Abstract
The study monitored the effect of differentiated mineral nutrition of microgreens species by solution of sodium selenate (2 mg Se/L) on the content of Se, chlorophylls, and other minerals. Chlorophylls were measured spectrophotometrically, Se by electrothermal atomic absorption method (ETAAS) with Zeeman-effect background and elements’ concentration was performed by a dual Inductively coupled plasma atomic emission spectroscopy (ICP-OES) iCAP7600 instrument. The content of selenium in fresh weight moved on average from 0.013 to 12.556 μg/g. Selenisation increased the content of Se in all tested species significantly (p < 0.05) without impacting yield. The content of chlorophyll a moved from 249.9 mg/kg (Mizuna) to 604.4 mg/kg (Arugula) with significant differences between the species, without significance (p ≤ 0.05) between tested variants. The influence of selenisation on other minerals significantly differed (p < 0.05) due to the genetic variability. A significant (p ≤ 0.05) increase in Ca was observed in green basil (10.7%) and cress (20.9%); of Fe in green basil (1.6%) and cress (40.9%); of K in arugula (1.6%), green basil (3.9%) and cress (2.8%); of Zn in arugula (2.6%), green basil (8.6%), cress (2.7%) and radish (5.9%); and of Ba in green basil (5.6%) and cress (23.9%).
1. Introduction
Microgreens are gaining increasing recognition among consumers, acclaimed for their freshness and health-promoting properties associated with densely fortified secondary metabolites [1]. Young plants, also called “microgreens”, are different kinds of vegetables, grains and herbs grown to the phenological phase of cotyledons or to the development of the first pair of true leaves [2,3]. At harvest, plant height is from 2.5 to 8 cm depending on the species [4]. They differ from sprouts because they require light and a growing medium and have a longer growth cycle (7–28 days) [5]. Based on sensory and health criteria, countless species are used to produce microgreens including commercial and local varieties, which belong to the following botanical families: Amarillydaceae, Amaranthaceae, Apiaceae, Asteraceae, Brassicaeae, Cucurbitaceae, Fabaceae, Lamiaceae, Oxalidaceae, Poaceae, Polygonaceae and Portulacaceae [1]. Microgreens garner immense potential for improving the nutritional value of the human diet, considering their high content of healthy compounds. On the other hand, they are gaining more and more interest not only for their nutritional value but also for their interesting organoleptic traits and commercial potential [6]. Overall, since microgreens are relatively new specialty commodities, the research in their nutritional quality and health benefits is also at dawn. They are rich in vitamins (e.g., vitamin C), minerals (e.g., copper and zinc) and phytochemicals, including carotenoids and phenolic compounds, which act as antioxidants in human body [7]. These and other health-promoting compounds in various species of microgreens have been confirmed by several authors [8,9,10]. It has been reported that many species of microgreens are more saturated with micronutrients than the adult versions of the same plants [11]. To increase the nutritional value and levels of essential minerals in vegetable food, microgreens are promising targets [10]. Their nutritional value can be improved by biofortification, which increases micronutrient levels during plant growth. Because selenium (Se) plays a significant role in antioxidant defence, biofortification with Se is a good way of improving the nutritional quality of sprouts and microgreens [3]. Organic selenium in plant foods is an efficient and safe source of Se supplements that support human nutrition and health [12]. Selenium (Se), as an essential micronutrient, has a biological activity that it performs mainly through selenoproteins. These specific proteins are responsible for thyroid hormone management, fertility, the aging process and immunity, but their key role is to maintain a redox balance in cells [13]. The Food and Nutrition Board at the Institute of Medicine of the National Academies, US, has recommended, for 19–50-year-old men and women, 45 micrograms (μg) of Se/day as the estimated average requirement (EAR), 55 μg of Se/day as the recommended dietary allowance (RDA) and 400 μg of Se/day as the tolerable upper intake level (UL) [14]. Selenium is generally taken up from the diet through food or other forms of external supplementation. Dietary selenium is obtained in the form of selenomethionine (SeMet), selenocysteine (Sec), selenite and selenate [15]. Therefore, biofortification strategies applied to produce Se-enriched foods could help overcome Se deficiency and its implications in human health and improve the nutraceutical value of food. Despite several scientific works that have dealt with Se-biofortification strategies, the production of Se-enriched foods suitable for animal and human consumption is still challenging [16]. The positive effect on the increased selenium content in fortified plants, as well as on some other monitored phytochemicals (antioxidants) was confirmed by authors Mezeyová, Kápolna, Andrejiová, Puccinelli and Smolen [3,17,18,19,20,21,22]. Experiments conducted on broccoli, radish, alfalfa and mung bean have shown that microgreens can be successfully biofortified with iron [23] and magnesium [24].
The research investigated whether fortification with an aqueous solution of sodium selenate may increase selenium content in chosen microgreens species, as well as whether selenisation impacts the yield and content of other physiologically active components, including chlorophylls and certain minerals. This paper represents unique study because selenium fortification in microgreens has not been investigated yet according to available resources, except for basil [3,25], coriander and tatsoi [25].
