Determination of Minimum Doses of Imazamox for Controlling Xanthium strumarium L. and Chenopodium album L. in Bean (Phaseolus vulgaris L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design and Data Collection
2.3. Data Analysis
3. Results and Discussions
3.1. Effect on Plant Heights
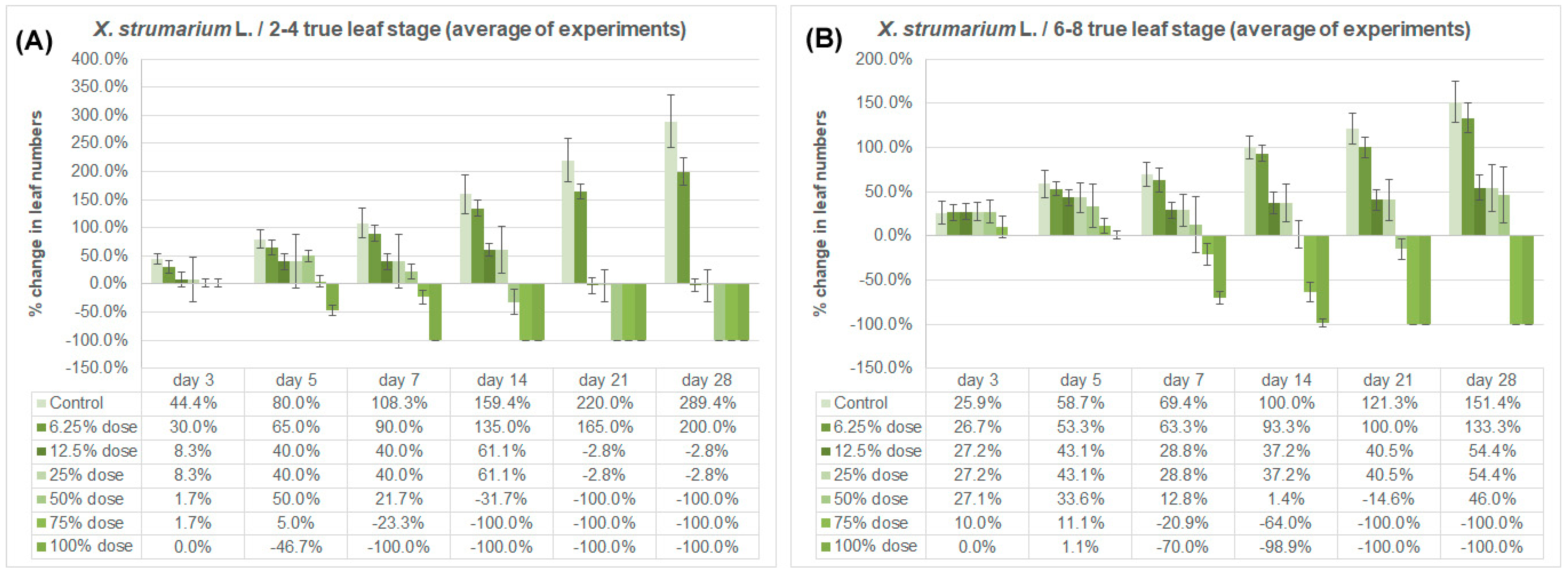
