Soil Greenhouse Gas Emissions in Different Pastures Implemented as a Management Strategy for Climate Change
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experiment Design
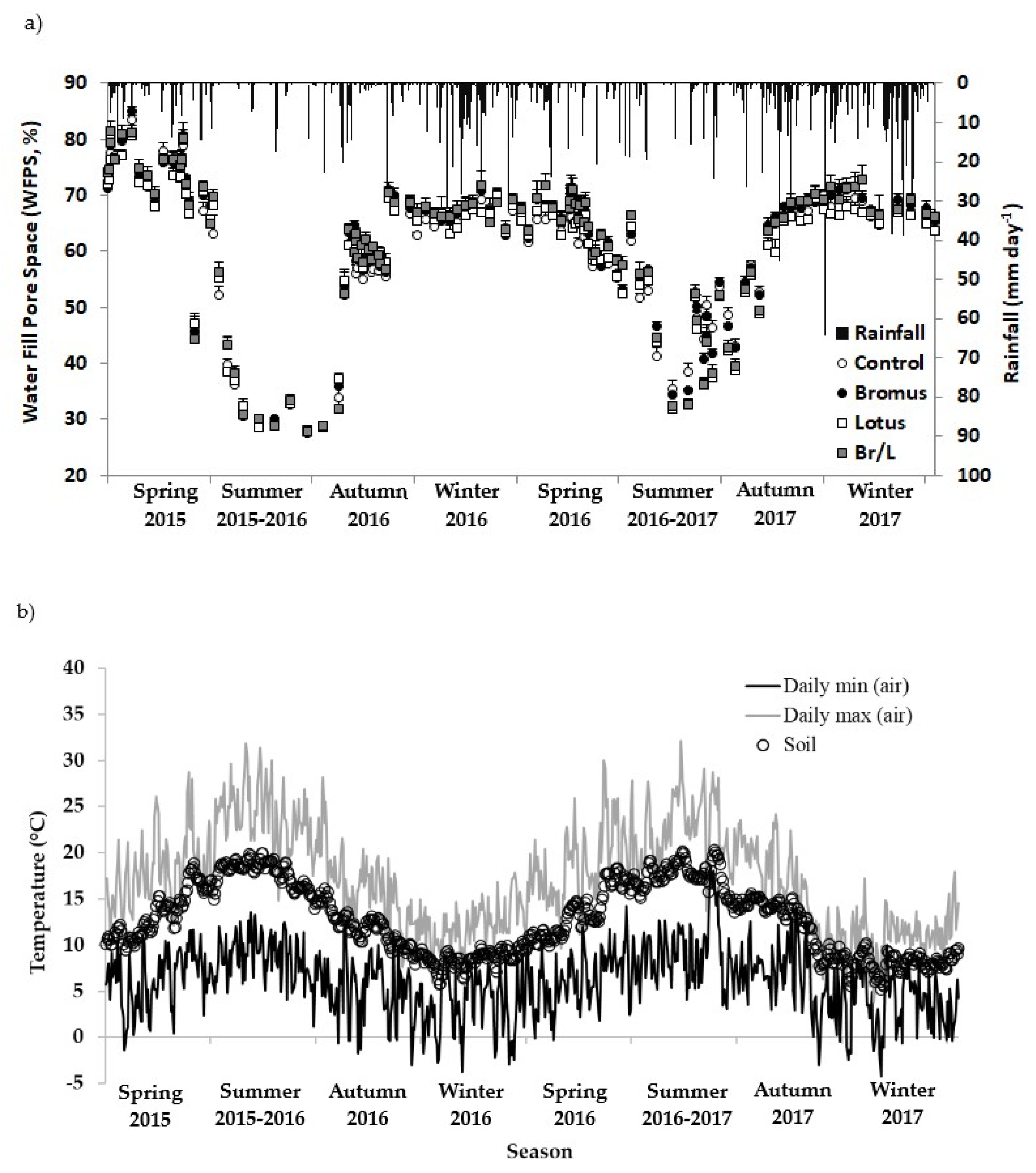
2.3. Soil Environmental and Climatic Measurements
2.4. Soil and Plant Analysis
2.5. Nitrous Oxide (N2O) and Methane (CH4) Flux Measurements
2.6. Flux Calculations
2.7. Equivalent CO2 Emissions (CO2−eq)
2.8. Emission Intensity
2.9. Statistical Analysis
3. Results
3.1. Soil Environmental and Climatic Measurements
3.2. Soil and Plant Analysis
Soil
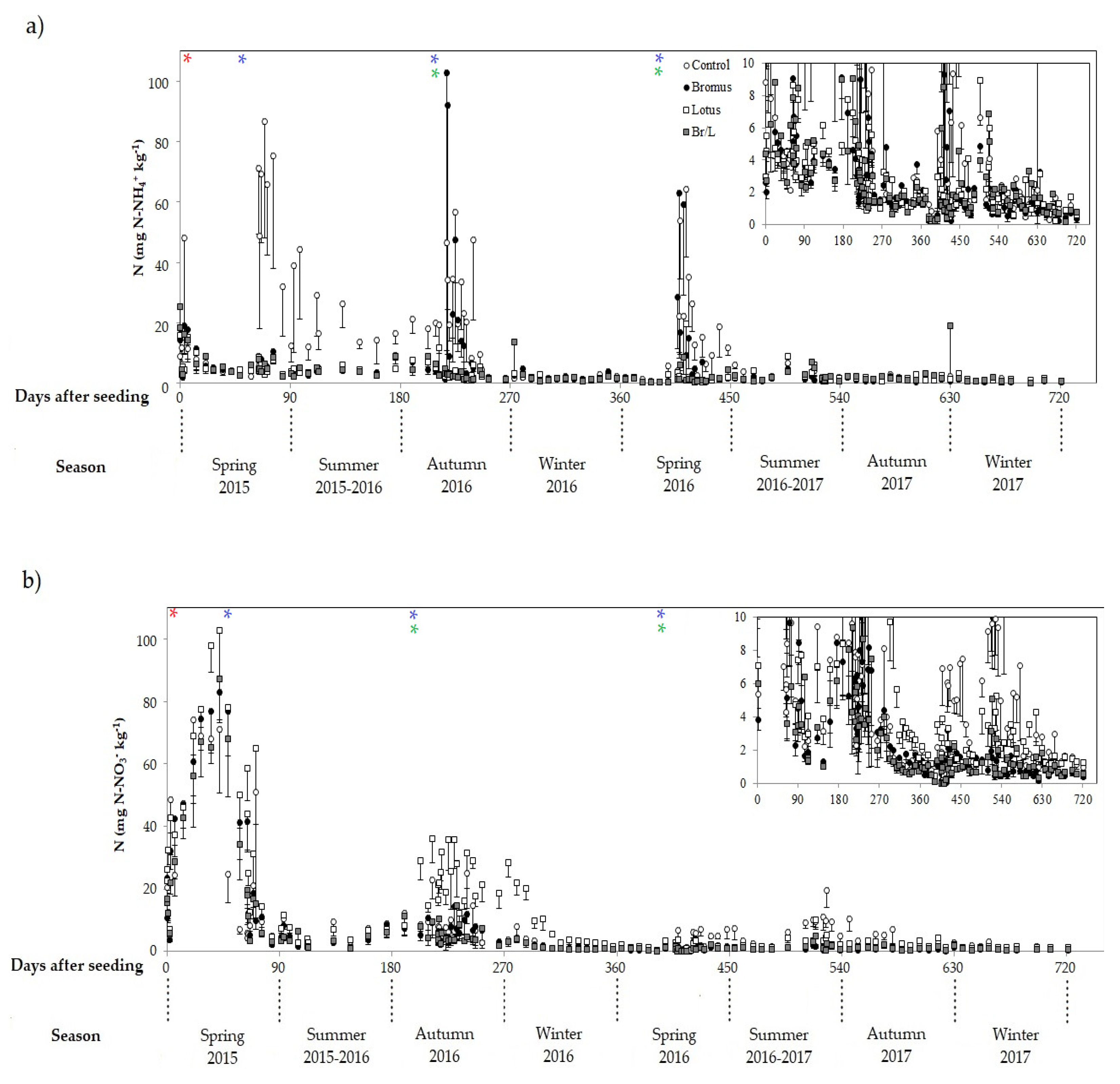
3.3. Fluxes of N–N2O, C–CH4 and CO2 during First Year of Experiment
3.4. Fluxes of N–N2O, C–CH4 and CO2 during Second Year of Experiment
3.5. Average Fluxes of N–N2O, C–CH4 and CO2 during Both Years of the Experiment
3.6. Pasture Yield, Emission Intensity and Cumulative Emissions of N–N2O, C–CH4 and CO2 during the First and Second Years of the Experiment
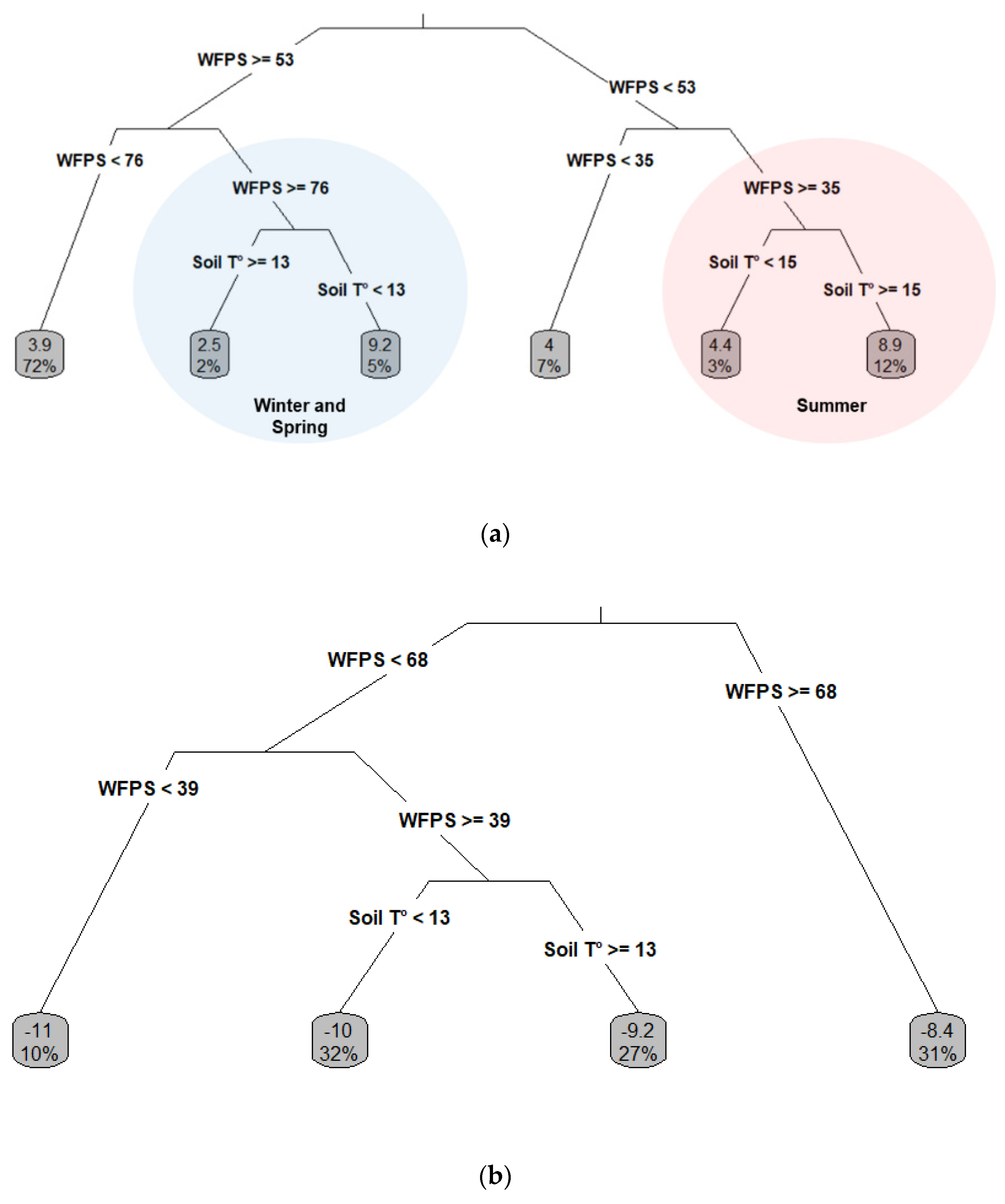
3.7. Key Variables Determining N-N2O and C-CH4 Fluxes
4. Discussion
4.1. Key Variables Driving The N-N2O and C-CH4 Fluxes
4.2. Effects of Pasture Treatment on Methane and Nitrous Oxide Emissions
4.3. Effects of Pasture Treatments on Pasture Yield and Chemical Composition (N Uptake)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brown, M.E.; Funk, C.C. Food Security Under Climate Change. Science 2008, 319, 580–581. [Google Scholar] [CrossRef] [PubMed]
- Anjos, L.J.S.; de Toledo, P.M. Measuring resilience and assessing vulnerability of terrestrial ecosystems to climate change in South America. PLoS ONE 2018, 13, e0194654. [Google Scholar] [CrossRef]
- Bates, B.; Kundzewicz, Z.; Wu, S.; Palutikof, J. Climate Change and Water; Technical Paper of the Intergovernmental Panel on Climate Change, IPCC Secretariat: Geneva, Switzerland, 2008. [Google Scholar]
- Quintana, J.M.; Aceituno, P. Changes in the rainfall regime along the extratropical west coast of South America (Chile): 30–43° S. Atmósfera 2012, 25, 1–22. [Google Scholar]
- Magrin, G.O.; Marengo, J.A.; Boulanger, J.P.; Buckeridge, M.S.; Castellanos, E.; Poveda, G.; Scarano, F.R.; Vicuña, S. Central and South America. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B:Regional Aspects. Con-tribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA,; New York, NY, USA,, 2014. [Google Scholar]
