Abstract
The search for new sources of plant protein for food and animal feed is driven by an increasing demand in developing countries and the interest in healthy alternatives to animal protein. Seeds from 23 different wild legumes belonging to tribes Gallegeae, Trifolieae, and Loteae were collected in southern Spain and their total amino acid composition was analyzed, by reverse phase-high performance liquid chromatography (RP-HPLC), in order to explore their nutritional value. Protein content in the seeds ranged from 15.5% in Tripodium tetraphyllum to 37.9% and 41.3% in Medicago minima and Medicago polymorpha, respectively. Species belonging to tribe Trifolieae, such as Melilotus elegans and Trifolium spp., showed the most equilibrated amino acid composition and the best theoretical nutritional values, although all species were deficient in sulfur amino acids. The amino acid composition of the seeds from some of these legumes was characterized by high levels of the anticancer non-proteic amino acid canavanine This amino acid was found free in the seeds from some of the species belonging to each of the three tribes included in the present work. Astragalus pelecinus in tribe Gallegea, Trifolium angustifolium in tribe Trifolieae, and Anthyllis vulneraria in tribe Loteae have 3.2%, 3.7%, and 7.2% canavanine, respectively. Seeds from Anthyllis vulneraria, Hymenocarpus lotoides, and Hymenocarpos cornicina have the highest contents in canavanine overall. In conclusion, the seeds from some of these legumes could be used for human consumption and for feeding animals because they contain protein of good nutritional quality. These plants could be useful in domestication and breeding programs for production of new varieties with improved nutritional and functional properties. In addition, some of these species may be of interest as a source of the bioactive compound canavanine.
1. Introduction
Plants represent an ever-growing source of protein for human nutrition because they are necessary to satisfy the increasing population of developing and third world countries. In addition, demand for plants as a source of quality protein is also increasing in developed countries because plants represent a healthier and more environmentally sound resource than animal protein. Thus, animal protein is more expensive to produce and implies a higher intake of saturated fats and cholesterol in comparison with plant protein. An inverse correlation between the consumption of plant foods and diseases—such as diabetes, stroke, cancer, and cardiovascular diseases (CVD)—is well established [1,2].
Legume seeds are in general rich in protein and fiber and poor in fat, with the exception of oilseeds such as soybean and peanut. Legumes and cereals constitute the main source of plant protein in human nutrition, although pulses have twice as much protein as cereals such as wheat, oat, and barley, and three times more than rice [3]. Pulses are also rich in minerals including magnesium, potassium, iron, and zinc. They are also rich in B vitamins and have a low content in sodium [3]. In addition, seed legumes are rich in secondary compounds with health promoting properties that include polyphenols, free amino acids, and proteins with antioxidant and antiproliferative activity [4,5]. Consumption of legumes also reduces the risk of suffering from CVD, which has been related with their benefical effect on risk factors such as cholesterol level, blood pressure, glycemic index, and insulin resistance [6].
Leguminous seeds are an important part of the human diet in most regions of the world, and both the seeds and green parts from many legumes are also used to feed animals. When used for human consumption, leguminous seeds, known as pulses, are usually subjected to softening by soaking in water before cooking [7]. These include pulses like beans, chickpeas, and lentils. Legumes are also grown to feed liverstock, including the green parts of species belonging to genera Medicago, Trifolium, and Vicia [8,9]. Soybean is a very important oilseed crop, and extraction of soybean oil renders a protein rich byproduct [10]. This protein byproduct can be subjected to alkaline extraction and purification for production of protein concentrates and isolates that have very good functional and nutritional properties and are extensively used in the food insustry. Leguminous seeds other than soybean can also be processed to make protein rich food products, facilitating the use of undervalued legumes for human consumption [11]. This is the case for instance of Vicia sativa L., which has been used for production of protein rich extruded products ready for human consumption [12].
Legumes in general have very good agronomic properties, and are able to thrive in harsh conditions that would not allow the use of many other crops. These properties include resistance to thermal and hydric stresses. In addition, nitrogen fixation by legumes reduces de need for fertilizers. Pulses also enjoy a very long shelf life, which greatly facilitates storage and distribution to consumers [13,14,15].
