Suitability of Early Blight Forecasting Systems for Detecting First Symptoms in Potato Crops of NW Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Period and Study Area
2.2. Weather Monitoring
2.3. Phenological Monitoring and First Early Blight Symptoms Detection
2.4. Description of the Forecasting Models Used
2.4.1. Plant-Based Models
2.4.2. Pathogen-Based Models
2.4.3. Plant-Pathogen-Based Model
2.5. Statistical Analysis
3. Results
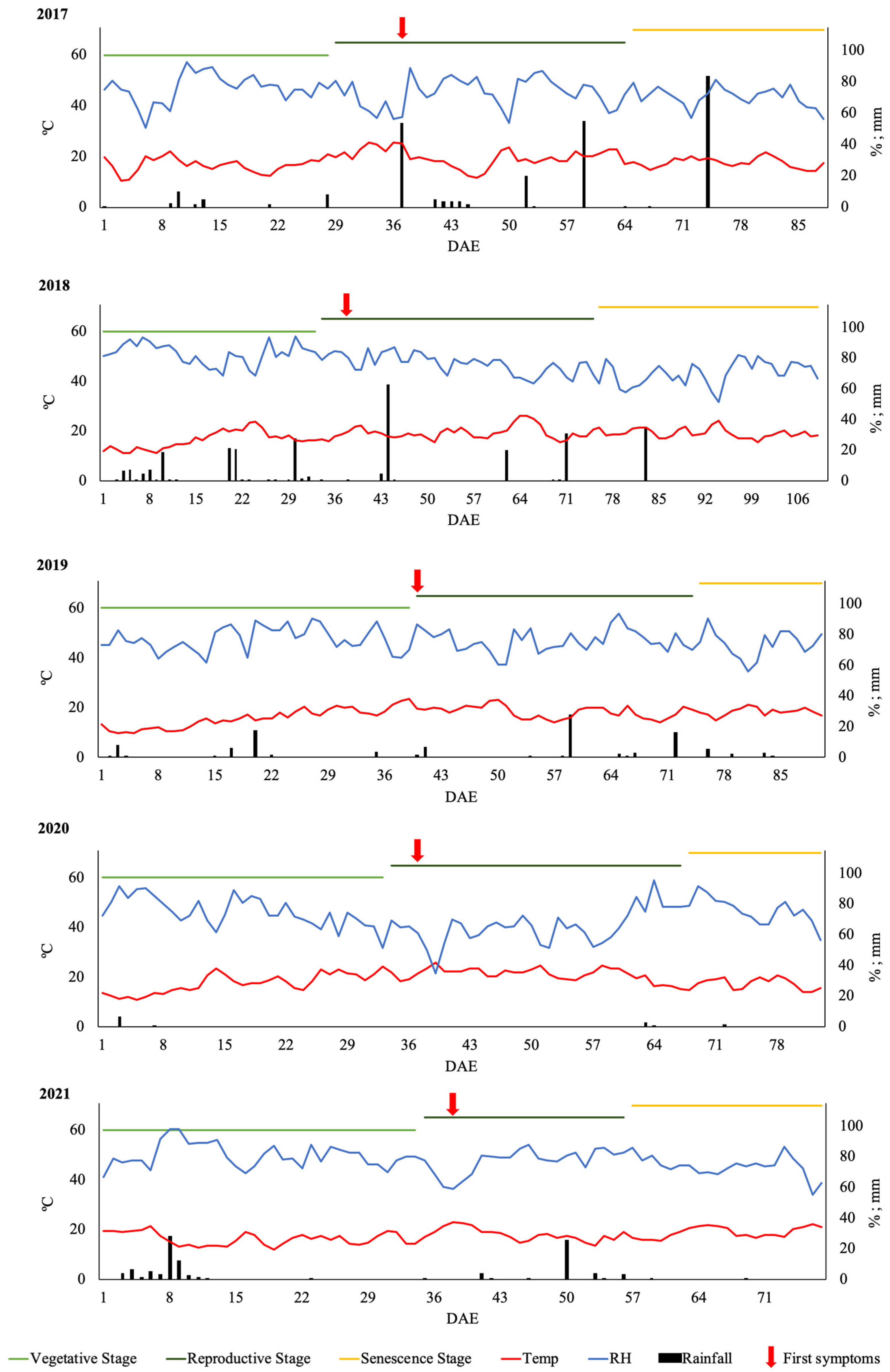
3.1. Phenological Development, First Symptoms of Early Blight and Weather Parameters during Potato Growing Seasons
3.2. Warning Risk of Early Blight Models According to First Symptoms
3.3. Accuracy of Forecasting Models for Determining the Onset of First Symptoms of Early Blight
3.4. Improving Accuracy of Early Blight Models in A Limia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soleimani, M.J.; Kirk, W. Enhance resistance to Alternaria alternata causing potato brown leaf spot disease by using some plant defense inducers. J. Plant Prot. Res. 2012, 52, 83–90. [Google Scholar] [CrossRef]
- Shtienberg, D. Alternaria diseases of potatoes: Epidemiology and management under Israeli conditions. In Fourteenth Euroblight Workshop Limassol, Cyprus. PPO–Spec. Rep. 2014, 16, 169–180. [Google Scholar]
- Tsedaley, B. Review on early blight (Alternaria spp.) of potato disease and its management options. J. Biol. Agric. Health 2014, 4, 191–199. [Google Scholar]
- Abuley, I.K.; Nielsen, B.J. Evaluation of models to control potato early blight (Alternaria solani) in Denmark. J. Crop Prot. 2017, 102, 118–128. [Google Scholar] [CrossRef]
- Escuredo, O.; Seijo-Rodríguez, A.; Meno, L.; Rodríguez-Flores, M.S.; Seijo, M.C. Seasonal dynamics of Alternaria during the potato growing cycle and the Influence of weather on the early blight disease in North-West Spain. Am. J. Potato Res. 2019, 96, 532–540. [Google Scholar] [CrossRef]
- Jindo, K.; Evenhuis, A.; Kempenaar, C.; Pombo, C.; Zhan, X.; Goitom, M.; Kessel, G. Holistic pest management against early blight disease towards sustainable agriculture. Pest Manag. Sci. 2021, 77, 3871–3880. [Google Scholar] [CrossRef]
- Rotem, J. The Genus Alternaria. Biology, Epidemiology and Pathogenicity; American Phytopathological Society: St. Paul, MN, USA, 1994; 326p. [Google Scholar]
- Van der Waals, J.E.; Korsten, L.; Aveling, T.A.S.; Denner, F.D.N. Influence of environmental factors on field concentrations of Alternaria solani conidia above a South African potato crop. Phytoparasitica 2003, 31, 353–364. [Google Scholar] [CrossRef]
- Escuredo, O.; Seijo, M.C.; Fernández-González, M.; Iglesias, I. Effects of meteorological factors on the levels of Alternaria spores on a potato crop. Int. J. Biometeorol. 2011, 55, 243–252. [Google Scholar] [CrossRef]