2. Materials and Methods
2.1. Plant Material and Growth Conditions
The experiment took place in the laboratory of the Agrobiotech Research Centre, Slovak University of Agriculture (SUA) in Nitra in January 2021. Seeds of commonly used species listed in Table 1 were sown in densities of 1 g per 53 cm2 (189 g/m2). The biological material was provided by obtaining commercially available certified fresh seeds intended for growing microgreens. All variants were repeated 10 times. In the control variant, the seeds of selected plant species were pre-soaked in 200 mL of deionized water for 30 min. The seeds were pre-soaked for 30 min in 200 mL of an aqueous solution of sodium selenate with a selenium content of 2 mg Se/L in the selenised variant. The concentration of selenium was based on a previous experiment, where 5 concentrations were tested (1, 2, 3, 4 and 5 mg Se/L). In the case of the dosage of 2 mg Se/L, the selected species achieved the highest yield. Seeds of microgreens species were cultivated in trays filled with Grodan growing mat EXPERT-composed of basalt wool–rockwool. It is an excellent medium due to its ideal absorption properties and a balanced water/air ratio. The experiment was conducted in the climatic chamber with phytotron system KK 750 FIT P (POL-EKO-APARATURA, Wodzislaw Slaski, Poland), with forced air convection and chamber capacity 749 L and controller microprocessor PID. The artificial lighting was provided through a 12/12 h photoperiod by fluorescent lamps, MASTER TL5 HO 39W/840 SLV/20 (Philips N.V. Philips N.V., The Netherlands), Color Code 840 (4000 K), Luminous Flux 3500 lm. These lights are integrated in the growth chamber from the factory, with a maximum illumination power of 15,000 lux. Day/night temperatures and relative humidity were set at 24/18 °C and 60%/70%, respectively, according to Caracciolo et al., 2020 [1].

Table 1.
Quantitative parameters and growing cycle of microgreen species.
Microgreens were harvested when the cotyledon leaves were fully formed, BBCH 10, (Figure 1) in an order according to their growing cycle (Table 1) using sanitized scissors, cut just above the substrate level. Immediately after harvesting, the fresh biomass was weighed. Analyses of chlorophylls were performed in fresh weight, and selenium and selected micro- and macroelements in dry weight. The plant material was dried at low temperatures (up to 35 °C) using a regulated humidity stream of air. Plants that were sufficiently dry were placed in a tightly covered glass jar (glass did not mist for several hours, the plants were sufficiently dried). Because the recommended daily allowance (RDA) for selenium is expressed in fresh mass, the content was converted from dry to fresh weight.

Figure 1.
Microgreens in the phase of fully developed cotyledon leaves (BBCH 10): (a) Mizuna, (b) Arugula, (c) Green Basil, (d) Cress, (e) Radish, (f) the appearance of the first true leaf in mizuna.
2.2. Quality Parameters Estimation
Chlorophylls—1 g of average fresh vegetable sample was weighted and homogenised by Heidolph Silent Crusher M (Heidolph Instruments, Schwabach, Germany) in acetone. After absolute homogenisation, acetone extract was carefully poured into the fritted glass filters. The pure extract was quantitatively poured into the volumetric flask (50 mL) and filled up with acetone to the final volume. The intensity of extract colour was measured at wave lengths of 649 nm (chlorophyll a) and 665 nm (chlorophyll b) on a spectrophotometer PHARO 200. The zero position was controlled by pure acetone at 750 nm. The possible dispersion value was deducted from individual absorbance values. Calculation of chlorophyll content was provided according to Hegedusova, et al., 2018 [26], in mg/dm3:
in mg/kg:
where:
df = dilution factor (if it is needed);
w = sample weight.
Selenium—An amount of 0.5 g of plant material weighed into a mineralization vessel was mineralized in a microwave mineralizer of the “CEM Mars X” type (microwave digester). After wetting with 1 mL double distilled water, 5 mL of conc. HNO3 and 1 mL of H2O2 was added. The product was mineralized at 150 °C for 20 min and then refilled into volumetric flask up to 25 mL. Electrothermal atomic absorption method (ETAAS) method with Zeeman-effect background have been applied to reduce spectral interference in the case of quantitative selenium analysis. The total selenium content was estimated by using of atomic absorption spectrometer SpectrAA240FS (Varian, Mulgrave Virginia, Australia) [27]. The operating conditions were as follows: cathode selenium lamp—current 10 mA, wavelength 196 nm, slit width of 1.0 nm. The graphite cuvette heated at 2600 °C was used as the atomizing medium. Sample injection volume was 10 μL. Palladium modifier Pd (NO3)2 with a concentration of 0.1 mol·dm−3 was injected into the graphite tube prior to adding the sample and 1% m/V L-ascorbic acid as the reducing agent. Calibration curve was used for determination of tested compounds concentration in an aqueous solution [28].