- Soni, S.; Rathore, A.; Sheoran, R.; Singh, S.; Dagar, H.; Loura, D.; Kumar, S.; Paras. Impact of climate change on forage and pasture production and strategies for its mitigation—A review. Forage Res. 2020, 46, 105–113. [Google Scholar]
- Rosenzweig, C.; Tubiello, F.N. Adaptation and mitigation strategies in agriculture: An analysis of potential synergies. Mitig. Adapt. Strateg. Glob. Chang. 2007, 12, 855–873. [Google Scholar] [CrossRef]
- Hopkins, A.; Del Prado, A. Implications of climate change for grassland in Europe: Impacts, adaptations and mitigation options: A review. Grass Forage Sci. 2007, 62, 118–126. [Google Scholar] [CrossRef]
- Joyce, L.A.; Briske, D.D.; Brown, J.R.; Polley, H.W.; McCarl, B.A.; Bailey, D.W. Climate Change and North American Rangelands: Assessment of Mitigation and Adaptation Strategies. Rangel. Ecol. Manag. 2013, 66, 512–528. [Google Scholar] [CrossRef]
- Mora, C.; Caldwell, I.R.; Caldwell, J.M.; Fisher, M.R.; Genco, B.M.; Running, S.W. Suitable Days for Plant Growth Disappear under Projected Climate Change: Potential Human and Biotic Vulnerability. PLoS Biol. 2015, 13, e1002167. [Google Scholar] [CrossRef]
- Settele, J.; Scholes, R.; Betts, R.; Bunn, S.; Leadley, P.; Nepstad, D.; Overpeck, J.T.; Taboada, M.A. Impacts, Adaptation, and Vulnerability. In Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Ghahramani, A.; Howden, S.M.; del Prado, A.; Thomas, D.T.; Moore, A.D.; Ji, B.; Ates, S. Climate Change Impact, Adaptation, and Mitigation in Temperate Grazing Systems: A Review. Sustainability 2019, 11, 7224. [Google Scholar] [CrossRef]
- Luo, W.; Xu, C.; Ma, W.; Yue, X.; Liang, X.; Zuo, X.; Knapp, A.K.; Smith, M.D.; Sardans, J.; Dijkstra, F.A.; et al. Effects of extreme drought on plant nutrient uptake and resorption in rhizomatous vs bunchgrass-dominated grasslands. Oecologia 2018, 188, 633–643. [Google Scholar] [CrossRef]
- Balocchi, O.A.; Caballero, J.M.; Smith, R. Characterization and agronomic variability of ecotypes of Bromus valdivianus Phil. Collected from Valdivia province. Agrosur 2001, 29, 64–77. [Google Scholar] [CrossRef]
- Balocchi, O.A.; Teuber, N. Recursos Forrajeros en Producción de Leche: II. Novedades en Gramíneas y Leguminosas Forrajeras; Instituto de Investigaciones Agropecuarias: Osorno, Chile, 2003. [Google Scholar]
