At-ore1 Gene Induces Distinct Novel H2O2-NACs Signaling in Regulating the Leaf Senescence in Soybeans (Glycine max L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene Construction and Plant Transformation
2.2. Plant Material and Growth Condition
2.3. Effect of Hormones and Hydrogen Peroxide
2.4. Total Chlorophyll Content Measurement
2.5. ROS Production and Antioxidant Activity Assays
2.6. Redox Status Measurement
2.7. Phytohormone Analysis
2.8. Analysis of Protein Profiles in Gel-Staining and Thylakoid Membrane Proteins by BN-PAGE
2.9. RNA Extraction and Quantitative Real-Time PCR Analysis
2.10. Metabolic and Cis-Regulatory Element Analysis
2.11. Statistical Analysis
3. Results
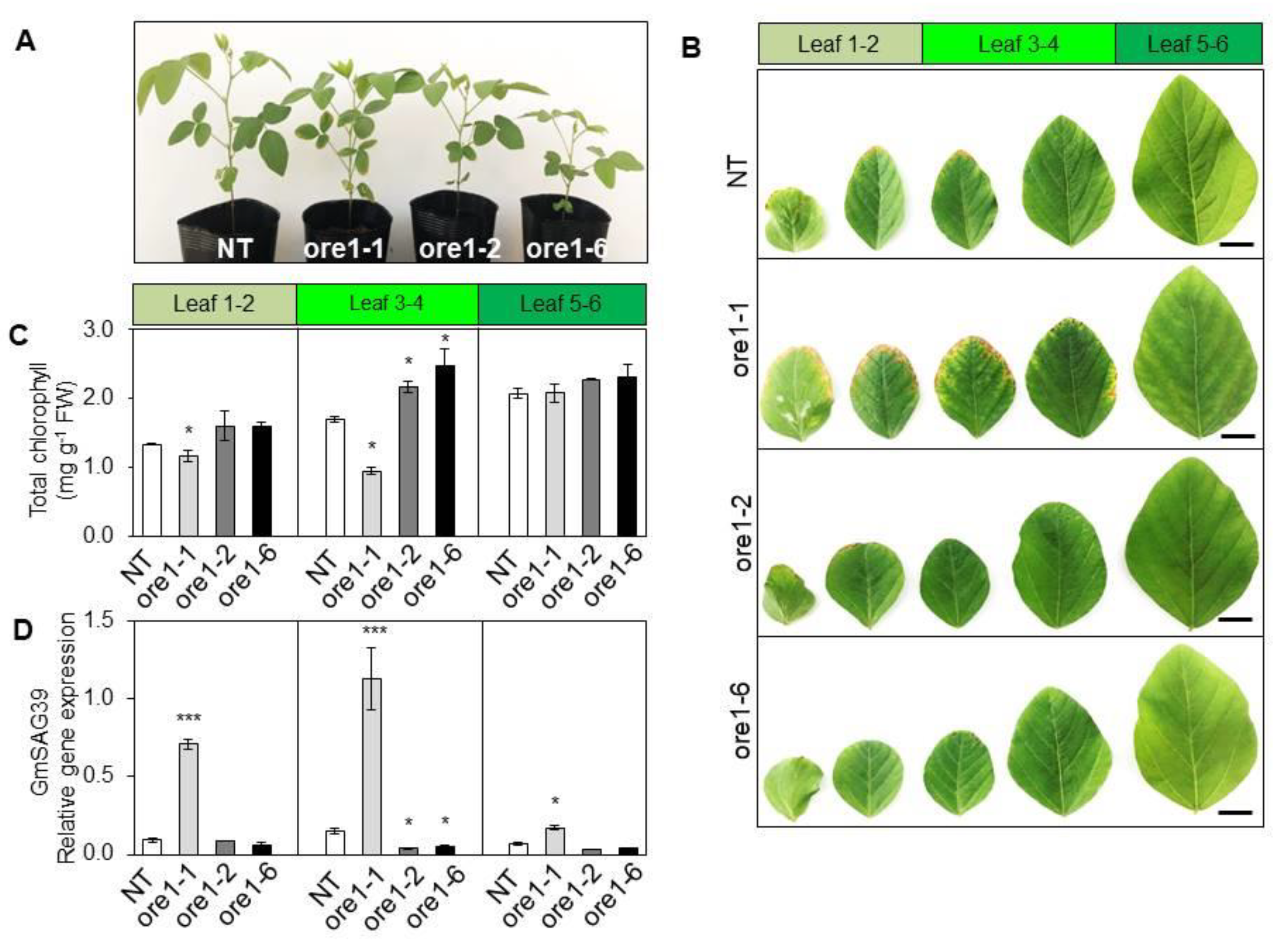
3.1. At-ore1 Gene Alters the Leaf Senescence Phenotype in Soybean
3.2. At-ore1 Confers Divergent Functions on Soybean Leaf Senescence by Regulating the Photosynthesis Complex
3.3. Expression of At-ore1 Defines Typical ABA/IAA Antagonistic Interaction
3.4. Delay of ABA-Induced Leaf Senescence in At-ore1 Soybean Transgenic Lines Is Mediated by Auxin
3.5. At-ore1 Does Not Inhibit Cell Death Typical of Hyper-ROS Accumulation in Soybeans
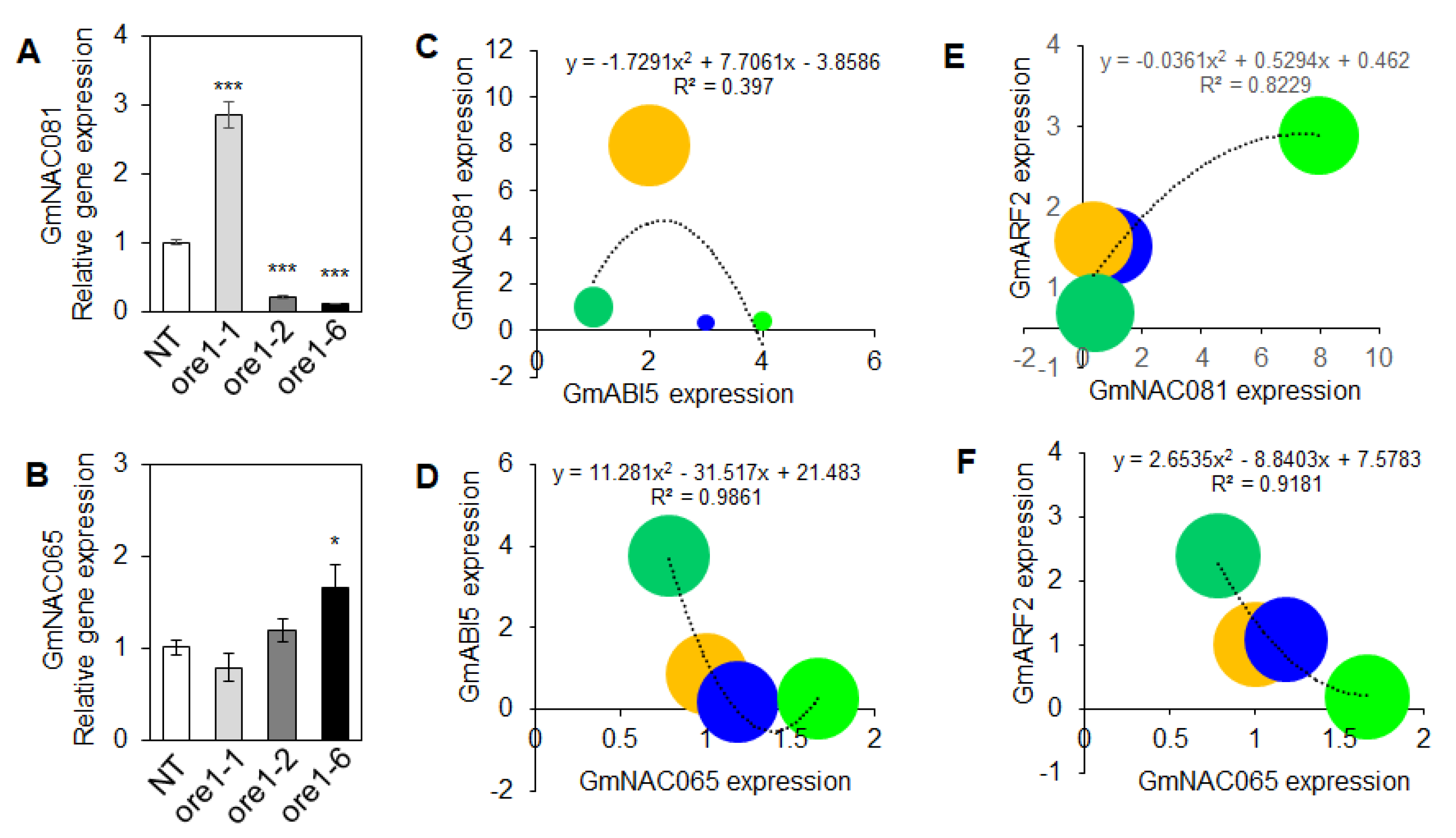
3.6. Downstream Events in the Transcription Factor NACs Regulate Leaf Senescence and Cell Death in the Atore1-Expressing Soybean
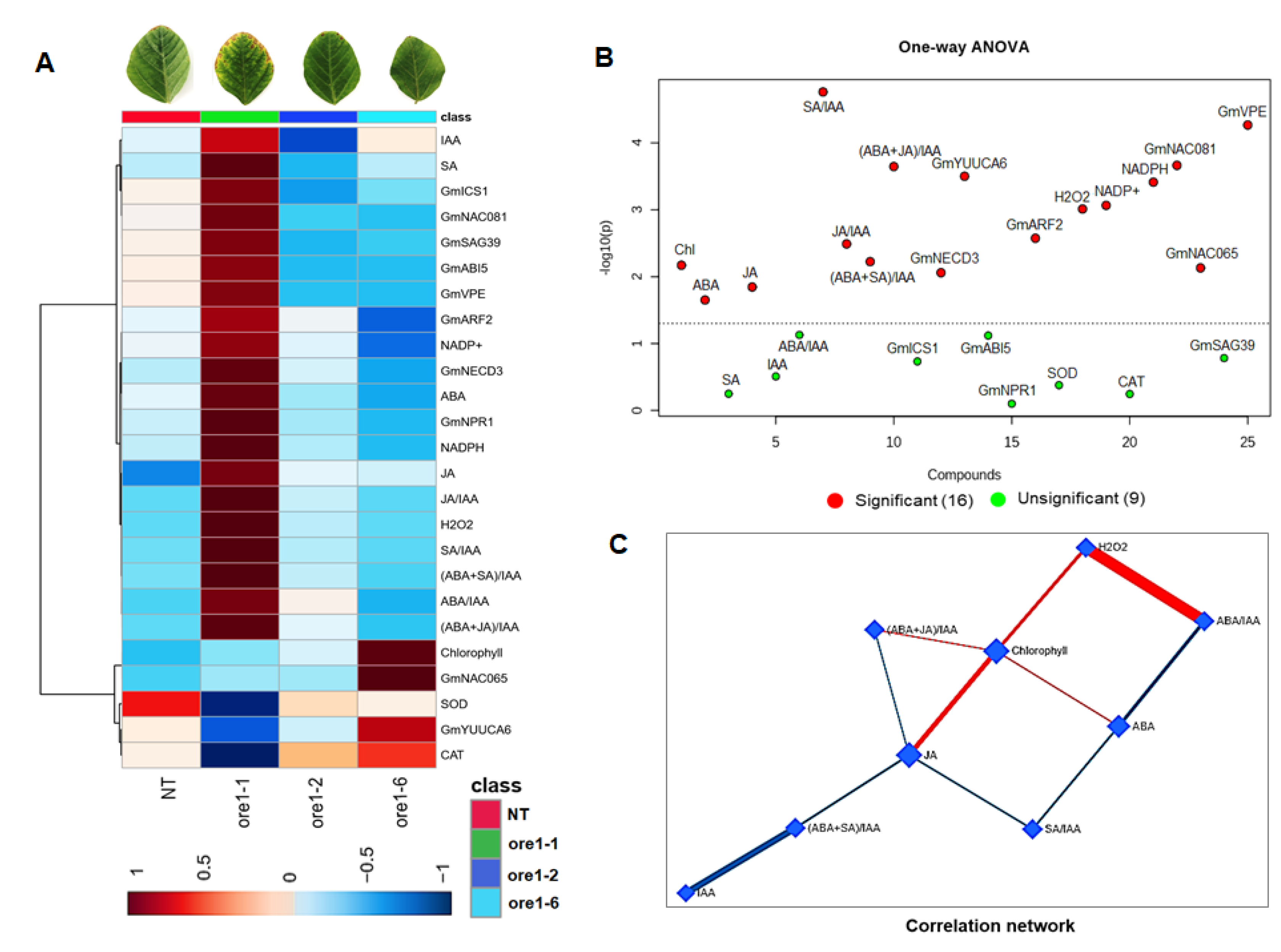
3.7. Identification of the Regulator Factors of Leaf Senescence in Atore1-Expressing Soybeans
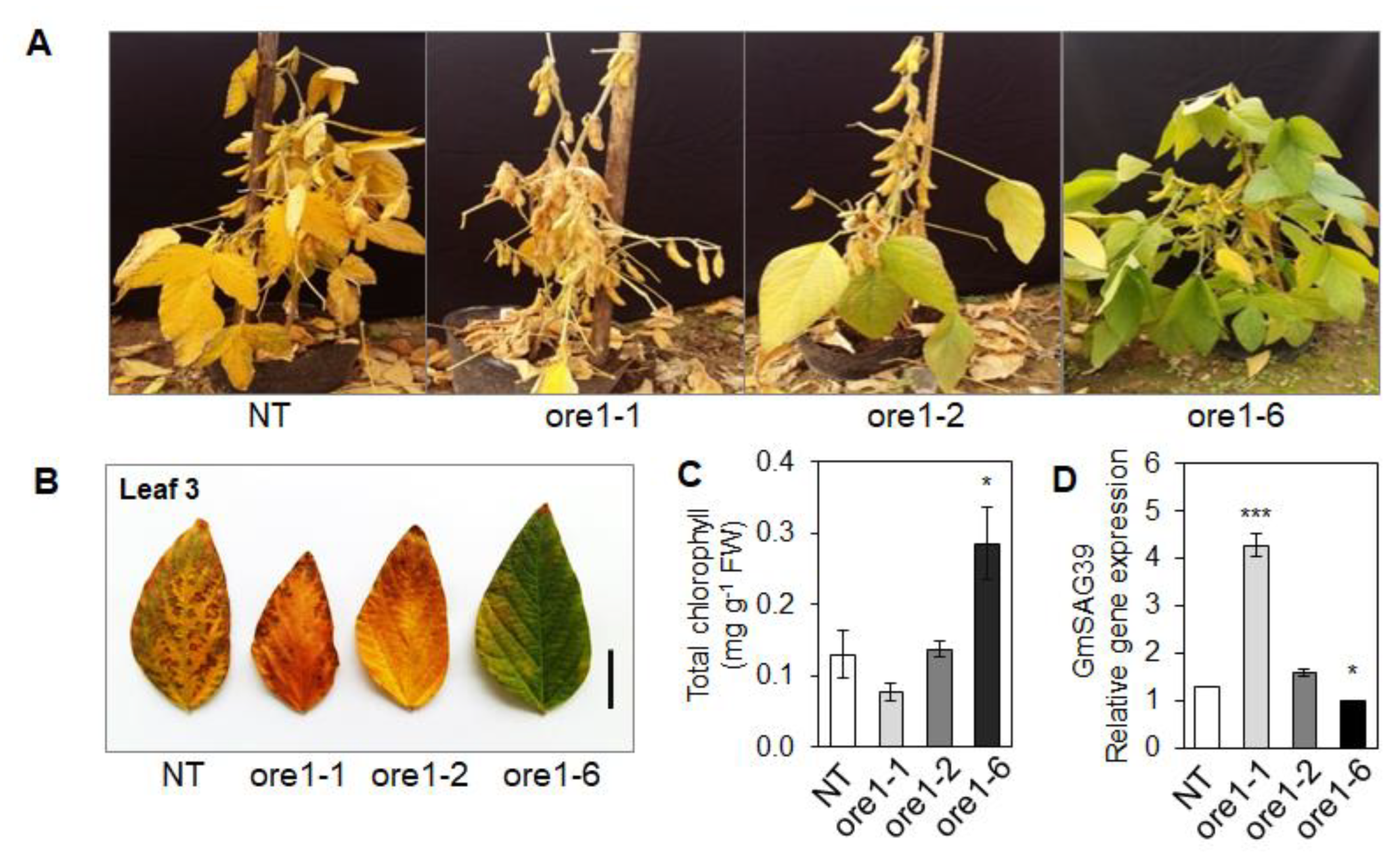
3.8. At-ore1 Induces Seed Yield in Soybeans by Modulating the Photosynthetic Complexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nooden, L.D.; Guiamet, J.J.; John, I. Senescence mechanisms. Physiol. Plant. 1997, 101, 746–753. [Google Scholar] [CrossRef]
- Gao, S.; Gao, J.; Zhu, X.; Song, Y.; Li, Z.; Ren, G.; Zhou, X.; Kuai, B. ABF2, ABF3, and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in Arabidopsis. Mol. Plant 2016, 9, 1272–1285. [Google Scholar] [CrossRef]
- Balazadeh, S.; Riaño-Pachón, D.M.; Mueller-Roeber, B. Transcriptionfactors regulating leaf senescence in Arabidopsis thaliana. Plant Biol. 2008, 1, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.-S.; Penfold, C.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, H.J.; Vu, Q.T.; Jung, S.; McClung, C.R.; Hong, S.H.; Nam, H.G. Circadian control of ORE1 by PRR9 positively regulates leaf senescence in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 8448–8453. [Google Scholar] [CrossRef] [PubMed]
