Agronomic Performance of Broomrape Resistant and Susceptible Faba Bean Accession
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Statistical Analysis
2.2.1. Variance Components
2.2.2. GEI Analysis
WAASB Index
WAASBY Superiority Index
Multi-Trait Stability Index (MTSI) Base on Factor Analysis
2.2.3. Correlations
3. Results
3.1. Mean Performance of Genotypes and Environments for Grain Yield
3.2. Pooled ANOVA
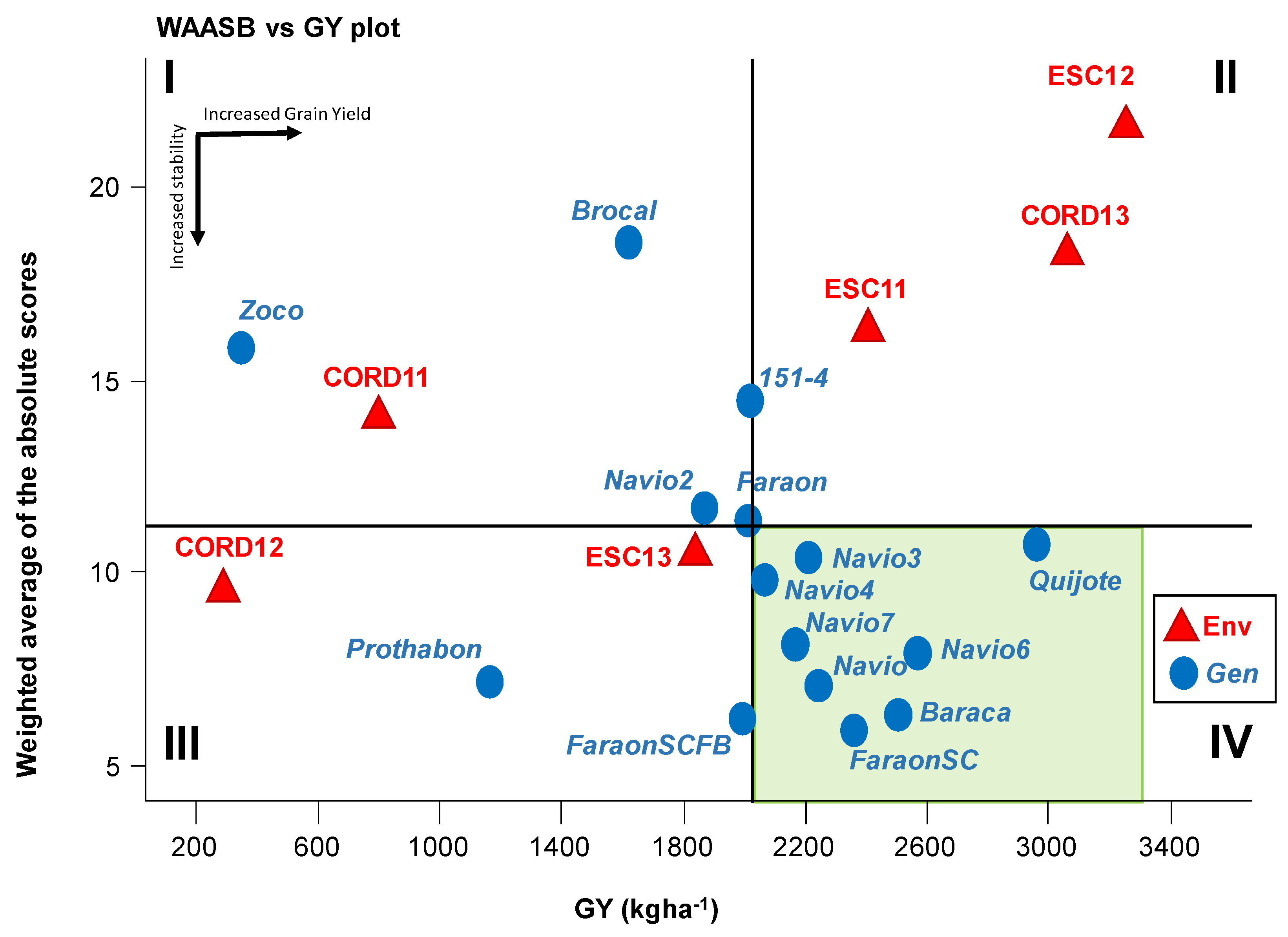
3.3. WAASB-Based Stability Analysis for Grain Yield
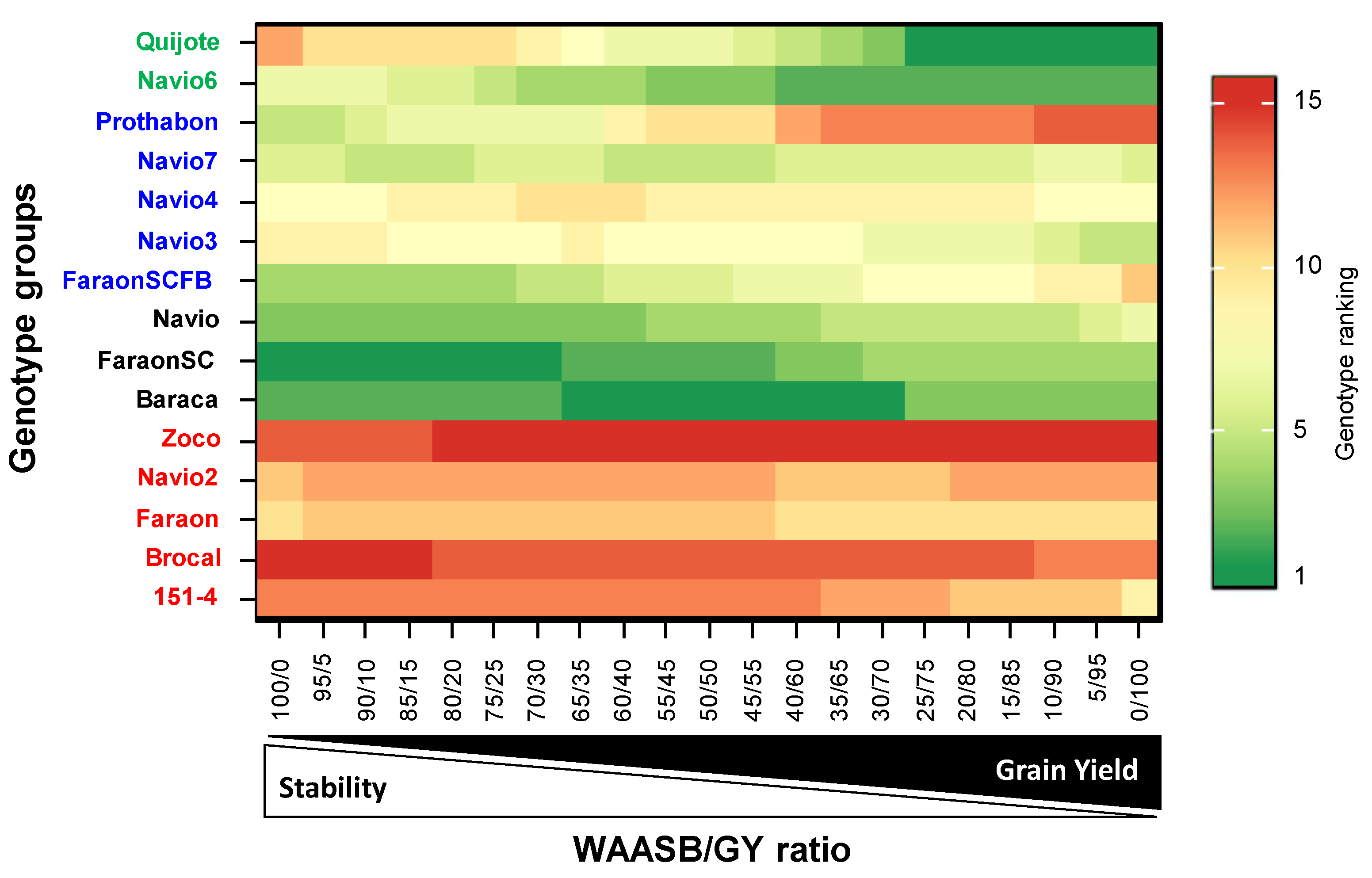
3.4. Genotype Ranking Based on WAASBY Index for Grain Yield
3.5. Mean Performance of Genotypes and Environments for Ascochyta Blight, Chocolate Spot and Broomrape Infection
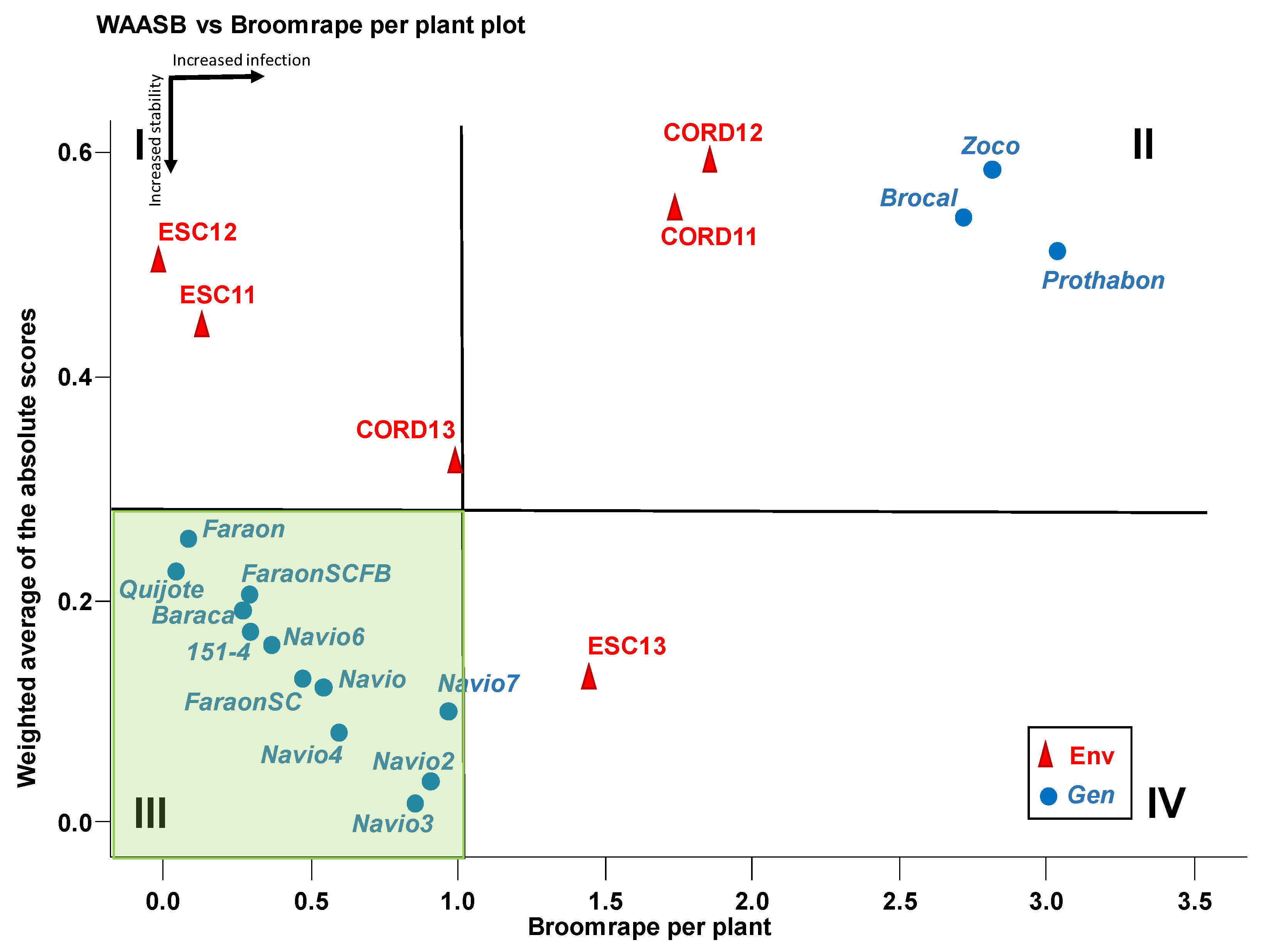
3.6. WAASB-Based Stability Analysis for Grain Yield
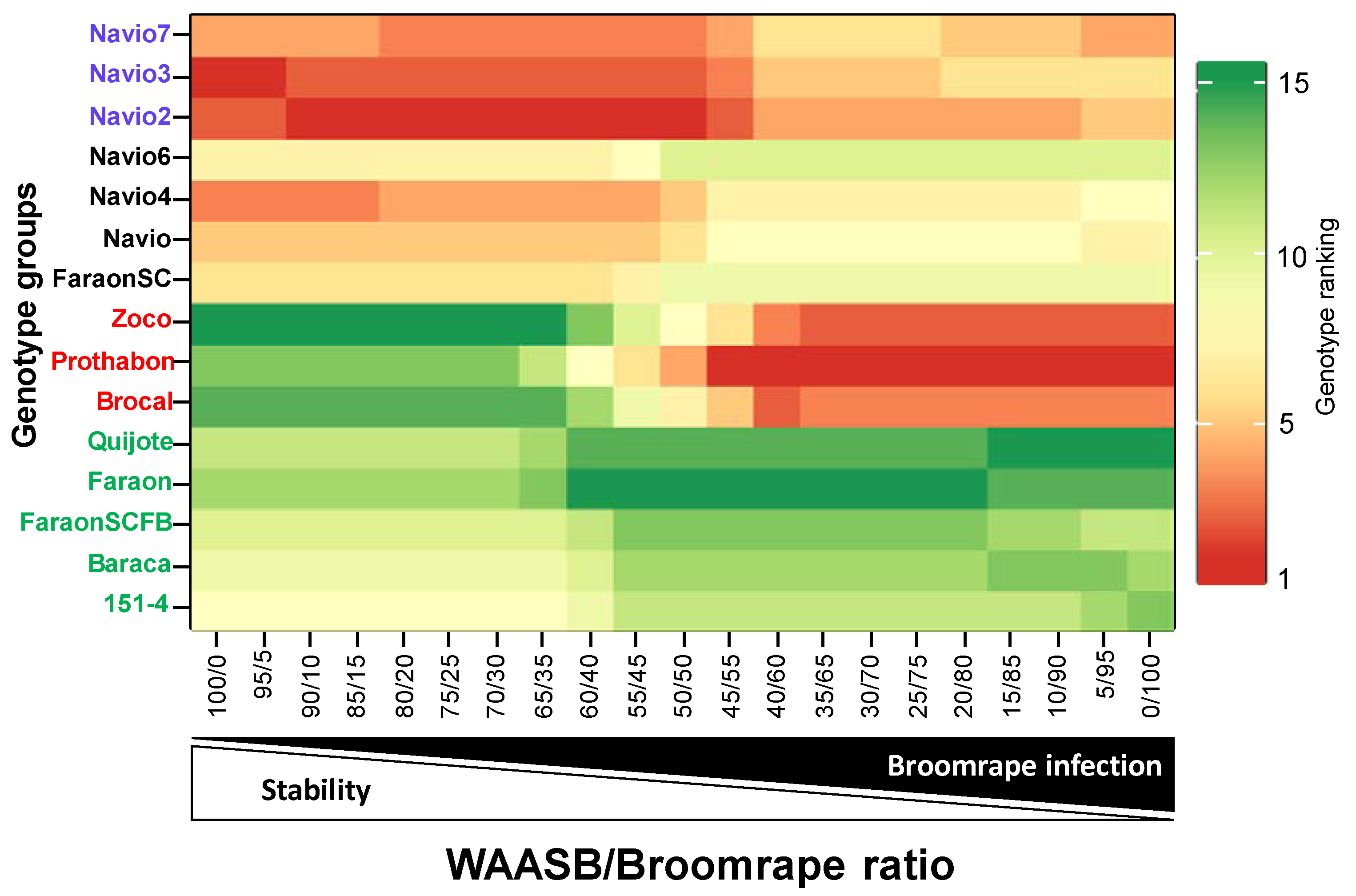
3.7. Genotype Ranking Based on WAASBY Index for Broomrape Infection
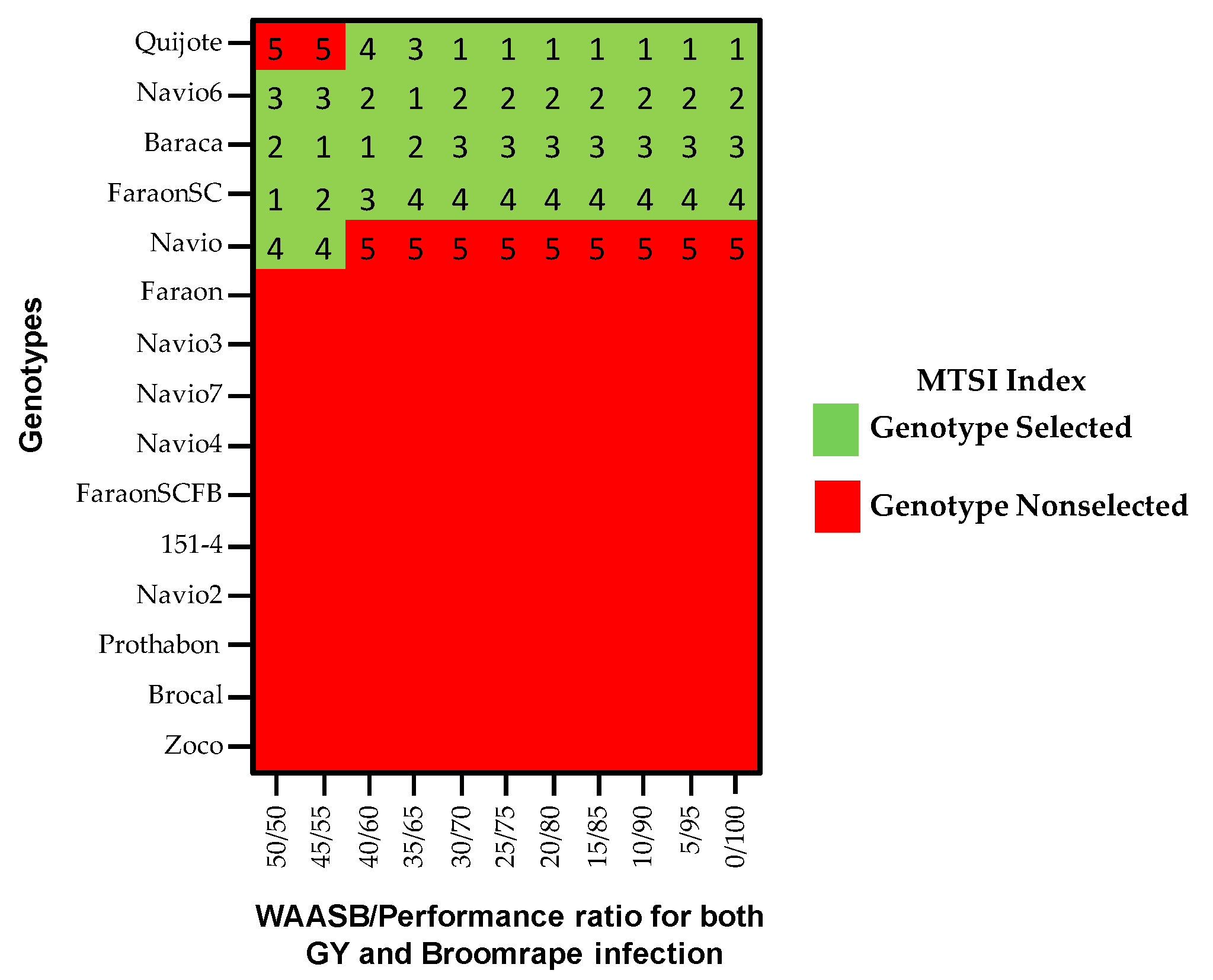
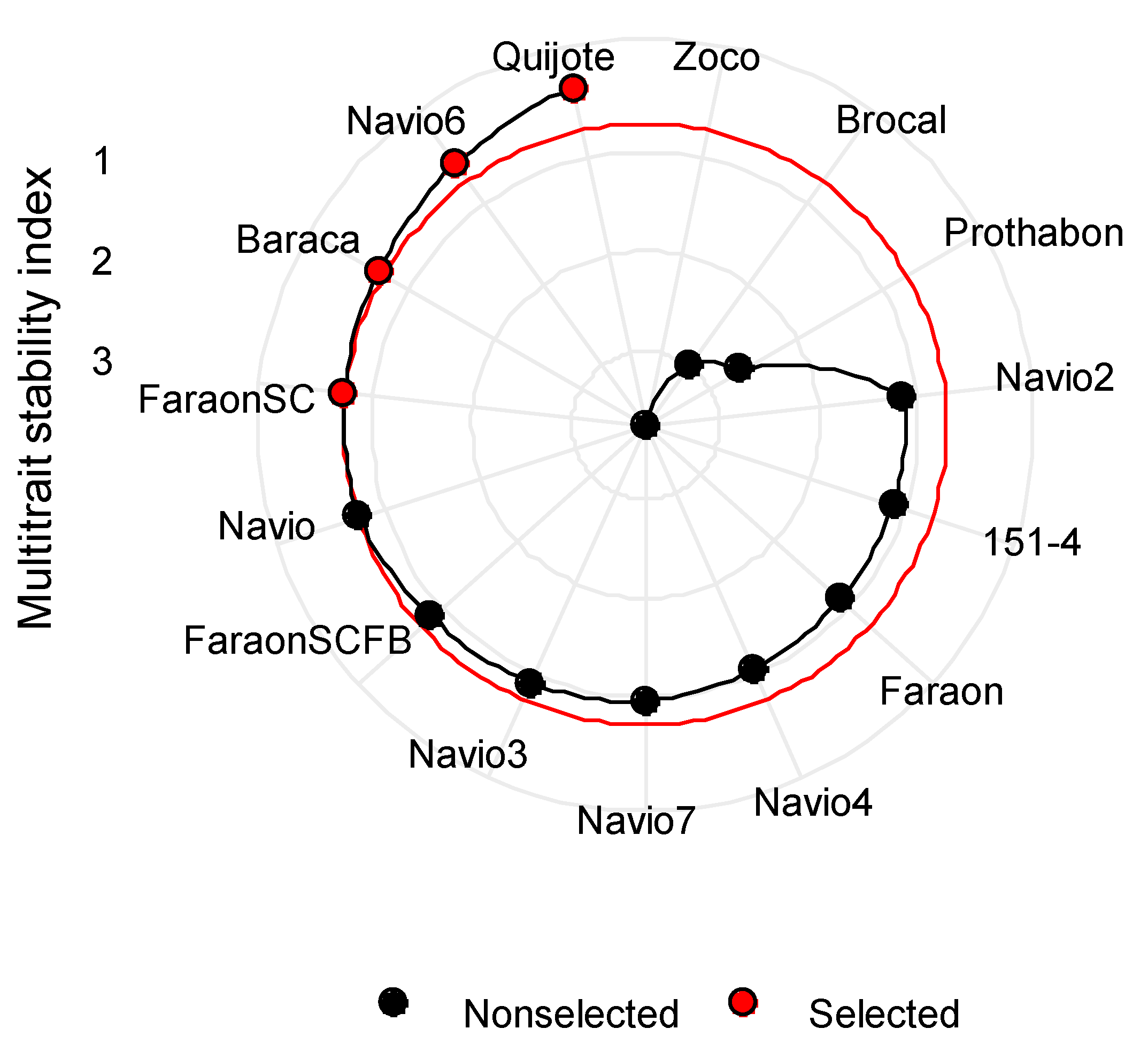
3.8. Multi-Trait Stability Index (MTSI)
3.9. Mean Performance of Genotypes and Environments for Precocity
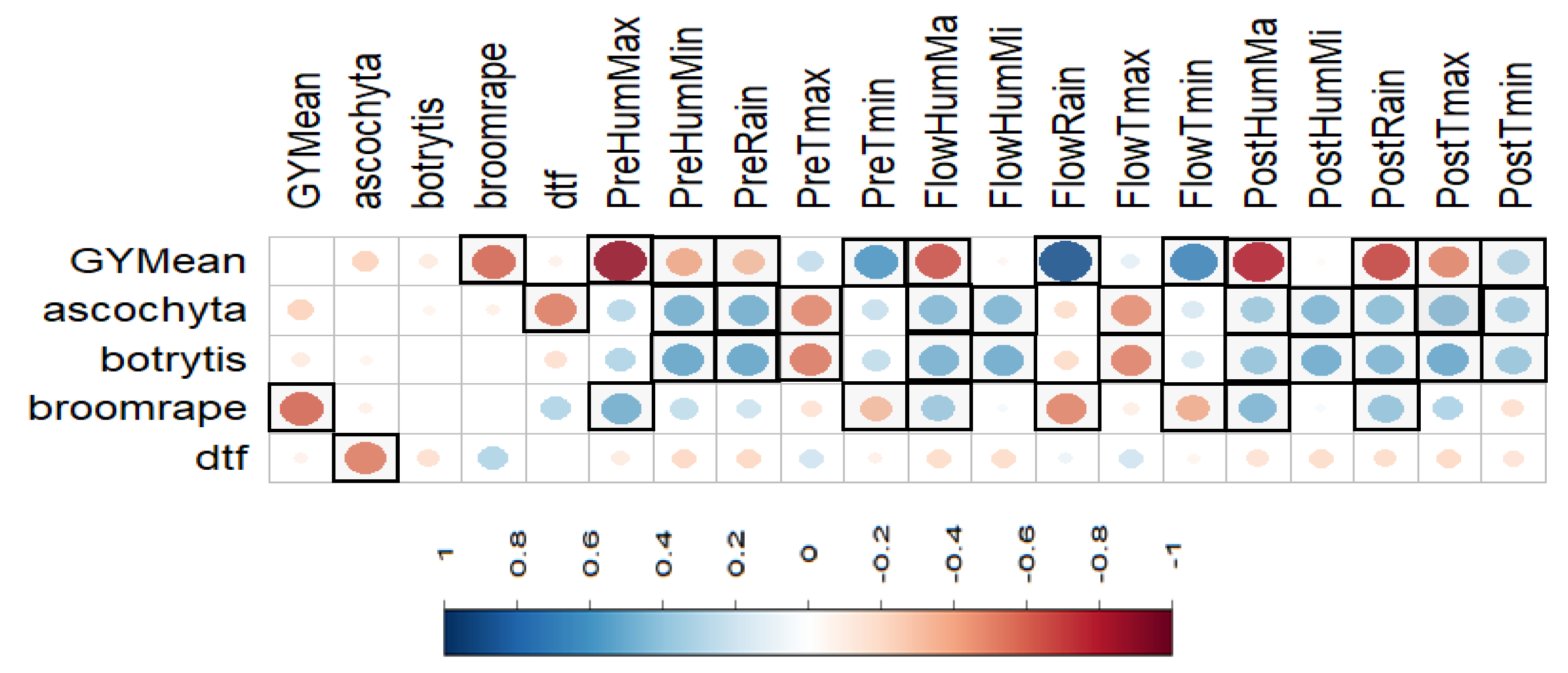
3.10. Correlations among Traits and Environmental Factors
4. Discussions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Flores, F.; Nadal, S.; Solis, I.; Winkler, J.; Sass, O.; Stoddard, F.L.; Link, W.; Raffiot, B.; Muel, F.; Rubiales, D. Faba bean adaptation to autumn sowing under European climates. Agron. Sustain. Dev. 2012, 32, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Mínguez, I.; Rubiales, D. Faba bean. In Crop Physiology: Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 453–482. ISBN 978-0-12-819194-1. [Google Scholar] [CrossRef]
- Duc, G.; Aleksić, J.M.; Marget, P.; Mikic, A.; Paull, J.; Redden, R.J.; Sass, O.; Stoddard, F.L.; Vandenberg, A.; Vishnyakova, M.; et al. Faba Bean. In Grain Legumes. Handbook of Plant Breeding; De Ron, A., Ed.; Springer: New York, NY, USA, 2015; Volume 10. [Google Scholar] [CrossRef]
- Bodner, G.; Kronberga, A.; Lepse, L.; Olle, M.; Vågen, I.M.; Rabante, L.; Fernández, J.A.; Ntatsi, G.; Balliu, A.; Rewald, B. Trait identification of faba bean ideotypes for Northern European environments. Eur. J. Agron. 2018, 96, 1–12. [Google Scholar] [CrossRef]
- Zong, X.; Yang, T.; Liu, R. Faba bean (Vicia faba L.) breeding. In Advances in Plant Breeding Strategies: Legumes; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Flores, F.; Hybl, M.; Knudsen, J.C.; Marget, P.; Muel, F.; Nadal, S.; Narits, L.; Raffiot, B.; Sass, O.; Solis, I.; et al. Adaptation of spring faba bean types across European climates. Field Crops Res. 2013, 145, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Link, W.; Balko, C.; Stoddard, F.L. Winter hardiness in faba bean: Physiology and breeding. Field Crops Res. 2010, 115, 287–296. [Google Scholar] [CrossRef]
