Boron Effects on Fruit Set, Yield, Quality and Paternity of Hass Avocado
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Sample Collection
2.3. Paternity Analysis
2.4. Mineral Nutrient Analysis
2.5. Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
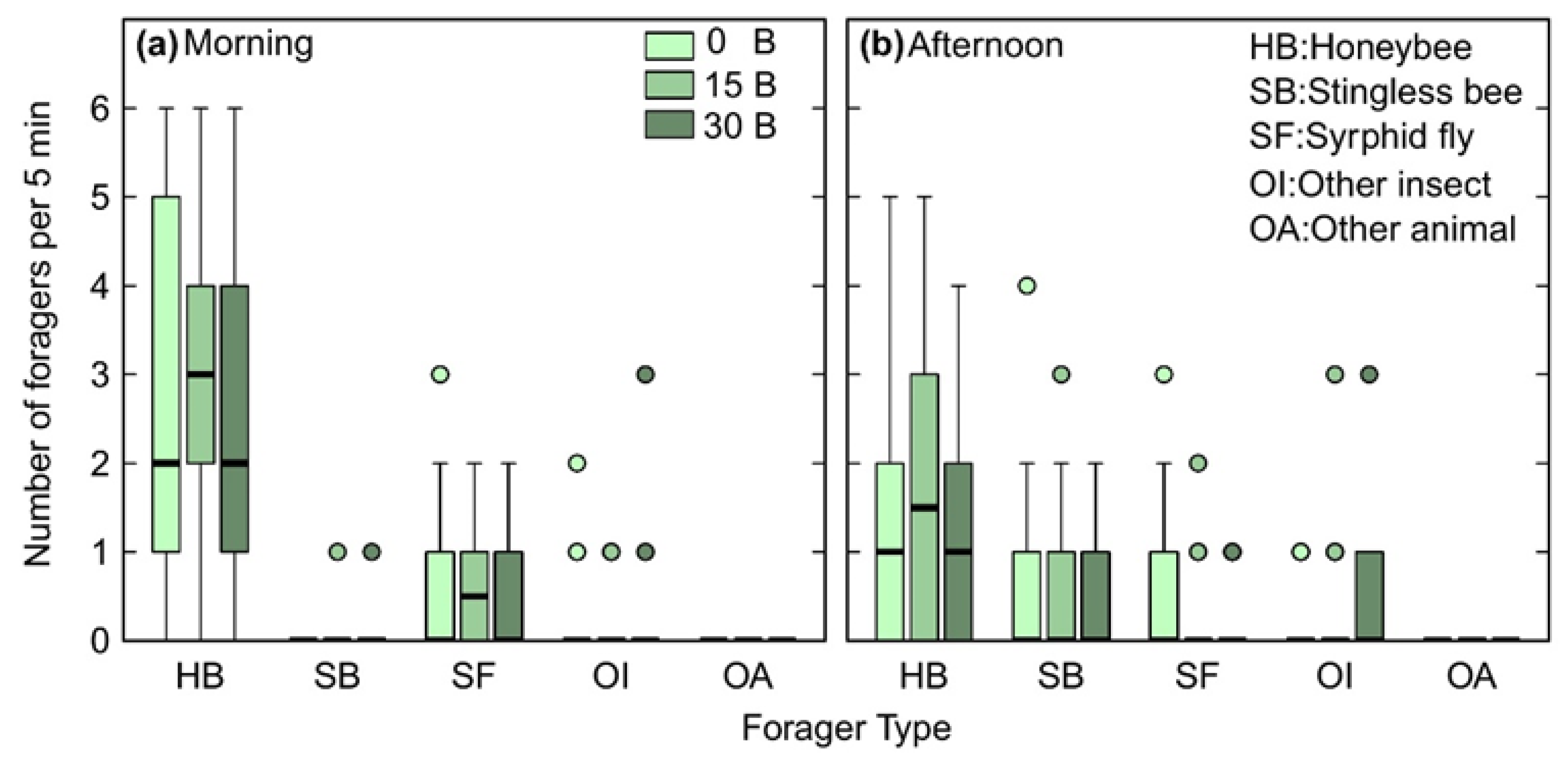
3.1. Flower Visitors
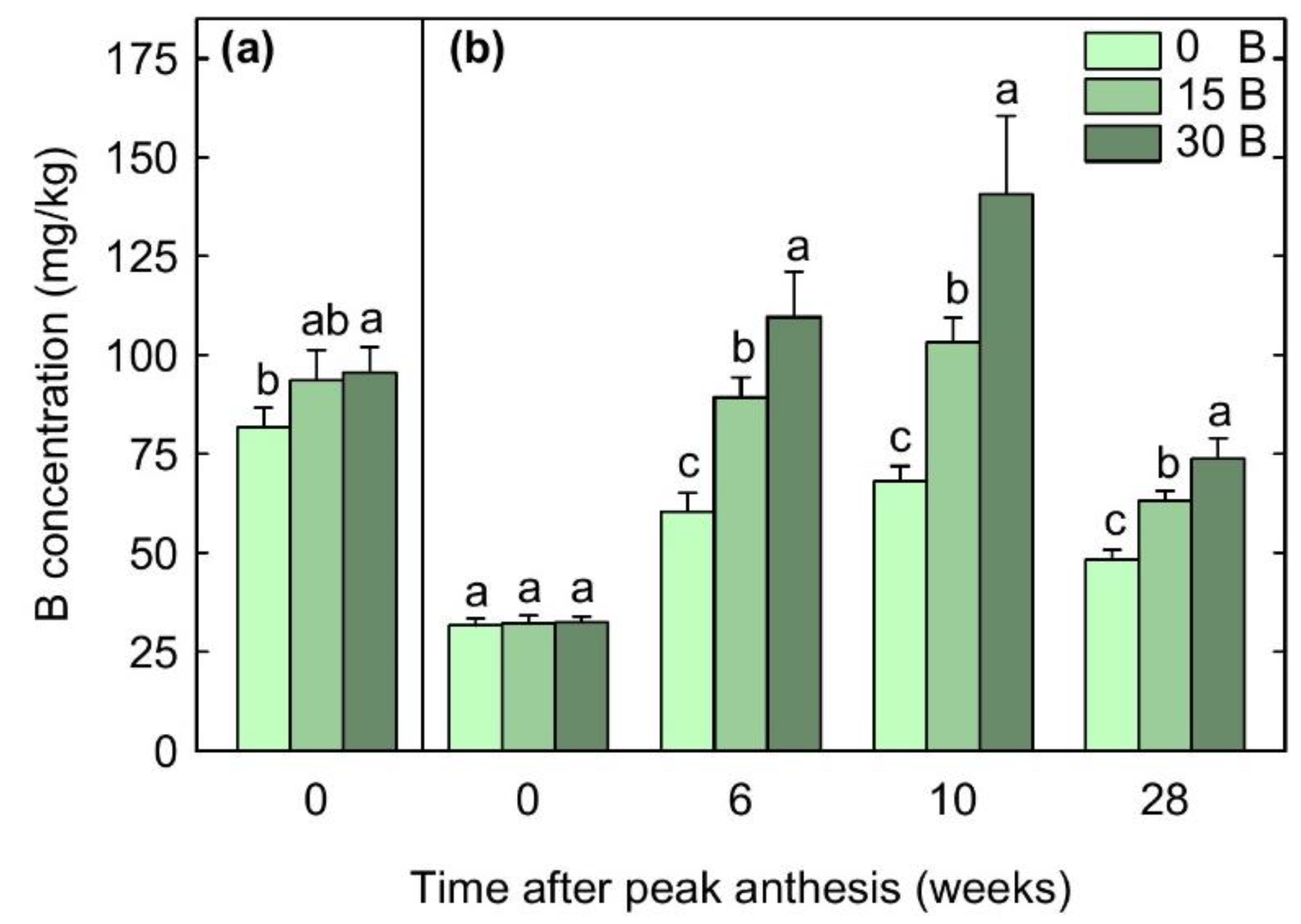