Minerals—The analysis of selected elements concentration was performed by inductively coupled plasma optical emission spectrometry (ICP-OES) iCAP7600 instrument (Thermo Scentific, Waltham, MA, USA). An EthosUp instrument (Milestone, Italy) was used for microwave mineralization of samples in a mixture of 5 mL HNO3 and 2 mL H2O2. Increasing the temperature to 200 °C lasted 15 min, the temperature of 200 °C was maintained for 15 min, and cooling performed for 30 min. The power of the radio frequency transmitter was 1150 W, the gas flow through the nebulizer was 0.45 L/min, the cooling gas flow was 12 L/min and auxiliary gas flow rate was 0.5 L/min. The exposure time at UV wavelengths was 15 s, at VIS wavelengths 5 s. The samples were repeatedly measured three times. Limit of Detection (LOD) and Limit of Quantification (LOQ) were calculated from the BEC (Background Equivalent Concentration) value determined from the condition of the ratio of the analytical signal intensity and the background intensity S/B = 1. A mixed standard of elements Al, Ag, Ba, Be, Bi, Ca, Cd, Co, Cr, Cs, Cu, Fe, Ga, In, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Rb, Sr, Tl, V and Zn (Multielement standard solution V for ICP, Sigma Aldrich, Merck SA, Darmstadt, Germany) was used for calibration of ICP-OES. The working gas for ICP-OES was argon with a purity of 99.999% (Messer Tatragas, Bratislava, Slovakia). The basic validation characteristics are given in Table 2.

Table 2.
The basic validation characteristics of the method.
A statistical analysis was performed by using of the Statgraphic Centurion XVII (StatPoint Inc., The Plains, VA, USA). Obtained results were evaluated by analysis of variance (ANOVA), and average values were tested by the least significant difference (LSD) test performed at the significance level of 95% (at p < 0.05).
3. Results and Discussion
3.1. Yield
The yield of microgreens species (fresh weight—fw) harvested in fully developed germination leaves (cotyledons) grown from 1 g of seeds moved from 4.42 g fw (green basil, control variant) to 7.61 g (cress, selenised variant). The differences between varieties were significant (p < 0.05) according to Table 3. Average values of fresh weight yield from all tested species were higher in the selenised variant, but the difference was not significant (p < 0.05) according to Figure 2. Newman et al., 2021 [30] used sodium selenate to biofortify basil, cilantro and scallion microgreens in hydroponic settings at different selenium concentrations. At 10.0 mg/L Se, scallion yield declined by 68.0 percent, but was not significantly affected at 5.0 mg/L Se. At 5.0 mg/L Se, basil yield reduced by 35.5 percent. The yield of cilantro, on the other hand, was unaffected by the Se treatments.

Table 3.
Yield of microgreens species after fortification with sodium selenate.
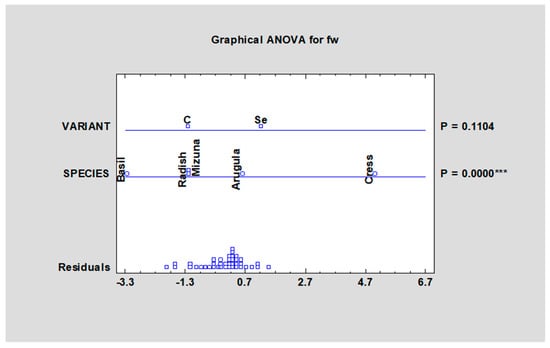
Figure 2.
Yield (fw) of microgreens species after fortification with sodium selenate: fw—fresh weight, control; SeAp—selenium application (2 mg Se/L), *** statistically significant differences at p ≤ 0.001 (***) by LSD in ANOVA (Statgraphic XVII).
The presence of Se in microgreens did not reduce the biomass production and induced a high antioxidant capacity in case of Puccinelli et al., 2019 [3], where basil plants were grown in a nutrient solution, containing 0 (control), 4 or 8 mg Se/L as sodium selenate, to full maturity. The seeds accumulated a high amount of Se and were then used to produce microgreens. Based on average data, rocket species showed higher yield (around 3 kg/m2) compared to the two basil varieties tested by Bulgari et al., 2021 [31], where their results showed high yield, from 2 to 3 kg/m2. A notably lower yield (<3.0 kg fw/m2) was produced by jute, green basil and cress; the highest yield was produced by radish (5.97 kg fw/m2), whereas the other nine microgreens species yielded 3.01–3.93 kg fw/m2 according to Kyriacou et al., 2019 [32]. The increased yield in their study can be attributable to the fact that they collected the microgreens at the stage of first true leaves, rather than cotyledons, as we did. According to Table 3, the yield of radish changed from 1600 (in control variant) to 1833 g/m2 (in the selenised variant), which is similar to the results of Li et al., 2021 [33], where Daikon radish produced the highest fresh shoot weight of 1.70 kg/m2, or 1.58 kg/m2 reported for the related “rapini” (Brassica rapa L.—Broccoletto group) microgreens by Gioia et al., 2017 [5]. Several critical aspects, such as seeding density, fertilization, weather conditions and, lastly, harvest stage, all contribute to differences. Kyriacou, M. et. al., 2021 [34] compared yield in two specific stages—from the appearance of the first (S1) to the second true leaf (S2)—of four Brassicaceae species, and Mizuna’s fw yield harvested in cotyledons was 1.56 kg/m2 in comparison to 2.81 kg/m2 in S2 stage.