- López, I.F.; Kemp, P.D.; Dörner, J.; Descalzi, C.A.; Balocchi, O.A.; García, S. Competitive Strategies and Growth of Neighbouring Bromus valdivianus Phil. and Lolium perenne L. Plants Under Water Restriction. J. Agron. Crop Sci. 2013, 199, 449–459. [Google Scholar] [CrossRef]
- Singh, D.K.; Bird, P.R.; Saul, G.R. Maximising the use of soil water by herbaceous species in the high rainfall zone of southern Australia: A review. Aust. J. Agric. Res. 2003, 54, 677–691. [Google Scholar] [CrossRef][Green Version]
- Stewart, A.V. Potential value of some Bromus species of the section Ceratochloa. N. Z. J. Agric. Res. 1996, 39, 611–618. [Google Scholar] [CrossRef]
- Capstaff, N.M.; Miller, A.J. Improving the Yield and Nutritional Quality of Forage Crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, A.; Moore, A.D. Impact of climate changes on existing crop-livestock farming systems. Agric. Syst. 2016, 146, 142–155. [Google Scholar] [CrossRef]
- Iannetta, P.P.M.; Young, M.; Bachinger, J.; Bergkvist, G.; Doltra, J.; Lopez-Bellido, R.J.; Monti, M.; Pappa, V.A.; Reckling, M.; Topp, C.F.E.; et al. A Comparative Nitrogen Balance and Productivity Analysis of Legume and Non-legume Supported Cropping Systems: The Potential Role of Biological Nitrogen Fixation. Front. Plant Sci. 2016, 7, 1700. [Google Scholar] [CrossRef]
- Preissel, S.; Reckling, M.; Schläfke, N.; Zander, P. Magnitude and farm-economic value of grain legume pre-crop benefits in Europe: A review. Field Crops Res. 2015, 175, 64–79. [Google Scholar] [CrossRef]
- Morris, P.; Carter, E.B.; Hauck, B.; Lanot, A.; Theodorou, M.K.; Allison, G. Responses of Lotus corniculatus to environmental change 3: The sensitivity of phenolic accumulation to growth temperature and light intensity and effects on tissue digestibility. Planta 2021, 253, 35. [Google Scholar] [CrossRef]
- Putnam, D.H.; Orloff, S.B. Forage Crops. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Oxford, MA, USA, 2014; pp. 381–405. [Google Scholar] [CrossRef]
- CIREN. Descripciones de Suelos, Materiales y Símbolos. Estudio Agrológico X Región. Tomo II; Centro de Información de Recursos Naturales (CIREN): Santiago, Chile, 2003. [Google Scholar]