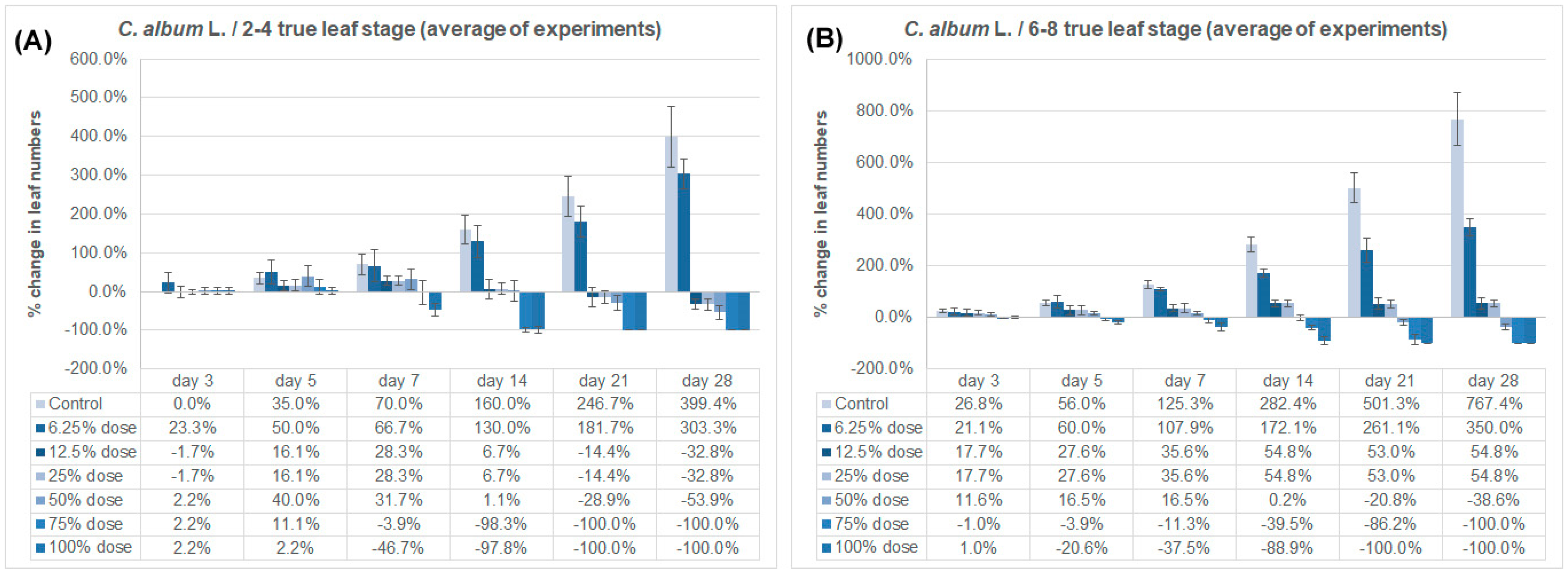
3.2. Effect on True Leaf Numbers
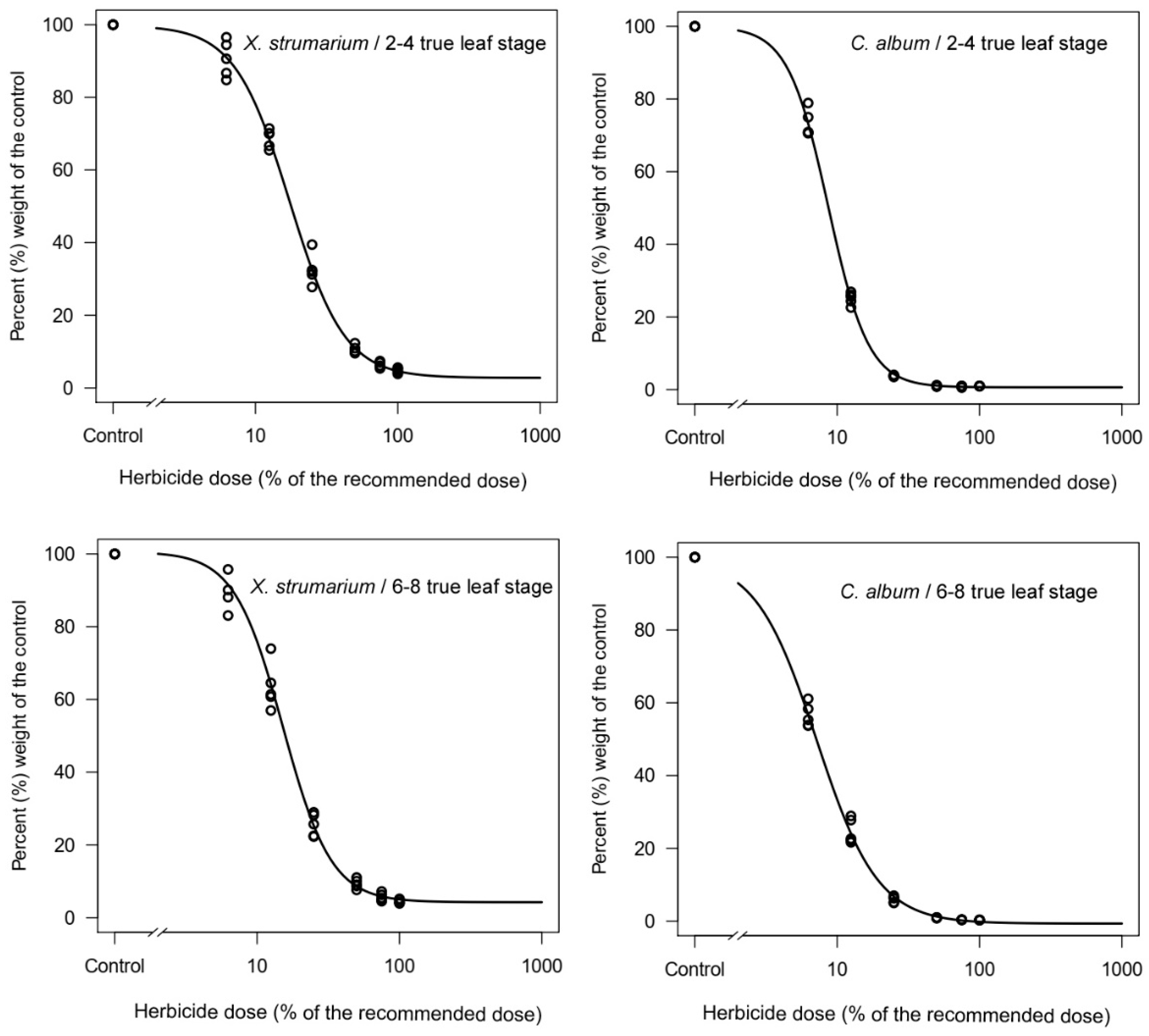
3.3. Four-Parameter Log-Logistic Model and Minimum Doses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Statistical Web Page. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 6 October 2021).