3.2. Chlorophylls
The content of chlorophyll a changed from 249.9 mg/kg fw (Mizuna, Se variant) to 604.4 mg/kg fw (Arugula, control variant), with significant differences between the species (Table 4) but without significance between tested variants (Figure 3).

Table 4.
Chlorophyll content in microgreens species after fortification with sodium selenate.
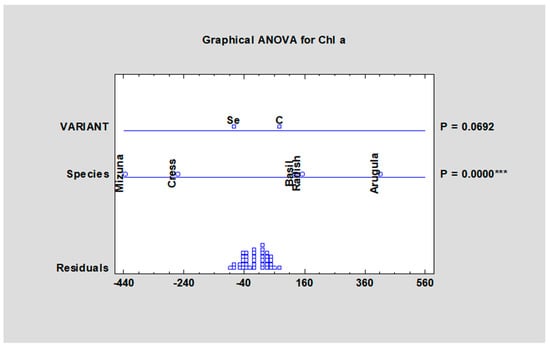
Figure 3.
Chlorophyll a content in microgreens species after fortification with sodium selenate. Fw—fresh weight, control; SeAp—selenium application (2 mg Se/L), *** statistically significant differences at p ≤ 0.001 (***) by LSD in ANOVA (Statgraphic XVII).
The situation was similar in case of chlorophyll b content (Table 4, Figure 4). A higher content of chlorophylls was observed in the microgreens of rocket than in both basil and Swiss chard, which did not show different values in total chlorophylls in the study by Bulgari et al., 2017 [35]. In the case of microgreens, a higher chlorophyll concentration is interesting from a visual appearance standpoint, as customers evaluate their fragrant cotyledons or first true leaves.
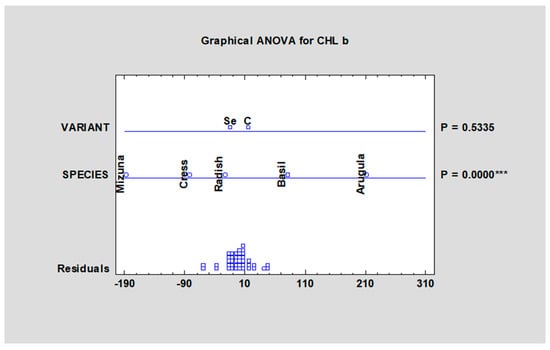
Figure 4.
Chlorophyll b content in microgreens species after fortification with sodium selenate. Fw—fresh weight, control; SeAp—selenium application (2 mg Se/L), *** statistically significant differences at p ≤ 0.001 (***) by LSD in ANOVA (Statgraphic XVII).
Kowitcharoen et al., 2021 [8] tested the bioactive composition of the microgreens, where the total chlorophyll content ranged from 12.35 (green pea) to 112.62 mg/100 g (lentil). The values of Brassicaceae species were as follows: in the case of Broccoli, 52.26; Chinese kale, 58.44; Purple radish, 49.80; and Radish, 59.21 mg/100 g. Although the contents of chlorophylls were significantly different between species, the chlorophyll a/b ratio was found to be similar, except for radish. According to Li et al., 2018 [36], the mean value of Chl a/b was in the range of 1.43–7.07 (mean: 2.47) across the 823 plant species tested on leaf chlorophyll content. According to Slosar, Mezeyova and Hegedusova, 2016 [37] an Chl a/b ratio of mizuna leaves was 2.41 in the case of arugula (2.87) and basil microgreens (2.67) by Bulgari et al., 2017 [35] and in the case of cress (3.97) by Keutgen et al., 2021 [38].
3.3. Selenium Content
Selenium content in fresh weight, moved from 0.0010 μg/g (green basil) to 0.0337 μg/g (cress) in the control variant and from 7.994 μg/g (green basil) to 17.507 μg/g (cress) in the selenised variant (Table 5). Cress incorporated the most selenium into its tissues, and the value in the selenised variant was 519 times higher compared to the control variant. The selenium content in basil reached the lowest value. On the contrary, a positive reaction of basil herb and seeds to biological enrichment through leaf fortification with selenium was found by the authors Mezeyova et al., 2018, 2020 and Puccinelli et al., 2017 [17,19,21]. According to our results, all tested species can be recommended for fortification programs in the form of selenised microgreens because selenisation in general significantly increased the content of Se in all tested species (Table 5, Figure 5).

Table 5.
Selenium content in microgreens species after fortification with sodium selenate.
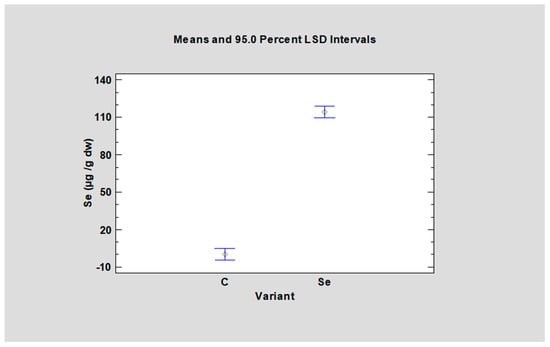
Figure 5.
Selenium content (dw—dry weight) in all tested microgreens species after fortification with sodium selenate. C—control variant; SeAp—selenium application (2 mg Se/L).
