Physiological Response of Soybean Plants to Seed Coating and Inoculation under Pot Experiment Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and the Course of the Pot Experiment
- A—control;
- B—bioinoculant;
- C—coated seeds: chitosan + alginate/jojoba oil/E;
- D—coated seeds: chitosan + alginate/PEG;
- C + B;
- D + B.
2.2. Seed Coating
2.3. Seed Inoculation
2.4. Measurement Gs and SPAD
2.5. Chlorophyll Fluorescence
2.6. Measurement of Gas Exchange
2.7. Statistical Analysis
3. Results
3.1. Plant Emergence and Nodulation
3.2. Green Mass
3.3. Physiological Measurements
3.4. Protein Content
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tamagno, S.; Sadras, V.O.; Haegele, J.W.; Armstrong, P.R.; Ciampitti, I.A. Interplay between nitrogen fertilizer and biological nitrogen fixation in soybean: Implications on seed yield and biomass allocation. Sci. Rep. 2018, 8, 17502. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, S.B.; Tajini, F.; Trabelsi, M.; Aouani, M.E.; Mhamdi, R. Competition for nodule formation between introduced strains of Mesorhizobium ciceri and the native populations of rhizobia nodulating chickpea (Cicer arietinum) in Tunisia. World J. Microbiol. Biotechnol. 2007, 23, 1195–1201. [Google Scholar] [CrossRef]
- Collino, D.J.; Salvagiotti, F.; Perticari, A.; Piccinetti, C.; Ovando, G.; Urquiaga, S.; Racca, R.W. 2015. Biological nitrogen fixation in soybean in Argentina: Relationships with crop, soil, and meteorological factors. Plant Soil 2015, 392, 239–252. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Sawah, A.M. The mode of integration between azotobacter and rhizobium affect plant growth, yield, and physiological responses of pea (Pisum sativum L.). J. Soil Sci. Plant Nutr. 2022, 155, 1–14. [Google Scholar] [CrossRef]
- Masciarelli, O.; Llanes, A.; Luna, V. A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res. 2014, 169, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ciampitti, I.A.; Salvagiotti, F. New insights into soybean biological nitrogen fixation. Agron. J. 2018, 110, 1185–1196. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; de Borja Reis, A.F.; Córdova, S.C.; Castellano, M.J.; Archontoulis, S.V.; Correndo, A.A.; De Almeida, L.F.A.; Moro Rosso, L.H. Revisiting biological nitrogen fixation dynamics in soybeans. Front. Plant Sci. 2021, 12, 727021. [Google Scholar] [CrossRef]
- Carciochi, W.D.; Rosso, L.H.M.; Secchi, M.A.; Torres, A.R.; Naeve, S.; Casteel, S.N.; Kovács, P.; Davidson, D.; Purcell, L.C.; Archontoulis, S.; et al. Soybean yield, biological N2 fixation and seed composition responses to additional inoculation in the United States. Sci. Rep. 2019, 9, 19908. [Google Scholar] [CrossRef]
- Santachiara, G.; Borrás, L.; Salvagiotti, F.; Gerde, J.A.; Rotundo, J.L. Relative importance of biological nitrogen fixation and mineral uptake in high yielding soybean cultivars. Plant Soil 2017, 418, 191–203. [Google Scholar] [CrossRef]
