Abstract
Unguided exploitation has impaired the sustainability of natural resources of agronomic non-wood forest plants from understory boglands in boreal forests. The extreme consequences of plant–soil interplay on medicinal plant communities under continuous interruptions need to be understood to implement strategies which can cope with possible ecological degradation. In this study, co-existing Ledum palustre and Vaccinium uliginosum communities were investigated after a four-year interruption of continuous removal of dominant species in stands at Xing’an Mountain. Nitrogen (N) availability was assessed by above-ground biomass and N content in nondominant plants and the biophysiological properties of rhizosphere soil. The removal treatment promoted soil mineral and organic N contents, but also reduced abundances of the soil communities of Rozellomycota phylum (by 82.76%), ericoid mycorrhiza of Meliniomyces varia (by 81.60%) and Phialocephala fort (by 69.54%). Vaccinium uliginosum overcame L. palustre through higher N utilization (biomass/%N) although the latter had higher abundances of soil Odiodendron maius and P. fort. The microbial community attributes accounted for a large proportion of N availability following the removal of dominance. In conclusion, our study demonstrates that understory agronomic plants in northern boglands should no longer be under continuous exploitation. Strategies should be considered to improve the promotion of N uptake by managing local soil microbial communities.
1. Introduction
Forests are a nature-based solution to global climate change, which is depriving the use of parts of obligate forests for timber production. This increases the dependences and incomes of millions of households on non-wood forest products (NWFPs). The Food and Agriculture Organization of the United Nations (FAO) defined NWFPs as “goods of biological origin other than wood derived from forests and other wooded land and trees outside forests” [1]. A general list of NWFPs includes raw materials for large-scale industrial processing of food, food additives, fibers, resins, gums, medicines, cosmetics, and handicrafts [2]. Significant efforts to study policies and strategies that encourage NWFP development in regions with bans on forest timber harvest are underway [3,4].
Banned logging is a major factor that encourages economic dependence on trading NWFPs, such as cases in regions of northeast Thailand [5], the Pacific Northwest [6], and forested lands in the Philippines [7]. In northeast China, natural forests were heavily exploited in the 1990s, with the intention of being reversed by the Natural Forest Conservation Program (NFCP) by 1998 through bans on commercial logging [8,9]. The performance of NFCP also contributed to the formation of sustainable forestry economies in northeast China moving from dependence on state-owned forestry enterprises to that on household livelihoods [10]. The sale of NWFPs has been a major source of income for local forestry households. The sustainable management of natural resources of NWFPs is a major issue of concern not only in northeast China but also in other forest regions with bans on logging.
Vaccinium uliginosum L. (bog bilberry) is an endemic berry that inhabits wet acidic soils in heathland, moorland, tundra, and peatland at the understory layer [11,12]. It is a typical NWFP species that contributes to a significant part of household livelihood through berry products in the northeastern forests of China [12]. Abundant anthocyanin content and the diverse composition of its berries are the major factors of value for merchants of northeastern forest resources [13]. Natural habitats of V. uliginosum support an annual yield of 0.6 million metric tons of berries using an area of 3.5 million hectares in forests of northeast China, accounting for ~15% of nationwide berry cultivation areas up to 2019 [14]. In understory bogs of boreal forests subjected to cold temperate climates, V. uliginosum usually co-exists with Ledum palustre L. in communities that dominate the understory communities [15,16]. Ledum palustre is a fragrant evergreen shrub dwelling in peat soils in northern Europe, Asia, and North America [17]. Since the 18th century, L. palustre has been used as an ethnomedicine to treat ailments [18,19]. Both V. uliginosum and L. palustre were exploited as NWFPs from their original habitats, but neither were derived and grown using planned management. Global warming is modifying soil carbon (C) and nitrogen (N) cycling and increasing wildfires which modify their communities [11,15,20]. These factors resulted in the over-consumption of natural reserves that threatened the development of current communities near the endangered red-line [15,21]. Permafrost thaw increases the understory soil hydrology of peat bogs where communities with these two plants exist [22]. This is driving the ecological degradation of bogland communities, which is further strengthened by anthropogenic activities [20,23,24]. It is necessary to ascertain strategies to cope with the ongoing degradation of these understory communities. The lack of sufficient understanding of stand characteristics generates uncertainties in making strategies.