- Meno, L.; Escuredo, O.; Rodríguez-Flores, M.S.; Seijo, M.C. Interrupted Wet Period (IWP) to forecast the aerial Alternaria in potato crops of A Limia (Spain). Agronomy 2019, 9, 585. [Google Scholar] [CrossRef] [Green Version]
- Meno, L.; Escuredo, O.; Rodríguez-Flores, M.S.; Seijo, M.C. Modification of the TOMCAST model with aerobiological data for management of potato early blight. Agronomy 2020, 10, 1872. [Google Scholar] [CrossRef]
- Meno, L.; Escuredo, O.; Rodríguez-Flores, M.S.; Seijo, M.C. Looking for a sustainable potato crop. Field assessment of early blight management. Agric. For. Meteorol. 2021, 308, 108617. [Google Scholar] [CrossRef]
- Van der Waals, J.E.; Korsten, L.; Aveling, T.A.S. A review of early blight of potato. Afr. Plant Prot. 2001, 7, 91–102. [Google Scholar]
- Spoelder, J.; Ellens, R.; Turkensteen, L.J.; Schepers, H. Comparing pathogenicity of Alternaria solani and Alternaria alternata in potato. In Fourteenth Euroblight Workshop Limassol, Cyprus. PPO–Spec. Rep. 2014, 16, 97–102. [Google Scholar]
- Leiminger, J.; Bäßler, E.; Knappe, C.; Bahnweg, G.; Hausladen, H. Quantification of disease progression of Alternaria spp. on potato using real-time PCR. Eur. J. Plant Pathol. 2015, 141, 295–309. [Google Scholar] [CrossRef]
- Horsfield, A.; Wicks, T.; Davies, K.; Wilson, D.; Paton, S. Effect of fungicide use strategies on the control of early blight (Alternaria solani) and potato yield. Australas. Plant Pathol. 2010, 39, 368–375. [Google Scholar] [CrossRef]
- Abuley, I.K.; Nielsen, B.J.; Labouriau, R. Resistance status of cultivated potatoes to early blight (Alternaria solani) in Denmark. Plant Pathol. 2018, 67, 315–326. [Google Scholar] [CrossRef]
- Ghahreman, N.; Varshaviana, V.; Javan-Nikkhahb, M.; Liaghata, A. Forecasting of potato early blight disease using different sets of meteorological data. Can. J. Appl. Sci. 2015, 3, 59–66. [Google Scholar]
- Franc, G.D.; Harrison, M.D.; Lahman, L.K. A simple day-degree model for initiating chemical control of potato early blight in Colorado. Plant Dis. 1988, 72, 851–854. [Google Scholar] [CrossRef]
- Gleason, M.L.; MacNab, A.A.; Pitblado, R.E.; Ricker, M.D.; East, D.A.; Latin, R.X. Disease-warning systems for processing tomatoes in eastern North America: Are we there yet? Plant Dis. 1995, 79, 113–121. [Google Scholar] [CrossRef]
- Harms, T.; Howard, R.; Konschuh, M. Evaluation of Early Blight (Alternaria solani) Prediction Techniques for Southern Alberta. n.d. Available online: http://www.demofarm.ca/pdf/early_blight_prediction.pdf (accessed on 15 September 2021).
- Abuley, I.K.; Nielsen, B.J. Integrating cultivar resistance into the TOMCAST model to control early blight of potato, caused by Alternaria solani. Crop Prot. 2019, 117, 69–76. [Google Scholar] [CrossRef]