- Córdova, S.C.; Castellano, M.J.; Dietzel, R.; Licht, M.A.; Togliatti, K.; Martinez-Feria, R.; Archontoulis, S.V. Soybean nitrogen fixation dynamics in Iowa, USA. Field Crops Res. 2019, 236, 165–176. [Google Scholar] [CrossRef]
- Bogino, P.; Nievas, F.; Banchio, E.; Giordano, W. Increased competitiveness and efficiency of biological nitrogen fixation in peanut via in-furrow inoculation of rhizobia. Eur. J. Soil Biol. 2011, 47, 188–193. [Google Scholar] [CrossRef]
- Ntambo, M.S.; Chilinda, I.S.; Taruvinga, A.; Hafeez, S.; Anwar, T.; Rahat Sharif, R.; Chambi, C.; Kies, L. The effect of rhizobium inoculation with nitrogen fertilizer on growth and yield of soybeans (Glycine max L.). Int. J. Biosci. 2017, 10, 163–172. [Google Scholar]
- Brockwell, J.; Bottomley, P.J.; Thies, J.E. Manipulation of rhizobia microflora for improving legume productivity and soil fertility: A critical assessment. Plant Soil 1995, 174, 143–180. [Google Scholar] [CrossRef]
- Pueppke, S.G. Nitrogen fixation by soybean in North America. In Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment. Nitrogen Fixation: Origins, Applications, and Research Progress; Werner, D., Newton, W.E., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 4, pp. 15–23. [Google Scholar] [CrossRef]
- Mpepereki, S.; Javaheri, F.; Davis, P.; Giller, K.E. Soyabeans and sustainable agriculture: Promiscuous soyabeans in southern Africa. Field Crops Res. 2000, 65, 137–149. [Google Scholar] [CrossRef]
- Asei, R.; Ewusi-Mensah, N.; Abaidoo, R.C. Response of soybean (Glycine max L.) to rhizobia inoculation and molybdenum application in the northern savannah zones of Ghana. J. Plant Sci. 2015, 3, 64–70. [Google Scholar] [CrossRef]
- Yates, R.J.; Howieson, J.G.; Reeve, W.G.; O’Hara, G.W. A re-appraisal of the biology and terminology describing rhizobial strain success in nodule occupancy of legumes in agriculture. Plant Soil 2011, 348, 255. [Google Scholar] [CrossRef]
- Kanonge-Mafaune, G.; Chiduwa, M.S.; Chikwari, E.; Pisa, C. Evaluating the effect of increased rates of rhizobial inoculation on grain legume productivity. Symbiosis 2018, 75, 217–227. [Google Scholar] [CrossRef]
- Lindström, K.; Murwira, M.; Willems, A.; Altier, N. The biodiversity of beneficial microbe-host mutualism: The case of rhizobia. Res. Microbiol. 2010, 161, 453–463. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Anand, A.; Dhar, B.; Vaishampayan, A. Genotypic characterization of phage-typed indigenous soybean Bradyrhizobia and their host range symbiotic effectiveness. Microb. Ecol. 2012, 63, 116–126. [Google Scholar] [CrossRef]
- Pedrozo, A.; de Oliveira, N.J.G.; Alberton, O. Biological nitrogen fixation and agronomic features of soybean (Glycine max (L.) Merr.) crop under different doses of inoculants. Acta Agronómica 2018, 67, 297–302. [Google Scholar] [CrossRef]
