en.scg.org.es/wp-content/uploads/2021/01/2-Mansilla-et-al.pdf (accessed on 22 May 2022).
- RRN (Red Rural Nacional). Grupos Operativos y Proyectos Innovadores. Sanidad Vegetal. Pg. 11; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2020; Available online: https://www.redruralnacional.es/sites/default/files/documents/4.-dossier_svegetal.pdf.pdf (accessed on 22 May 2022).
- MAPA (Ministerio de Agricultura, Pesca y Alimentación). Proyecto de Innovación del Grupo Operativo Supra-Autonómico de Sanidad Sobre Gonipterus en Eucalipto-GOSSGE; Gobierno de España: Madrid, Spain, 2018; Available online: https://www.profoas.com/pdf/proyectos/Gossge-Dossier.pdf (accessed on 10 September 2021).
- ASPAPEL (Asociación Española de Fabricantes de Pasta, Papel y Cartón). Prospección de Presencia y Grado de Afectación de Gonipterus Scutellatus Gyll; Comunicación Interna: Madrid, España, 2013. [Google Scholar]
- Stewart, D.W.; Shamdasani, P.N. Focus Groups: Theory and Practice, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- García-Ventura, C.; Bermejo, A.; González-García, C.; Grande-Ortíz, M.A.; Ayuga-Téllez, E.; Sánchez de Medina-Garrido, A.; Ramírez-Montoro, J.J. Analysis of Differences in the Choice of the Economic Value of Urban Trees in Madrid when Displayed in Situ and in Photographs. Agronomy 2020, 10, 311. [Google Scholar] [CrossRef] [Green Version]