The ‘Green Revolution’ started a process of substituion of many local crops by comercial, more genetically uniform crops. Nevertheless, diversification of cultivars and protection of biodiversity is neccesary to preserve healthy agricultural systems, and greatly increases the chances of crops to survive climate change [16]. The goal of this work was to study the potential of 23 local legumes as new sources of seed quality protein. Amino acid composition was used to determine theoretical nutritional quality. Some of these species are still used, or have been used in the past, as sources of food and animal feed, or even have a history of use in traditional medine.
2. Materials and Methods
2.1. Materials
Diethyl ethoxymethylenemanolate and D, L α-aminobutyric acid were purchased from Fluka and Sigma, respectively. All other chemicals were of analytical grade. Legume seeds were collected from the wild during May and June 2014 in Huelva and Sevilla provinces, Andalucía, Spain. For the identification of legume plants, Flora Iberica (1999) [17] was employed. Seeds at full maturity were collected from several plants in each population and completely dried in the laboratory at room temperature before storage at −20 °C. Voucher specimens of each population are deposited at the Instituto de la Grasa, C.S.I.C., Sevilla, Spain.
2.2. Amino Acid Analysis
Seed flour samples (500 mg) were hydrolyzed by incubation in 6 N HCl at 110 °C for 24 h. Amino acids were determined, in duplicate, after derivatization with diethyl ethoxymethylenemanolate by reverse phase-high performance liquid chromatography (RP-HPLC), according to the method described by Alaiz et al. [18]. using D, L α-aminobutyric acid as internal standard.
The RP-HPLC system (Beckman-Coulter, Inc., Fullerton, CA, USA) consisted of a 126 solvent module, 166 detector, and IBM personal computer. Data acquisition and processing were carried out using 32 Karat 7.0 version software (Beckman-Coulter). Samples (20μL) were injected in a reversed-phase column (Novapack C18, 300 mm × 3.9 mm i.d., 4 μm, Waters).
A binary gradient was used for elution with a flow of 0.9 mL/min. The solvents used were (A) sodium acetate (25 mM) containing sodium azide (0.02% w/v) pH 6.0 and (B) acetonitrile. Elution was as follows: time 0.0–3.0 min, linear gradient from A/B (91:9) to A/B (86/14); 3.0–13.0 min, elution with A/B (86/14); 13.0–30.0 min, linear gradient from A/B (86:14) to A/B (69/31); 30.0–35.0 min, elution with A/B (69:31). The column was maintained at 18 °C. Tryptophan was determined also by RP-HPLC after basic hydrolysis according to Yust et al., (2004) [19]. Protein content was based on amino acid analysis [20].
2.3. Determination of Nutritional Parameters
The amino acid composition of the seeds was used for determination of the following nutritional parameters:
- -
- Amino acid score (chemical score):
- (1)
- (% essential amino acids in sample/% essential amino acid recommended by FAO [21]) × 100
- -
- Protein efficiency ratio (PER) was calculated according to the following equations [22]:
- (2)
- PER1 = −0.684 + 0.456 × Leu −0.047 × Pro
- (3)
- PER2 = −0.468 + 0.454 × Leu −0.105 × Tyr
- (4)
- PER3 = −1.816 + 0.435 × Met + 0.78 × Leu + 0.211 × Hys −0.944 × Tyr
- -
- Predicted biological value (BV) was calculated using the following equation [23]:
- (5)
- BV = 102.15 × Lys0.41 × (Phe + Tyr)0.60 × (Met + Cys)0.77 × Thr0.24 × Trp0.21
where each amino acid symbol represents:
- % amino acid/% amino acid FAO pattern [21], when % amino acid ≤% amino acid FAO pattern [21], or:
- % amino acid FAO pattern [21]/% amino acid, when % amino acid ≥% amino acid FAO pattern [21].
3. Results and Discussion
3.1. Protein Content
As shown in Table 1, protein content in the seeds ranged from 15.5% in Tripodium tetraphyllum (L.) Fourr. to 37.9% and 41.3% in Medicago minima (L.) L. and Medicago polymorpha L., respectively. This is consistent with previous reports for legumes in general [24] and for other wild Mediterranean legumes such as those belonging to genera Vicia [25] and Lathyrus [26]. The highest protein content was found in genus Medicago, and the average protein content in Tribes Gallegeae, Trifolieae, and Loteae were 29.1%, 32.5%, and 27.6%, respectively. The protein content in many of the species in Table 1 was even higher than those reported for commercial legumes such as chickpea (24.7%) [11] and Vicia faba L. (26.6%) [27] although lower than the reported for lupins [28].