- Jarecki, W.; Buczek, J.; Jańczak-Pieniążek, M. Soybean (Glycine max (L.) Merr.) response to commercial inoculation of Bradyrhizobium japonicum. Appl. Ecol. Environ. Res. 2020, 18, 6713–6724. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Faroog, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Elgawad, H.A.; Xiong, Y.C.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Hamound, Y.A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant 2021, 172, 2153–2169. [Google Scholar] [CrossRef] [PubMed]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Daly, P.; Sharma, A.; Shaghaleh, H.; Hamound, Y.A.; El-Esawi, M.A.; Pan, R.; Wan, Q.; et al. Seed priming and foliar application with jasmonic acid enhance salinity stress tolerance of soybean (Glycine max, L.) seedlings. J. Sci. Food Agric. 2021, 101, 2027–2041. [Google Scholar] [CrossRef]
- 26. Ning, L.H.; Du, W.K.; Song, H.N.; Shao, H.B.; Qi, W.C.; Sheteiwy, M.S.; Yu, D.Y. Identification of responsive miRNAs involved in combination stresses of phosphate starvation and salt stress in soybean root. Environ. Exp. Bot. 2019, 167, 103823. [Google Scholar] [CrossRef]
- Sharma, K.K.; Singh, U.S.; Sharma, P.; Kumar, A.; Sharma, L. Seed treatments for sustainable agriculture—A review. J. Appl. Nat. Sci. 2015, 7, 521–539. [Google Scholar] [CrossRef]
- Penn, H.J.; Dale, A.M. Imidacloprid seed treatments affect individual ant behavior and community structure but not egg predation, pest abundance or soybean yield. Pest Manag. Sci. 2017, 73, 1625–1632. [Google Scholar] [CrossRef]
- Smalling, K.L.; Hladik, M.L.; Sanders, C.J.; Kuivila, K.M. Leaching and sorption of neonicotinoid insecticides and fungicides from seed coatings. J. Environ. Sci. Health B 2018, 53, 176–183. [Google Scholar] [CrossRef]
- Calvo-Agudo, M.; Dregni, J.; González-Cabrera, J.; Dicke, M.; Heimpel, G.E.; Tena, A. Neonicotinoids from coated seeds toxic for honeydew-feeding biological control agents. Environ. Pollut. 2021, 289, 117813. [Google Scholar] [CrossRef]
- Poliserpi, M.B.; Cristos, D.S.; Brodeur, J.C. Imidacloprid seed coating poses a risk of acute toxicity to small farmland birds: A weight-of-evidence analysis using data from the grayish baywing Agelaioides badius. Sci. Total Environ. 2021, 763, 142957. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern seed technology: Seed coating delivery systems for enhancing seed andcrop performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Zeng, D.-F.; Zhang, L. A novel environmentally friendly soybean seed-coating agent. Acta Agric. Scand. B Soil Plant Sci. 2010, 60, 545–551. [Google Scholar] [CrossRef]
- Santos, V.M.; Oliveira, T.C.; Mendes, M.G.; Yamanaka, C.H.; Macedo, W.R. Soybean seed chemical treatment associated with inoculants: Physiological and agronomical analyses. Plant Physiol. Rep. 2021, 26, 247–255. [Google Scholar] [CrossRef]
- Balboa, G.R.; Ciampitti, I.A. Estimating biological nitrogen fixation in field-grown soybeans: Impact of B value. Plant Soil 2020, 446, 195–210. [Google Scholar] [CrossRef]