- Gharzeddin, K.; Maalouf, F.; Khoury, B.; Abou-Khater, L.; Christmann, S.; El-Dine, N.A.J. Efficiency of different breeding strategies in improving the faba bean productivity for sustainable agriculture. Euphytica 2019, 215, 203. [Google Scholar] [CrossRef]
- Papastylianou, P.; Vlachostergios, D.N.; Dordas, C.; Tigka, E.; Papakaloudis, P.; Kargiotidou, A.; Pratsinakis, E.; Koskosidis, A.; Pankou, C.; Kousta, A.; et al. Genotype X Environment Interaction Analysis of Faba Bean (Vicia faba L.) for Biomass and Seed Yield across Different Environments. Sustainability 2021, 13, 2586. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Iannucci, A. Breeding strategy for faba bean in southern Europe based on cultivar responses across climatically contrasting environments. Crop Sci. 2008, 48, 983–991. [Google Scholar] [CrossRef]
- Skovbjerg, C.K.; Knudsen, J.N.; Füchtbauer, W.; Stougaard, J.; Stoddard, F.L.; Janss, L.; Andersen, S.U. Evaluation of yield, yield stability, and yield–protein relationship in 17 commercial faba bean cultivars. Legum. Sci. 2020, 2, 39. [Google Scholar] [CrossRef]
- Ruisi, P.; Amato, G.; Badagliacca, G.; Frenda, A.S.; Giambalvo, D.; Di Micel, G. Agro-ecological benefits of faba bean for rainfed Mediterranean cropping systems. Ital. J. Agron. 2017, 12, 865. [Google Scholar] [CrossRef] [Green Version]
- Abou-Khater, L.; Maalouf, F.; Rubiales, D. Status of faba bean (Vicia faba L.) in the Mediterranean and East African countries. In Developing Climate-Resilient Food and Fodder Legumes; Jha, U.C., Nayyar, H., Kumar, S., Siddique, K., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Sellami, M.H.; Lavini, A.; Calandrelli, D.; De Mastro, G.; Pulvento, C. Evaluation of Genotype, Environment, and Management Interactions on Fava Beans under Mediterranean Field Conditions. Agronomy 2021, 11, 1088. [Google Scholar] [CrossRef]
- Stoddard, F.L.; Nicholas, A.H.; Rubiales, D.; Thomas, J.; Villegas, A.M. Integrated pest management in faba bean. Field Crops Res. 2010, 115, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Rubiales, D.; Fernández-Aparicio, M. Innovations in parasitic weeds management in legume crops. A review. Agron. Sustain. Dev. 2012, 32, 433–449. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. The effect of Orobanche crenata infection severity in faba bean, field pea and grass pea productivity. Front. Plant Sci. 2016, 7, 1409. [Google Scholar] [CrossRef] [Green Version]
- Rubiales, D.; Fernández-Aparicio, M.; Wegmann, K.; Joel, D.M. Revisiting strategies for reducing the seedbank of Orobanche and Phelipanche spp. Weed Res. 2009, 49, 23–33. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Eizenberg, H.; Grenz, J.H.; Sillero, J.C.; Ávila, C.M.; Sauerborn, J.; Rubiales, D. Broomrape management in faba bean. Field Crops Res. 2010, 115, 319–328. [Google Scholar] [CrossRef]