- Sadzawka, A.; Carrasco, M.A.; Grez, R.; Mora, M.L.; Flores, H.; Neuman, A. Métodos De Análisis Recomendados Para Los Suelos Chilenos. Revisión 2006; Instituto de Investigaciones Agropecuarias INIA: Santiago, Chile, 2006; p. 164. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Applications; Addison Wesley Longman Limited: Essex, UK, 1997. [Google Scholar]
- Sadzawka, A.; Carrasco, M.A.; Demanet, R.; Flores, H.; Grez, R.; Mora, M.L.; Neuman, A. Métodos De Análisis De Tejidos Vegetales, 2nd ed.; Serie Actas N40; Instituto de Investigaciones Agropecuarias INIA: Santiago, Chile, 2007; p. 139. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Análisis de Fibra de Forrajes; Universidad Agraria La Molina: Lima, Perú, 1972. [Google Scholar]
- Saggar, S.; Andrew, R.M.; Tate, K.R.; Hedley, C.B.; Rodda, N.J.; Townsend, J.A. Modelling nitrous oxide emissions from dairy-grazed pastures. Nutr. Cycl. Agroecosystems 2004, 68, 243–255. [Google Scholar] [CrossRef]
- Vistoso, E.; Alfaro, M.; Saggar, S.; Salazar, F. Effect of Nitrogen Inhibitors on Nitrous Oxide Emissions and Pasture Growth After an Autumn Application in Volcanic Soil. Chilean journal of agricultural research 2012, 72, 133–139. [Google Scholar] [CrossRef][Green Version]
- Barton, L.; Kiese, R.; Gatter, D.; Butterbach-Bahl, K.; Buck, R.; Hinz, C.; Murphy, D.V. Nitrous oxide emissions from a cropped soil in a semi-arid climate. Glob. Chang. Biol. 2008, 14, 177–192. [Google Scholar] [CrossRef]
- IPCC. Informe de Síntesis. Contribución de los Grupos de trabajo I, II y III al Quinto Informe de Evaluación del Grupo ntergubernamental de Expertos sobre el Cambio Climático; IPCC: Ginebra, Switzerland, 2014. [Google Scholar]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Lesschen, J.P.; Velthof, G.L.; de Vries, W.; Kros, J. Differentiation of nitrous oxide emission factors for agricultural soils. Environ. Pollut. 2011, 159, 3215–3222. [Google Scholar] [CrossRef]
- Cosentino, V.R.N.; Figueiro Aureggui, S.A.; Taboada, M.A. Hierarchy of factors driving N2O emissions in non-tilled soils under different crops. Eur. J. Soil Sci. 2013, 64, 550–557. [Google Scholar] [CrossRef]
- Da Silva Cardoso, A.; Junqueira, J.B.; Reis, R.A.; Ruggieri, A.C. How do greenhouse gas emissions vary with biofertilizer type and soil temperature and moisture in a tropical grassland? Pedosphere 2020, 30, 607–617. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E.; Livesley, S.J.; Richards, G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: A review. Plant Soil 2008, 309, 43–76. [Google Scholar] [CrossRef]