- Montanha, G.S.; Dias, M.A.N.; Corrêa, C.G.; Pereira de Carvalho, H.W. Unfolding the fate and effects of micronutrients supplied to soybean (Glycine max (L.) Merrill) and maize (Zea mays L.) through seed treatment. J. Soil Sci. Plant. Nutr. 2021, 21, 3194–3202. [Google Scholar] [CrossRef]
- Jamilah, J.; Irawan, N.; Thesiwati, A.S.; Ernita, M. Soybean seed [Glycine max L.] coated by fertile soil-applied sodium bicarbonate at alluvial soil. IOP Conf. Ser. Earth Environ. Sci. 2020, 497, 012039. [Google Scholar] [CrossRef]
- Montanha, G.S.; Rodrigues, E.S.; Marques, J.P.R. Zinc nanocoated seeds: An alternative to boost soybean seed germination and seedling development. SN Appl. Sci. 2020, 2, 857. [Google Scholar] [CrossRef]
- Ludwig, E.J.; Nunes, U.R.; Prestes, O.D.; Fagundes, L.K.; Fernandes, T.S.; Saibt, N. Polymer coating in soybean seed treatment and their relation to leaching of chemicals. Rev. Ambient. Água 2020, 15, e2602. [Google Scholar] [CrossRef]
- Kuchlan, P.; Kuchlan, M.K.; Ansari, M.M. Efficient application of Trichoderma viride on soybean [Glycine max (L.) Merrill] seed using thin layer polymer coating. Legume Res. 2019, 42, 250–259. [Google Scholar] [CrossRef]
- Tang, C.C.; Li, Y.; Kurnaz, L.B.; Li, J. Development of eco-friendly antifungal coatings by curing natural seed oils on wood. Prog. Org. Coat. 2021, 161, 106512. [Google Scholar] [CrossRef]
- Soares, C.M.; Ludwig, M.P.; Soares Rother, C.M.; Decarli, L. 2019. Seed quality and crop performance of soybeans submitted to different forms of treatment and seed size. J. Seed Sci. 2019, 41, 69–75. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Zhu, X.; Liu, R.; Xiang, P.; Chen, J.; Liu, X.; Duan, Y.; Chen, L. Management of the soybean cyst nematode Heterodera glycines with combinations of different rhizobacterial strains on soybean. PLoS ONE 2017, 12, e0182654. [Google Scholar] [CrossRef] [PubMed]
- Ambika, S.; Manonmani, V.; Bhaskaran, M.; Deepika, S. Influence of polymer coated KSL 441 (op) soybean seed on productivity under moisture stress conditions. Legume Res. 2017, 40, 150–154. [Google Scholar] [CrossRef]
- Tripathi, B.; Pandey, A.; Bhatia, R.; Walia, S.; Yadav, A.K. Improving soybean seed performance with natural colorant-based novel seed-coats. J. Crop Improv. 2015, 29, 301–318. [Google Scholar] [CrossRef]
- Jeyabal, A.; Kuppuswamy, G.; Lakshmanan, A. Effect of seed coating on yield attributes and yield of soybean (Glycine max L.). J. Agron. Crop Sci. 1992, 169, 145–150. [Google Scholar] [CrossRef]
- Faqir, Y.H.; Ma, J.H.; Chai, Y.L. Chitosan in modern agriculture production. Plant Soil Environ. 2021, 67, 679–699. [Google Scholar] [CrossRef]
- Li, J.; Han, A.; Zhang, L.; Meng, Y.; Xu, L.; Ma, F.; Liu, R. Chitosan oligosaccharide alleviates the growth inhibition caused by physcion and synergistically enhances resilience in maize seedlings. Sci. Rep. 2022, 12, 162. [Google Scholar] [CrossRef]