- Sillero, J.C.; Villegas-Fernández, A.m.; Thomas, J.; RojasMolina, M.M.; Emeran, A.A.; Fernández-Aparicio, M.; Rubiales, D. Faba bean breeding for disease resistance. Field Crops Res. 2010, 115, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Rubiales, D.; Khazaei, H. Advances in disease and pest resistance in faba bean. Theor. Appl. Genet. 2022, on line first. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multienvironment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef] [Green Version]
- Stroup, W.W.; Mulitze, D.K. Nearest neighbour adjusted best linear unbiased prediction. Am. Stat. 1991, 45, 194–200. [Google Scholar]
- Piepho, H.P.; Möhring, J.; Melchinger, A.E.; Büchse, A. BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 2008, 161, 209–228. [Google Scholar] [CrossRef]
- Piepho, H.P. Best Linear Unbiased Prediction (BLUP) for regional yield trials: A comparison to additive main effects and multiplicative interaction (AMMI) analysis. Theor. Appl. Genet. 1994, 89, 647–654. [Google Scholar] [CrossRef]
- Flores, F.; Moreno, M.T.; Cubero, J.I. A comparison of univariate and multivariate methods to analyze G × E interaction. Field Crops Res. 1998, 56, 271–286. [Google Scholar] [CrossRef]
- Elias, A.A.; Robbins, K.R.; Doerge, R.W.; Tuinstra, M.R. Half a century of studying genotype × environment interactions in plant breeding experiments. Crop Sci. 2016, 56, 2090–2105. [Google Scholar] [CrossRef]
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Yang, R.C.; Crossa, J.; Cornelius, P.L.; Burgueño, J. Biplot analysis of genotype × environment interaction: Proceed with caution. Crop Sci. 2009, 49, 1564–1576. [Google Scholar] [CrossRef] [Green Version]
- Olivoto, T. Índices de Estabilidade Genotípica e Seleção Simultânea Multivariada: Uma Nova Abordagem. Ph.D. Thesis, Universidade Federal de Santa Maria, Santa Maria, Brazil, 2019. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C.; da Silva, J.A.G.; Marchioro, V.S.; de Souza, V.Q.; Jost, E. Mean Performance and Stability in Multi-Environment Trials I: Combining Features of AMMI and BLUP Techniques. Agron. J. 2019, 111, 2949–2960. [Google Scholar] [CrossRef]
- Smith, A.B.; Cullis, B.R.; Thompson, R. The analysis of crop cultivar breeding and evaluation trials: An overview of current mixed model approaches. J. Agric. Sci. 2005, 143, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Olivoto, T.; Lúcio, A.D.C. Metan: An R package for multienvironment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Nadal, S.; Moreno, M.T.; Cubero, J.I. Registration of ‘Baraca” faba bean. Crop Sci. 2004, 44, 1864–1865. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Moral, A.; Kharrat, M.; Rubiales, D. Resistance against broomrapes (Orobanche and Phelipanche spp.) in faba bean (Vicia faba) based in low induction of broomrape seed germination. Euphytica 2012, 186, 897–905. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Kisugi, T.; Xie, X.; Rubiales, D.; Yoneyama, K. Low strigolactone root exudation: A novel mechanism of broomrape (Orobanche and Phelipanche spp.) resistance available for faba bean breeding. J. Agric. Food Chem. 2014, 62, 7063–7071. [Google Scholar] [CrossRef] [PubMed]