Table 1.
Protein content and amino acid composition in seeds. Data expressed as g/100 g protein are the average ± sd of two determinations.
3.2. Amino Acid Composition
The amino acid composition of the legume seeds was within the expected range [29]. All the analyzed species were deficient in the sulfur amino acids Met and Cys. In addition, other deficiencies were found in some of the seeds. Thus, seeds from Astragalus species within tribe Galegeae were deficient in Thr and Lys; Ononis natrix L. and Medicago species belonging to tribe Trifolieae, and species in tribe Loteae were deficient in Lys; Trifolium stellatum L. and Hippocrepis ciliata Willd. were deficient in Thr; Anthyllis vulneraria L. was deficient in His; and Hymenocarpos lotoides (L.) Vis. and Tripodium tethraphyllum (L.) Fourr. were deficient in Leu. The seeds from Melilotus elegans Salzm. ex Ser. and Trifolium species belonging to tribe Trifolieae, with the exception of T. stellatum, did not have any deficiency other than Met and Cys, and provided the amino acid compositions that more closly resembled FAO requirements. Most importantly, the content in Lys exceeded FAO requirements, ranging from 6.2% in Trifolium repens to 6.8% in T. stellatum. The contents in sulfur amino acids and Lys are characteristicaly low in legumes and cereals, respectively. Trp contents were high, in comparison, for example, with wild Lathyrus, ranging from 0.5% to 0.8% [26].
In addition to the 20 amino acids that are present in proteins, many legumes accumulate in their seeds important amounts of free non-proteinogenic amino acids (NPAA) [30]. These legumes are clustered in the NPAA clade that includes most of the herbaceous legumes and is subdivided in several additional subclades [31]. One of these subclades is the inverted-repeat (IR) lacking clade that includes the three legume tribes included in the present study. Species in the IR-lacking clade are caracterized by the absence of an inverted repeat sequence in their chloroplast DNA [32].
Canavanine, an analoge of arginine (Figure 1), is one of the most common NPAA in legume seeds [33].
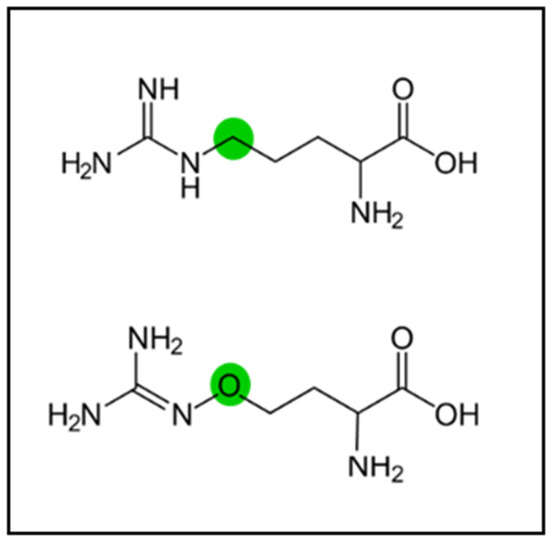
Figure 1.
Chemical structures of arginine (up) and canavanine (down); green dots highlight the differential structural features.
Canavanine has traditionally been considered an antinutritional compound because it can subtitute for arginine in newly synthesized proteins [34]. It is also considered a source of nitrogen for seed germination and seedling growth for some species in which it is very abundant. More recently, some interesting bioactive properties have been attributed to canavanine—including antiproliferative, chemopreventive [35], chemosensitizing, and radiosensitizing [36] activities. Additionally, in vivo studies of the combined effect of canavanine and 5-fluorouracil have been developed [37]. Thus, canavanine is now considered to be a bioactive compound with potential health promoting and therapeutic properties. Canavanine was found in the seeds from some of the species belonging to each of the three tribes included in the present work, and many of them are actually very rich in this NPAA (Table 1). Astragalus pelecinus (L.) Barneby in tribe Gallegea, Trifolium angustifolium L. in tribe Trifolieae, and Anthyllis vulneraria in tribe Loteae have 3.2%, 3.7%, and 7.2% canavanine, respectively. Seeds from Anthyllis vulneraria, Hymenocarpus lotoides and Hymenocarpos cornicina (L.) Vis. have the highest contents in canavanine overall. Thus, the seeds from this group of local, mediterranean legumes include species that represent not only a good source of nutritious protein, but also a source of canavanine as a bioactive compound. Proper removal of canavanine can be readily accomplished in order to prevent possible anti-nutritional effects [38].