- Matallana-Ramirez, L.P.; Rauf, M.; Farage-Barhom, S.; Dortay, H.; Xue, G.-P.; Dröge-Laser, W.; Lers, A.; Balazadeh, S.; Mueller-Roeber, B. NAC transcription factor ORE1 and senescence-induced BIFUNCTIONAL NUCLEASE1 (BFN1) constitute a regulatory cascade in Arabidopsis. Mol. Plant 2013, 6, 1438–1452. [Google Scholar] [CrossRef]
- Balazadeh, S.; Siddiqui, H.; Allu, A.D.; Matallana-Ramirez, L.P.; Caldana, C.; Mehrnia, M.; Zanor, M.I.; Köhler, B.; Mueller-Roeber, B. A generegulatory network controlled by the NAC transcription factorANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 2010, 62, 250–264. [Google Scholar] [CrossRef]
- Kim, J.H.; Woo, H.R.; Kim, J.; Lim, P.O.; Lee, I.C.; Choi, S.H.; Hwang, D.; Nam, H.G. Trifurcate feed-forward regulation of age-dependent cell deathinvolving miR164 in Arabidopsis. Science 2009, 323, 1053–1057. [Google Scholar] [CrossRef]
- Rauf, M.; Arif, M.; Dortay, H.; Matallana-Ramírez, L.P.; Waters, M.T.; Gil Nam, H.; Lim, P.; Mueller-Roeber, B.; Balazadeh, S. ORE1 balances leaf senescence against maintenance by antagonizing G2-like-mediated transcription. EMBO Rep. 2013, 14, 382–388. [Google Scholar] [CrossRef]
- Peng, Z.; Li, J.; Wen, X.; Guo, H. Ethylene-insensitive 3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell 2013, 25, 3311–3328. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, J.H.; Gil Nam, H.; Lim, P.O. The delayed leaf senescence mutants of Arabidopsis, ore1, ore3, and ore9 are tolerant to oxidative stress. Plant Cell Physiol. 2004, 45, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hong, S.H.; Kim, Y.W.; Lee, I.H.; Jun, J.H.; Phee, B.-K.; Rupak, T.; Jeong, H.; Lee, Y.; Hong, B.S.; et al. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 4023–4036. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Li, Z.; Yang, Z.; Chen, J.; Wu, S.; Zhu, X.; Gao, S.; Ren, G.; Kuai, B.; Zhou, X. EIN3 and ORE1 accelerate de-greening during ethylene-mediated leaf senescence by directly activating chlorophyll catabolic genes in Arabidopsis. PLoS Gene. 2015, 11, 1005–1399. [Google Scholar] [CrossRef]
- Asad, M.A.U.; Zakari, S.A.; Zhao, Q.; Zhou, L.; Ye, Y.; Cheng, F. Abiotic stresses intervene with aba signaling to induce de-structive metabolic pathways leading to death: Premature leaf senescence in plants. Inter. J. Mol. Sci. 2019, 20, 256. [Google Scholar] [CrossRef]
- Lee, I.C.; Hong, S.W.; Whang, S.S.; Lim, P.O.; Gil Nam, H.; Koo, J.C. Age-Dependent Action of an ABA-Inducible receptor kinase, RPK1, as a positive regulator of senescence in Arabidopsis leaves. Plant Cell Physiol. 2011, 52, 651–662. [Google Scholar] [CrossRef]