3.2. Floral and Foliar Nutrient Concentrations
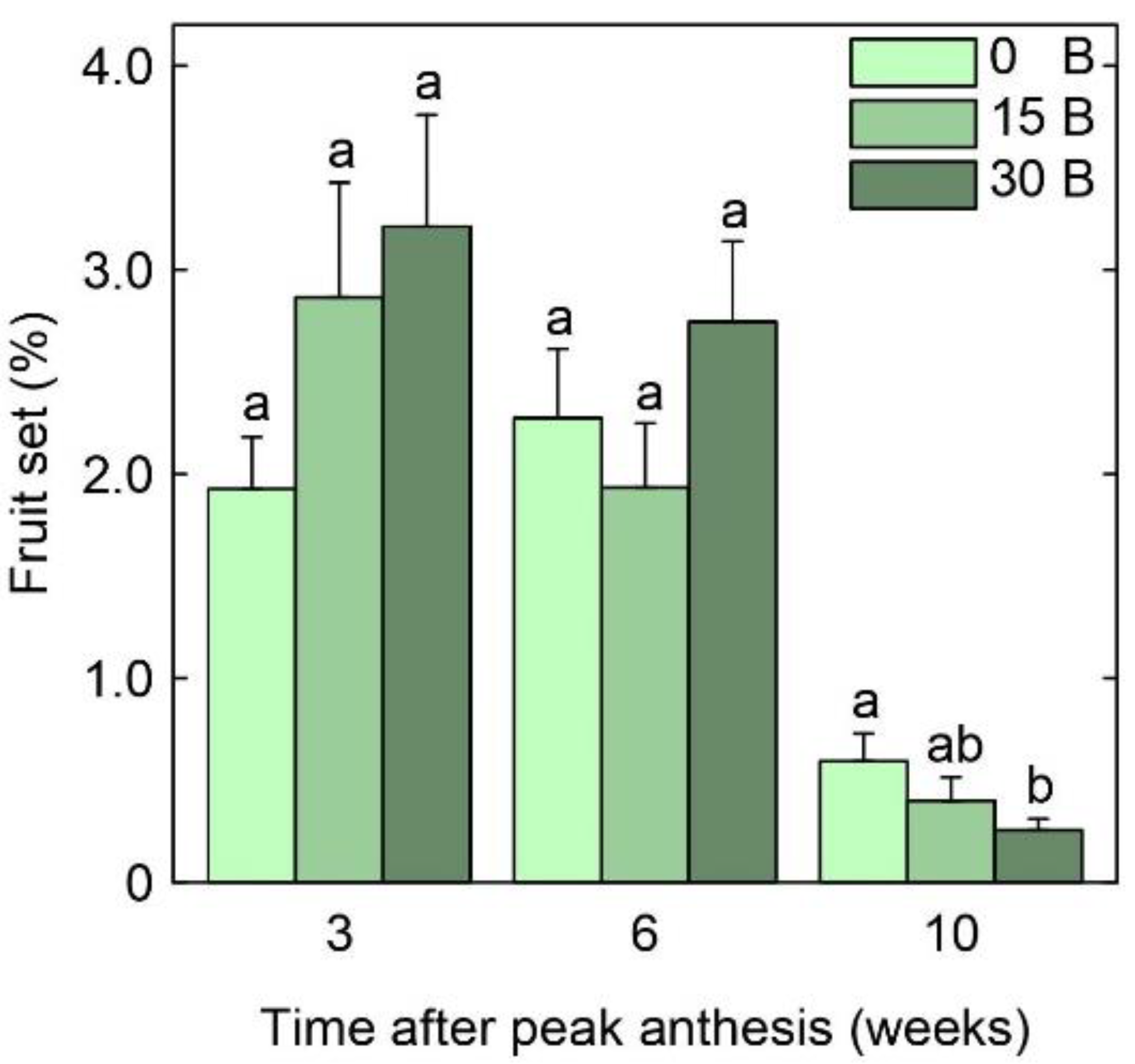
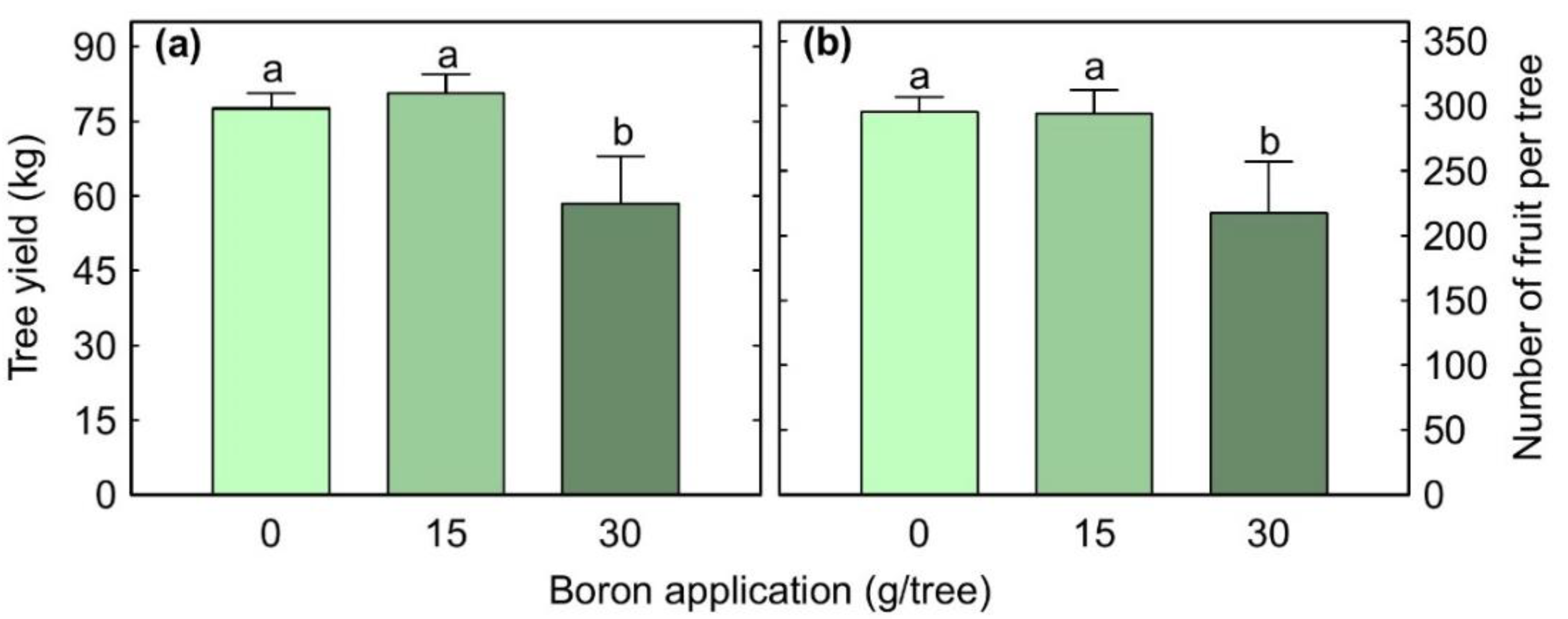
3.3. Fruit Set and Yield
3.4. Levels of Self- and Cross-Paternity
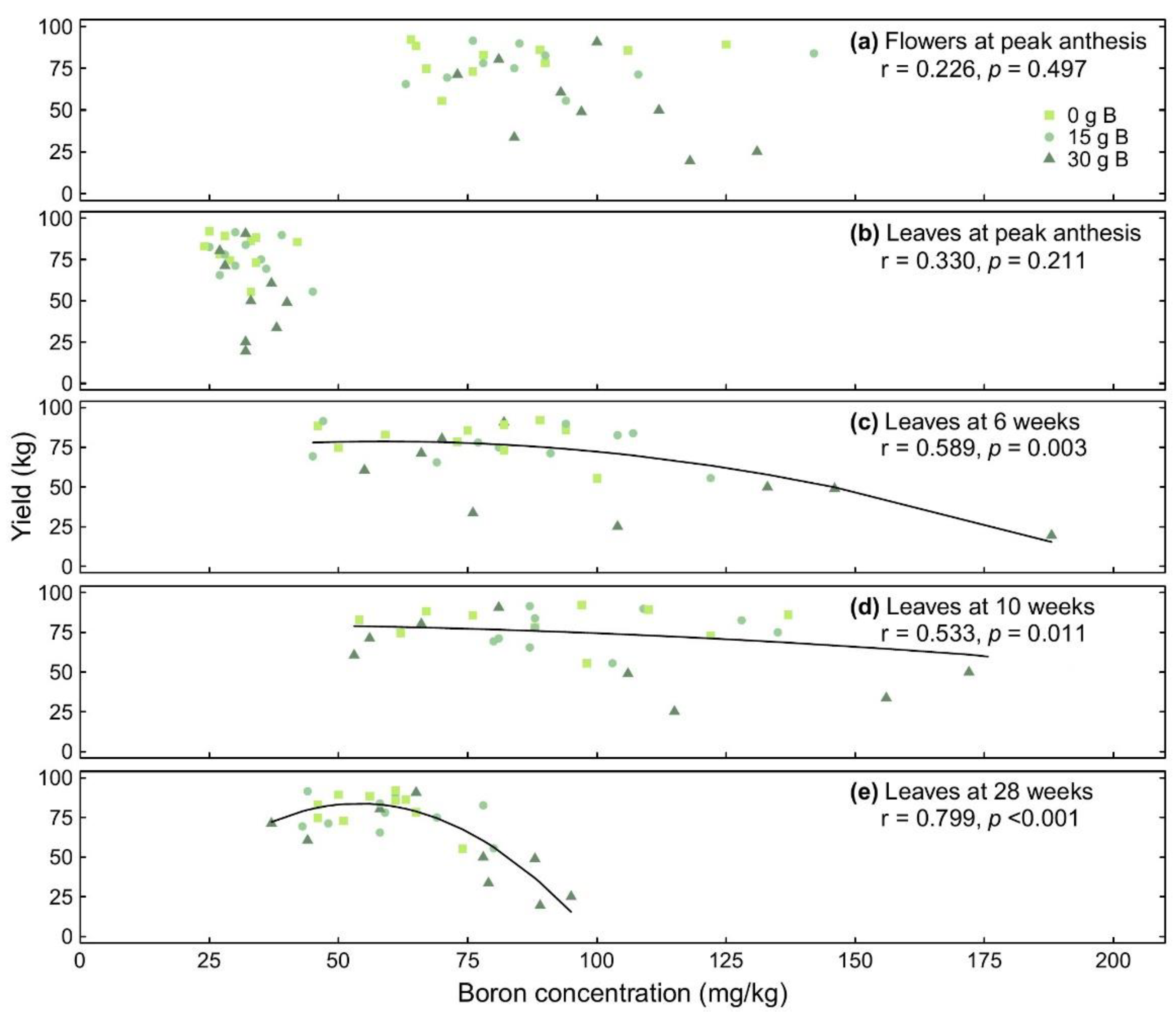
3.5. Relationships between Yield and Boron Concentrations
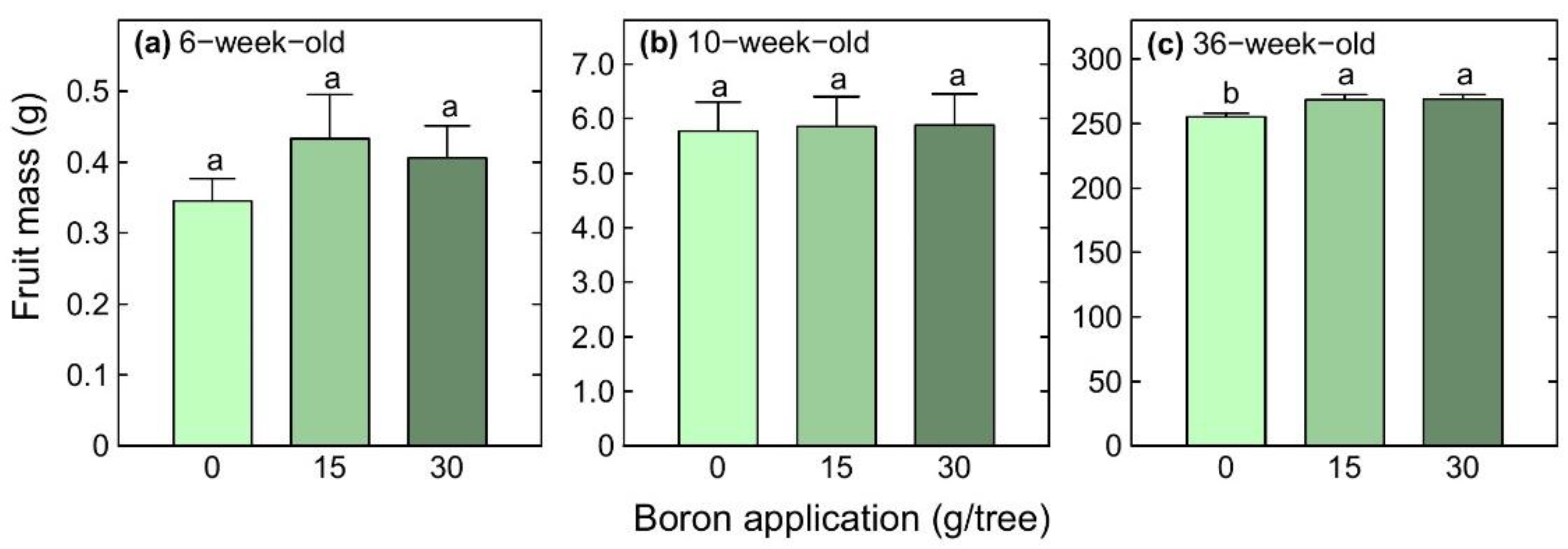
3.6. Fruit Size
3.7. Mineral Nutrient Concentrations
3.8. Fatty Acid Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- International Nut and Dried Fruit Council. Tree Nut and Dried Fruit Production to Add Up to 4.5 Million and 3.3 Million Metric Tons, Respectively. Available online: https://www.nutfruit.org/industry/news/detail/tree-nut-and-dried-fruit-productions-to-add-up-to-4-5-million-and-3-3-million-metric-tons-respectively (accessed on 24 September 2021).
- Food and Agriculture Organisation (FAO). Fruit and Vegetables—Your Dietary Essentials. The International Year of Fruits and Vegetables, 2021, Background Paper; Food and Agriculture Organisation: Rome, Italy, 2020. [Google Scholar]
- Statista. Global Fruit Production in 2020, by Selected Variety. Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 28 April 2022).