- Fu, Y.; Bhunia, A.K.; Yao, Y. Alginate-based antimicrobial coating reduces pathogens on alfalfa seeds and sprouts. Food Microbiol. 2022, 103, 103954. [Google Scholar] [CrossRef]
- Jarecki, W.; Wietecha, J. Effect of seed coating on the yield of soybean Glycine max (L.) Merr. Plant Soil Environ. 2021, 67, 468–473. [Google Scholar] [CrossRef]
- Jarecki, W. Soybean Response to Seed Coating with Chitosan + Alginate/PEG and/or Inoculation. Agronomy 2021, 11, 1737. [Google Scholar] [CrossRef]
- Wiatrak, P. Effect of polymer seed coating with micronutrients on soybeans in Southeastern Coastal Plains. Am. J. Agric. Biol. Sci. 2013, 8, 302–308. [Google Scholar] [CrossRef][Green Version]
- Vollmann, J.; Walter, H.; Sato, T.; Schweiger, P. Digital image analysis and chlorophyll metering for phenotyping the effects of nodulation in soybean. Comput. Electron. Agric. 2011, 75, 190–195. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schweitzer, L.E.; Nelson, R.L. Association of specific leaf weight, an estimate of chlorophyll, and chlorophyll concentration with apparent photosynthesis in soybean. Photosynth. Res. 1996, 49, 1–10. [Google Scholar] [CrossRef]
- Yu, M.; Ding, G.; Gao, G.; Zhao, Y.; Sai, K. Leaf temperature fluctuations of typical psammophytic plants and their application to stomatal conductance estimation. Forests 2018, 9, 313. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. In Word Reference Base for Soil Resources 2014, Update 2015; Word Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 192. [Google Scholar]
- Fotyma, M.; Kęsik, K.; Lipiński, W.; Filipiak, K.; Purchała, L. Soil tests as the basis of fertilizer advisory. Stud. Rep. IUNG-PIB Puławy 2015, 42, 9–51. (In Polish) [Google Scholar]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018. [Google Scholar] [CrossRef]
- PN-EN ISO 20483:2014-02 and PN-EN ISO 20483:2014-02/Ap1. Polish Committee for Standardization. Available online: https://www.pkn.pl/en (accessed on 29 April 2022).
- Alotaibi, M.O.; Saleh, A.M.; Sobrinho, R.L.; Sheteiwy, M.S.; El-Sawah, A.M.; Mohammed, A.E.; Elgawad, H.A. Arbuscular mycorrhizae mitigate aluminum toxicity and regulate proline metabolism in plants grown in acidic soil. J. Fungi 2021, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Ahmed, M.; Mohammed, A.E.; Alotaibi, M.O.; Yehia, R.S.; Selim, S.; Saleh, A.M.; Beemster, G.T.; Sheteiwy, M.S. Increasing atmospheric CO2 differentially supports arsenite stress mitigating impact of arbuscular mycorrhizal fungi in wheat and soybean plants. Chemosphere 2022, 296, 134044. [Google Scholar] [CrossRef]
- Sessitsch, A.; Howieson, J.G.; Perret, X.; Antoun, H.; Martínez-Romero, E. Advances in rhizobium research. Crit. Rev. Plant Sci. 2002, 21, 323–378. [Google Scholar] [CrossRef]
- Ogunkanmi, L.; MacCarthy, D.S.; Adiku, S.G.K. Impact of extreme temperature and soil water stress on the growth and yield of soybean (Glycine max (L.) Merrill). Agriculture 2022, 12, 43. [Google Scholar] [CrossRef]