The influence of genetical variability in response to fortification was confirmed, as in the control variant, the species created a homogenous group according to Table 5, but after selenisation, they significantly differed (p < 0.05). A similar result was also found by Pannico et al., 2020 [25], where the optimal Se dose that guarantees the effectiveness of Se biofortification and improves the content of bioactive compounds was 16 μM in coriander and tatsoi and 8 μM in green and purple basil. The dose of 2 mg/L can be used for fortification without reducing yield. The selenised variant had higher average fresh mass yield of all examined species (although without significant difference). Se content increased (p ≤ 0.05) with increasing Se treatments for three culinary herbs (basil, cilantro and scallions) according to Newman et al., 2021 [30], where the Se content of fresh scallions increased 98, 202 and 507 times for doses of 2.5, 5.0 and 10.0 mg/L Se, respectively; the Se content of fresh basil increased 64 and 155 times at doses of 2.5 and 5.0 mg/L Se, respectively; and the Se content of fresh cilantro increased 18 and 40 times at 2.5 and 5.0 mg/L Se treatments, respectively. The increased Se content values in our study might be the result of a different method of selenium solution application and a different stage of microgreen harvest. Our plants were harvested at an early stage, and the smaller biomass of microgreens may concentrate the Se content [25] as a “dilution effect” of minerals has been observed as basil plants mature and biomass increases [21]. Application to seeds in laboratory conditions, which is characteristic for growing of microgreens, is suitable because, in this way, there is no contamination of the soil, but the incorporation of selenium into the plant occurs to a large extent. It is very important to maintain an adequate level of Se—both deficiency and excess can be dangerous for human health [13]. According to our findings, 1 g (fw) of fortified microgreens of Mizuna, Arugula, Green Basil, Cress or Radish will cover 23%, 25%, 15%, 32% and 20% of the Recommended Dietary Allowance (RDA), respectively. All microgreen genotypes exhibited an increase in the Se content in response to the biofortification treatments, thereby satisfying the recommended daily allowance for Se (RDA-Se) from 20% to 133% according to Pannico et al., 2020 [25], as they applied sodium selenate solution at three concentrations (0.8 and 16 μM Se) on the bioactive compounds and mineral content of coriander, green basil, purple basil and tatsoi microgreens grown in soilless cultivation. Our findings suggest that 1 g of microgreens provides a proportion of RDA-Se ranging from 14.53 percent to 31.83 percent. Plants that have been fortified are merely a dietary supplement. Because a balanced all-day diet allows a person to receive additional selenium dosages, it is not necessary to adjust one’s entire diet just to consume plant microgreens.
3.4. Mineral Elements
The influence of sodium selenate fortification on other mineral compounds significantly differed (p < 0.05) due to the genetic variability in the tested species (Table 6). The calcium content ranged from 3379.63 μg/g (radish) to 7130.48 μg/g (green basil) in the selenised variants. Green basil had the highest content of Al also in control variant (6440.63 μg/g). Calcium content significantly decreased after sodium selenate fortification in mizuna, arugula and radish. On the contrary, an increase was observed in the case of green basil (about 10.7%) and cress (about 20.9%) in comparison to control. Calcium as a nutrient is most associated with the formation and metabolism of bone. In the circulatory system, extracellular fluid, muscle, and other tissues are critical for mediating vascular contraction and vasodilatation, muscle function, nerve transmission, intracellular signalling, and hormonal secretion [39]. Following Di Gioa, Renna and Santamaria, 2017 [5] analysing the content of the main minerals in few microgreens, it is possible to observe that microgreens represent a good source of potassium and calcium. The contents of Ca and Mg were not affected by Se biofortification in basil or cilantro microgreens; however, both minerals increased in scallions at 10.0 mg/L Se by 59.8% for Ca and 60.6% for Mg according to Newman et al., 2021 [30]. The values of magnesium content in our study moved from 5538.37 μg/g (mizuna, selenised variant) to 18219.51 μg/g (cress, control variant). Mg content was significantly lower in the selenised variant in case of all tested species, except for radish.

Table 6.
Mineral elements content in dry weight (dw) in microgreens species after fortification with sodium selenate.
The significant influence (p < 0.05) of genetical variability in response to fortification with sodium selenate was also detected in the case of iron. The values moved from 81.9 radish (selenised variant) to 513.2 μg/g (cress, selenised variant). Iron is an essential element for almost all living organisms, as it participates in a wide variety of metabolic processes. Disorders of iron metabolism are among the most common diseases of humans and encompass a broad spectrum of diseases with diverse clinical manifestations, ranging from anaemia to neurodegenerative diseases [40]. The increases in the case of green basil (about 1.6%) and cress (about 40.9%) were significant, as were the decreases in the Fe content in mizuna, arugula and radish after fortification. The contents of Fe, Mn, Zn and Ba in scallions were the highest (p ≤ 0.05) in the 10.0 mg/L Se treatment compared with the other Se doses in the study by Newman et al., 2021 [30]. Potassium values ranged in intervals from 3374.25 (radish) to 5593.42 μg/g dw (green basil) in the selenised variants. Significant (p < 0.05) increases were found in arugula (about 1. 6%), green basil (about 3.9%) and in cress (about 2.8%). Radish and arugula demonstrated decreases after selenisation.