- Guerra, M.E.; López-Corrales, M.; Wünsch, A.; Rodrigo, J. Evaluation of the reproductive process as the cause for low fruit set in two Japanese plum cultivars. Acta Hortic. 2012, 932, 37–42. [Google Scholar] [CrossRef]
- Brunetto, G.; Melo, G.W.B.D.; Toselli, M.; Quartieri, M.; Tagliavini, M. The role of mineral nutrition on yields and fruit quality in grapevine, pear and apple. Rev. Bras. Frutic. 2015, 37, 1089–1104. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. An improved fruit transcriptome and the identification of the candidate genes involved in fruit abscission induced by carbohydrate stress in litchi. Front. Plant Sci. 2015, 6, 439. [Google Scholar] [CrossRef] [Green Version]
- Garner, L.C.; Lovatt, C.J. Physiological factors affecting flower and fruit abscission of ‘Hass’ avocado. Sci. Hortic. 2016, 199, 32–40. [Google Scholar] [CrossRef]
- Pathak, T.B.; Maskey, M.L.; Dahlberg, J.A.; Kearns, F.; Bali, K.M.; Zaccaria, D. Climate change trends and impacts on California agriculture: A detailed review. Agronomy 2018, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Katayama, N.; Bouam, I.; Koshida, C.; Baba, Y.G. Biodiversity and yield under different land-use types in orchard/vineyard landscapes: A meta-analysis. Biol. Conserv. 2019, 229, 125–133. [Google Scholar] [CrossRef]
- Trueman, S.J.; Kämper, W.; Nichols, J.; Ogbourne, S.M.; Hawkes, D.; Peters, T.; Hosseini Bai, S.; Wallace, H.M. Pollen limitation and xenia effects in a cultivated mass-flowering tree, Macadamia integrifolia (Proteaceae). Ann. Bot. 2022, 129, 135–146. [Google Scholar] [CrossRef]
- Gibbs, P.E. Late-acting self-incompatibility—The pariah breeding system in flowering Plants. New Phytol. 2014, 203, 717–734. [Google Scholar] [CrossRef]
- Christopher, D.A.; Mitchell, R.J.; Karron, J.D. Pollination intensity and paternity in flowering plants. Ann. Bot. 2020, 125, 1–9. [Google Scholar] [CrossRef]
- Sedgley, M.; Griffin, A.R. Sexual Reproduction of Tree Crops; Academic Press Limited: San Diego, CA, USA, 1989. [Google Scholar]
- Stephenson, A.G. Flower and fruit abortion: Proximate causes and ultimate functions. Annu. Rev. Ecol. Syst. 1981, 12, 253–279. [Google Scholar] [CrossRef]
- Trueman, S.J.; Turnbull, C.G.N. Fruit set, abscission and dry matter accumulation on girdled branches of macadamia. Ann. Bot. 1994, 74, 667–674. [Google Scholar] [CrossRef]
- Pérez, V.; Herrero, M.; Hormaza, J.I. Self-fertility and preferential cross-fertilization in mango (Mangifera indica). Sci. Hortic. 2016, 213, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Conner, P.J.; Funderburk, J.F.; Price, J.G. Effects of foliar-applied boron on fruit retention, fruit quality, and tissue boron concentration of pecan. HortScience 2008, 43, 696–699. [Google Scholar] [CrossRef] [Green Version]
- Ganie, M.A.; Akhter, F.; Bhat, M.A.; Malik, A.R.; Junaid, J.M.; Abas Shah, M.; Bhat, A.H.; Bhat, T.A. Boron—A critical nutrient element for plant growth and productivity with referance to temperate fruits. Curr. Sci. 2013, 104, 76–85. [Google Scholar]
- Silber, A.; Naor, A.; Cohen, H.; Bar-Noy, Y.; Yechieli, N.; Levi, M.; Noy, M.; Peres, M.; Duari, D.; Narkis, K.; et al. Avocado fertilization: Matching the periodic demand for nutrients. Sci. Hortic. 2018, 241, 231–240. [Google Scholar] [CrossRef]
- De Silva, A.L.; Kämper, W.; Wallace, H.M.; Ogbourne, S.M.; Hosseini Bai, S.; Nichols, J.; Trueman, S.J. Boron effects on fruit set, yield, quality and paternity of macadamia. Agronomy 2022, 12, 684. [Google Scholar] [CrossRef]
- Whiley, A.W.; Wolstenholme, B.N. Carbohydrate management in avocado trees for increased production. SAAGA Yearb. 1990, 13, 25–27. [Google Scholar]
- Lahav, E.; Whiley, A.W. Irrigation and mineral nutrition. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 259–298. [Google Scholar]
- Wolstenholme, B.N.; Whiley, A.W. What do carbohydrate reserves tell us about avocado orchard management? SAAGA Yearb. 1997, 20, 63–67. [Google Scholar]
- Escobar, J.V.; Cortes, M.; Correa, G.; Rondon, T.; Rodríguez, P. ‘Hass’ avocado internal disorders under simulated export conditions and its relationship with flesh mineral content and preharvest variables. Horticulturae 2021, 7, 77. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Dreher, M.; Davenport, A.J. Avocado consumption is associated with better diet quality and nutirent intake and lower metabolic syndrome risk in US adults. Nutr. J. 2013, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Dreher, M.L.; Davenport, A.J. Hass avocado composition and potential health effects. Crit. Rev. Food Sci. Nutr. 2013, 53, 738–750. [Google Scholar] [CrossRef] [Green Version]
- Dreher, M.L.; Cheng, F.W.; Ford, N.A. A comprehensive review of Hass avocado clinical trials, observational studies, and biological mechanisms. Nutrients 2021, 13, 4376. [Google Scholar] [CrossRef]
- Coronado, J.A.; Bijman, J.; Omta, O.; Oude Lansink, A. A case study of the Mexican avocado industry based on transaction costs and supply chain management practices. Econ. Teoría Práct. 2015, 42, 137–165. [Google Scholar] [CrossRef] [Green Version]
- Juma, I.; Fors, H.; Hovmalm, H.P.; Nyomora, A.; Fatih, M.; Geleta, M.; Carlsson, A.S.; Ortiz, R.O. Avocado production and local trade in the southern highlands of Tanzania: A case of an emerging trade commodity from horticulture. Agronomy 2019, 9, 749. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz, M.L.; Hormaza, J.I. Fruit set in avocado: Pollen limitation, pollen load size, and selective fruit abortion. Agronomy 2021, 11, 1603. [Google Scholar] [CrossRef]
- Wolstenholme, B. Developments in the world avocado industry, and their relevance to the South African and African industries. Acta Hortic. 2013, 1007, 7. [Google Scholar] [CrossRef]
- Joyce, D. Supply Chain Quality Improvement—Technologies and Practices to Reduce Bruising; Horticulture Innovation Australia Limited: Sydney, Australia, 2018. [Google Scholar]