- Landschoot, S.; De Reu, J.; Audenaert, K.; Vanhaverbeke, P.; Haesaert, G.; De Baets, B.; Waegeman, W. Potentials and limitations of existing forecasting models for Alternaria on potatoes: Challenges for model improvement. Potato Res. 2017, 60, 61–76. [Google Scholar] [CrossRef]
- Sands, P.J.; Hackett, C.; Nix, H.A. A model of the development and bulking of potatoes (Solanum tuberosum L.) I. Derivation from well-managed field crops. Field Crops Res. 1979, 2, 309–331. [Google Scholar] [CrossRef]
- Pscheidt, J.W.; Stevenson, W.R. Comparison of forecasting methods for control of potato early blight in Wisconsin. Plant Dis. 1986, 70, 915–920. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Engel, T. Simulation of phenological development of wheat crops. Agric. Syst. 1998, 58, 1–24. [Google Scholar] [CrossRef]
- Streck, N.A.; de Paula, F.L.M.; Bisognin, D.A.; Heldwein, A.B.; Dellai, J. Simulating the development of field grown potato (Solanum tuberosum L.). Agric. For. Meteorol. 2007, 142, 1–11. [Google Scholar] [CrossRef]
- Madden, L.; Pennypacker, S.P.; MacNab, A.A. FAST, a forecast system for Alternaria solani on tomato. Phytopathology 1978, 68, 1354–1358. [Google Scholar] [CrossRef] [Green Version]
- Gent, D.H.; Schwartz, H.F. Validation of potato early blight disease forecast models for Colorado using various sources of meteorological data. Plant Dis. 2003, 87, 78–84. [Google Scholar] [CrossRef]
- Olanya, O.M.; Honeycutt, C.W.; Larkin, R.P.; Griffin, T.S.; He, Z.; Halloran, J.M. The effect of cropping systems and irrigation management on development of potato early blight. Plant Pathol. J. 2009, 75, 367–375. [Google Scholar] [CrossRef]
- Iglesias, I.; Rodríguez-Rajo, F.J.; Méndez, J. Evaluation of the different Alternaria prediction models on a potato crop in A Limia (NW of Spain). Aerobiologia 2007, 23, 27–34. [Google Scholar] [CrossRef]
- Cowgill, W.P.; Maletta, M.H.; Manning, T.; Tietjen, W.H.; Johnston, S.A.; Nitzsche, P.J. Early blight forecasting systems: Evaluation, modification, and validation for use in fresh-market tomato production in northern New Jersey. HortScience 2005, 40, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Forsythe, W.C.; Rykiel, E.J.; Stahl, R.S.; Wu, H.I.; Schoolfield, R.M. A model comparison for daylength as a function of latitude and day of year. Ecol. Modell. 1995, 80, 87–95. [Google Scholar] [CrossRef]
- Pscheidt, J.W.; Stevenson, W.R. The critical period for control of early blight (Alternaria solani) of potato. Am. Potato J. 1988, 65, 425–438. [Google Scholar] [CrossRef]


| 2017 | 2018 | 2019 | 2020 | 2021 | |
|---|---|---|---|---|---|
| Planting | 22 April | 15 May | 16 May | 26 May | 19 May |
| End of senescence | 11 August | 17 September | 1 September | 31 August | 25 August |
| Model | Risk Units | Geographical Area | Ref. |
|---|---|---|---|
| Plant-based | |||
| P-Days | 300 | Wisconsin (USA) | [25] |