- Kharrat, M.; Abbes, Z.; Amri, M. A new faba bean small seeded variety Najeh tolerant to orobanche registered in the Tunisian catalogue. Tunis. J. Plant Prot. 2010, 5, 125–130. [Google Scholar]
- Amri, M.; Trabelsi, I.; Abbes, Z.; Kharrat, M. Release of a new faba bean variety “Chourouk” resistant to the parasitic plants Orobanche foetida and O. crenata in Tunisia. Int. J. Agric. Biol. 2019, 21, 499–505. [Google Scholar]
- Ghalwash, A.M.; Soliman, I.E.; Khaffagy Azza, E. Performance of some faba bean (Vicia faba, L.) cultivars under numerous broomrape (Orobanche crenata Forsk.) control treatments. J. Agric. Sci. Mansoura Univ. 2008, 33, 2439–2448. [Google Scholar] [CrossRef]
- Rubiales, D.; Sillero, J.C.; Rojas-Molina, M.M. Characterization resistance mechanisms in faba bean (Vicia faba) against broomrape species (Orobanche and Phelipanche spp.). Front. Plant Sci. 2016, 7, 1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villegas-Fernández, A.M.; Sillero, J.C.; Emeran, A.A.; Winkler, J.; Raffiot, B.; Tay, J.; Flores, F.; Rubiales, D. Identification and multi-environment validation of resistance to Botrytis fabae in Vicia faba. Field Crops Res. 2009, 114, 84–90. [Google Scholar] [CrossRef]
- Rubiales, D.; Avila, C.M.; Sillero, J.C.; Hybl, M.; Narits, L.; Sass, O.; Flores, F. Identification and multi-environment validation of resistance to Ascochyta fabae in faba bean (Vicia faba). Field Crops Res. 2012, 126, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Red de Información Agroclimática de Andalucía (RIA). Available online: https://www.juntadeandalucia.es/agriculturaypesca/ifapa/riaweb/web/ (accessed on 2 March 2022).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- RAEA. Resultados de Ensayos de Variedades de Habas en Andalucía, Campaña 2014/2015. 2015. Available online: https://www.juntadeandalucia.es/agriculturaypesca/ifapa/servifapa/registro-servifapa/37d4d136-e654-4e4c-a587-dea7ad944cba (accessed on 17 March 2022).
- RAEA. Resultados de Ensayos de Variedades de Habas en Andalucía, Campaña 2015/2016. 2016. Available online: https://www.juntadeandalucia.es/agriculturaypesca/ifapa/servifapa/registro-servifapa/8e98eefe-86e6-4b49-b8e5-bbf136756810 (accessed on 17 March 2022).
- FAOSTAT. 2021. Available online: https://www.fao.org/faostat (accessed on 3 May 2022).
- Cernay, C.; Ben-Ari, T.; Pelzer, E.; Meynard, J.M.; Makoswki, D. Estimating variability in grain legume yields across Europe and the Americas. Sci. Rep. 2015, 5, 11171. [Google Scholar] [CrossRef]