- Newett, S. Achieving More Consistent Yields of Quality Fruit in the Australian Avocado Industry; Horticulture Innovation Australia Limited: Sydney, Australia, 2018. [Google Scholar]
- Blanke, M.M.; Lovatt, C.J. Anatomy and transpiration of the avocado inflorescence. Ann. Bot. 1993, 71, 543–547. [Google Scholar] [CrossRef]
- Gazit, S.; Degani, C. Reproductive biology. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 101–133. [Google Scholar]
- Kämper, W.; Ogbourne, S.M.; Hawkes, D.; Trueman, S.J. SNP markers reveal relationships between fruit paternity, fruit quality, and distance from a cross-pollen source in avocado orchards. Sci. Rep. 2021, 11, 20043. [Google Scholar] [CrossRef]
- Knight, R. History, Distribution and uses. In The Avocado: Botany, Production and Uses; Whiley, A., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 1–12. [Google Scholar]
- Dixon, J.; Elmsly, T.A.; Greenwood, A.C. Differences in initial fruit set on determinate and indeterminate flowering shoots. N. Z. Avocado Grow. Assoc. Annu. Res. Rep. 2007, 7, 31–40. [Google Scholar]
- Evans, L.J.; Goodwin, R.M.; McBrydie, H.M. Factors affecting ‘Hass’ avocado (Persea americana) fruit set in New Zealand. N. Z. Plant Prot. 2010, 63, 214–218. [Google Scholar] [CrossRef]
- Ish-Am, G. Avocado pollination—A review. In Flowering, Fruit Set and Yield, Proceedings of the New Zealand and Australia Avocado Grower’s Conference, Tauranga, New Zealand, 20–22 September 2005; Avocados Australia: Rocklea, Australia, 2005. [Google Scholar]
- Lovatt, C.J. Hass Avocado Nutrition Research in California; University of California: Riverside, CA, USA, 2013. [Google Scholar]
- Souza, F.B.M.d.; Pio, R.; Tadeu, M.H.; Zambon, C.R.; Reighard, G.L. Boric acid in germination of pollen grains and fruit set of peach cultivars in subtropical region. Rev. Ciênc. Agron. 2017, 48, 496–500. [Google Scholar] [CrossRef]
- Sharafi, Y.; Raina, M. Effect of boron on pollen attributes in different cultivars of Malus domestica L. Natl Acad. Sci. Lett. 2021, 44, 189–194. [Google Scholar] [CrossRef]
- Perica, S.; Brown, P.H.; Connell, J.H.; Nyomora, A.M.S.; Dordas, C.; Hu, H.; Stangoulis, J. Foliar boron application improves flower fertility and fruit set of olive. HortScience 2001, 36, 714–716. [Google Scholar] [CrossRef] [Green Version]
- Boldingh, H.L.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, J.I. Carbohydrate and boron content of styles of ‘Hass’ avocado (Persea americana Mill.) flowers at anthesis can affect final fruit set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Smith, T.E.; Stephenson, R.A.; Asher, C.J.; Hetherington, S.E. Boron deficiency of avocado: Effects on pollen viability and fruit set. In Boron in Soils and Plants; Bell, R.W., Rerkasem, B., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 131–133. [Google Scholar]
- Minchin, P.E.H.; Thorp, T.G.; Boldingh, H.L.; Gould, N.; Cooney, J.M.; Negm, F.B.; Focht, E.; Arpaia, M.L.; Hu, H.; Brown, P. A possible mechanism for phloem transport of boron in ‘Hass’ avocado (Persea americana Mill.) trees. J. Hortic. Sci. Biotechnol. 2012, 87, 23–28. [Google Scholar] [CrossRef]
- Coetzer, L.A.; Robbertse, P.J.; Janse van Vuuren, B.P.H. The role of boron in avocados: Theory, practise and reality. SAAGA Yearb. 1993, 16, 2–4. [Google Scholar]
- Williams, J.H.; Reese, J.B. Evolution of development of pollen performance. Curr. Top. Dev. Biol. 2019, 131, 299–336. [Google Scholar] [CrossRef]
- Lovatt, C.; Zheng, Y.; Khuong, T.; Campisi-Pinto, S.; Crowley, D.; Rolshausen, P. Yield Characteristics of ‘Hass’ Avocado Trees under California Growing Conditions. In Proceedings of the VIII World Avocado Congress, Lima, Peru, 13–18 September 2015. [Google Scholar]
- Avocados Australia. Avocados Australia Best Practice Resource-Packaging for Export; Avocados Australia: Rocklea, Australia, 2018. [Google Scholar]
- Hass Avocado Board (HAB). Consumer Tracking Study. 2021. Available online: https://hassavocadoboard.com/research/consumer-tracking-study-2021/ (accessed on 21 April 2022).
- Bybordi, A.; Malakouti, M.J. Effects of foliar applications of nitrogen, boron and zinc on fruit setting and quality of almonds. Acta Hortic. 2006, 726, 351–358. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M.; Klamkowski, K. Response of apple trees to boron fertilization under conditions of low soil boron availability. Sci. Hortic. 2008, 116, 58–64. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, A.S.; Malik, A.U.; Afzal, I.; Shahid, M.; Razzaq, K. Foliar application of boron influences the leaf mineral status, vegetative and reproductive growth, yield and fruit quality of ‘Kinnow’ mandarin (Citrus reticulata Blanco.). J. Plant Nutr. 2012, 35, 2067–2079. [Google Scholar] [CrossRef]
- Smith, T.E.; Stephenson, R.A.; Asher, C.J.; Hetherington, S.E. Boron deficiency of avocado: Effects on fruit size and ripening. In Boron in Soils and Plants; Bell, R.W., Rerkasem, B., Eds.; Kluwer Academic Publishers: Amsterdam, Netherlands, 1997; pp. 135–137. [Google Scholar]
- Bard, Z.J.; Wolstenholme, B.N. Soil boron application for the control of boron deficiency in KwaZulu-Natal avocado orchards. SAAGA Yearb. 1997, 20, 13–15. [Google Scholar]
- Abdel-Karim, H.A.; Nehad, M.A.; El-Rouby, K.M.; Roshdy, K.A. Effect of foliar application of boron and zinc on fruit set, yield and some fruit characteristics of Fuerte avocado. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 443–449. [Google Scholar]
- Newett, S.; Perkins, M.; Coates, L.; Irvine-Brown, S.; Joyce, D. Review of pre-harvest mineral nutrition for post-harvest quality. Talk. Avocados 2021, 32, 54–57. [Google Scholar]
- Bureau of Meteorology (BOM). Climate Data Online. Available online: http://www.bom.gov.au/climate/data/ (accessed on 24 March 2021).