The variable response of various species was also found in the trial of Newman et al., 2019 [41], where, at the highest Se treatment for basil and cilantro, 5.0 mg/L, basil increased in potassium, phosphorus, sulphur, total phenolic compounds (102.6%) and antioxidant capacity (68.6%), but plant yield decreased by 35.5%. Cilantro demonstrated increased sodium, total phenolic compounds (50.3%) and antioxidant capacity (66.0%) without an effect on plant yield. According to Table 6, a significant increase in zinc content was found in arugula (2.6%), in green basil (8.6%), in cress (2.7%) and in radish (5.9%). Barium demonstrated a significant (p < 0.05) increase in the case of green basil (5.6%) and cress (23.9%). Mizuna, arugula and radish content were significantly (p < 0.05) decreased after selenisation. In case of Newman et al., 2021 [30], the contents of Fe, Mn, Zn and Ba in scallions were the highest (p ≤ 0.05) in the 10.0 mg/L Se treatment compared with the other Se doses. Basil and cilantro did not demonstrate significant changes in Cu, Fe, Mn, Zn or Ba. Sodium has a specific position within the tested elements, as a reduction in dietary sodium not only decreases the blood pressure and the incidence of hypertension but is also associated with a reduction in morbidity and mortality from cardiovascular diseases [42]. Decrease after selenisation in mizuna (about 10.9%), arugula (about 12.8%) and radish (about 6.1%) is favourable. The effect of selenisation on mineral elements content was very variable, and when comparing the data of all tested species together, the differences were not significant (p < 0.05), as shown in Figure 6. When examining the correlation relationships between the individual elements in the control variant, no positive or negative correlation was identified between any pair of elements. A positive correlation between selenium and zinc concentration was found in the selenised form, when the average Zn concentration increased by 4.9 percent, from 75.10 mg/kg to 78.84 mg/kg (about 4.9%). As a result, it can be concluded that selenisation at the applied dose significantly increases the Se content while also increasing the Zn content. There was no noticeable change in the content of any other element as a result of the application of Se. Statistically significant (p < 0.05) synergistic relationships were shown in pairs of Al and Fe and Ba and Ca after the Se fortification of oyster mushroom in Golian at al., 2022 [29].The results of Pinto et al., 2015 [43] showed that microgreens possess a higher content of most minerals (Ca, Mg, Fe, Mn, Zn, Se and Mo) and a lower NO3– content than mature lettuces. According to Xiao et al., 2012 [4], compared to their mature-leaf counterparts, microgreens contain higher amounts of important phytonutrients (ascorbic acid, β-carotene, α-tocopherol, and phylloquinone). The nutraceutical profile of this popular food can be improved by biofortification with sodium selenate, which has a significant effect on selenium content.
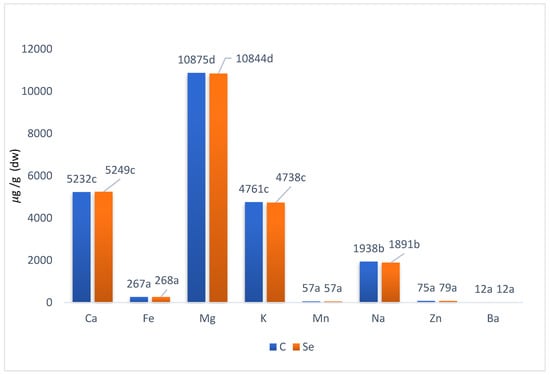
Figure 6.
Mineral elements content (dw—dry weight) in all tested microgreens species after fortification with sodium selenate. C—control variant; Se—selenium application (2 mg Se/L). Values are the average content of mineral substances within all tested species (SD), the different letters represent statistically significant differences between the observed varieties and variants at p < 0.05 by LSD in ANOVA (Statgraphic XVII).
4. Conclusions
The obtained results reveal new potential for the fortification of five microgreens species by raising the concentration of an essential element, such as selenium, without affecting yield (at the specified and verified sodium selenate dose of 2 mg Se/L). Slovak soils are poor in selenium. In this context, crop fortification appears to be a suitable alternative way of increasing Se intake in the human diet. The plant microgreens studied (mizuna, arugula, cress, green basil and radish) showed significant (p < 0.05) potential for selenium incorporation, making them ideal for this purpose. Mizuna has the highest Se content (17.507 g/g fw), and its benefit is a shorter duration from seed to harvest, especially when compared to basil. The recommended daily dose allowance (RDA) of selenium for an adult (55 μg) will be covered by 4.4 g of fresh mizuna microgreens, 3.1 g cress, 5.0 g radish, 4.0 g arugula and 6.9 g green basil harvested in cotyledons after the selenisation of their seeds with sodium selenate in a dose of 2 mg Se/L. The content of chlorophylls (fw) was the highest in case of arugula, with significant differences between the species but without significance between tested variants. Cress reached the highest values of tested mineral elements (dw) in the case of Al, Fe and Mg and green basil in Ca, K, Mn, Zn and Ba. As a response of the monitored species to selenisation, there was a significant (p < 0.05) increase in all monitored minerals in the case of basil; in the case of cress, there was an increase in all minerals except magnesium; and in other species, the results were variable. The selenium content of all studied species was significantly higher (p < 0.05) than the control, and as the microgreen plants are suitable for direct consumption without any post-harvest treatment, with a wide range of flavours and smells, it is an interesting form of nutritional enrichment.