- Villegas-Fernández, A.M.; Rubiales, D. Trends and perspectives for faba bean production in the Mediterranean Basin. Legum. Perspect. 2015, 10, 31–32. [Google Scholar]
- Bishop, J.; Potts, S.G.; Jones, H.E. Susceptibility of Faba Bean (Vicia faba L.) to Heat Stress During Floral Development and Anthesis. J. Agron. Crop Sci. 2016, 202, 508–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maalouf, F.; Abou-Khater, L.; Babiker, Z.; Jighly, A.; Alsamman, A.M.; Hu, J.; Ma, Y.; Rispail, N.; Balech, R.; Hamweih, A.; et al. Genetic Dissection of Heat Stress Tolerance in Faba Bean (Vicia faba L.) Using GWAS. Plants 2022, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.H.; Alghamdi, S.S.; Migdadi, H.M.; El-Harty, E.H.; Adhikari, K.N. Evaluation of Frost Damage and Pod Set in Faba Bean (Vicia faba L.) under Field Conditions. Plants 2021, 10, 1925. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Sillero, J.C.; Moral, A.; Cubero, J.I.; Rubiales, D. Effect of sowing date and host resistance on the establishment and development of Orobanche crenata in faba bean and common vetch. Weed Res. 2004, 44, 282–288. [Google Scholar] [CrossRef]
- Rubiales, D.; Alcántara, C.; Pérez-de-Luque, A.; Gil, J.; Sillero, J.C. Infection of chickpea (Cicer arietinum) by crenate broomrape (Orobanche crenata) as influenced by sowing date and weather conditions. Agronomie 2003, 23, 359–362. [Google Scholar] [CrossRef]
- Rubiales, D.; Pérez-de-Luque, A.; Fernández-Aparicio, M.; Sillero, J.C.; Román, B.; Kharrat, M.; Khalil, S.; Joel, D.M.; Riches, C. Screening techniques and sources of resistance against parasitic weeds in grain legumes. Euphytica 2006, 147, 187–199. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. Escape and true resistance to crenate broomrape (Orobanche crenata Forsk.) in grass pea (Lathyrus sativus L.) germplasm. Field Crops Res. 2012, 125, 92–97. [Google Scholar] [CrossRef]
- Rubiales, D.; Moreno, M.T.; Sillero, J.C. Search for resistance to crenate broomrape (Orobanche crenata Forsk.) in pea germplasm. Gen. Res. Crop Evol. 2005, 52, 853–861. [Google Scholar] [CrossRef]
- Abd El-Fatah, B.E.S.; Nassef, D.M.T. Inheritance of faba bean resistance to broomrape, genetic diversity and QTL mapping analysis. Mol. Biol. Rep. 2020, 47, 11–32. [Google Scholar] [CrossRef]
- Gutiérrez, N.; Palomino, C.; Satovic, Z.; Ruiz-Rodríguez, M.D.; Vitale, S.; Gutiérrez, M.V.; Rubiales, D.; Kharrat, M.; Amri, M.; Emeran, A.A.; et al. QTLs for Orobanche spp. resistance in faba bean: Identification and validation across different environments. Mol. Breed. 2013, 32, 909–992. [Google Scholar] [CrossRef]
- Román, B.; Torres, A.M.; Rubiales, D.; Cubero, J.I.; Satovic, Z. Mapping of quantitative trait loci controlling broomrape (Orobanche crenata Forsk.) resistance in faba bean (Vicia faba L.). Genome 2002, 45, 1057–1063. [Google Scholar] [CrossRef]