3.3. Nutritional Properties
The amino acid composition of proteins is a very good predictor of nutritional quality. It readly affords valuable nutritional information for a large number of samples that can be later confirmed using more time consuming in vitro and in vivo methods [39]. Four different parameters have being calculated using data on the amino acid compositions shown in Table 1. The theoretical protein efficiency ratio (PER) based on the amino acid composition shows a high correlation with real PER, which is determined by calculating weight gain in feeding trials. Theoretical PER values depend on the amount of Leu (PER1); Leu and Tyr (PER2); and Met, Leu, His, and Tyr (PER3). PER values below 1.5 are indicative of low-quality protein and PER values above 2 are characteristics of high quality protein. Calculated PER values were higher in tribe Trifolieae, especially in Trifolium repens L., and the two species of Medicago (Table 2). In general, values in tribe Trifolieae were within the range reported for cultivated legumes such as soybean [40] and chickpea [41], and higher than those reported for wild lupinus [42], peanut [43], and Vigna radiata (L.) R. Wilczek [44]. PER values were also higher than those reported for cereals such as rice and wheat [45]. The lowest PER values were found in genus Astragalus and in Tripodium tetraphyllum. Additionally, genus Scorpiurus showed the lowest PER3 values. In general, the PER values that have been determined in this study are lower than those reported for other wild legumes such as Vicia [25].

Table 2.
Theorethycal nutritional parameters of seed protein based on amino acomposition.
The biological value (BV) for a protein constitutes a theoretical calculation of the amount of ingested protein that is incorporated into the organism [46]. The highest BVs, 52.2 and 52.6, were observed in Astragalus pelecinus (L.) Barneby and A. cymbaecarpos Brot. respectively. These values are lower than BV reported for wild Vicia species such as V. benghalensis L. (67) [25]. The lowest BV was observed in Tripodium tetraphyllum (10.9).
The amino acid score (AAS) and the ratio of essential amino acids to total amino acids (% EAS) are considered to be better indicators of protein quality than BV, even though they are also approximations to the real nutritional value that do not account for amino acid digestibility and availability [47]. In general, tribe Trifolieae showed the highest AAS and % EAS values (Table 2). These two parameters were highest in Trifolium stellatum (123 and 43.3, respectively). On the contrary, Astragalus hamosus L. and A. cymbaecarpos had the lowest AAS and % EAS. These results are similar to those reported for other mediterranean wild legumes such as Vicia [25] and Lathyrus [26]. In summary, species belonging to tribe Trifolieae have the best nutritional profile according to PER, BV, AAS, and % EAS. Species in tribe Gallegeae had the worst PER, AAS, and % EAS values.
3.4. Multivariate Analysis
A multivariate analysis was carried out in order to clasify the legumes according to the seed amino acid composition. As shown in Figure 2, this clasification partially matches taxonomic clasification, and resulted in clades that resemble the distribution in tribes and genera. Nevertheless, the match with taxonomic classification was not perfect. For example, Astragalus species, included in tribe Gallegeae clustered in a single clade, while species in tribe Trifoleae clustered in a single clade that also includes Coronilla glauca L. and Ornithopus compressus L. Several species of the same genera also clustered together, including Astragalus, Scorpiurus, Hymenocarpos, and Medicago species. It is northworthy that Hymenocarpos is correctly resolved from Anthyllis and Dorycnopsis. These genera were until recently included in genus Anthyllis [48]. Hence, our results support the division of Anthyllis in these three genera. In summary, multivariate analysis indicates that data on amino acid composition is consistent with the taxonomic classification of the wild legumes included in the present study.
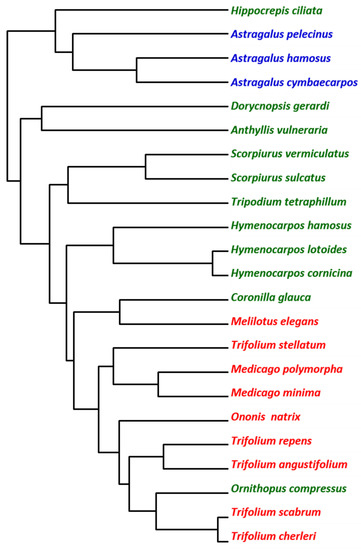
Figure 2.