- Chaurasia, S. Green Beans. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; pp. 289–300. [Google Scholar]
- Stagnari, F.; Pisante, M. The critical period for weed competition in French bean (Phaseolus vulgaris L.) in Mediterranean areas. Crop Prot. 2011, 30, 179–184. [Google Scholar] [CrossRef]
- Hu, M.; Hou, N.; Li, Y.; Liu, Y.; Zhang, H.; Zeng, D.; Tan, H. The Effect of Microplastics on Behaviors of Chiral Imidazolinone Herbicides in the Aquatic Environment: Residue, Degradation and Distribution. J. Hazard. Mater. 2021, 418, 126176. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Wołejko, E.; Kaczyński, P.; Konecki, R.; Iwaniuk, P.; Drągowski, W.; Jablońska-Trypuć, A. Effect of microorganism on behaviour of two commonly used herbicides in wheat/soil system. Appl. Soil Ecol. 2021, 162, 103879. [Google Scholar] [CrossRef]
- Lamb, B.T.; McCrea, A.A.; Stoodley, S.H.; Dzialowski, A.R. Monitoring and water quality impacts of an herbicide treatment on an aquatic invasive plant in a drinking water reservoir. J. Environ. Manag. 2021, 288, 112444. [Google Scholar] [CrossRef]
- Kudsk, P.; Streibig, J.C. Herbicides—A two-edged sword. Weed Res. 2003, 43, 90–102. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Uygur, F.N. The effects of reduced doses and application timing of metribuzin on redroot pigweed (Amaranthus retroflexus L.) and wild mustard (Sinapis arvensis L.). Turk. J. Agric. For. 2010, 34, 467–474. [Google Scholar] [CrossRef]
- de Freitas Souza, M.; Lins, H.A.; de Mesquita, H.C.; da Silva Teófilo, T.M.; Reginaldo, L.T.R.T.; Pereira, R.K.V.; Silva, D.V. Can irrigation systems alter the critical period for weed control in onion cropping? Crop Prot. 2020, 147, 105457. [Google Scholar] [CrossRef]
- Chowdhury, I.F.; Doran, G.S.; Stodart, B.J.; Chen, C.; Wu, H. Trifluralin and Atrazine Sensitivity to Selected Cereal and Legume Crops. Agronomy 2020, 10, 587. [Google Scholar] [CrossRef]
- Voronichev, B.A.; Zadorin, A.M.; Titov, V.N.; Razumov, V.V.; Tolkacheva, M.A. On the issue of the possibility of expanding the range of herbicides for use in forage beans cenoses. Legumes Groat Crops 2020, 4, 78–85. [Google Scholar]
- Luqman, L.; Hussain, Z.; Ilyas, M.; Khan, I.A.; Bakht, T. Influence of sowing orientation and intercropping of chilies on onion yield and its associated weeds in Peshawar, Pakistan. Pak. J. Bot. 2020, 52, 95–100. [Google Scholar] [CrossRef]
- Oveisi, M.; Kaleibar, B.P.; Mashhadi, H.R.; Müller-Schärer, H.; Bagheri, A.; Amani, M.; Masoumi, D. Bean cultivar mixture allows reduced herbicide dose while maintaining high yield: A step towards more eco-friendly weed management. Eur. J. Agron. 2021, 122, 126173. [Google Scholar] [CrossRef]
- Swinton, S.M.; Buhler, D.D.; Forcella, F.; Gunsolus, J.L.; King, R.P. Estimation of crop yield loss due to interference by multiple weed species. Weed Sci. 1994, 42, 103–109. [Google Scholar] [CrossRef]
- Villette, S.; Maillot, T.; Guillemin, J.P.; Douzals, J.P. Simulation-aided study of herbicide patch spraying: Influence of spraying features and weed spatial distributions. Comput. Electron. Agric. 2021, 182, 105981. [Google Scholar] [CrossRef]
- Retta, A.; Vanderlip, R.L.; Higgins, R.A.; Moshier, L.J.; Feyerherm, A.M. Suitability of corn growth models for incorporation of weed and insect stresses. Agron. J. 1991, 83, 757–765. [Google Scholar] [CrossRef]
- Evans, S.P.; Knezevic, S.Z.; Lindquist, J.L.; Shapiro, C.A.; Blankenship, E.E. Nitrogen application influences the critical period for weed control in corn. Weed Sci. 2003, 51, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Weaver, S.E.; Hamill, A.S. Risks and Reliability of Using Herbicides at Below-Labeled Rates1. Weed Technol. 2000, 14, 106–115. [Google Scholar] [CrossRef]
- Monteiro, A.; Santos, S. Sustainable Approach to Weed Management: The Role of Precision Weed Management. Agronomy 2022, 12, 118. [Google Scholar] [CrossRef]
- Dogan, M.N.; Hurle, K. Influence of growth stage and some environmental factors after application on the effectiviness of the reduced doses of nicosulfuron on Amaranthus retroflexus L. Türkiye II. Bitki Koruma Kongr. 1997, 1, 99–106. [Google Scholar]
- Walker, S.R.; Medd, R.W.; Robinson, G.R.; Cullis, B.R. Improved management of Avena ludoviciana and Phalaris paradoxa with more densely sown wheat and less herbicide. Weed Res. 2002, 42, 257–270. [Google Scholar] [CrossRef]
- Auskalnis, A.; Kadzys, A. Effect of timing and dosage in herbicide application on weed biomass in spring wheat. Agron. Res. 2006, 4, 133–136. [Google Scholar]
- Barros, J.F.; Basch, G.; de Carvalho, M. Effect of reduced doses of a post-emergence herbicide to control grass and broad-leaved weeds in no-till wheat under Mediterranean conditions. Crop Prot. 2007, 26, 1538–1545. [Google Scholar] [CrossRef]
- Kahramanoglu, I. Assessment of pre-planting Pendimethalin’s minimum dose on redroot pigweed (Amaranthus retroflexus L.). Int. J. Agric. Sci. 2014, 4, 210–213. [Google Scholar]
- Zengin, H. Studies on weeds and their intensity, frequency and association in bean fields in Erzurum provinces. Turk. J. Agric. For. 1999, 23, 69–74. [Google Scholar]
- Zengin, H.; Çoruh, İ. Role of Two Irrigation Water Sources in Composing Weed Flora of Bean Fields in Erzincan Province. Türkiye Herboloji Derg. 2010, 13, 3–7. [Google Scholar]
- Abd El-Ghani, M.; Soliman, A.; Hamdy, R.; Bennoba, E. Weed flora in the reclaimed lands along the northern sector of the Nile Valley in Egypt. Turk. J. Bot. 2013, 37, 464–488. [Google Scholar] [CrossRef]
- CABI. Chenopodium Album (Fat Hen). Invasive Species Compendium. 2021. Available online: https://www.cabi.org/isc/datasheet/12648#tosummaryOfInvasiveness (accessed on 6 November 2021).