- Maalouf, F.; Khalil, S.; Ahmed, S.; Akintunde, A.N.; Kharrat, M.; El Shama’a, K.; Hajjar, S.; Malhotra, R.S. Yield stability of faba bean lines under diverse broomrape prone production environments. Field Crops Res. 2011, 124, 288–294. [Google Scholar] [CrossRef]
- Rubiales, D.; Flores, F.; Emeran, A.A.; Kharrat, M.; Amri, M.; Rojas-Molina, M.M.; Sillero, J.C. Identification and multi-environment validation of resistance against broomrapes (Orobanche crenata and O. foetida) in faba bean (Vicia faba). Field Crops Res. 2014, 166, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Trabelsi, I.; Yoneyama, K.; Abbes, A.; Amri, M.; Xie, X.; Kisugi, T.; Kim, H.I.; Kharrat, M.; Yoneyama, K. Characterization of strigolactones produced by Orobanche foetida and Orobanche crenata resistant faba bean (Vicia faba L.) genotypes and effects of phosphorous, nitrogen, and potassium deficiencies on strigolactone production. S. Afr. J. Bot. 2017, 108, 15–22. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Lozano, M.D.; Moreno, M.T.; Testillano, P.S.; Rubiales, D. Resistance to broomrape (Orobanche crenata) in faba bean (Vicia faba): Cell wall changes associated with pre-haustorial defensive mechanisms. Ann. Appl. Biol. 2007, 151, 89–98. [Google Scholar] [CrossRef]
- Rubiales, D. Can we breed for durable resistance to broomrapes? Phytopathol. Mediterr. 2018, 57, 170–185. [Google Scholar]
| Accessions | Reported Broomrape Response | Origin/Derived from Accession No. | Reference |
|---|---|---|---|
| Baraca | Resistant | Minor type, broomrape-resistant cultivar released in Spain, derived from Alameda × VF1071 (derived from F402) | [35] |
| 151-4 | Resistant | Minor type, derived from Baraca × VF1273, sister line of cv. Omeya | [21] |
| Quijote | Resistant | Minor type, selection made at Córdoba from Tunisian XBJ90.04-6-2-1-1, itself derived from Sel88Lat.18035xPOL27-3 | [36,37] |
| Navio | Resistant | Minor type, selection made at Córdoba from Tunisian XBJ90.03-16-1-1-1, sister line of cv. Najeh released in Tunisia | [36,37,38,39] |
| Navio2 | Resistant | Minor type, selection made at Córdoba from Navio | |
| Navio3 | Resistant | Minor type, selection made at Córdoba from Navio (light seed cuticle, normal color flowers) | |
| Navio4 | Resistant | Minor type, selection made at Córdoba from Navio (brown seed cuticle, normal color flowers) | |
| Navio6 | Resistant | Minor type, selection made at Córdoba from Navio (brown seed cuticle, normal color flowers) | |
| Navio7 | Resistant | Minor type, selection made at Córdoba from Navio (brown seed cuticle, normal color flowers) | |
| Faraon | Resistant | Minor type, selection made at Córdoba from Misr-1 (brown seed cuticle, normal color flowers) | [40] |
| FaraonSC | Resistant | Minor type, selection made at Córdoba from Misr-1 (light seed cuticle, normal color flowers) | |
| FaraonSCFB | Resistant | Minor type, further selection made at Córdoba from FaraonSC, with white flower | |