- Quiles, M.J.; García, C.; Cuello, J. Differential effects of abscisic acid and methyl jasmonate on endo-proteinases in senescing barley leaves. Plant Growth Regul. 1995, 16, 197–204. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef]
- Mao, C.; Lu, S.; Lv, B.; Zhang, B.; Shen, J.; He, J.; Luo, L.; Xi, D.; Chen, X.; Ming, F. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef]
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954. [Google Scholar] [CrossRef]
- He, X.-J.; Mu, R.-L.; Cao, W.-H.; Zhang, Z.-G.; Zhang, J.-S.; Chen, S.-Y. AtNAC2, A transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J. 2005, 44, 903–916. [Google Scholar] [CrossRef]
- Nguyen, T.D.; La, V.H.; Nguyen, V.D.; Bui, T.T.; Nguyen, T.T.; Je, Y.H.; Chung, Y.S.; Ngo, X.B. Convergence of Bar and Cry1Ac Mutant Genes in Soybean Confers Synergistic Resistance to Herbicide and Lepidopteran Insects. Front. Plant Sci. 2021, 12, 698–882. [Google Scholar] [CrossRef] [PubMed]
- Olhoft, P.M.; Flagel, L.E.; Donovan, C.M.; Somers, D.A. Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 2003, 216, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Hanafy, M.S.; Deguchi, M.; Hasegawa, H.; Terakawa, T.; Kitamura, K.; Ishimoto, M. Generation and characterization of herbicide-resistant soybean plants expressing novel Phosphinothricin N-acetyltransferase Genes. Breed. Sci. 2009, 59, 245–251. [Google Scholar] [CrossRef][Green Version]
- Richardson, A.D.; Duigan, S.P.; Graeme, P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Lee, B.R.; Li, L.S.; Jung, W.J.; Jin, Y.L.; Avice, J.C.; Qurry, A.; Kim, T.H. Water deficit-induced oxidative stress and the activa-tionof antioxidant enzymes in white clover leaves. Biol. Plant. 2009, 53, 505–510. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.R.; Islam, M.T.; Park, S.H.; Jung, H.I.; Bae, D.W.; Kim, T.H. characterization of salicylic acid-mediated mod-ulation of the drought stress responses: Reactive oxygen species, proline, and redox state in brassica napus. Environ. Exp. Bot. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Lee, B.R.; Muneer, S.; Park, S.H.; Zhang, Q.; Kim, T.H. Ammonium-induced proline and sucrose accumulation, and their sig-nificance in antioxidative activity and osmotic adjustment. Act. Physiol. Plant 2013, 35, 2655–2664. [Google Scholar] [CrossRef]