- Saeed, A.; Hussain, A.; Khan, M.I.; Arif, M.; Maqbool, M.M.; Mehmood, H.; Elshikh, M.S. The influence of environmental factors on seed germination of Xanthium strumarium L.: Implications for management. PLoS ONE 2020, 15, e0241601. [Google Scholar] [CrossRef] [PubMed]
- Fogliatto, S.; Serra, F.; Patrucco, L.; Milan, M.; Vidotto, F. Effect of Different Water Salinity Levels on the Germination of Imazamox-Resistant and Sensitive Weedy Rice and Cultivated Rice. Agronomy 2019, 9, 658. [Google Scholar]
- Beck, L.; Marsalis, M.; Lauriault, L.; Serena, M. Efficacy of Various Herbicides for the Control of Perennial Plantago spp. and Effects on Alfalfa Damage and Yield. Agronomy 2020, 10, 1710. [Google Scholar] [CrossRef]
- BKU. Aktif Madde Detay—40 G/L IMAZAMOX. 2021. Available online: https://bku.tarimorman.gov.tr/AktifMadde/Details/14?csrt=14601147293078804708&undefined=undefined (accessed on 6 November 2021).
- ADAMA. The Grower’s Guide to Imazamox. 2021. Available online: https://www.adama.com/canada/en/news/the-growers-guide-to-imazamox (accessed on 6 December 2021).
- Kaur, P.; Kaur, P.; Kaur, N.; Jain, D.; Singh, K.; Bhullar, M.S. Dissipation and phytotoxicity of imazethapyr and imazamox in soils amended with β-cyclodextrin-chitosan biocomposite. Sci. Total Environ. 2020, 735, 139566. [Google Scholar] [CrossRef]
- Soltani, N.; Nurse, R.E.; Van Eerd, L.L.; Vyn, R.J.; Shropshire, C.; Sikkema, P.H. Weed control, environmental impact and profitability with trifluralin plus reduced doses of imazethapyr in dry bean. Crop Prot. 2010, 29, 364–368. [Google Scholar] [CrossRef]
- Streibig, J.C.; Rudermo, M.; Jensen, J.E. Dose–Response Curves and Statistical Models. In Herbicide Bioassays; Streibig, J.C., Kudsk, P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 30–55. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defelice, S.L.; Sims, B.D. Integrating reduced doses of post emergence herbicides and cultivation for broadleaf weed control in soybeans (Glycine max). Weed Sci. 1990, 38, 541–545. [Google Scholar]
- Vitta, J.I.; Faccini, D.E.; Nisensohn, L.A. Control of Amaranthus quitensis in soybean crops in Argentina: An alternative to reduce herbicide use. Crop Prot. 2000, 19, 511–513. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Jaffer, I.; Khaliq, A. Reducing isoproturon dose in combination with Sorgaab for weed control in wheat. Pak. J. Weed Sci. Res. 2003, 9, 153–160. [Google Scholar]
- Hess, F.D.; Falk, R.H. Herbicide deposition on leaf surfaces. Weed Sci. 1990, 38, 280–288. [Google Scholar] [CrossRef]
- Chachalis, D.; Reddy, K.N.; Elmore, C.D.; Steele, M.L. Herbicide efficacy, leaf structure, and spray droplet contact angle among Ipomoea species and small flower morning glory. Weed Sci. 2001, 49, 628–634. [Google Scholar] [CrossRef]
- Sterling, T.M. Mechanisms of herbicide absorption across plant membranes and accumulation in plant cells. Weed Sci. 1994, 42, 263–276. [Google Scholar] [CrossRef]
- Kudsk, P.; Kristensen, J.L. Effect of environmental factors on herbicide performance. In Proceedings of the first international weed control congress, Melbourne, Australia, 17–21 February 1992; Weed Science Society of Victoria: Victoria, Australia, 1992; Volume 99, pp. 173–186. [Google Scholar]
- Brady, T.M.; Cross, B.; Doehner, R.F.; Finn, J.; Ladner, D.L. The Discovery of Imazamox, a New Broad-Spectrum Imidazolinone Herbicide. In Synthesis and Chemistry of Agrochemicals; Symposium Series; ACS Publications: Washington, DC, USA, 1998; pp. 30–37. [Google Scholar]