Dendrogram based on the seed amino acid composition.
4. Conclusions
The analysis of the amino acid composition of the seeds from 23 wild legumes show interesting characteristics from a nutritional and functional point of view. Hence, protein in the seeds of some of these plants is of good nutritional quality and may constitute a valuable resource for human consumption and as animal feed. Species belonging to tribe Trifoliae have the highest content in protein, and also the best nutritional quality in terms of theoretical nutritional parameters. Although some of the species included in the present work have a record of being used as animal feed or for human consumption (see the last column in Table 2), their potential is far greater than currently acknowledged. Some of the species also have a high content in canavanine, and may represent an interesting source of this NPAA with potential pharmacological applications. Further characterization of the nutritional value of the protein by determination of digestibility in vitro (protein corrected amino acids score) or in vivo would be of great interest. These results may constitute the basis for revalorization of some of these legumes, which are a valuable resource for domestication and breeding programmes, as constituents in foods and animal feeds, and for production of canavanine.
Author Contributions
Conceptualization, R.P.F.G. and J.V.; Methodology, Y.E., M.A. and J.V.; Software, Y.E. and J.V.; Formal analysis, Y.E. and J.V.; Investigation, Y.E. and J.V; Resources, Y.E. and J.V.; Data curation, Y.E. and J.V; Writing—original draft preparation, Y.E., J.G.-C. and J.V.; Writing—review and editing, Y.E., M.A., J.G.-C., R.P.F.G. and J.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by C.S.I.C. (Spain). The APC was funded by FCT—Foundation for Science and Technology, I.P. (Portugal), within the scope of the project ref. no. UIDB/00681/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was supported by the FCT—Foundation for Science and Technology, I.P. (Portugal), within the scope of the project ref. no. UIDB/00681/2020. Furthermore, we would like to thank the CERNAS Research Centre and the Polytechnic Institute of Viseu for their support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296. [Google Scholar] [CrossRef]
- Vanamala, J. Food systems approach to cancer prevention. Crit. Rev. Food Sci. Nutr. 2017, 57, 2573–2588. [Google Scholar] [CrossRef]
- FAO. Pulses: Nutritions Seeds for Sustainable Future; Food and Agriculture Organization: Rome, Italy; United Nations: New York, NY, USA, 2016; pp. 1–196. Available online: http://www.fao.org/3/a-i5528e.pdf (accessed on 16 December 2021).
- Rochfort, S.; Panozzo, J. Phytochemicals for Health, the Role of Pulses. J. Agric. Food Chem. 2007, 55, 7981–7994. [Google Scholar] [CrossRef]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Roca-Tor, A.; Aloy-Garcia, M.; Mattivi, F.; Llorach, R.; Lacueva-Andres, C.; Sarda-Urpi, M. Phytochemicals in Legumes: A Qualitative Reviewed Analysis. J. Agric. Food Chem. 2020, 68, 13486–13496. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Pathania, S.; Brites, C.; Rosa, E.A.S.; Barros, A.I.B. Potential of legumes: Nutritional value, bioactive properties, innovative food products, and application of eco-friendly tools for their assessment. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Hawkins, C.; Yu, L.X. Recent progress in alfalfa (Medicago sativa L.) genomics and genomic selection. Crop J. 2018, 6, 565–575. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J. The use of red clover (Trifolium pratense) in soil fertility-building: A review. Field Crops Res. 2018, 221, 38–49. [Google Scholar] [CrossRef]
- Erickson, D.R. Practical Handbook of Soybean Processing and Utilization; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar] [CrossRef]