- Corley, J.C.; Lantschner, M.V.; Martínez, A.S.; Fischbein, D.; Villacide, J.M. Management of Sirex noctilio populations in exotic pine plantations: Critical issues explaining invasion success and damage levels in South America. J. Pest Sci. 2019, 92, 131–142. [Google Scholar] [CrossRef]
- Ayuga Téllez, E.; González García, C.; Martín Fernández, S.; Martínez Falero, E.; Pardo Méndez, M. Técnicas de Muestreo en Ciencias Forestales y Ambientales, 1st ed.; Bellisco: Madrid, España, 1999. [Google Scholar]
- Williams, J.R.; Moutia, L.A.; Hermelin, P.R. The Biological Control of Gonipterus scutellatus Gyll. (Col. Curculionidae) in Mauritius. Bull. Entomol. Res. 1951, 42, 23–28. [Google Scholar] [CrossRef]
- Schröder, M.L.; Nahrung, H.F.; de Souza, N.M.; Lawson, S.A.; Slippers, B.; Wingfield, M.J.; Hurley, B.P. Distribution of Gonipterus Species and their Egg Parasitoids in Australia: Implications for Biological Control. Forests 2021, 12, 969. [Google Scholar] [CrossRef]
- Stone, C.; Coops, N.C. Assessment and monitoring of damage from insects in Australian eucalypt forests and commercial plantations. Aust. J. Entomol. 2004, 43, 283–292. [Google Scholar] [CrossRef]
- Ceia, R.S.; Faria, N.; Lopes, P.B.; Alves, J.; da Silva, A.A.; Valente, C.; Gonçalves, C.I.; Mata, V.A.; Santos, S.A.P.; Azevedo-Pereira, H.M.V.S.; et al. Local and landscape effects on the occurrence and abundance of the Eucalyptus weevil Gonipterus platensis (Coleoptera: Curculionidae). For. Ecol. Manag. 2021, 500, 119618. [Google Scholar] [CrossRef]