- Trezzi, M.M.; Alcántara-de la Cruz, R.; Rojano-Delgado, A.M.; Alcántara, E.; Pagnoncelli Jr, F.D.B.; Viecelli, M.; De Prado, R. Influence of temperature on the retention, absorption and translocation of fomesafen and imazamox in Euphorbia heterophylla. Pesticide Biochem. Physiol. 2021, 173, 104794. [Google Scholar] [CrossRef]
- Fipke, M.V.; Vidal, R.A. Influence of density and development stage of ryegrass on glyphosate effectiveness. Planta Daninha 2019, 37. [Google Scholar] [CrossRef] [Green Version]
- Panozzo, S.; Collavo, A.; Sattin, M. Sensitivity Analysis of Italian Lolium spp. to Glyphosate in Agricultural Environments. Plants 2020, 9, 165. [Google Scholar] [CrossRef] [Green Version]


| Weed Species/Growing Stage | Imazamox Doses | Plant Heights (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| X. strumarium/2–4 true leaf stage (1st experiment) | Control | 26.0 a | 28.0 a | 30.0 a | 31.0 a | 33.0 a | 35.0 a | 39.0 a |
| 25.00% dose | 20.0 b | 21.0 b | 22.0 bc | 23.0 bc | 18.0 b | 15.0 b | 15.0 b | |
| 50.00% dose | 24.0 ab | 25.0 ab | 26.0 ab | 26.0 ab | 21.0 b | 17.0 b | 15.0 b | |
| 75.00% dose | 19.0 b | 20.0 b | 21.0 bc | 21.0 bc | 20.0 b | 15.0 b | 13.0 b | |
| 100.00% dose | 20.0 b | 20.0 b | 18.0 c | 18.0 c | 15.0 b | 5.0 c | 5.0 c | |
| X. strumarium/2–4 true leaf stage (2nd experiment) | Control | 25.0 ab | 28.0 a | 29.0 a | 30.0 a | 32.0 a | 34.0 a | 38.0 a |
| 25.00% dose | 22.0 b | 23.0 ab | 23.0 ab | 25.0 abc | 18.0 b | 14.0 b | 16.0 b | |
| 50.00% dose | 22.0 b | 22.0 b | 24.0 ab | 27.0 ab | 20.0 b | 15.0 b | 15.0 b | |
| 75.00% dose | 22.0 b | 22.0 b | 22.0 b | 22.0 bc | 18.0 b | 12.0 bc | 10.0 bc | |
| 100.00% dose | 28.0 a | 28.0 a | 20.0 b | 19.0 c | 15.0 b | 6.0 c | 6.0 c | |
| X. strumarium/2–4 true leaf stage (3rd experiment) | Control | 25.2 a | 27.6 a | 29.0 a | 30.2 a | 32.2 a | 34.2 a | 38.0 a |
| 6.25% dose | 25.8 a | 26.6 b | 26.4 b | 27.4 b | 29.2 b | 31.0 b | 34.0 b | |
| 12.50% dose | 24.8 ab | 24.4 b | 24.0 bc | 23.0 c | 24.2 c | 26.0 c | 28.6 c | |
| 25.00% dose | 21.2 c | 21.6 c | 22.0 c | 23.6 c | 17.8 d | 14.0 d | 15.2 d | |
| 50.00% dose | 22.8 c | 23.2 bc | 24.6 bc | 26.4 b | 20.4 d | 15.8 d | 14.6 d | |
| 75.00% dose | 20.4 c | 20.6 c | 21.2 c | 21.2 cd | 18.6 d | 13.0 d | 11.0 e | |
| 100.00% dose | 23.6 bc | 23.6 bc | 18.8 d | 18.0 d | 14.4 e | 5.4 e | 5.4 f | |
| X. strumarium/6–8 true leaf stage (1st experiment) | Control | 38.0 a | 39.0 a | 40.0 a | 41.0 a | 43.0 a | 45.0 a | 49.0 a |
| 25.00% dose | 31.0 ab | 32.0 b | 32.0 b | 33.0 b | 34.0 b | 34.0 b | 34.0 b | |
| 50.00% dose | 30.0 b | 31.0 b | 32.0 b | 32.0 b | 31.0 b | 28.0 c | 24.0 c | |
| 75.00% dose | 31.0 ab | 32.0 b | 31.0 b | 24.0 c | 18.0 c | 14.0 d | 10.0 d | |
| 100.00% dose | 33.0 ab | 34.0 ab | 30.0 b | 25.0 c | 17.0 c | 12.0 d | 7.0 d | |
| X. strumarium/6–8 true leaf stage (2nd experiment) | Control | 35.0 a | 36.0 a | 41.0 a | 42.0 a | 43.0 a | 45.0 a | 47.0 a |
| 25.00% dose | 28.0 a | 28.5 a | 28.5 b | 30.0 b | 32.0 b | 34.0 b | 34.0 b | |
| 50.00% dose | 28.0 a | 29.0 a | 29.0 b | 29.0 b | 28.0 b | 27.0 b | 23.0 c | |
| 75.00% dose | 30.0 a | 31.0 a | 31.0 b | 24.0 b | 17.0 c | 13.0 c | 10.0 d | |
| 100.00% dose | 35.0 a | 36.0 a | 30.0 b | 24.0 b | 16.0 c | 11.0 c | 6.0 d | |
| X. strumarium/6-8 true leaf stage (3rd experiment) | Control | 37.0 a | 37.8 a | 40.6 a | 41.6 a | 43.0 a | 45.0 a | 48.2 a |
| 6.25% dose | 36.8 a | 36.2 a | 37.6 b | 38.6 b | 40.0 b | 42.0 ab | 45.0 ab | |
| 12.50% dose | 37.8 a | 37.6 a | 37.8 b | 38.6 b | 39.4 b | 40.8 b | 43.0 b | |