- Queval, G.; Noctor, G.A. Plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue ex-tracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 2007, 363, 58–69. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Zhang, Q.; Lee, B.-R.; Park, S.-H.; Zaman, R.; Bae, D.-W.; Kim, T.-H. Evidence of salicylic acid-mediated protein degradation and amino acid transport in mature leaves of Brassica napus. J. Plant Growth Regul. 2015, 34, 684–689. [Google Scholar] [CrossRef]
- Järvi, S.; Suorsa, M.; Paakkarinen, V.; Aro, E.-M. Optimized native gel systems for separation of thylakoid protein complexes: Novel super- and mega-complexes. Biochem. J. 2011, 439, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.; Foyer, C.H.; Kunert, K.J. Two new cysteine proteinases with specific expression patterns in mature and senescent tobacco (Nicotiana tabacum L.) leaves. J. Exp. Bot. 2006, 57, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Jibran, R.; Hunter, D.; Dijkwel, P.P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Livak, J.K.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lim, P.O.; Lee, I.C.; Kim, J.; Kim, H.J.; Ryu, J.S.; Woo, H.R.; Nam, H.G. Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J. Exp. Bot. 2010, 61, 1419–1430. [Google Scholar] [CrossRef]
- Kim, J.I.; Murphy, A.S.; Baek, D.; Lee, S.-W.; Yun, D.-J.; Bressan, R.A.; Narasimhan, M.L. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 3981–3992. [Google Scholar] [CrossRef]
- Faria, J.A.; Reis, P.A.; Reis, M.T.; Rosado, G.L.; Pinheiro, G.L.; Mendes, G.C.; Fontes, E.P. The NAC domain-containing protein, GmNAC6, is a downstream component of the ER stress- and osmotic stress-induced NRP-mediated cell-death signaling pathway. BMC Plant Biol. 2011, 11, 129. [Google Scholar] [CrossRef]
- Fraga, O.; de Melo, B.; Quadros, I.; Reis, P.; Fontes, E. Senescence-Associated Glycine max (Gm)NAC Genes: Integration of Natural and stress-induced leaf senescence. Int. J. Mol. Sci. 2021, 22, 8287. [Google Scholar] [CrossRef]
- Mendes, G.C.; Reis, P.A.B.; Calil, I.P.; Carvalho, H.H.; Aragão, F.J.L.; Fontes, E.P.B. GmNAC30 and GmNAC81 integrate the endoplasmic reticulum stress- and osmotic stress-induced cell death responses through a vacuolar processing enzyme. Proc. Natl. Acad. Sci. USA 2013, 110, 19627–19632. [Google Scholar] [CrossRef]
- Pimenta, M.R.; Silva, P.A.; Mendes, G.C.; Alves, J.R.; Caetano, H.D.N.; Machado, J.P.B.; Brustolini, O.J.B.; Carpinetti, P.A.; Melo, B.P.; Silva, J.C.F.; et al. The stress-induced soybean NAC transcription factor GmNAC81 plays a positive role in developmentally pro-grammed leaf senescence. Plant Cell Physiol. 2016, 57, 1098–1114. [Google Scholar] [CrossRef]