Author Contributions
Conceptualization, I.M. and M.G.; methodology, I.M. and A.H.; validation, A.H.; formal analysis, J.M.; investigation, I.M.; resources, I.M. and M.Š.; writing—original draft preparation, I.M.; writing—review and editing, I.M. and A.H.; supervision, A.H.; funding acquisition, A.H., J.M., A.A. and M.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by KEGA, grant number KEGA 004SPU-4/2022, “Interactive Classroom for Horticulture study program in the Context of Innovation of the Current Student’s Teaching Process” and the project, Development of Theoretical Knowledge, and Practical Skills of Students for Teaching of Subject Vegetable Production, KEGA 018SPU-4/2020.
Data Availability Statement
Institute of Horticulture, Faculty of Horticulture and Landscape Engineering, Slovak University of Agriculture, Nitra 94901, Slovakia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; de Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]
- Andrejiová, A.; Hegedűsová, A.; Mezeyová, I.; Kóňová, E. Content of Selected Bioactive Substances in Dependence on Lighting in Microgreens. Acta Hortic. Regiotect. 2017, 20, 6–10. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Production of Selenium-Biofortified Microgreens from Selenium-Enriched Seeds of Basil. J. Sci. Food Agric. 2019, 99, 5601–5605. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of Vitamin and Carotenoid Concentrations of Emerging Food Products: Edible Microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Springer: Boston, MA, USA, 2017; pp. 403–432. [Google Scholar] [CrossRef]
- Renna, M.; Paradiso, V.M. Ongoing Research on Microgreens: Nutritional Properties, Shelf-Life, Sustainable Production, Innovative Growing and Processing Approaches. Foods 2020, 9, 826. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional Quality and Health Benefits of Microgreens, a Crop of Modern Agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Kowitcharoen, L.; Phornvillay, S.; Lekkham, P.; Pongprasert, N.; Srilaong, V. Bioactive Composition and Nutritional Profile of Microgreens Cultivated in Thailand. Appl. Sci. 2021, 11, 7981. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.R.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chen, P.; Yu, L.; Stommel, J.R. Microgreens of Brassicaceae: Genetic Diversity of Phytochemical Concentrations and Antioxidant Capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Duchovskis, P. Nutrient Levels in Brassicaceae Microgreens Increase Under Tailored Light-Emitting Diode Spectra. Front. Plant Sci. 2019, 10, 1475. [Google Scholar] [CrossRef]
- Tan, L.; Nuffer, H.; Feng, J.; Kwan, S.H.; Chen, H.; Tong, X.; Kong, L. Antioxidant Properties and Sensory Evaluation of Microgreens from Commercial and Local Farms. Food Sci. Hum. Wellness 2020, 9, 45–51. [Google Scholar] [CrossRef]
- Ye, M.; Li, J.; Yu, R.; Cong, X.; Huang, D.; Li, Y.; Chen, S.; Zhu, S. Selenium Speciation in Selenium-Enriched Plant Foods. Food Anal. Methods 2022, 1–13. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef] [PubMed]
- Stoffaneller, R.; Morse, N. A Review of Dietary Selenium Intake and Selenium Status in Europe and the Middle East. Nutrients 2015, 7, 1494–1537. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.-S.; Kim, J.-H. The Role of Selenium Metabolism and Selenoproteins in Cartilage Homeostasis and Arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Mezeyová, I.; Hegedűsová, A.; Hegedűs, O.; Farkaš, J.; Šlosár, M. Qualitative Parameters of Less Grown Basils Depending on Nutrition in the Form of Selenium. Int. J. Agric. For. Life Sci. 2018, 2, 164–170. [Google Scholar]
- Kápolna, E.; Laursen, K.H.; Husted, S.; Larsen, E.H. Bio-Fortification and Isotopic Labelling of Se Metabolites in Onions and Carrots Following Foliar Application of Se and 77Se. Food Chem. 2012, 133, 650–657. [Google Scholar] [CrossRef]
- Mezeyová, I.; Hegedusová, A.; Hegedus, O.; Vargová, A.; Timoracká, M.; Šlosár, M.; Andrejiová, A.; Juríková, T.; Mezey, J. Basil Seeds as a Source of Antioxidants Affected by Fortification with Selenium. Folia Horticulturae 2020, 32, 11–20. [Google Scholar] [CrossRef]
- Andrejiová, A.; Hegedűsová, A.; Mezeyová, I. Effect of Genotype and Selenium Biofortification on Content of Important Bioactive Substances in Tomato (Lycopersicon Esculentum Mill.) fruits. J. Int. Sci. Publ. 2016, 4, 8–18. [Google Scholar]