Habitats of co-existing V. uliginosum and L. palustre communities are mostly concentrated in boreal forests [12,25,26,27] where soils are apt to suffer low N availability caused by permafrost [16]. Nitrogen is a key element that is heavily needed by NWFP plants [28,29,30] and their co-existing communities [31,32,33,34]. Frozen soils restrict both root proliferation and N uptake through a combination of cold and drought stresses [35,36,37]. Local shrubs have to acclimate to low N [11,25], which will be reversed by climate warming and cause ecosystem degradation [23]. Warming can directly promote permafrost melting, converting forests to boglands accordingly. Litters decompose on soils with high efficiency and an increment of active layer depth, during which soil N compounds are mobilized to higher availabilities [11,16,23]. Wildfires occur at a higher frequency under climate warming and fire can also promote N uptake efficiency in V. uliginosum [38]. Nitrogen utilization is a key parameter used to assess the ability of assimilation and the absorption of N by juvenile plants in understory layers [39,40,41,42,43,44,45]. To the best of our knowledge, N utilization has not been well revealed in co-existing V. uliginosum and L. palustre communities that are subjected to intensive interruptions.
When an ecosystem has become prone to being interrupted by anthropogenic activities, the objective communities may lose their ecological niche and functions if the interruption is continuous [46]. Climate warming is reshaping the ecosystem structure of understory in boreal bogland, which may be strengthened by anthropogenic activities and develop into deeper ecological degradation. Therefore, it is a common approach to remove the objective plants from ecosystems exposed to interruption and assess the consequences compared to a reference without any removal treatment [47,48]. Xing’an Mountain is an ecological barrier in northeast China, where permafrost has decreased by 35–37% since the 1970s [24]. In this study, co-existing V. uliginosum and L. palustre communities were studied in the natural understory ecosystems of Xing’an Mountain in northeast China. Stands were chosen with either species as the community’s dominant species which underwent the treatment of dominant species removal. Our goal was to assess N utilization and stand characteristics that impact N uptake in communities which had their dominant species removed. We hypothesized that: (i) communities dominated by V. uliginosum vs. L. palustre would not result in a difference in N utilization, (ii) which will be reduced by the removal of dominant species.
2. Materials and Methods
2.1. Study Site and Condition
This study was conducted in two stands at Xing’an Mountain of the Heilongjiang province in northeast China. One stand was set in Hanjiayuan Forest Farm in the Great Khingan region (52°04′48″ N, 125°22′30″ E) (elevation: 376 m), with the other in Xinqing Forest Farm in the Lesser Khingan region (48°21′12″ N, 129°36′23″ E) (elevation: 351 m). Undergrowth marsh fields were chosen as stands to investigate peatlands forested by Abies nephrolepis (Trautv. ex Maxim.) Maxim., Betula platyphylla Sukaczev, Larix gmelinii (Rupr.) Rupr., Pinus koraiensis Siebold and Zucc., and deciduous broadleaf mixed species [49]. Vegetations distributed on the moss layer were dominated by a combination of co-existing V. uliginosum and L. palustre forming a richness of 80–90%. The remaining herbs mainly included species of Eriophorum vaginatum L., Carex schmidtii Meinsh, and Juncus effusus L. Other co-existing shrubs included Chamaedaphne calyculata (L.) Moench, Duschekia fruticosa, and Betula nana.
2.2. Study Placement and Design
To predict the extreme consequence of vegetative communities subjected to interruption, plants of dominant species were removed from objective communities. The dominant species with the highest abundance was removed in two stand locations. Either V. uliginosum or L. palustre was chosen as the dominant species of the understory communities as soon as the abundance was found to be higher than 50%, leaving the other at 30–40%. A total of 12 understory communities were chosen with six dominated by V. uliginosum and the other six by L. palustre. Six communities were targeted in the stand at Great Khingan with three dominated by V. uliginosum and the other three by L. palustre. Similar communities with the same dominant species were repeated in another stand at Lesser Khingan. Every community was outlined by global positioning system (GPS) signal tracking and regions were mapped to facilitate plot placement. Within the central area of each community, two plots were set with each being 100 m × 100 m. A total of 15 sub-plots (2 m × 2 m) were randomly placed therein. Any two randomly adjacent plots were separated by a distance of at least 100 m to avoid interactions. One plot received the treatment of removing the dominant species (V. uliginosum or L. palustre) and the other received no interruption and was taken as the control. Therefore, two factors were involved in this study: one was of the dominant species (V. uliginosum vs. L. palustre) and the other was the interruption of treatment (removal vs. control). Every basic unit of treatment was replicated in six plots with each combination of species (V. uliginosum vs. L. palustre) and interruption treatment (removal vs. control). The removal treatment began during the summertime of 2013 and removals were repeated every June for four years.
The removal treatment was implemented by mowing all the above-growing organs of objective species in sub-plots. To avoid horizontal influence, all sub-plots were sheltered by nylon screens since the first mow. Shelters were torn down every year before the annual removal treatment and then were reconstructed after the plants were mowed. Shelters were fixed to a height of about 1.5 m which prevented weed competition from above-ground organs (woody stems, newly growing branches, twigs, flowers, and fruits) while allowing for airflow and organisms and insects in the air.