- Ivanova, N.; Fazekas, A.; Hebert, P. Semi-automated, membrane-based protocol for DNA isolation from plants. Plant Mol. Biol. Rep. 2008, 26, 186–198. [Google Scholar] [CrossRef]
- Kämper, W.; Trueman, S.J.; Cooke, J.; Kasinadhuni, N.; Brunton, A.J.; Ogbourne, S.M. Single-nucleotide polymorphisms that uniquely identify cultivars of avocado (Persea americana). Appl. Plant Sci. 2021, 9, e11440. [Google Scholar] [CrossRef]
- Mcgeehan, S.L.; Naylor, D.V. Automated instrumental analysis of carbon and nitrogen in plant and soil samples. Commun. Soil Sci. Plant Anal. 1988, 19, 493–505. [Google Scholar] [CrossRef]
- Rayment, G.; Higginson, F. Australian Laboratory Handbook of Soil and Water Chemical Methods; Inkata Press: Melbourne, Australia, 1992. [Google Scholar]
- Martinie, G.D.; Schilt, A.A. Investigation of the wet oxidation efficiencies of perchloric acid mixtures for various organic substances and the identities of residual matter. Anal. Chem. 1976, 48, 70–74. [Google Scholar] [CrossRef]
- Munter, R.; Grande, R. Plant tissue and soil extract analysis by ICP-atomic emission spectrometry. In Developments in Atomic Plasma Spectrochemical Analysis; Byrnes, R.M., Ed.; Heyden: London, UK, 1981; pp. 653–672. [Google Scholar]
- Kämper, W.; Trueman, S.J.; Tahmasbian, I.; Hosseini Bai, S. Rapid determination of nutrient concentrations in Hass avocado fruit by vis/nir hyperspectral imaging of flesh or skin. Remote Sens. 2020, 12, 3409. [Google Scholar] [CrossRef]
- Richards, T.E.; Kämper, W.; Trueman, S.J.; Wallace, H.M.; Ogbourne, S.M.; Brooks, P.R.; Nichols, J.; Hosseini Bai, S. Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plants 2020, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Degani, C.; Goldring, A.; Gazit, S. Pollen parent effect on outcrossing rate in ‘Hass’ and ‘Fuerte’ avocado plots during fruit development. J. Amer. Soc. Hortic. Sci. 1989, 114, 106–111. [Google Scholar]
- Degani, C.; El-Batsri, R.; Gazit, S. Outcrossing rate, yield, and selective fruit abscission in ‘Ettinger’ and ‘Ardith’ avocado plots. J. Amer. Soc. Hortic. Sci. 1997, 122, 813–817. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Influence of physical distance between cultivars on yield, outcrossing rate and selective fruit drop in avocado (Persea americana, Lauraceae). Ann. Appl. Biol. 2011, 158, 354–361. [Google Scholar] [CrossRef]
- Alcaraz, M.L.; Hormaza, J.I. Avocado pollination and fruit set—A perspective from Spain. Calif. Avocado Soc. Yearb. 2009, 92, 113–135. [Google Scholar]
- Kobayashi, M.; Lin, J.Z.; Davis, J.; Francis, L.; Clegg, M.T. Quantitative analysis of avocado outcrossing and yield in California using RAPD markers. Sci. Hortic. 2000, 86, 135–149. [Google Scholar] [CrossRef]
- Whiley, A.W. Avocado production in Australia. In Avocado Production in Asia and the Pacific; Papademetriou, M., Ed.; Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific: Bangkok, Thailand, 2000; pp. 1–8. [Google Scholar]
- Whiley, A.W. Crop management. In The Avocado: Botany, Production and Uses; Whiley, A.W., Schaffer, B., Wolstenholme, B.N., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 231–253. [Google Scholar]
- Gamble, J.; Harker, F.; Jaeger, S.; White, A.; Bava, C.; Beresford, M.; Stubbings, B.; Wohlers, M.; Hofman, P.; Marques, R.; et al. The impact of dry matter, ripeness and internal defects on consumer perceptions of avocado quality and intentions to purchase. Postharvest Biol. Technol. 2010, 57, 35–43. [Google Scholar] [CrossRef]
- Shaaban, M.M. Role of boron in plant nutrition and human health. Am. J. Plant Physiol. 2010, 5, 224–240. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, F.H. Update on human health effects of boron. J. Trace Elem. Med. Biol. 2014, 28, 383–387. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Trace Elements in Human Nutrition and Health; World Health Organisation: Geneva, Switzerland, 1996. [Google Scholar]