- Hube, S.; Alfaro, M.A.; Scheer, C.; Brunk, C.; Ramírez, L.; Rowlings, D.; Grace, P. Effect of nitrification and urease inhibitors on nitrous oxide and methane emissions from an oat crop in a volcanic ash soil. Agric. Ecosyst. Environ. 2017, 238, 46–54. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Kotowska, A.; Bubier, J.; Dise, N.B.; Crill, P.; Hornibrook, E.R.C.; Minkkinen, K.; Moore, T.R.; Myers-Smith, I.H.; Nykänen, H.; et al. A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Glob. Chang. Biol. 2014, 20, 2183–2197. [Google Scholar] [CrossRef]
- Mazzetto, A.M.; Barneze, A.S.; Feigl, B.J.; Van Groenigen, J.W.; Oenema, O.; Cerri, C.C. Temperature and moisture affect methane and nitrous oxide emission from bovine manure patches in tropical conditions. Soil Biol. Biochem. 2014, 76, 242–248. [Google Scholar] [CrossRef]
- Azam, F.; Piccinin, G.G.; Dan, L.G.M.; Braccini, A.L.E.; Mariano, D.C.; Okumura, R.S. Agronomic Efficiency of Azospirillum brasilense in Physiological Parameters and Yield Components in Wheat Crop. J. Agron. 2011, 10, 132–135. [Google Scholar]
- Dunfield, P.F. The soil methane sink. In Greenhouse Gas Sinks; Reay, D., Hewitt, C.N., Smith, K.A., Grace, J., Eds.; CAB International: Cambridge, UK, 2007; pp. 152–170. [Google Scholar]
- Shah, A. Determination of Biological Nitrogen Fixation Induced N2O Emission from Arable Soil by Using a Closed Chamber Technique. Appl. Environ. Soil Sci. 2014, 2014, 685168. [Google Scholar] [CrossRef]
- Beltran, I.E.; Gregorini, P.; Morales, A.; Balocchi, O.A.; Pulido, R.G. Interaction between herbage mass and time of herbage allocation modifies milk production, grazing behaviour and nitrogen partitioning of dairy cows. Anim. Prod. Sci. 2019, 59, 1837–1846. [Google Scholar] [CrossRef]
- Beltran, I.E.; Calvache, I.; Cofre, R.; Salazar, F.; Keim, J.P.; Morales, A.; Pulido, R.G.; Alfaro, M. Nitrogen Intake and Its Partition on Urine, Dung and Products of Dairy and Beef Cattle in Chile. Agronomy 2022, 12, 15. [Google Scholar] [CrossRef]
- Parga, J.; Torres, A. Cultivos Forrajeros Para Sistemas Lecheros; Instituto de Investigaciones Agropecuarias: Osorno, Chile, 1993. [Google Scholar]