| 25.00% dose | 29.8 b | 30.4 b | 30.4 c | 31.6 c | 33.4 c | 34.4 c | 34.4 c | |
| 50.00% dose | 29.4 b | 30.2 b | 30.8 c | 30.8 c | 29.6 c | 27.6 d | 23.8 d | |
| 75.00% dose | 30.8 b | 31.8 b | 31.4 c | 24.4 d | 17.8 d | 13.6 e | 10.0 e | |
| 100.00% dose | 34.2 ab | 35.2 ab | 30.2 c | 24.6 d | 16.6 d | 11.8 e | 6.8 f | |
| Weed Species/Growing Stage | Imazamox Doses | Plant Heights (cm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| C. album/2–4 true leaf stage (1st experiment) | Control | 2.9 a | 3.2 a | 3.5 a | 4.0 a | 5.1 a | 7.0 a | 11.1 a |
| 25.00% dose | 2.4 b | 2.6 bc | 2.8 b | 3.0 b | 3.0 b | 3.0 b | 3.0 b | |
| 50.00% dose | 2.2 b | 2.3 c | 2.4 c | 2.4 c | 2.2 c | 2.2 c | 2.2 c | |
| 75.00% dose | 2.4 b | 2.5 c | 2.5 bc | 2.5 c | 2.0 c | 1.9 c | 1.9 c | |
| 100.00% dose | 2.9 a | 2.9 ab | 2.8 b | 2.7 bc | 2.2 c | 2.2 c | 2.2 c | |
| C. album/2–4 true leaf stage (2nd experiment) | Control | 2.9 a | 3.1 a | 3.4 a | 3.8 a | 5.0 a | 7.0 a | 11.1 a |
| 25.00% dose | 2.4 b | 2.6 bc | 2.8 b | 3.0 b | 3.0 b | 3.0 b | 3.0 b | |
| 50.00% dose | 2.2 b | 2.3 d | 2.4 c | 2.4 c | 2.1 c | 2.1 c | 2.1 c | |
| 75.00% dose | 2.4 b | 2.4 cd | 2.5 c | 2.4 c | 2.0 c | 2.0 c | 2.0 c | |
| 100.00% dose | 2.8 a | 2.9 ab | 2.9 b | 2.8 bc | 2.2 c | 2.2 c | 2.2 c | |
| C. album/2–4 true leaf stage (3rd experiment) | Control | 2.7 a | 2.9 a | 3.3 a | 3.7 a | 4.9 a | 6.8 a | 10.9 a |
| 6.25% dose | 2.6 a | 2.7 a | 2.8 b | 3.2 b | 4.1 b | 5.8 b | 9.3 b | |
| 12.50% dose | 2.5 a | 2.5 a | 2.8 b | 3.2 b | 4.1 b | 5.4 b | 4.9 c | |
| 25.00% dose | 2.2 b | 2.4 ab | 2.6 b | 2.8 bc | 2.8 c | 2.8 c | 2.8 d | |
| 50.00% dose | 2.0 b | 2.1 b | 2.2 c | 2.2 c | 1.9 d | 1.9 d | 1.9 e | |
| 75.00% dose | 2.2 b | 2.3 b | 2.3 c | 2.3 c | 1.8 d | 1.7 d | 1.7 e | |
| 100.00% dose | 2.6 a | 2.7 a | 2.7 b | 2.5 c | 2.0 d | 2.0 d | 2.0 e | |
| C. album/6–8 true leaf stage (1st experiment) | Control | 6.0 a | 6.5 a | 7.5 a | 9.6 a | 16.0 a | 22.0 a | 32.0 a |
| 25.00% dose | 4.0 b | 5.0 b | 5.1 bc | 4.5 bc | 4.5 b | 4.0 b | 3.0 b | |
| 50.00% dose | 5.7 ab | 5.9 ab | 5.6 b | 5.0 b | 4.0 b | 3.0 b | 2.0 b | |
| 75.00% dose | 5.0 ab | 5.2 ab | 4.8 bc | 4.8 b | 2.0 c | 0.0 c | 0.0 c | |
| 100.00% dose | 5.1 ab | 5.1 ab | 4.0 c | 3.2 c | 0.0 d | 0.0 c | 0.0 c | |
| C. album/6–8 true leaf stage (2nd experiment) | Control | 8.0 a | 9.6 a | 12.0 a | 16.0 a | 25.6 a | 28.4 a | 35.0 a |
| 25.00% dose | 7.2 bc | 8.0 b | 8.0 b | 8.0 b | 8.0 b | 8.0 b | 8.0 b | |
| 50.00% dose | 6.8 bc | 6.8 bc | 6.8 b | 6.8 b | 6.8 b | 6.8 b | 6.6 b | |
| 75.00% dose | 6.4 c | 6.4 c | 6.4 b | 6.4 b | 6.4 b | 6.4 b | 6.4 b | |
| 100.00% dose | 8.0 a | 8.0 b | 8.0 b | 8.0 b | 8.0 b | 8.0 b | 8.0 b | |
| C. album/6–8 true leaf stage (3rd experiment) | Control | 7.2 a | 8.4 a | 9.8 a | 13.0 a | 20.8 | 25.6 a | 33.8 a |
| 6.25% dose | 7.2 a | 7.4 b | 8.8 b | 11.0 b | 17.8 a | 21.6 b | 28.8 b | |
| 12.50% dose | 7.2 a | 6.8 c | 7.8 c | 7.4 c | 9.4 b | 10.2 c | 10.8 c | |
| 25.00% dose | 5.6 b | 6.8 c | 6.8 d | 6.4 c | 6.4 c | 6.2 d | 5.8 d | |
| 50.00% dose | 6.6 ab | 6.6 c | 6.4 d | 6.0 c | 5.6 c | 5.2 d | 4.4 d | |
| 75.00% dose | 6.2 b | 6.2 d | 6.2 d | 6.2 c | 4.4 c | 3.2 d | 3.2 d | |
| 100.00% dose | 7.0 a | 7.0 c | 6.0 e | 6.0 c | 4.0 c | 4.0 d | 4.0 d | |
| Weed Species/Growing Stage | Imazamox Doses | Leaf Numbers | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| X. strumarium/2–4 true leaf stage (1st experiment) | Control | 4.0 a | 6.0 a | 7.0 a | 8.0 a | 10.0 a | 12.4 a | 14.8 a |
| 25.00% dose | 3.6 a | 3.6 b | 4.8 c | 4.8 b | 5.6 b | 3.0 b | 3.0 b | |