- Gonçalves, C.I.; Vilas-Boas, L.; Branco, M.; Rezende, G.D.; Valente, C. Host susceptibility to Gonipterus platensis (Coleoptera: Curculionidae) of Eucalyptus species. Ann. For. Sci. 2019, 76, 63. [Google Scholar] [CrossRef]
- Hudgins, E.J.; Liebhold, A.M.; Leung, B. Predicting the spread of all invasive forest pests in the United States. Ecol. Lett. 2017, 20, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Tomé, M.; Almeida, M.H.; Barreiro, S.; Branco, M.R.; Deus, E.; Pinto, G.; Silva, J.S.; Soares, P.; Rodríguez-Soalleiro, R. Opportunities and challenges of Eucalyptus plantations in Europe: The Iberian Peninsula experience. Eur. J. For. Res. 2021, 140, 489–510. [Google Scholar] [CrossRef]
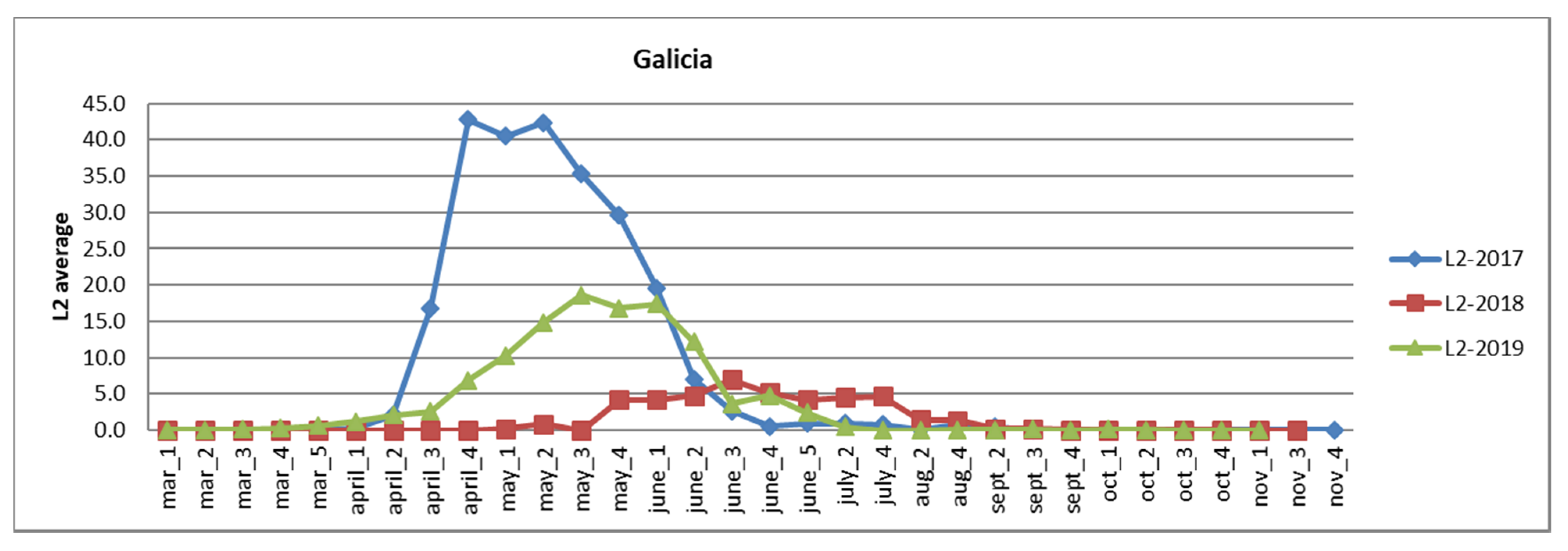
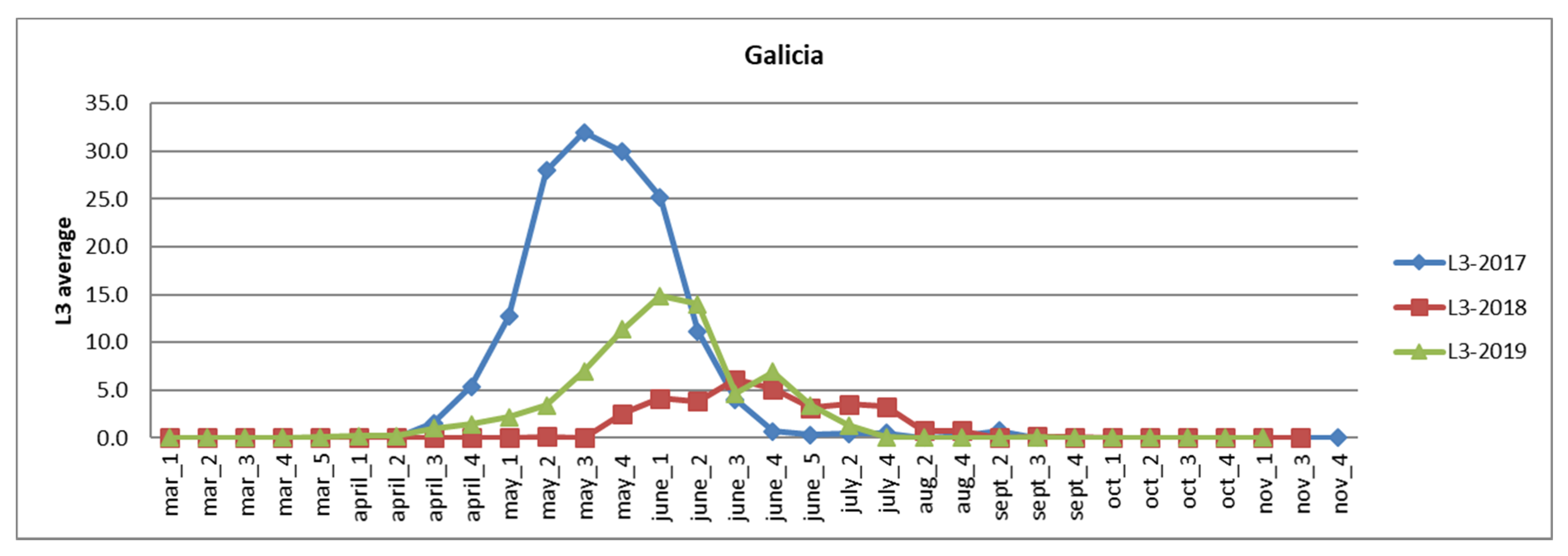
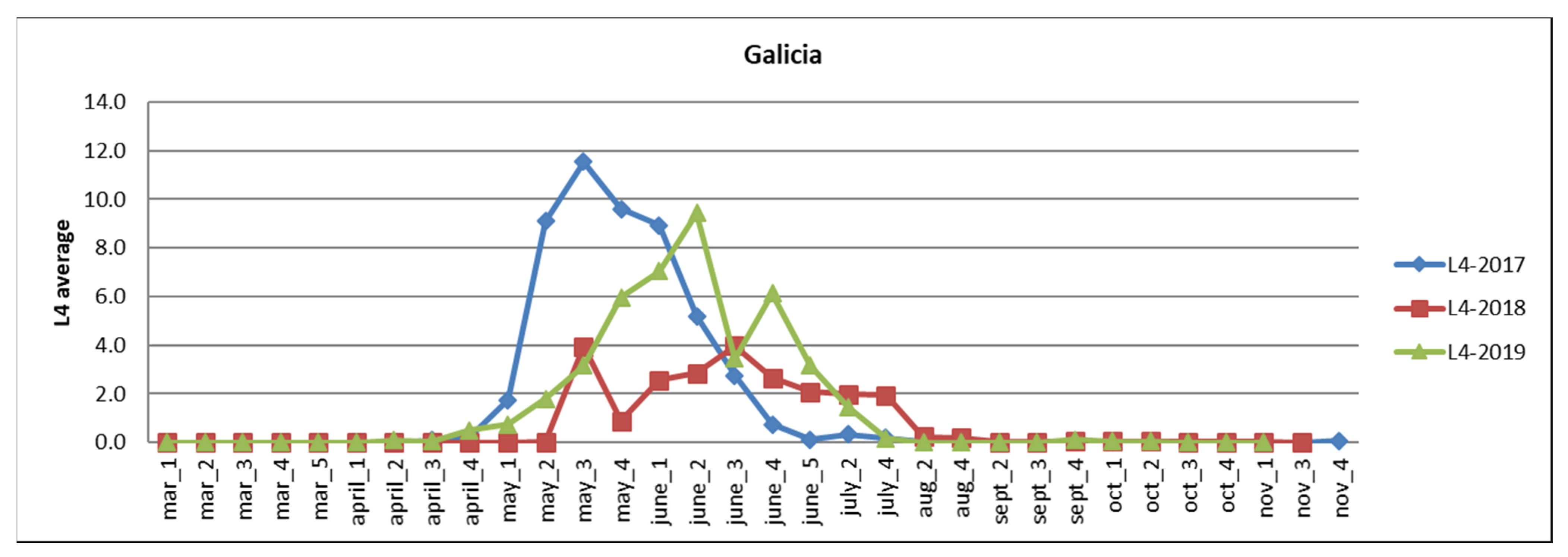
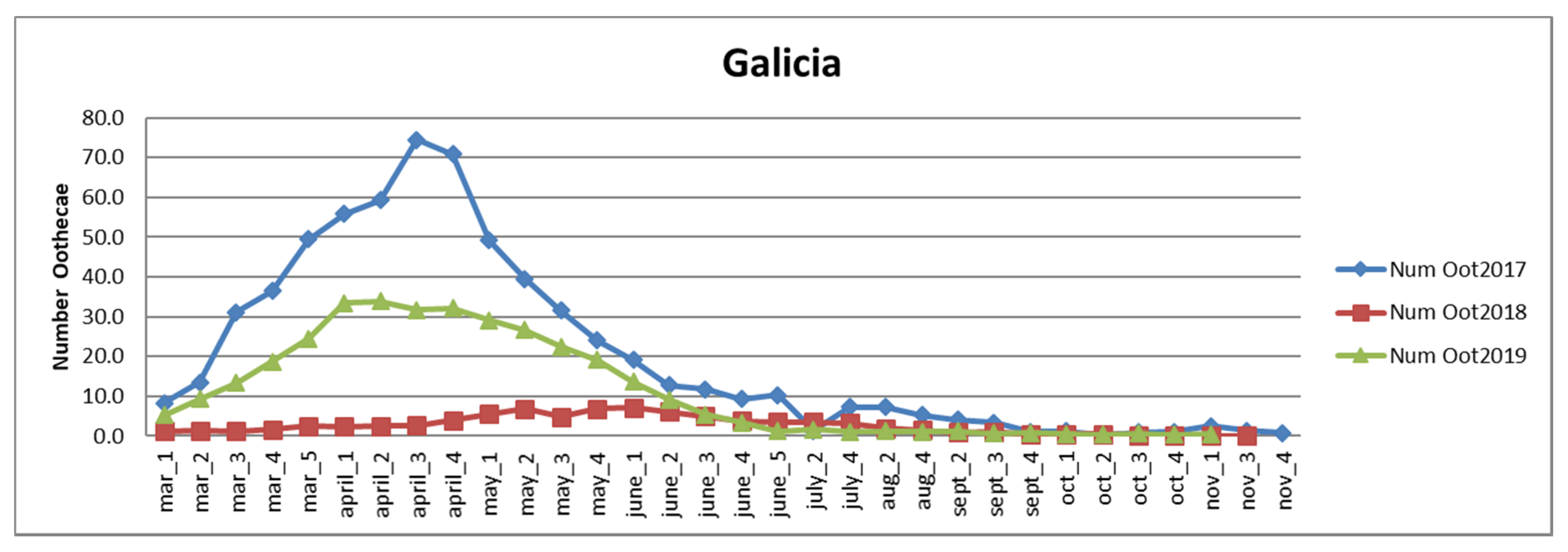
| Autonomous Region | Area of Eucalyptus CCC ≥ 70% | Affected Area Degree 1 | Affected Area Degree 2–3 |
|---|---|---|---|
| GALICIA | 163,664 | 96,493 | 17,457 |
| ASTURIAS | 42,345 | 19,412 | 5950 |
| TOTAL | 206,009 | 115,905 | 23,407 |
| Autonomous Region | n0 | N | n |
|---|---|---|---|
| Galicia | 153 | 174 | 82 |
| Asturias | 100 | 90 | 48 |
| Degree of Defoliation | Tree Crown Damage |
|---|---|
| 0 | 0–10% |
| 1 | ≥−25% |
| 2.1 | 26–45% |
| 2.2 | 46–60% |
| 3 | 61–90% |
| 4 | >90% |
| Degree of Defoliation | A Coruña (%) | Lugo (%) | Pontevedra (%) | Total (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2019 | Spring 2020 | 2019 | Spring 2020 | 2019 | Spring 2020 | 2019 | Spring 2020 | |