- Hafner, S.D.; Pacholski, A.; Bittman, S.; Burchill, W.; Bussink, W.; Chantigny, M.; Carozzi, M.; Génermont, S.; Häni, C.; Hansen, M.N.; et al. The ALFAM2 database on ammonia emission from field-applied manure: Description and illustrative analysis. Agric. For. Meteorol. 2018, 258, 66–79. [Google Scholar] [CrossRef]
- López-Aizpún, M.; Horrocks, C.A.; Charteris, A.F.; Marsden, K.A.; Ciganda, V.S.; Evans, J.R.; Chadwick, D.R.; Cárdenas, L.M. Meta-analysis of global livestock urine-derived nitrous oxide emissions from agricultural soils. Glob. Chang. Biol. 2020, 26, 2002–2013. [Google Scholar] [CrossRef]
- Acuña, H.; Concha, A.; Figueroa, M. Condensed Tannin Concentrations of Three Lotus Species Grown in Different Environments. Chil. J. Agric. Res. 2008, 68, 31–41. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Turner, L.R.; Donaghy, D.J.; Lane, P.A.; Rawnsley, R.P. Effect of defoliation management, based on leaf stage, on perennial ryegrass (Lolium perenne L.), prairie grass (Bromus Willdenowii Kunth.) and cocksfoot (Dactylis Glomerata L.) under dryland conditions. 1. Regrowth, tillering and water-soluble carbohydrate concentration. Grass Forage Sci. 2006, 61, 164–174. [Google Scholar] [CrossRef]

| Parameters | Initial | Final | |||||
|---|---|---|---|---|---|---|---|
| Control | Bromus | Lotus | Br/L | SEM | p-Value | ||
| pH H2O (soil:water, 1:2.5) | 5.77 ± 0.04c | 5.94a | 5.96a | 5.91ab | 5.86b | 0.02 | <0.01 |
| pH CaCl2 (soil:CaCl2, 1:2.5) | 4.85 ± 0.04b | 5.13a | 5.14a | 5.12a | 5.07a | 0.02 | <0.01 |
| Organic Matter, g kg−1 | 234.9 ± 7.88 | 229.7 | 237.2 | 235.3 | 242.3 | 4.58 | 0.36 |
| Available N, mg kg−1 | 14.50 ± 2.22 | 18. 75 | 17.40 | 22.60 | 21.24 | 1.66 | 0.22 |
| Olsen P, mg kg−1 | 5.90 ± 0.34c | 16.14b | 17.09a | 11.60a | 13.10b | 0.45 | <0.01 |
| Available S, mg kg−1 | 8.37 ± 0.97a | 3.0b | 4.81b | 3.59b | 4.40b | 0.67 | <0.01 |
| Exchangeable Ca, cmol (+) kg−1 | 2.29 ± 0.16b | 7.01a | 6.99a | 6.61a9a | 6.99a | 0.19 | <0.01 |
| Exchangeable Mg, cmol (+) kg−1 | 0.38 ± 0.04b | 1.06a | 1.09a | 1.00a | 1.0.9a | 0.05 | <0.01 |
| Exchangeable K, cmol (+) kg−1 | 0.29 ± 0.03bc | 0.43bc | 0.52a | 0.32ab | 0.27c | 0.03 | <0.01 |
| Exchangeable Na, cmol (+) kg−1 | 0.10 ± 0.01ab | 0.16a | 0.08b | 0.096b | 0.13ab | 0.02 | <0.01 |
| Al Saturation, % 1 | 4.65 ± 0.67a | 3.90b | 3.39b | 1.5b | 1.30b | 0.27 | <0.01 |
| Bulk density, g cm−3 | 0.68 ± 0.007a | 0.59b | 0.01 | <0.01 | |||