| 50.00% dose | 4.0 a | 4.0 b | 6.0 b | 4.8 b | 3.0 c | 0.0 c | 0.0 c | |
| 75.00% dose | 4.0 a | 4.0 b | 4.0 d | 3.0 c | 0.0 d | 0.0 c | 0.0 c | |
| 100.00% dose | 4.0 a | 4.0 b | 2.0 e | 0.0 d | 0.0 d | 0.0 c | 0.0 c | |
| X. strumarium/2–4 true leaf stage (2nd experiment) | Control | 3.6 a | 5.0 a | 6.8 a | 8.0 a | 10.0 a | 12.0 a | 15.0 a |
| 25.00% dose | 3.6 a | 3.6 b | 4.6 b | 4.6 b | 5.6 b | 4.0 b | 4.0 b | |
| 50.00% dose | 4.0 a | 4.0 b | 6.0 a | 5.0 b | 2.0 c | 0.0 c | 0.0 c | |
| 75.00% dose | 4.0 a | 4.0 b | 4.4 b | 3.0 c | 0.0 d | 0.0 c | 0.0 c | |
| 100.00% dose | 4.0 a | 4.0 b | 2.4 c | 0.0 d | 0.0 d | 0.0 c | 0.0 c | |
| X. strumarium/2–4 true leaf stage (3rd experiment) | Control | 4.0 a | 5.8 a | 7.0 a | 8.0 a | 9.8 a | 12.4 a | 15.0 a |
| 6.25% dose | 4.0 a | 5.2 a | 6.6 b | 7.6 a | 9.4 a | 10.6 b | 12.0 b | |
| 12.50% dose | 4.0 a | 4.6 b | 5.6 c | 6.6 b | 7.2 b | 7.4 c | 7.2 c | |
| 25.00% dose | 3.6 a | 3.8 b | 4.8 c | 4.8 c | 5.4 c | 3.0 d | 3.0 d | |
| 50.00% dose | 4.0 a | 4.2 b | 6.0 b | 4.8 c | 3.2 d | 0.0 e | 0.0 e | |
| 75.00% dose | 4.0 a | 4.2 b | 4.2 cd | 3.2 d | 0.0 e | 0.0 e | 0.0 e | |
| 100.00% dose | 4.0 a | 4.0 b | 2.0 d | 0.0 e | 0.0 e | 0.0 e | 0.0 e | |
| X. strumarium/6–8 true leaf stage (1st experiment) | Control | 7.0 a | 8.0 a | 10.0 a | 12.0 a | 14.0 a | 15.2 a | 16.4 a |
| 25.00% dose | 7.0 a | 8.0 a | 9.0 a | 8.0 b | 8.6 b | 8.6 b | 9.0 b | |
| 50.00% dose | 7.0 a | 8.0 a | 7.0 b | 5.0 c | 6.0 c | 6.4 c | 7.2 c | |
| 75.00% dose | 7.0 a | 7.0 ab | 7.0 b | 5.0 c | 2.0 d | 0.0 d | 0.0 d | |
| 100.00% dose | 6.0 a | 6.0 b | 6.0 b | 2.0 d | 0.0 e | 0.0 d | 0.0 d | |
| X. strumarium/6–8 true leaf stage (2nd experiment) | Control | 6.0 a | 8.0 a | 10.0 a | 10.0 a | 12.0 a | 13.4 a | 15.6 a |
| 25.00% dose | 6.0 a | 8.0 a | 9.0 a | 8.0 b | 8.4 b | 9.0 b | 10.0 b | |
| 50.00% dose | 6.0 a | 8.0 a | 9.0 a | 8.0 b | 6.4 c | 5.0 c | 10.0 b | |
| 75.00% dose | 6.0 a | 7.0 ab | 7.0 b | 5.0 c | 2.4 d | 0.0 d | 0.0 c | |
| 100.00% dose | 6.0 a | 6.0 b | 6.0 b | 1.6 d | 0.0 e | 0.0 d | 0.0 c | |
| X. strumarium/6–8 true leaf stage (3rd experiment) | Control | 6.0 a | 7.8 a | 10.0 a | 10.2 a | 12.0 a | 13.4 a | 15.6 a |
| 6.25% dose | 6.0 a | 7.6 a | 9.2 b | 9.8 a | 11.6 a | 12.0 b | 14.0 a | |
| 12.50% dose | 6.0 a | 7.4 a | 8.4 c | 8.6 b | 9.0 b | 10.0 c | 10.8 b | |
| 25.00% dose | 6.0 a | 8.0 a | 9.0 b | 8.2 b | 8.8 b | 8.8 d | 10.0 b | |
| 50.00% dose | 6.0 a | 8.0 a | 9.0 b | 8.0 b | 6.6 c | 4.8 e | 10.0 b | |
| 75.00% dose | 6.0 a | 6.8 a | 7.0 d | 5.0 c | 2.4 d | 0.0 f | 0.0 c | |
| 100.00% dose | 6.0 a | 6.0 a | 6.2 d | 1.8 d | 0.2 e | 0.0 f | 0.0 c | |
| Weed Species/Growing Stage | Imazamox Doses | Leaf Numbers | ||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| C. album/2–4 true leaf stage (1st experiment) | Control | 3.6 a | 3.6 a | 4.8 b | 6.0 a | 9.2 a | 12.0 a | 17.2 a |
| 25.00% dose | 3.6 a | 3.6 a | 4.0 b | 4.6 b | 3.6 b | 3.0 b | 2.6 b | |
| 50.00% dose | 4.0 a | 4.0 a | 6.0 a | 5.4 ab | 4.2 b | 3.0 b | 2.0 b | |
| 75.00% dose | 4.0 a | 4.0 a | 4.4 b | 4.4 b | 0.0 c | 0.0 c | 0.0 c | |
| 100.00% dose | 4.0 a | 4.0 a | 4.0 b | 2.0 c | 0.0 c | 0.0 c | 0.0 c | |
| C. album/2–4 true leaf stage (2nd experiment) | Control | 3.6 a | 3.6 a | 4.8 ab | 6.0 a | 9.0 a | 12.4 a | 17.0 a |
| 25.00% dose | 4.0 a | 4.0 a | 5.0 a | 5.4 a | 5.0 b | 4.0 b | 2.6 b | |
| 50.00% dose | 3.6 a | 3.6 a | 4.0 b | 4.0 b | 3.0 c | 2.2 c | 1.2 bc | |
| 75.00% dose | 4.0 a | 4.0 a | 4.0 b | 2.6 c | 0.0 d | 0.0 d | 0.0 c | |