| Brocal | Susceptible | Minor type, commercial cultivar | |
| Prothabon | Susceptible | Minor type, commercial cultivar | [41] |
| Zoco | Susceptible | Major type, light cuticle, gourmet market class |
| Environment | Season | Site | Soil Type | Soil pH | Average Tmax (°C) | Average Tmin (°C) | Rain (mm) | Supplementary Irrigation (mm) at Flowering |
|---|---|---|---|---|---|---|---|---|
| Cor-11 | 2010–2011 | Córdoba | Cambisol | 6.5–7 | 19.8 | 8.6 | 514 | 0 |
| Cor-12 | 2011–2012 | Córdoba | Cambisol | 6.5–7 | 20.8 | 5.6 | 145 | 80 |
| Cor-13 | 2012–2013 | Córdoba | Cambisol | 6.5–7 | 18.3 | 7.5 | 497 | 0 |
| Esc-11 | 2010–2011 | Escacena | Fluvisol | 7–7.5 | 19.2 | 9.9 | 534 | 0 |
| Esc-12 | 2011–2012 | Escacena | Fluvisol | 7–7.5 | 20.4 | 8.8 | 134 | 0 |
| Esc-13 | 2012–2013 | Escacena | Fluvisol | 7–7.5 | 18.2 | 8.7 | 472 | 0 |
| Accessions | Cord11 | Cord12 | Cord13 | Esc11 | Esc12 | Esc13 | Average over Environments | SE * |
|---|---|---|---|---|---|---|---|---|
| Quijote | 2300 | 1582 | 4015 | 3593 | 3020 | 2626 | 2856 | 233 |
| Navio6 | 2071 | 423 | 3507 | 3758 | 3778 | 2060 | 2599 | 356 |
| Baraca | 1693 | 878 | 3942 | 2502 | 3473 | 2269 | 2460 | 289 |
| FaraonSC | 1047 | 633 | 3949 | 2851 | 3180 | 2227 | 2315 | 310 |
| Navio3 | 467 | 151 | 3431 | 2771 | 4756 | 2084 | 2277 | 422 |
| Navio7 | 200 | 493 | 3240 | 3120 | 3967 | 2238 | 2210 | 358 |
| Navio | 1338 | 346 | 2775 | 2882 | 3924 | 1949 | 2203 | 329 |
| Navio4 | 1009 | 403 | 3320 | 1433 | 4078 | 2284 | 2088 | 344 |
| 151-4 | 1090 | 655 | 3980 | 2269 | 2007 | 2082 | 2014 | 275 |
| Faraon | 444 | 186 | 3998 | 1329 | 3811 | 2231 | 2000 | 386 |
| FaraonSCFB | 282 | 153 | 3287 | 2760 | 2800 | 2540 | 1970 | 329 |
| Navio2 | 160 | 171 | 3755 | 1727 | 4069 | 1482 | 1894 | 388 |
| Brocal | 221 | 82 | 1556 | 3260 | 3989 | 567 | 1613 | 373 |
| Prothabon | 309 | 80 | 1527 | 1942 | 2836 | 953 | 1275 | 248 |
| Zoco | 159 | 51 | 874 | 676 | 971 | 250 | 497 | 103 |
| Mean | 853 | 419 | 3144 | 2458 | 3377 | 1856 | 2018 | |
| SE | 113 | 69 | 165 | 141 | 192 | 144 | 345 |
| Trait | Random Effects | Estimate | Standard Error | Pr > Chisq | % of Total Variance Explained by |
|---|---|---|---|---|---|
| Grain yield (kg/ha) | E | 1,405,583 | 921,411 | <0.0001 | 59 |
| Block(E) | 74,686 | 40,140 | <0.0001 | 3 | |
| G | 254,266 | 121,579 | <0.0001 | 11 | |
| GEI | 279,434 | 68,173 | <0.0001 | 12 | |
| Residual | 350,352 | 38,180 | <0.0001 | 15 | |
| Broomrape infection (Oc/plant) | E | 0.4879 | 0.3275 | <0.0001 | 20 |
| Block(E) | 0 | 0 | |||
| G | 0.9173 | 0.3752 | <0.0001 | 38 | |
| GEI | 0.1551 | 0.0820 | 0.0287 | 6 | |
| Residual | 0.8824 | 0.0930 | <0.0001 | 36 |
| Accessions | Cord11 | Cord12 | Cord13 | Esc11 | Esc12 | Esc13 | Mean | SE |
|---|---|---|---|---|---|---|---|---|
| Quijote | 0.51 | 0.51 | 0.01 | 0.01 | 0.00 | 0.32 | 0.23 | 0.07 |