- Melo, B.P.; Lourenço-Tessutti, I.T.; Fraga, O.T.; Pinheiro, L.B.; de Jesus Lins, C.B.D.J.; Morgante, C.V.; Engler, J.A.; Reis, P.A.B.; Grossi-De-Sá, M.F.; Fontes, E.P.B. Contrasting roles of GmNAC065 and GmNAC085 in natural senescence, plant development, multiple stresses and cell death responses. Sci. Rep. 2021, 11, 11178. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, J.S.; Seo, J.S.; Park, B.S.; Chua, N. Arabidopsis ubiquitin-specific proteases UBP12 and UBP13 shape ORE1 levels during leaf senescence induced by nitrogen deficiency. New Phytol. 2019, 223, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, W.; Huang, C.; Yang, J.; Jin, M.; Chen, J.; Pang, D.; Chang, Y.; Li, Y.; Wang, Z. Exogenous abscisic acid coordinating leaf senescence and transport of assimilates into wheat grains under drought stress by regulating hormones homeostasis. Crop. J. 2020, 9, 901–914. [Google Scholar] [CrossRef]
- Teale, W.; Ditengou, F.; Dovzhenko, A.; Li, X.; Molendijk, A.; Ruperti, B.; Paponov, I.; Palme, K. Auxin as a model for the integration of hormonal signal processing and transduction. Mol. Plant 2008, 1, 229–237. [Google Scholar] [CrossRef]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin Response Factor2 (ARF2) and Its Regulated Homeo-Domain Gene Hb33 Mediate Abscisic Acid Response in Arabidopsis. PLoS Gene. 2011, 7, e1002172. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Kim, H.J.; Gil Nam, H.; Lim, P.O. Regulatory network of NAC transcription factors in leaf senescence. Curr. Opin. Plant Biol. 2016, 33, 48–56. [Google Scholar] [CrossRef]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Ann. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef]
- Yang, J.; Worley, E.; Udvardi, M. A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis Leaves. Plant Cell 2014, 26, 4862–4874. [Google Scholar] [CrossRef]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, mendel’s green cotyledon gene, encodes magnesium-dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef]
- Sakuraba, Y.; Han, S.-H.; Lee, S.-H.; Hörtensteiner, S.; Paek, N.-C. Arabidopsis NAC016 promotes chlorophyll breakdown by directly upregulating STAYGREEN1 transcription. Plant Cell Rep. 2015, 35, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Dal Bosco, C.; Lezhneva, L.; Biehl, A.; Leister, D.; Strotmann, H.; Wanner, G.; Meurer, J. Inactivation of the chloroplast ATP synthase gamma subunit results in high non-photochemical fluorescence quenching and altered nuclear gene expression in Arabidopsis Thaliana. J. Biol. Chem. 2003, 279, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Piao, W.; Lim, J.-H.; Han, S.-H.; Kim, Y.-S.; An, G.; Paek, N.-C. Rice ONAC106 inhibits leaf senescence and increases salt tolerance and tiller angle. Plant Cell Physiol. 2015, 56, 2325–2339. [Google Scholar] [CrossRef]