- Sánchez-Vioque, R.; Clemente, A.; Vioque, J.; Bautista, J.; Millán, F. Protein isolates from chickpea (Cicer arietinum L.): Chemical composition, functional properties and protein characterization. Food Chem. 1999, 64, 237–243. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Drago, S.R.; González, R.J.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Physical and nutritional properties of extruded products based on whole grain with the addition of wild legumes (Vicia lutea subsp. lutea var. hirta and Vicia sativa subsp. sativa). Int. J. Food Sci. Technol. 2013, 48, 1949–1955. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Scheben, A.; Edwards, D.; Spillane, C.; Ortiz, R. Assessing and exploiting functional diversity in germplasm pools to enhance abiotic stress adaptation and yield in cereals and food legumes. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Tani, E.; Abraham, E.; Chachalis, D.; Travlos, I. Molecular, genetic and agronomic approaches to utilizing pulses as cover crops and green manure into cropping systems. Int. J. Mol. Sci. 2017, 18, 1202. [Google Scholar] [CrossRef]
- Jacob, C.; Carrasco, B.; Schwember, A.R. Advances in breeding and biotechnology of legume crops. Plant Cell Tissue Organ Cult. 2016, 127, 561–584. [Google Scholar] [CrossRef]
- Dash, P.K.; Rai, R. Green revolution to grain revolution: Florigen in the frontiers. J. Biotechnol. 2022, 343, 38–46. [Google Scholar] [CrossRef]
- Talavera, S.; Aedo, C.; Castroviejo, S.; Zarco-Romero, C.; Sáez, L.; Salgueiro, F.J.; Velayos, M. Leguminosae, Vol. 7(I). In Flora Ibérica; Castroviejo, S., Ed.; Real Jardín Botánico, CSIC: Madrid, Spain, 1999. [Google Scholar]
- Alaiz, M.; Navarro, J.L.; Girón, J.; Vioque, E. Amino acid analysis by high-performance liquid chromatography after derivatization with diethyl ethoxymethylenemalonate. J. Chromatogr. 1992, 591, 181–186. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Calle-Girón, J.; Vioque, J.; Millán, F.; Alaiz, M. Determination of tryptophan by high-performance liquid chromatography of alkaline hydrolysates with spectrophotometric detection. Food Chem. 2004, 85, 317–320. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Alaiz, M.; Zamora, R. Determination of Peptides and Proteins in Fats and Oils. Anal. Chem. 2000, 73, 698–702. [Google Scholar] [CrossRef]
- Joint FAO/WHO/UNU. Expert Consultation on Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Swiztherland, 1985; 206p. [Google Scholar]
- Alsmeyer, R.H.; Cunnigham, A.E.; Happich, M.L. Equations Predict PER from Amino Acid Analysis. Food Technol. 1974, 28, 34–40. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201303166075 (accessed on 14 December 2021).
- New Method for Prediction of Protein Value from Essential Amino Acid Pattern [Biological Value of Foods, Nitrogen Balance]. Available online: https://agris.fao.org/agris-search/search.do?recordID=US19760098012 (accessed on 13 December 2021).
- Aparna, K.; Khatoon, N.; Prakash, J. Cooking quality and in vitro digestibility of legumes cooked in different media. J. Food Sci. Technol. 2000, 37, 169–173. [Google Scholar]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Nutritional characteristics of seed proteins in 28 Vicia species (Fabaceae) from Southern Spain. LWT—J. Food Sci. 2011, 76, C1118–C1124. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Nutritional characteristics of seed proteins in 15 Lathyrus species (fabaceae) from Southern Spain. LWT—Food Sci. Technol. 2011, 44, 1059–1064. [Google Scholar] [CrossRef]
- Vioque, J.; Alaiz, M.; Giron-Calle, J. Nutritional and functional properties of Vicia faba protein isolates and related fractions. Food Chem. 2012, 132, 67–72. [Google Scholar] [CrossRef]
- Johnson, S.K.; Clements, J.; Villarino, C.B.J.; Coorey, R. Chapter 9. Lupins: Their unique nutritional and health-promoting attributes. In The Gluten-Free Ancient Grains: Cereals, Pseudocereals and Legumes—Sustainable, Nutritious and Health-Promoting Foods for the 21st Century; Taylor, J.R.N., Awika, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility−Digestibility Relationship of Legume Proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]