| South Alberta (Canada) | [21] | ||
| Colorado (USA) | [29] | ||
| Maine (USA) | [30] | ||
| Irán | [18] | ||
| 330 | Denmark | [4] | |
| 585–830 | Flanders (Belgium) | [23] | |
| GDDs | 361–417 | San Luis Valley (USA) | [19] |
| 625 | Irán | [18] | |
| 1000 | Wisconsin (USA) | [25] | |
| WE | 1 | Brazil | [27] |
| Pathogen-based | |||
| IWP | 6 | Spain | [31] |
| South Africa | [8] | ||
| TOMCAST | 10 | Spain | [11,12] |
| 15 | Maine (USA) | [30] | |
| 15–20–25 | Denmark | [4,17] | |
| 17 | South Alberta (Canada) | [21] | |
| 20 | Ontario (Canada) | [20] | |
| 20–25 | New Jersey (USA) | [32] | |
| Plant-pathogen-based | |||
| TOMCAST + Maturity | 25 + 330 | Denmark | [4] |
| Date (DAE *) | |||||
|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | |
| Emerging stage | 16 May (1) | 1 June (1) | 4 June (1) | 10 June (1) | 10 June (1) |
| Reproductive stage | 13 June (29) | 4 July (34) | 13 July (40) | 13 July (34) | 14 July (35) |
| Senescence stage | 19 July (65) | 15 August (76) | 17 August (75) | 14 August (66) | 7 August (59) |
| First symptoms | 21 June (38) | 8 July (38) | 13 July (40) | 16 July (37) | 17 July (38) |
| 2017 | 2018 | 2019 | 2020 | 2021 | Mean (CI) | |
|---|---|---|---|---|---|---|
| DAE of first symptoms | 37 | 38 | 40 | 37 | 38 | |
| Plant-based models | ||||||
| P-days | 260 | 290 | 265 | 257 | 281 | 271 (258–283) |
| GDDs | 413 | 385 | 351 | 393 | 376 | 384 (364–404) |
| WE model | 1.37 | 1.39 | 1.47 | 1.37 | 1.38 | 1.40 (1.36–1.43) |
| Pathogen-based models | ||||||
| IWP | 18 | 17 | 27 | 25 | 34 | 24 (18–29) |
| IWP (88%) | 31 | 34 | 35 | 32 | 32 | 33 (32–34) |
| TOMCAST | 17 | 29 | 18 | 10 | 34 | 22 (13–30) |
| Plant-pathogen-based model | ||||||
| TOMCAST + Maturity | 17 + 260 | 29 + 290 | 18 + 265 | 10 + 257 | 34 + 281 | 22 + 271 (13 + 258–30 + 283) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meno, L.; Abuley, I.K.; Escuredo, O.; Seijo, M.C. Suitability of Early Blight Forecasting Systems for Detecting First Symptoms in Potato Crops of NW Spain. Agronomy 2022, 12, 1611. https://doi.org/10.3390/agronomy12071611
Meno L, Abuley IK, Escuredo O, Seijo MC. Suitability of Early Blight Forecasting Systems for Detecting First Symptoms in Potato Crops of NW Spain. Agronomy. 2022; 12(7):1611. https://doi.org/10.3390/agronomy12071611
Chicago/Turabian StyleMeno, Laura, Isaac Kwesi Abuley, Olga Escuredo, and M. Carmen Seijo. 2022. "Suitability of Early Blight Forecasting Systems for Detecting First Symptoms in Potato Crops of NW Spain" Agronomy 12, no. 7: 1611. https://doi.org/10.3390/agronomy12071611
APA StyleMeno, L., Abuley, I. K., Escuredo, O., & Seijo, M. C. (2022). Suitability of Early Blight Forecasting Systems for Detecting First Symptoms in Potato Crops of NW Spain. Agronomy, 12(7), 1611. https://doi.org/10.3390/agronomy12071611