- Kulkarni, K.P.; Tayade, T.; Asekova, S.; Song, J.T.; Shannon, J.G.; Lee, J.D. Harnessing the potential of forage legumes, alfalfa, soybean, and cowpea for sustainable agriculture and global food security. Fron. Plant Sci. 2018, 9, 1314. [Google Scholar] [CrossRef]
- Lentola, A.; Giorio, C.; Petrucco Toffolo, E.; Girolami, V.; Tapparo, A. A new method to assess the acute toxicity toward honeybees of the abrasion particles generated from seeds coated with insecticides. Environ. Sci. Eur. 2020, 32, 93. [Google Scholar] [CrossRef]
- Lentola, A.; Giorio, C.; Bogialli, S.; Roverso, M.; Marzaro, M.; Girolami, V.; Tapparo, A. Methiocarb metabolites are systemically distributed throughout corn plants grown from coated seeds. Environ. Chem. Lett. 2021, 19, 1887–1892. [Google Scholar] [CrossRef]
- Han, R.; Wu, Z.; Huang, Z.; Man, X.; Teng, L.; Wang, T.; Liu, P.; Wang, W.; Zhao, X.; Hao, J.; et al. Tracking pesticide exposure to operating workers for risk assessment in seed coating with tebuconazole and carbofuran. Pest Manag. Sci. 2021, 77, 2820–2825. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoration ready. Restor. Ecol. 2020, 28, 266–275. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating: A tool for delivering beneficial microbes to agricultural crops. Fron. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Ehsanfar, S.; Modarres-Sanavy, S.A.M. Crop protection by seed coating. Commun. Agric. Appl. Boil. Sci. 2005, 70, 225–229. [Google Scholar]
- Chachalis, D.; Smith, M.L. Hydrophobic-polymer application reduces imbibition rate and partially improves germination of emergence of soybean seedlings. Seed Sci. Technol. 2001, 29, 91–98. [Google Scholar]
- Sharratt, B.S.; Gesch, R.W. Emergence of polymer-coated corn and soybean influenced by tillage and sowing date. Agron. J. 2008, 100, 585–590. [Google Scholar] [CrossRef]
- Schogolev, A.S.; Raievska, I.M. Role of nitrogen deficiency on growth and development near isogenic by E genes lines of soybean co-inoculated with nitrogen-fixing bacteria. Regul. Mech. Biosyst. 2021, 12, 326–334. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Applications of absorbent polymers for sustainable plant protection and crop yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Korbecka-Glinka, G.K.; Wiśniewska-Wrona, M.; Kopania, E. The use of natural polymers for treatments enhancing sowing material. Polimery 2021, 66, 11–20. [Google Scholar] [CrossRef]
- Kintl, A.; Huňady, I.; Vymyslický, T.; Ondrisková, V.; Hammerschmiedt, T.; Brtnický, M.; Elbl, J. Effect of Seed Coating and PEG-Induced Drought on the Germination Capacity of Five Clover Crops. Plants 2021, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Raeta, R.; Vannacci, G. Seeds encapsulation in calcium alginate pellets. Seed Sci. Technol. 2004, 32, 649–661. [Google Scholar] [CrossRef]
- Zeng, D.; Luo, X.; Tu, R. Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2012, 2012, 104565. [Google Scholar] [CrossRef]