- World Health Organisation (WHO). Environmental Health Criteria for Boron; World Health Organisation: Geneva, Switzerland, 1998. [Google Scholar]
- Woolf, A.; Wong, M.; Eyres, L.; McGhie, T.; Lund, C.; Olsson, S.; Wang, Y.; Bulley, C.; Wang, M.; Friel, E.; et al. Avocado oil. In Gourmet and Health-Promoting Specialty Oils, 1st ed.; Moreau, R.A., Kamal-Eldin, A., Eds.; American Oil Chemists’ Society Press: Urbana, IL, USA, 2009; pp. 73–125. [Google Scholar]


| Fruit Parameter | Boron Application (g/tree) | ||
|---|---|---|---|
| 0 | 15 | 30 | |
| Length (cm) | 9.94 ± 0.05 a | 9.92 ± 0.06 a | 9.97 ± 0.06 a |
| Diameter (cm) | 7.24 ± 0.03 b | 7.40 ± 0.04 a | 7.39 ± 0.04 a |
| Flesh mass (g) | 179.5 ± 2.9 b | 195.4 ± 4.4 a | 193.6 ± 3.8 a |
| Seed mass (g) | 42.1 ± 0.8 a | 40.1 ± 1.0 a | 40.6 ± 1.0 a |
| Sample Type | Nutrient | Boron Application (g/tree) | ||
|---|---|---|---|---|
| 0 | 15 | 30 | ||
| Flesh | Boron (B) | 2.46 ± 0.09 c | 3.81 ± 0.13 b | 4.10 ± 0.12 a |
| Carbon (C) | 15.3 ± 0.2 a | 15.4 ± 0.2 a | 15.7 ± 0.2 a | |
| Nitrogen (N) | 443 ± 13 a | 473 ± 13 a | 480 ± 13 a | |
| Phosphorous (P) | 56.6 ± 1.1 b | 59.6 ± 1.4 ab | 60.9 ± 1.2 a | |
| Potassium (K) | 575 ± 11 a | 570 ± 12 a | 598 ± 11 a | |
| Aluminium (Al) | 0.071 ± 0.010 a | 0.054 ± 0.004 b | 0.046 ± 0.003 b | |
| Calcium (Ca) | 7.18 ± 0.21 a | 6.65 ± 0.21 ab | 6.33 ± 0.23 b | |
| Copper (Cu) | 0.560 ± 0.030 a | 0.650 ± 0.037 a | 0.667 ± 0.036 a | |
| Iron (Fe) | 0.624 ± 0.025 a | 0.661 ± 0.026 a | 0.605 ± 0.022 a | |
| Magnesium (Mg) | 28.52 ± 0.39 a | 28.21 ± 0.38 a | 28.11 ± 0.38 a | |
| Manganese (Mn) | 0.510 ± 0.020 a | 0.520 ± 0.015 a | 0.485 ± 0.016 a | |
| Sodium (Na) | 8.12 ± 0.39 a | 8.28 ± 0.47 a | 7.87 ± 0.49 a | |
| Sulphur (S) | 29.6 ± 1.3 a | 29.0 ± 1.4 a | 29.3 ± 1.2 a | |
| Zinc (Zn) | 0.744 ± 0.015 b | 0.799 ± 0.015 a | 0.774 ± 0.015 ab | |
| Seed | Boron (B) | 1.36 ± 0.09 b | 2.67 ± 0.16 a | 2.82 ± 0.16 a |
| Carbon (C) | 20.5 ± 0.2 a | 19.7 ± 0.3 a | 20.1 ± 0.3 a | |
| Nitrogen (N) | 483 ± 17 a | 515 ± 16 a | 516 ± 15 a | |
| Phosphorous (P) | 65.4 ± 1.4 a | 70.2 ± 1.7 a | 65.0 ± 2.2 a | |
| Potassium (K) | 571 ± 10 a | 584 ± 12 a | 554 ± 13 a | |
| Aluminium (Al) | 0.050 ± 0.004 a | 0.053 ± 0.004 a | 0.051 ± 0.004 a | |
| Calcium (Ca) | 8.45 ± 0.60 a | 9.94 ± 0.98 a | 8.28 ± 0.60 a | |
| Copper (Cu) | 0.406 ± 0.016 a | 0.400 ± 0.012 a | 0.367 ± 0.015 a | |
| Iron (Fe) | 1.06 ± 0.03 a | 1.07 ± 0.03 a | 1.02 ± 0.03 a | |
| Magnesium (Mg) | 38.8 ± 1.2 a | 39.1 ± 1.3 a | 35.4 ± 1.3 a | |
| Manganese (Mn) | 0.623 ± 0.032 b | 0.799 ± 0.040 a | 0.595 ± 0.027 b | |
| Sodium (Na) | 0.490 ± 0.060 a | 0.429 ± 0.052 a | 0.530 ± 0.060 a | |
| Sulphur (S) | 34.9 ± 1.1 a | 35.3 ± 1.3 a | 36.2 ± 1.4 a | |
| Zinc (Zn) | 0.605 ± 0.019 a | 0.646 ± 0.017 a | 0.603 ± 0.019 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron Effects on Fruit Set, Yield, Quality and Paternity of Hass Avocado. Agronomy 2022, 12, 1479. https://doi.org/10.3390/agronomy12061479
Hapuarachchi NS, Kämper W, Wallace HM, Hosseini Bai S, Ogbourne SM, Nichols J, Trueman SJ. Boron Effects on Fruit Set, Yield, Quality and Paternity of Hass Avocado. Agronomy. 2022; 12(6):1479. https://doi.org/10.3390/agronomy12061479
Chicago/Turabian StyleHapuarachchi, Nimanie S., Wiebke Kämper, Helen M. Wallace, Shahla Hosseini Bai, Steven M. Ogbourne, Joel Nichols, and Stephen J. Trueman. 2022. "Boron Effects on Fruit Set, Yield, Quality and Paternity of Hass Avocado" Agronomy 12, no. 6: 1479. https://doi.org/10.3390/agronomy12061479
APA StyleHapuarachchi, N. S., Kämper, W., Wallace, H. M., Hosseini Bai, S., Ogbourne, S. M., Nichols, J., & Trueman, S. J. (2022). Boron Effects on Fruit Set, Yield, Quality and Paternity of Hass Avocado. Agronomy, 12(6), 1479. https://doi.org/10.3390/agronomy12061479