| Faraon | 0.24 | 0.35 | 0.04 | 0.00 | 0.00 | 0.90 | 0.26 | 0.09 |
| 151-4 | 0.61 | 0.62 | 0.47 | 0.00 | 0.00 | 0.72 | 0.40 | 0.08 |
| Baraca | 0.24 | 0.89 | 0.58 | 0.00 | 0.00 | 0.73 | 0.41 | 0.12 |
| FaraonSCFB | 0.29 | 0.81 | 0.15 | 0.00 | 0.00 | 1.24 | 0.42 | 0.12 |
| Navio6 | 0.59 | 0.81 | 0.39 | 0.00 | 0.00 | 1.05 | 0.47 | 0.12 |
| FaraonSC | 1.16 | 0.87 | 0.45 | 0.02 | 0.00 | 0.96 | 0.58 | 0.13 |
| Navio4 | 1.30 | 1.32 | 0.49 | 0.04 | 0.00 | 0.77 | 0.65 | 0.15 |
| Navio | 0.89 | 1.18 | 0.54 | 0.02 | 0.00 | 1.36 | 0.67 | 0.14 |
| Navio3 | 1.51 | 1.61 | 0.73 | 0.09 | 0.00 | 1.23 | 0.86 | 0.19 |
| Navio2 | 1.38 | 1.91 | 0.61 | 0.07 | 0.00 | 1.67 | 0.94 | 0.23 |
| Navio7 | 2.29 | 1.54 | 0.18 | 0.06 | 0.00 | 1.81 | 0.98 | 0.24 |
| Brocal | 4.88 | 3.67 | 3.26 | 0.66 | 0.31 | 4.00 | 2.79 | 0.58 |
| Zoco | 4.13 | 4.94 | 4.01 | 0.73 | 0.29 | 3.17 | 2.88 | 0.53 |
| Prothabon | 4.12 | 5.75 | 3.27 | 1.23 | 1.65 | 2.37 | 3.06 | 0.56 |
| Mean | 1.61 | 1.79 | 1.01 | 0.19 | 0.15 | 1.49 | 1.04 | |
| SE | 0.23 | 0.32 | 0.20 | 0.05 | 0.09 | 0.22 | 0.54 |
| Trait | Factor | XO | XS | SD | h2 | SG (%) |
|---|---|---|---|---|---|---|
| Grain yield | FA1 | 2018 | 2557 | 539 | 0.79 | 21.23 |
| Broomrape infection | FA1 | 1.04 | 0.42 | −0.62 | 0.92 | 55.00 |
| Accessions | Cord11 | Cord12 | Esc12 | Mean | SE |
|---|---|---|---|---|---|
| Faraon | 97 | 110 | 112 | 106 | 2.6 |
| Navio2 | 100 | 106 | 112 | 106 | 1.7 |
| FaraonSC | 97 | 118 | 112 | 109 | 3.3 |
| FaraonSCFB | 102 | 115 | 112 | 109 | 2.3 |
| Navio3 | 100 | 115 | 112 | 109 | 2.3 |
| Brocal | 110 | 115 | 112 | 112 | 2.0 |
| Baraca | 100 | 125 | 112 | 112 | 5.7 |
| Quijote | 107 | 117 | 117 | 114 | 2.2 |
| Navio4 | 120 | 117 | 115 | 117 | 1.8 |
| Navio6 | 122 | 117 | 115 | 118 | 1.5 |
| Navio7 | 122 | 117 | 117 | 119 | 1.3 |
| Navio | 122 | 117 | 125 | 121 | 1.4 |
| Prothabon | 126 | 117 | 127 | 123 | 1.7 |
| 151-4 | 129 | 120 | 122 | 124 | 2.1 |
| Zoco | 145 | 138 | 144 | 142 | 1.5 |
| Mean | 113 | 118 | 118 | 116 | |
| SE | 2.2 | 1.4 | 1.4 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiales, D.; Moral, A.; Flores, F. Agronomic Performance of Broomrape Resistant and Susceptible Faba Bean Accession. Agronomy 2022, 12, 1421. https://doi.org/10.3390/agronomy12061421
Rubiales D, Moral A, Flores F. Agronomic Performance of Broomrape Resistant and Susceptible Faba Bean Accession. Agronomy. 2022; 12(6):1421. https://doi.org/10.3390/agronomy12061421
Chicago/Turabian StyleRubiales, Diego, Ana Moral, and Fernando Flores. 2022. "Agronomic Performance of Broomrape Resistant and Susceptible Faba Bean Accession" Agronomy 12, no. 6: 1421. https://doi.org/10.3390/agronomy12061421
APA StyleRubiales, D., Moral, A., & Flores, F. (2022). Agronomic Performance of Broomrape Resistant and Susceptible Faba Bean Accession. Agronomy, 12(6), 1421. https://doi.org/10.3390/agronomy12061421