- Hung, K.T.; Kao, C.H. Hydrogen peroxide is necessary for abscisic acid-induced senescence of rice leaves. J. Plant Physiol. 2004, 161, 1347–1357. [Google Scholar] [CrossRef]
- Li, Z.; Wang, F.; Lei, B.; Cao, Z.; Pan, G.; Cheng, F. Genotypic-dependent alteration in transcriptional expression of various CAT Isoenzyme genes in esl mutant rice and its relation to H2O2-induced leaf senescence. Plant Growth Regul. 2013, 73, 237–248. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Tan, X.L.; Chen, J.W.; Liu, Z.L.; Kuang, J.; Lu, W.J.; Chen, W.; Shan, J.Y. BrNAC055, a novel transcriptional ac-tivator, regulates leaf senescence in Chinese flowering cabbage by modulating reactive oxygen species production and chlorophyll degradation. J. Agric. And. Food Chem. 2018, 66, 9399–9408. [Google Scholar] [CrossRef]
- Garapati, P.; Xue, G.-P.; Munné-Bosch, S.; Balazadeh, S. Transcription factor ATAF1 in Arabidopsis promotes senescence by direct regulation of key chloroplast maintenance and senescence transcriptional cascades. Plant Physiol. 2015, 168, 1122–1139. [Google Scholar] [CrossRef]
- Jensen, M.K.; Lindemose, S.; de Masi, F.; Reimer, J.J.; Nielsen, M.; Perera, V.; Workman, C.T.; Turck, F.; Grant, M.R.; Mundy, J.; et al. ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana. FEBS Open Bio 2013, 3, 321–327. [Google Scholar] [CrossRef]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef]
- Balazadeh, S.; Kwasniewski, M.; Caldana, C.; Mehrnia, M.; Zanor, M.I.; Xue, G.-P.; Mueller-Roeber, B. ORS1, an H2O2-Responsive NAC Transcription Factor, Controls Senescence in Arabidopsis thaliana. Mol. Plant 2011, 4, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.S.; Xie, Q.; Fei, J.F.; Chua, N.H. MicroRNA Directs mRNA Cleavage of the Transcription Factor NAC1 to Downregu-Lateauxin Signals for Arabidopsis Lateral Root Development. Plant Cell 2005, 17, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.P.; Fraga, O.T.; Silva, J.C.F.; Ferreira, D.D.O.; Brustolini, O.J.B.; Carpinetti, P.A.; Machado, J.P.B.; Reis, P.A.B.; Fontes, E.P.B. Revisiting the soybean GmNAC Superfamily. Front. Plant Sci. 2018, 9, 1864. [Google Scholar] [CrossRef]
- Ferreira, D.O.; Fraga, O.T.; Pimenta, M.R.; Caetano, H.D.N.; Machado, J.P.B.; Carpinetti, P.A.; Brustolini, O.J.B.; Quadros, I.P.S.; Reis, P.A.B.; Fontes, E.P.B. GmNAC81 inversely modulates leaf senescence and drought tolerance. Front. Genet. 2020, 11, 601876. [Google Scholar] [CrossRef] [PubMed]