- Puccinelli, M.; Malorgio, F.; Rosellini, I.; Pezzarossa, B. Uptake and Partitioning of Selenium in Basil (Ocimum Basilicum L.) Plants Grown in Hydroponics. Scientia Horticulturae 2017, 225, 271–276. [Google Scholar] [CrossRef]
- Smoleń, S.; Skoczylas, Ł.; Ledwożyw-Smoleń, I.; Rakoczy, R.; Kopeć, A.; Piątkowska, E.; Bieżanowska-Kopeć, R.; Koronowicz, A.; Kapusta-Duch, J. Biofortification of Carrot (Daucus Carota L.) with Iodine and Selenium in a Field Experiment. Front. Plant Sci. 2016, 7, 730. [Google Scholar] [CrossRef] [PubMed]
- Przybysz, A.; Wrochna, M.; Małecka-Przybysz, M.; Gawrońska, H.; Gawroński, S.W. Vegetable Sprouts Enriched with Iron: Effects on Yield, ROS Generation and Antioxidative System. Sci. Hortic. 2016, 203, 110–117. [Google Scholar] [CrossRef]
- Przybysz, A.; Wrochna, M.; Małecka-Przybysz, M.; Gawrońska, H.; Gawroński, S.W. The Effects of Mg Enrichment of Vegetable Sprouts on Mg Concentration, Yield and ROS Generation. J. Sci. Food Agric. 2016, 96, 3469–3476. [Google Scholar] [CrossRef] [PubMed]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Hegedusova, A.; Slosar, M.; Mezeyova, I.; Hegedus, O.; Andrejiova, A.; Szarka, K. Methods for Estimation of Selected Biologically Active Substances; Slovak University of Agriculture: Nitra, Slovakia, 2018. [Google Scholar]
- Arumona, A.E.; Garhwal, A.; Khunnam, W.; Youplao, P.; Ray, K.; Yupapin, P. Electron Cloud Zeeman Effect Sensors Using Silver Bars Embedded Microring Resonator. Opt. Quantum Electron. 2022, 54, 140. [Google Scholar] [CrossRef]
- Hegedűs, O.; Hegedűsová, A.; Šimková, S.; Pavlík, V.; Jomová, K. Evaluation of the ET-AAS and HG-AAS Methods of Selenium Determination in Vegetables. J. Biochem. Biophys. Methods 2008, 70, 1287–1291. [Google Scholar] [CrossRef]
- Golian, M.; Hegedűsová, A.; Mezeyová, I.; Chlebová, Z.; Hegedűs, O.; Urminská, D.; Vollmannová, A.; Chlebo, P. Accumulation of Selected Metal Elements in Fruiting Bodies of Oyster Mushroom. Foods 2021, 11, 76. [Google Scholar] [CrossRef]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of Sodium Selenate Improves Dietary Mineral Contents and Antioxidant Capacity of Culinary Herb Microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef]
- Bulgari, R.; Negri, M.; Santoro, P.; Ferrante, A. Quality Evaluation of Indoor-Grown Microgreens Cultivated on Three Different Substrates. Horticulturae 2021, 7, 96. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Pannico, A.; Soteriou, G.A.; Giordano, M.; Ritieni, A.; de Pascale, S.; Rouphael, Y. Functional Quality in Novel Food Sources: Genotypic Variation in the Nutritive and Phytochemical Composition of Thirteen Microgreens Species. Food Chem. 2019, 277, 107–118. [Google Scholar] [CrossRef]
- Li, T.; Lalk, G.T.; Bi, G. Fertilization and Pre-Sowing Seed Soaking Affect Yield and Mineral Nutrients of Ten Microgreen Species. Horticulturae 2021, 7, 14. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Zarrelli, A.; Soteriou, G.A.; Kyratzis, A.; Antoniou, C.; Pizzolongo, F.; Romano, R.; et al. Ontogenetic Variation in the Mineral, Phytochemical and Yield Attributes of Brassicaceous Microgreens. Foods 2021, 10, 1032. [Google Scholar] [CrossRef]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and Quality of Basil, Swiss Chard, and Rocket Microgreens Grown in a Hydroponic System. N. Zealand J. Crop Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Slosar, M.; Mezeyova, I.; Hegedusova, A. Less-Known Leaf Vegetables Grown in Slovak Republic Conditions: New Sources of Antioxidants. J. Cent. Eur. Agric. 2016, 17, 695–706. [Google Scholar] [CrossRef][Green Version]
- Keutgen, N.; Hausknecht, M.; Tomaszewska-Sowa, M.; Keutgen, A.J. Nutritional and Sensory Quality of Two Types of Cress Microgreens Depending on the Mineral Nutrition. Agronomy 2021, 11, 1110. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The Roles of Iron in Health and Disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Newman, R.G. The Effect of Sodium Selenate Biofortification on Plant Yield, Mineral Content, and Antioxidant Capacity of Culinary Herb Microgreens. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2019. [Google Scholar] [CrossRef]
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium Intake and Hypertension. Nutrients 2019, 11, 1970. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.; Ferreira, I.M.P.L.V.O. Comparison between the Mineral Profile and Nitrate Content of Microgreens and Mature Lettuces. J. Food Compos. Anal. 2015, 37, 38–43. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).