| Particle density, g cm−3 | 2.41 ± 0.068 | No sampling | - | - | |||
| Treatments (T) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Control | Bromus | Br/L 1 | Lotus | SEM | T | Time | TxTime |
| Nitrogen, % | 2.44b | 2.48a | 2.63a | 2.67a | 0.06 | 0.04 | <0.01 | <0.01 |
| Digestibility, % | 78.3a | 75.5b | 75.9ab | 74.9b | 0.644 | <0.01 | <0.01 | <0.01 |
| ME 2, Mcal kg DM−1 | 2.54a | 2.44b | 2.45b | 2.41b | 0.02 | <0.01 | <0.01 | <0.01 |
| Treatment (T) | Season (S) 3 | p Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters 1 | Control | Bromus | Br/L | Lotus | SEM 2 | Aut | Win | Spr | Sum | SEM | T | S | TxS |
| First year | |||||||||||||
| N2O, mg N–N2O m−2 d−1 | 0.404 | 0.419 | 0.404 | 0.460 | 0.031 | 0.320b | 0.334b | 0.621a | 0.412b | 0.027 | 0.54 | <0.01 | 0.07 |
| CH4, mg C–CH4 m−2d−1 | −0.975 | −1.047 | −0.945 | −1.023 | 0.038 | −0.793a | −1.072b | −1.068b | −1.057b | 0.029 | 0.25 | <0.01 | 0.06 |
| CO2−eq, g CO2 m−2 d−1 | 0.128 | 0.134 | 0.13 | 0.151 | 0.012 | 0.091b | 0.098b | 0.227a | 0.126b | 0.011 | 0.54 | <0.01 | 0.03 |
| Second year | |||||||||||||
| N2O, mg N–N2O m−2 d−1 | 0.532a | 0.50a | 0.345b | 0.530a | 0.021 | 0.589b | 0.287d | 0.405c | 0.693a | 0.021 | <0.01 | <0.01 | <0.01 |
| CH4, mg C–CH4 m−2d−1 | −0.853 | −0.886 | −0.909 | −0.941 | 0.042 | −0.881a | −0.841a | −0.891a | −0.976b | 0.028 | 0.5 | <0.01 | 0.09 |
| CO2−eq, g CO2 m−2 d−1 | 0.188 | 0.175 | 0.181 | 0.175 | 0.009 | 0.210b | 0.098c | 0.123c | 0.288a | 0.008 | 0.76 | <0.01 | 0.77 |
| Overall average | |||||||||||||
| N2O, mg N–N2O m−2 d−1 | 0.471 | 0.462 | 0.476 | 0.495 | 0.02 | 0.431c | 0.330d | 0.508b | 0.635a | 0.019 | 0.69 | <0.01 | 0.37 |
| CH4, mg C–CH4 m−2d−1 | −0.92 | −0.969 | −0.932 | −0.99 | 0.034 | −0.990b | −0.959b | −0.839a | −1.021b | 0.022 | 0.46 | <0.01 | 0.31 |
| CO2−eq, g CO2 m−2 d−1 | 0.159 | 0.155 | 0.158 | 0.162 | 0.008 | 1.41c | 0.100c | 0.178b | 0.215a | 0.008 | 0.93 | <0.01 | 0.56 |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Parameters 1 | Control | Bromus | Br/L | Lotus | SEM 2 | p Value 3 |
| First year | ||||||
| N–N2O, kg N ha−1 | 1.49 | 1.55 | 1.48 | 1.67 | 0.09 | 0.39 |
| C–CH4, kg C ha−1 | −3.7 | −3.89 | −3.59 | −3.84 | 0.15 | 0.51 |
| CO2-eq, kg C ha−1 | 997 | 1093 | 1074 | 1113 | 44.8 | 0.34 |
| EI, kg CO2−eq t−1 DM−1 | 88.6d | 180.6c | 228.9b | 298.7a | 11.1 | <0.01 |