- Hara-Nishimura, I.; Hatsugai, N.; Nakaune, S.; Kuroyanagi, M.; Nishimura, M. Vacuolar processing enzyme: An executor of plant cell death. Curr. Opin. Plant Biol. 2005, 8, 404–408. [Google Scholar] [CrossRef]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Ham, L.H.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Differential gene expression in soybean leaf tissues at late developmental stages under drought stress revealed by genome-wide transcriptome analysis. PLoS ONE 2012, 7, e49522. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, A.; Yáñez, A.; Matus, I.A.; Tapia, G.; Castillo, D.; Sanchez-Jardón, L.; Araus, J.L. Physiological traits associated with wheat yield potential and performance under water-stress in a mediterranean environment. Front. Plant Sci. 2016, 7, 987. [Google Scholar] [CrossRef]
- Vadez, V.; Deshpande, S.P.; Kholova, J.; Hammer, G.; Borrell, A.K.; Talwar, H.S.; Hash, C.T. Stay-green quantitative trait loci’s effects on water extraction, transpiration efficiency and seed yield depend on recipient parent background. Funct. Plant Biol. 2011, 38, 553–566. [Google Scholar] [CrossRef]
- Kang, K.; Shim, Y.; Gi, E.; An, G.; Paek, N.-C. Mutation of ONAC096 Enhances Grain Yield by Increasing Panicle Number and Delaying Leaf Senescence during Grain Filling in Rice. Int. J. Mol. Sci. 2019, 20, 5241. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Turečková, V.; Xue, G.-P.; Fernie, A.R.; Mueller-Roeber, B.; Balazadeh, S. The NAC transcription factor SlNAP2 regulates leaf senescence and fruit yield in tomato. Plant Physiol. 2018, 177, 1286–1302. [Google Scholar] [CrossRef]





| Trangenics Lines | Hormone Status | ||||
|---|---|---|---|---|---|
| ABA | SA | JA | IAA | GA3 | |
| NT | 540.712 ± 15.047 b | 25.048 ± 0.784 b | 2.577 ± 0.197 c | 26.857 ± 1.087 b | 11.589 ± 0.438 a |
| ore1-1 | 910.968 ± 29.478 a | 96.824 ± 3.392 a | 6.400 ± 0.238 a | 7.479 ± 0.391 c | 23.381 ± 0.737 b |
| ore1-2 | 485.014 ± 20.767 c | 23.391 ± 0.962 b | 3.388 ± 0.212 b | 13.767 ± 0.641 b | 22.222 ± 0.891 b |
| ore1-6 | 391.326 ± 18.260 d | 24.874 ± 0.649 b | 3.196 ± 0.296 b | 158.01 ± 4.615 a | 17.304 ± 0.848 b |
| Hormone Ratios | ABA/IAA | SA/IAA | JA/IAA | (ABA+SA)/IAA | (ABA+JA)/IAA |
| NT | 20.201 ± 0.386 c | 0.938 ± 0.024 c | 0.096 ± 0.004 c | 21.134 ± 0.410 c | 20.297 ± 0.390 c |
| ore1-1 | 121.884 ± 1.319 a | 12.953 ± 0.113 a | 0.856 ± 0.007 a | 134.836 ± 1.406 a | 122.739 ± 1.324 a |