| 0 | 88.30 | 96.08 | 93.14 | 93.75 | 0.33 | 11.40 | 75 | 84.67 |
| 1 | 11.66 | 1.38 | 6.86 | 5.43 | 37.90 | 24.68 | 14.8 | 5.49 |
| 2.1 and 2.2 | 0.04 | 1.80 | 0.00 | 0.82 | 61.78 | 63.41 | 10.2 | 9.40 |
| 3 and 4 | 0.00 | 0.74 | 0.00 | 0.00 | 0.00 | 0.51 | 0 | 0.44 |
| Degree of Defoliation | Spring 2019 | Autumn 2019 | Spring 2020 |
|---|---|---|---|
| 0 | 11.24% | 28.87% | 24.75% |
| 1 | 57.30% | 41.24% | 52.97% |
| 2.1 | 26.97% | 27.84% | 18.81% |
| 2.2 | 4.49% | 2.06% | 2.97% |
| 3 and 4 | 0.00% | 0.00% | 0.50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuga-Téllez, E.; García-Iruela, A.; Rielo, J.C.; González-García, C. Actions for Monitoring the Gonipterus Pest in Eucalyptus on the Cantabrian Coast. Agronomy 2022, 12, 1692. https://doi.org/10.3390/agronomy12071692
Ayuga-Téllez E, García-Iruela A, Rielo JC, González-García C. Actions for Monitoring the Gonipterus Pest in Eucalyptus on the Cantabrian Coast. Agronomy. 2022; 12(7):1692. https://doi.org/10.3390/agronomy12071692
Chicago/Turabian StyleAyuga-Téllez, Esperanza, Alberto García-Iruela, José Causí Rielo, and Concepción González-García. 2022. "Actions for Monitoring the Gonipterus Pest in Eucalyptus on the Cantabrian Coast" Agronomy 12, no. 7: 1692. https://doi.org/10.3390/agronomy12071692
APA StyleAyuga-Téllez, E., García-Iruela, A., Rielo, J. C., & González-García, C. (2022). Actions for Monitoring the Gonipterus Pest in Eucalyptus on the Cantabrian Coast. Agronomy, 12(7), 1692. https://doi.org/10.3390/agronomy12071692