| 100.00% dose | 4.0 a | 4.0 a | 4.0 b | 2.0 c | 0.0 d | 0.0 d | 0.0 c | |
| C. album/2–4 true leaf stage (3rd experiment) | Control | 3.4 b | 3.4 b | 4.6 b | 5.8 a | 9.0 a | 11.8 a | 17.8 a |
| 6.25% dose | 3.8 a | 4.6 a | 5.6 a | 6.2 a | 8.6 a | 10.6 b | 15.2 b | |
| 12.50% dose | 3.6 a | 3.8 a | 4.4 b | 5.0 b | 4.6 b | 3.4 c | 2.8 c | |
| 25.00% dose | 3.8 a | 3.6 ab | 4.2 b | 4.6 b | 3.6 b | 2.8 c | 2.4 c | |
| 50.00% dose | 3.8 a | 4.0 a | 5.8 a | 5.4 ab | 4.2 b | 2.8 c | 2.0 c | |
| 75.00% dose | 3.8 a | 4.0 a | 4.6 b | 4.2 bc | 0.2 c | 0.0 d | 0.0 d | |
| 100.00% dose | 3.8 a | 4.0 a | 4.0 b | 2.2 c | 0.2 c | 0.0 d | 0.0 d | |
| C. album/6–8 true leaf stage (1st experiment) | Control | 7.6 a | 9.6 a | 11.8 a | 17.0 a | 28.8 a | 45.2 a | 65.0 a |
| 25.00% dose | 6.4 ab | 7.8 b | 7.8 b | 9.2 b | 10.2 b | 9.2 b | 9.2 b | |
| 50.00% dose | 6.0 b | 6.5 bc | 7.0 b | 7.0 c | 6.0 c | 5.0 c | 4.0 c | |
| 75.00% dose | 7.2 ab | 7.2 bc | 7.4 b | 7.4 bc | 4.0 cd | 3.0 c | 0.0 d | |
| 100.00% dose | 6.0 b | 6.0 c | 5.0 c | 5.0 d | 2.0 d | 0.0 d | 0.0 d | |
| C. album/6–8 true leaf stage (2nd experiment) | Control | 7.6 ab | 9.6 a | 11.8 a | 17.0 a | 28.8 a | 45.2 a | 65.0 a |
| 25.00% dose | 6.8 ab | 7.9 bc | 9.2 b | 10.0 b | 11.1 b | 11.0 b | 11.0 b | |
| 50.00% dose | 6.8 ab | 7.8 bc | 8.0 b | 8.0 c | 6.9 c | 5.4 c | 4.0 c | |
| 75.00% dose | 6.4 b | 6.4 c | 6.0 c | 5.4 d | 4.2 d | 0.0 d | 0.0 d | |
| 100.00% dose | 8.0 a | 8.0 b | 6.0 c | 4.0 d | 0.0 e | 0.0 d | 0.0 d | |
| C. album/6–8 true leaf stage (3rd experiment) | Control | 7.6 a | 9.6 a | 11.8 a | 17.0 a | 28.8 a | 45.2 a | 65.0 a |
| 6.25% dose | 7.8 a | 9.4 a | 12.4 a | 16.2 a | 21.2 b | 28.0 b | 35.0 b | |
| 12.50% dose | 7.6 a | 8.6 b | 9.8 b | 11.0 b | 13.8 c | 15.2 c | 17.2 c | |
| 25.00% dose | 7.0 a | 8.0 b | 8.6 b | 8.0 c | 9.8 d | 10.6 d | 11.0 d | |
| 50.00% dose | 7.0 a | 7.8 bc | 8.0 b | 8.0 c | 6.9 e | 5.2 e | 4.1 e | |
| 75.00% dose | 6.6 a | 6.4 c | 6.0 c | 5.2 d | 4.0 f | 0.0 f | 0.0 f | |
| 100.00% dose | 7.8 a | 8.0 b | 6.2 c | 4.2 d | 0.0 g | 0.0 f | 0.0 f | |
| Weed Species | Parameters | ED90 | p-Value | |||
|---|---|---|---|---|---|---|
| C | D | b | ED50 | |||
| X. strumarium/2–4 true leaf stage | 0.23 | 8.41 | 2.25 | 17.36 | 46.18 | 0.9487 ns |
| X. strumarium/6–8 true leaf stage | 0.79 | 19.21 | 2.52 | 15.10 | 36.11 | 0.9157 ns |
| C. album/2–4 true leaf stage | 0.00 | 1.02 | 3.07 | 8.66 | 17.69 | 0.9336 ns |
| C. album/6–8 true leaf stage | −0.01 | 2.95 | 2.03 | 7.17 | 21.21 | 0.7314 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gürbüz, R.; Yentürk, Ö. Determination of Minimum Doses of Imazamox for Controlling Xanthium strumarium L. and Chenopodium album L. in Bean (Phaseolus vulgaris L.). Agronomy 2022, 12, 1557. https://doi.org/10.3390/agronomy12071557
Gürbüz R, Yentürk Ö. Determination of Minimum Doses of Imazamox for Controlling Xanthium strumarium L. and Chenopodium album L. in Bean (Phaseolus vulgaris L.). Agronomy. 2022; 12(7):1557. https://doi.org/10.3390/agronomy12071557
Chicago/Turabian StyleGürbüz, Ramazan, and Ömer Yentürk. 2022. "Determination of Minimum Doses of Imazamox for Controlling Xanthium strumarium L. and Chenopodium album L. in Bean (Phaseolus vulgaris L.)" Agronomy 12, no. 7: 1557. https://doi.org/10.3390/agronomy12071557
APA StyleGürbüz, R., & Yentürk, Ö. (2022). Determination of Minimum Doses of Imazamox for Controlling Xanthium strumarium L. and Chenopodium album L. in Bean (Phaseolus vulgaris L.). Agronomy, 12(7), 1557. https://doi.org/10.3390/agronomy12071557