| ore1-2 | 35.243 ± 0.480 b | 1.700 ± 0.030 b | 0.246 ± 0.003 b | 36.943 ± 0.511 b | 35.489 ± 0.477 b |
| ore1-6 | 7.709 ± 0.049 d | 0.490 ± 0.003 c | 0.062 ± 0.001 c | 8.199 ± 0.048 d | 7.772 ± 0.049 d |
| Motif Name | Logo | Position | Sequence (5′-3′) | Function |
|---|---|---|---|---|
| ABRE-motif |  | +972, +1097 | ACGTG | Cis-acting element involved in the abscisic acid responsiveness |
| TGA-element |  | +1317 | AACGAC | Auxin-responsive element |
| GATA box |  | +1387 | GATA | Chlorophyll a/b binding protein |
| TGACG-motif | 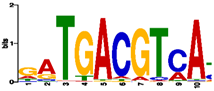 | +1494 | TGACG | Cis-acting regulatory element involved in the MeJA-responsiveness |
| Transgenic Lines | Antioxidant Activity | Redox Status | |||
|---|---|---|---|---|---|
| SOD | CAT | NADP+ | NADPH | NADP+/NADPH Ratio | |
| NT | 6.240 ± 0.98 b | 1.975 ± 0.24 a | 3.293 ± 0.02 b | 2.620 ± 0.05 b | 1.258 ± 0.01 b |
| ore1-1 | 12.839 ± 0.36 a | 1.971 ± 0.20 a | 5.875 ± 0.10 a | 3.749 ± 0.09 a | 1.569 ± 0.04 a |
| ore1-2 | 4.773 ± 0.36c | 2.174 ± 0.31 a | 3.080 ± 0.09 b | 2.592 ± 0.03 b | 1.188 ± 0.03 bc |
| ore1-6 | 4.231 ± 0.43 d | 2.342 ± 0.15 a | 2.564 ± 0.18 c | 2.357 ± 0.04 b | 1.091 ± 0.09 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, V.H.; Nguyen, T.H.A.; Ngo, X.B.; Tran, V.D.; Khuat, H.T.; Bui, T.T.; Tran, T.T.H.; Chung, Y.S.; Nguyen, T.D. At-ore1 Gene Induces Distinct Novel H2O2-NACs Signaling in Regulating the Leaf Senescence in Soybeans (Glycine max L.). Agronomy 2022, 12, 2110. https://doi.org/10.3390/agronomy12092110
La VH, Nguyen THA, Ngo XB, Tran VD, Khuat HT, Bui TT, Tran TTH, Chung YS, Nguyen TD. At-ore1 Gene Induces Distinct Novel H2O2-NACs Signaling in Regulating the Leaf Senescence in Soybeans (Glycine max L.). Agronomy. 2022; 12(9):2110. https://doi.org/10.3390/agronomy12092110
Chicago/Turabian StyleLa, Van Hien, Trinh Hoang Anh Nguyen, Xuan Binh Ngo, Van Dien Tran, Huu Trung Khuat, Tri Thuc Bui, Thi Thu Ha Tran, Young Soo Chung, and Tien Dung Nguyen. 2022. "At-ore1 Gene Induces Distinct Novel H2O2-NACs Signaling in Regulating the Leaf Senescence in Soybeans (Glycine max L.)" Agronomy 12, no. 9: 2110. https://doi.org/10.3390/agronomy12092110
APA StyleLa, V. H., Nguyen, T. H. A., Ngo, X. B., Tran, V. D., Khuat, H. T., Bui, T. T., Tran, T. T. H., Chung, Y. S., & Nguyen, T. D. (2022). At-ore1 Gene Induces Distinct Novel H2O2-NACs Signaling in Regulating the Leaf Senescence in Soybeans (Glycine max L.). Agronomy, 12(9), 2110. https://doi.org/10.3390/agronomy12092110