- Aboalfayah, R.; Samara, R. Antifeedants impact of plant essential oil on green peach aphid on potato crops. J. Ecol. Eng. 2022, 23, 274–285. [Google Scholar] [CrossRef]
- Evangelista, J.R.E.; Oliveira, J.A.; Botelho, F.J.E.; Oliveira, R.M.E.; Pereira, C.E. Performance of film coated soybean seeds in soil different water contents. Ciênc. Agrotec. 2007, 31, 994–999. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Bioch. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Stecca, J.D.L.; Martin, T.N.; Lúcio, A.D.; Deak, E.A.; Fipke, G.M.; Bruning, L.A. Inoculation of soybean seeds coated with osmoprotector in diferents soil pH’s. Acta Sci. Agron. 2019, 41, e39482. [Google Scholar] [CrossRef]
- Procházka, P.; Štranc, P.; Vostřel, J.; Řehoř, J.; Křováček, J.; Brinar, J.; Pazderů, K. The influence of effective soybean seed treatment on root biomass formation and seed production. Plant Soil Environ. 2019, 65, 588–593. [Google Scholar] [CrossRef]
- Procházka, P.; Štranc, P.; Pazderů, K.; Štranc, J.; Vostřel, J. Effects of biologically active substances used in soybean seed treatment on oil, protein and fibre content of harvested seeds. Plant Soil Environ. 2017, 63, 564–568. [Google Scholar] [CrossRef]
- Kasper, S.; Christoffersen, B.; Soti, P.; Racelis, A. Abiotic and biotic limitations to nodulation by leguminous cover crops in South Texas. Agriculture 2019, 9, 209. [Google Scholar] [CrossRef]
- Ortez, O.A.; Tamagno, S.; Salvagiotti, F.; Prasad, P.V.V.; Ciampitti, I.A. Soybean nitrogen sources and demand during the seed-filling period. Agron. J. 2019, 111, 1779–1787. [Google Scholar] [CrossRef]
- Gesch, R.W.; Archer, D.W.; Spokas, K. Can using polymer-coated seed reduce the risk of poor soybean emergence in no-tillage soil? Field Crops Res. 2012, 125, 109–116. [Google Scholar] [CrossRef]
- Meghvansi, M.K.; Prasad, K.; Harwani, D.; Mahna, S.K. Response of soybean cultivars toward inoculation with three arbuscular mycorrhizal fungi and Bradyrhizobium japonicum in the alluvial soil. Eur. J. Soil Biol. 2008, 44, 316–323. [Google Scholar] [CrossRef]
- Fritschi, F.B.; Ray, J.D. Soybean leaf nitrogen, chlorophyll content, and chlorophyll a/b ratio. Photosynthetica 2007, 45, 92–98. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Avelar, S.A.G.; Baudet, L.; de Oliveira, S.; Ludwig, M.P.; Crizel, R.L.; Rigo, G.A. Soybean seed treatment and coating with liquid and powdered polymer. Interciencia 2015, 40, 133–137. [Google Scholar]
- Jańczak-Pieniążek, M.; Buczek, J.; Bobrecka-Jamro, D.; Szpunar-Krok, E.; Tobiasz-Salach, R.; Jarecki, W. Morphophysiology, productivity and quality of soybean (Glycine max (L.) Merr.) cv. Merlin in response to row spacing and seeding systems. Agronomy 2021, 11, 403. [Google Scholar] [CrossRef]
- Matiru, V.N.; Dakora, F.D. Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces of African cereal crops. Afr. J. Biotechnol. 2004, 3, 1–7. [Google Scholar] [CrossRef]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed coating: Science or marketing spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]