- Bell, E.A. Nonprotein Amino Acids of Plants: Significance in Medicine, Nutrition, and Agriculture. J. Agric. Food Chem. 2003, 51, 2854–2865. [Google Scholar] [CrossRef]
- Wojciechowski, M.F.; Lavin, M.; Sanderson, M.J. A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. Am. J. Bot. 2004, 91, 1846–1862. [Google Scholar] [CrossRef]
- Cardoso, D.; Pennington, R.T.; de Queiroz, L.P.; Boatwright, J.S.; Van Wyk, B.E.; Wojciechowski, M.F.; Lavin, M. Reconstructing the deep-branching relationships of the papilionoid legumes. S. Afr. J. Bot. 2013, 89, 58–75. [Google Scholar] [CrossRef]
- Rosenthal, G.A. L-Canavanine: A higher plant insecticidal allelochemical. Amino Acids 2001, 21, 319–330. [Google Scholar] [CrossRef]
- Rosenthal, G.A.; Dahlman, D.L. Incorporation of L-canavanine into proteins and the expression of its antimetabolic effects. J. Agric. Food Chem. 2002, 39, 987–990. [Google Scholar] [CrossRef]
- Rosenthal, G.A. L-Canavanine: A Potential Chemotherapeutic Agent for Human Pancreatic Cancer. Pharm. Biol. 1998, 36, 194–201. [Google Scholar] [CrossRef][Green Version]
- Bence, A.K.; Adams, V.R.; Crooks, P.A. L-Canavanine as a radiosensitization agent for human pancreatic cancer cells. Mol. Cell. Biochem. 2003, 244, 37–43. [Google Scholar] [CrossRef]
- Swaffar, D.S.; Ang, C.Y.; Desai, P.B.; Rosenthal, G.A.; Thomas, D.A.; Crooks, P.A.; John, W.J. Combination therapy with 5-fluorouracil and L-canavanine—In-vitro and in-vivo studies. Anti-Cancer Drugs 1995, 6, 586–593. [Google Scholar] [CrossRef]
- Megías, C.; Cortés-Giraldo, I.; Giron-Calle, J.; Alaiz, M.; Vioque, J. Purification of canavanine from the legume Vicia disperma. Biocatal. Agric. Biotechnol. 2016, 5, 150–154. [Google Scholar] [CrossRef]
- Monsoor, M.A.; Yusuf, H.K.M. In vitro protein digestibility of lathyrus pea (Lathyrus sativus), lentil (Lens culinaris), and chickpea (Cicer arietinum). Int. J. Food Sci. Technol. 2002, 37, 97–99. [Google Scholar] [CrossRef]
- Wolzak, A.; Elias, L.G.; Bressan, R. Protein quality of vegetable proteins as determined by traditional biological methods and rapid chemical assays. J. Agric. Food Chem. 1981, 29, 1063–1068. [Google Scholar] [CrossRef]
- Newman, C.W.; Roth, N.J.; Newman, R.K.; Lockerman, R.H. Protein quality of chickpea (Cicer arietinum L.). Nutr. Rep. Int. 1987, 36, 1–8. [Google Scholar]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Analytical nutritional characteristics of seed proteins in six wild Lupinus species from Southern Spain. Food Chem. 2009, 117, 466–469. [Google Scholar] [CrossRef]
- Ghuman, P.K.; Mann, S.K.; Hira, C.K. Evaluation of protein quality of peanut (Arachis hypogaea) cultivars using Tetrahymena pyriformis. J. Sci. Food Agric. 1990. [Google Scholar] [CrossRef]
- Savage, G.P.; Deo, S. The Nutritional Value of Mung Bean and Urd (Vigna radiata var. aureus and var. mungo). Nutr. Abs. Rev. 1989, 59, 639–662. [Google Scholar]
- Friedman, M. Nutritional Value of Proteins from Different Food Sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Sarwar, G.; Blair, R.; Friedman, M.; Gumbmann, M.R.; Hackler, L.R.; Pellett, P.L.; Smith, T.K. Inter- and Intra-laboratory Variability in Rat Growth Assays for Estimating Protein Quality of Foods. J. AOAC Int. 1984, 67, 976–981. [Google Scholar] [CrossRef]
- Paul, M.J. Dietary Protein Quality in Human. AOAC Int. 2005, 88, 874–876. [Google Scholar]
- Degtjareva, G.V.; Valiejo-Roman, C.M.; Samigullin, T.H.; Guara-Requena, M.; Sokoloff, D.D. Phylogenetics of Anthyllis (Leguminosae: Papilionoideae: Loteae): Partial incongruence between nuclear and plastid markers, a long branch problem and implications for morphological evolution. Mol. Phylogenet. Evol. 2012, 62, 693–707. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).