| Pasture yield, t DM ha−1 | 11.3a | 6.1b | 4.8b | 3.74c | 0.31 | <0.01 |
| Second year | ||||||
| N–N2O, kg N ha−1 | 1.84 | 1.66 | 1.7 | 1.69 | 0.08 | 0.41 |
| C–CH4, kg C ha−1 | −3.03 | −3.17 | −3.23 | −3.29 | 0.15 | 0.66 |
| CO2−eq, kg CO2 ha−1 | 1125 | 1046 | 1083 | 1048 | 30.5 | 0.27 |
| EI, kg CO2−eq t−1 DM−1 | 141.1ab | 123.8ab | 111.4b | 147.9a | 7.5 | 0.02 |
| Pasture yield, t DM ha−1 | 8.0bc | 8.7ab | 9.8a | 7.1c | 0.37 | <0.01 |
| Treatment (T) | Year (Y) | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter 1 | Control | Bromus | Br/L | Lotus | SEM 2 | 1 | 2 | SEM | T | Y | T*Y |
| N–N2O, kg N ha−1 | 1.67 | 1.61 | 1.59 | 1.68 | 0.058 | 1.55 | 1.73 | 0.05 | 0.63 | <0.01 | 0.24 |
| C–CH4, kg C ha−1 | −3.37 | −3.53 | −3.41 | −3.56 | 0.11 | −3.76 | −3.18 | 0.08 | 0.49 | <0.01 | 0.62 |
| CO2−eq, kg CO2 ha−1 | 1061.4 | 1064.4 | 1078.7 | 1080.6 | 29.1 | 1066.9 | 1075.8 | 19.2 | 0.95 | 0.75 | 0.08 |
| EI, kg CO2eq t−1 DM−1 | 114.9c | 152.2bc | 170.1b | 223.3a | 6.7 | 199.2 | 131.1 | 4.73 | <0.01 | <0.01 | <0.01 |
| Pasture yield, t DM ha−1 | 9.7a | 7.4b | 7.2b | 5.4c | 0.27 | 6.46 | 8.38 | 0.17 | <0.01 | <0.01 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro, M.; Hube, S.; Salazar, F.; Beltrán, I.; Rodriguez, M.; Ramírez, L.; Saggar, S. Soil Greenhouse Gas Emissions in Different Pastures Implemented as a Management Strategy for Climate Change. Agronomy 2022, 12, 1097. https://doi.org/10.3390/agronomy12051097
Alfaro M, Hube S, Salazar F, Beltrán I, Rodriguez M, Ramírez L, Saggar S. Soil Greenhouse Gas Emissions in Different Pastures Implemented as a Management Strategy for Climate Change. Agronomy. 2022; 12(5):1097. https://doi.org/10.3390/agronomy12051097
Chicago/Turabian StyleAlfaro, Marta, Sara Hube, Francisco Salazar, Ignacio Beltrán, Marion Rodriguez, Luis Ramírez, and Surinder Saggar. 2022. "Soil Greenhouse Gas Emissions in Different Pastures Implemented as a Management Strategy for Climate Change" Agronomy 12, no. 5: 1097. https://doi.org/10.3390/agronomy12051097
APA StyleAlfaro, M., Hube, S., Salazar, F., Beltrán, I., Rodriguez, M., Ramírez, L., & Saggar, S. (2022). Soil Greenhouse Gas Emissions in Different Pastures Implemented as a Management Strategy for Climate Change. Agronomy, 12(5), 1097. https://doi.org/10.3390/agronomy12051097