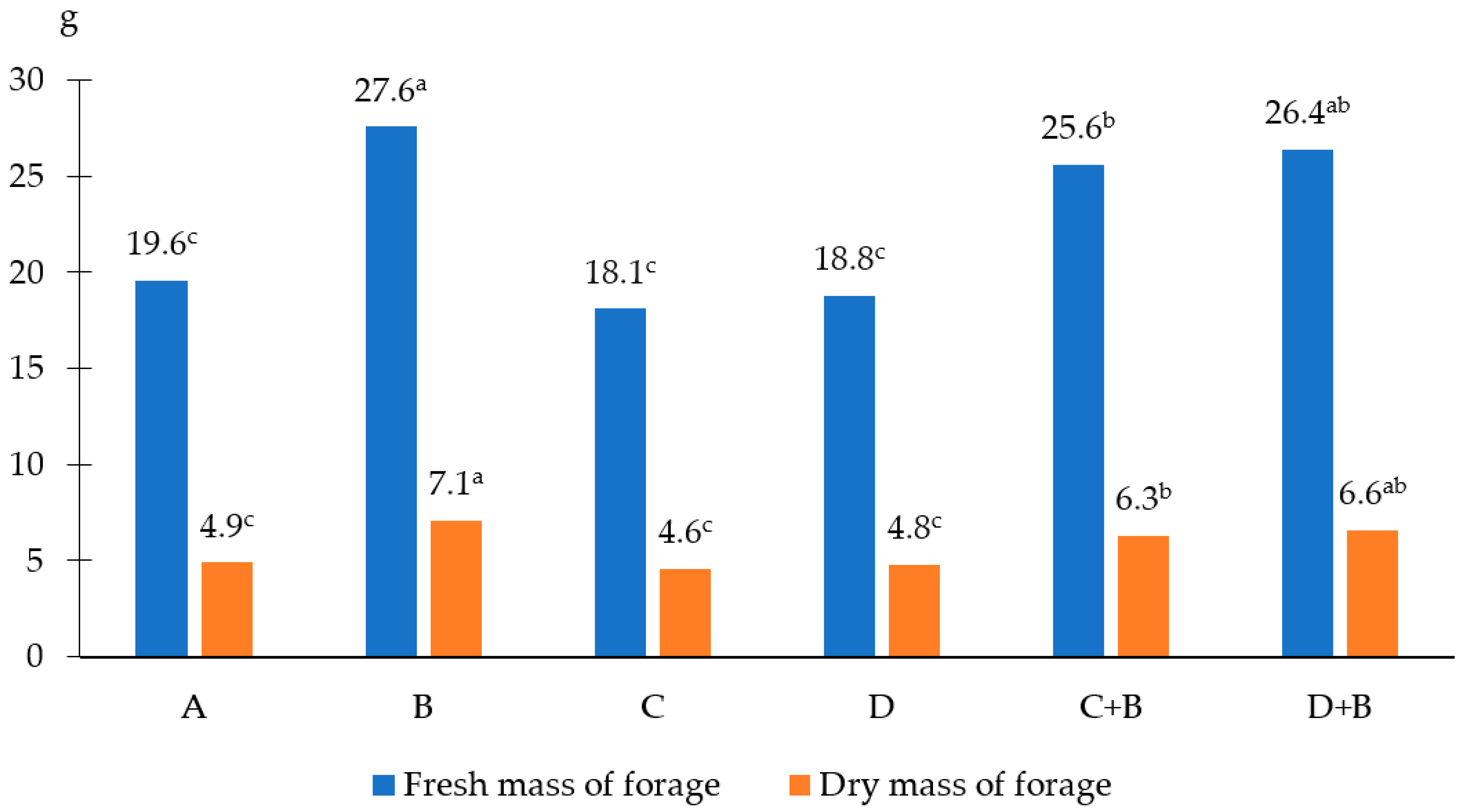
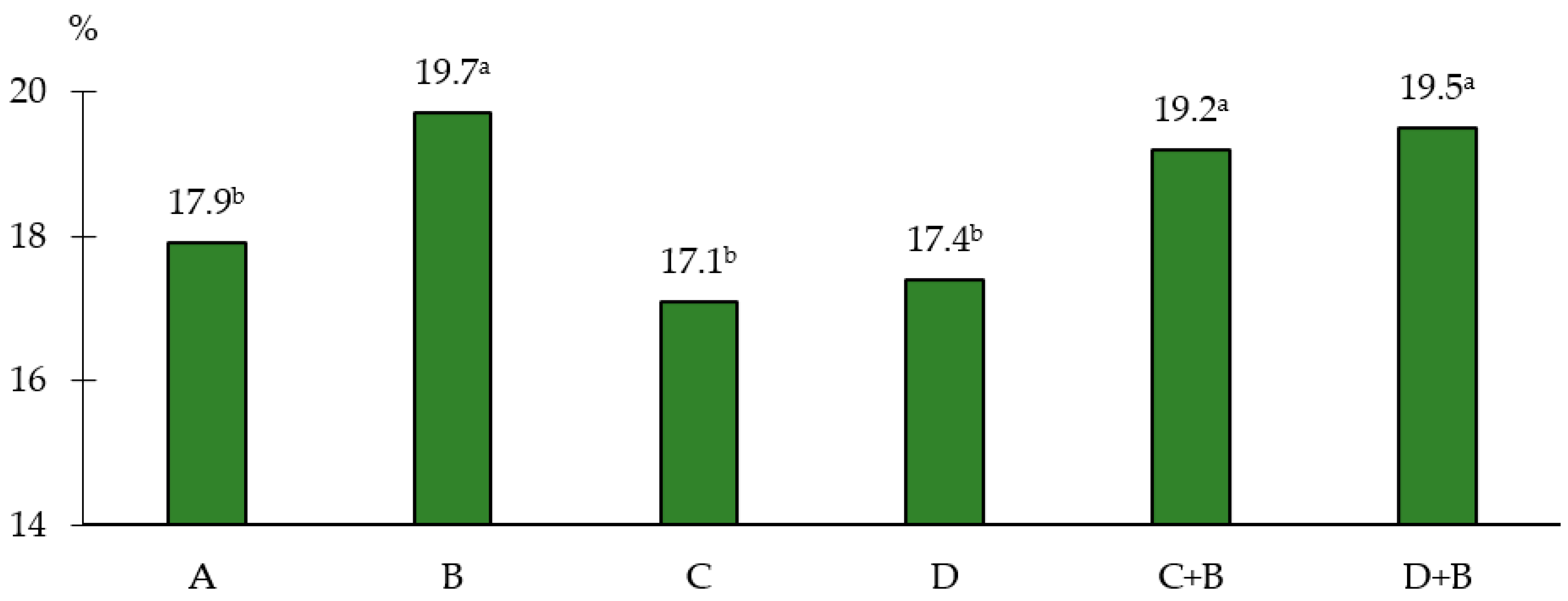
| Factor | Emergence (Days from the Date of Sowing) | Number of Plants after Emergence (Plants.·pot−1) |
|---|---|---|
| A | 14.50 b | 7.50 c |
| B | 14.50 b | 7.70 bc |
| C | 18.00 a | 8.80 ab |
| D | 17.25 a | 9.10 a |
| C + B | 18.00 a | 8.80 ab |
| D + B | 17.25 a | 8.90 a |
| Factor | Number of Nodules from the Plant | DW of Nodules from the Plant (g) |
| A | - | - |
| B | 25.8 a | 0.38 a |
| C | - | - |
| D | - | - |
| C + B | 23.8 a | 0.33 a |
| D + B | 24.4 a | 0.35 a |
| Factor | Developmental Phase on the BBCH Scale | ||
|---|---|---|---|
| 13 BBCH | 51 BBCH | 65 BBCH | |
| A | 27.3 a | 32.6 ab | 33.2 b |
| B | 27.6 a | 35.4 a | 36.2 a |
| C | 26.9 a | 31.5 b | 32.5 b |
| D | 27.1 a | 32.4 ab | 33.0 b |
| C + B | 27.5 a | 34.6 a | 35.8 a |
| D + B | 27.7 a | 35.1 a | 36.0 a |
| Factor | Developmental Phase on the BBCH Scale | ||
|---|---|---|---|
| 13 BBCH | 51 BBCH | 65 BBCH | |
| A | 329.3 a | 332.5 b | 295.5 d |
| B | 325.6 a | 411.8 a | 434.1 a |
| C | 315.3 ab | 380.3 a | 383.0 c |
| D | 285.5 b | 400.3 a | 409.5 abc |
| C + B | 324.8 a | 391.5 a | 395.5 bc |
| D + B | 296.2 ab | 414.6 a | 427.2 ab |
| Factor | Intensity of Photosynthesis Net (PN) (μmol(CO2)∙m−2∙s−1) | Transpiration Rate (E) (mmol(H2O)∙m−2∙s−1) | Intercellular CO2 Concentration (Ci) (mmol∙L−1) |
|---|---|---|---|
| A | 16.3 ab | 3.13 a | 68.5 a |
| B | 17.6 a | 3.31 a | 52.3 b |
| C | 16.1 b | 3.08 a | 63.2 ab |
| D | 15.9 b | 3.11 a | 64.6 ab |
| C + B | 17.1 ab | 3.26 a | 55.3 b |
| D + B | 17.3 ab | 3.29 a | 57.3 bc |
| Factor | Maximal Photochemical Efficiency of PSII (Fv/Fm) | Maximum Quantum Yield of Primary Photochemistry (Fv/F0) | Performance Index (PI) |
|---|---|---|---|
| A | 0.731 a | 2.28 b | 3.48 b |
| B | 0.768 a | 3.05 a | 4.29 a |
| C | 0.742 a | 2.33 b | 3.35 b |
| D | 0.746 a | 2.37 b | 3.41 b |
| C + B | 0.745 a | 2.88 a | 4.17 a |
| D + B | 0.753 a | 2.99 a | 4.23 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarecki, W. Physiological Response of Soybean Plants to Seed Coating and Inoculation under Pot Experiment Conditions. Agronomy 2022, 12, 1095. https://doi.org/10.3390/agronomy12051095
Jarecki W. Physiological Response of Soybean Plants to Seed Coating and Inoculation under Pot Experiment Conditions. Agronomy. 2022; 12(5):1095. https://doi.org/10.3390/agronomy12051095
Chicago/Turabian StyleJarecki, Wacław. 2022. "Physiological Response of Soybean Plants to Seed Coating and Inoculation under Pot Experiment Conditions" Agronomy 12, no. 5: 1095. https://doi.org/10.3390/agronomy12051095
APA StyleJarecki, W. (2022). Physiological Response of Soybean Plants to Seed Coating and Inoculation under Pot Experiment Conditions. Agronomy, 12(5), 1095. https://doi.org/10.3390/agronomy12051095