2.3. Field Sampling
Removal treatments were done every June from 2013 to 2017. One month after the last removal treatment was employed in 2017, plots were investigated at the sub-plot level where sampling was applied to co-existing populations. Three individuals of dominant plant species were randomly targeted in sub-plots to confirm the locations for soil sampling. The above-ground organs of the co-existing species (not the dominant one) were harvested with rhizosphere soils sampled in a volume of 5.5 cm in diameter and 15 cm in depth. An auger was used to collect soil samples for measuring bulk density (BD) 10–15 cm beneath the surface. Roots and rocks were picked and excluded from moist soils which were sieved through a 2 mm mesh and homogenized to a mixture of samples collected from 15 sub-plots. Soil samples were moved to the laboratory on ice (0–4 °C) within 48 h and divided into two halves per plot. One half of the soils were placed on paper and air-dried to measure physicochemical properties and phenolic acid. The other half was stored in an ultra-cold storage freezer at −80 °C until the microbial community’s attributes were determined.
After plant and soil sampling, two polyvinyl chloride (PVC) tubes (15 cm length and 5 cm inner diameter) were inserted into soils around targeted plants in all sub-plots to the depth of 15 cm. One tube was immediately excavated, and soils were moved to sampling bags until transportation to the laboratory. The other tube was sealed using a plastic cap and incubated for 130 d [50]. Post-incubation tubes were excavated from soils and soils were also moved to the laboratory in sampling bags.
2.4. Chemical Analysis
Soils underwent chemical analysis to determine the parameter contents of boglands studied in the current literature [23,24,25,38]. Air-dried soil samples were mixed in distilled water in a proportion of 1:2 (v/v) and measured for pH value. The soil content of total phenol compounds was determined by an ultraviolet spectrophotometer methodology [51]. A soil sample of 20 g was dissolved in 1000 mL distilled water and shaken for 20 h. Samples were filtered, then solutions were concentrated at 2000 rpm and received drips of nitric acid until the pH was lowered to 2.0. Diethyl-ether was used for extraction three times and residues were dissolved in 10 mL of 99% ethanol. Tannic acid was used as the standard reagent to quantify phenol compounds. Soil organic carbon (SOC) was determined by the dichromate oxidation method with the quantification using ferrous ammonium sulfate titration [52].
Soil soluble nitrogen (N) was extracted using 5 g of air-dried sample in 50 mL of 2 M KCl after being shaken for 1 h. Mineral N was calculated as the sum of ammonium-N and nitrate-N, which were determined using the flow injection system (Lachat Instrument, Hach Inc., Loveland, CO, USA). Soil N mineralization rate was calculated as the difference of mineral N concentration in PVC tubes at two times in situ. One time was on the day when tubes were inserted into soils and the following time was within 130 days [50]. Soil organic N (SON) was calculated as total soluble N minus mineral N. Soil total N (including both organic and inorganic) was digested using 0.2 g of air-dried sample in 5 mL sulfuric acid with hydrogen peroxide additives. Total N content in soils was determined by Kjeldahl’s method [53]. Soil total phosphorus (P) and potassium (K) contents were extracted by digesting soils with perchloric acid and determined by inductively coupled plasma spectroscopy (Model-Thermo iCAP6000 Series, ThermoFisher Scientific-China Branch, Shanghai, China). Soil microbial biomass C (MBC) and N (MBN) contents were determined by the chloroform-fumigation extraction method [54], wherein the coefficient of Kc was calculated as 0.38 [55].
Plant samples were dried in an oven at 70 °C for 72 h then dry weight was measured. Total N content was determined using smashed plant samples with the same Kjeldahl method as described for the soil samples above. The ectomycorrhizal colonization rate was measured by the method of Phillips and Hayman [56]. The plants’ fine roots were soaked in 10% (w/w) potassium hydroxide at 60 °C for 20 h, rinsed using distilled water, and cleaned using 1% (v/v) hydrochloric acid. Roots were stained using Trypan Blue and divided into segments at a length of 0.5 cm. Stained roots were examined at 100× magnification and the total percent of root tips from colonized segments was counted.
Soil microbial DNA was extracted from 0.5 g of fresh soil samples and determined using Fast DNA® Spin Kit (Soil-use Series, MP Biomedicals, Cleveland, OH, USA). Observed DNA fractions were dissolved in diethylstilbestrol buffers. Information about the total soil microbial DNA was determined using 1% agarose (w/w) in electrophoreses. DNA concentration was measured using RNA analyzer (NanoDrop 2000, ThermoScientific China-Branch, Shanghai, China). DNA samples were preserved at −20 °C until analysis of real-time quantitative PCR using the IQ-V Real-time PCR monitoring system (Bio-Rad Laboratories GmbH, Feldkirchen, Germany) which was implemented by Shenggong Biology Inc. (Shanghai, China). Extracted DNA was amplified using ITS1F/ITS4 primers [57]. The Primer Premier ver. 5.0 software was used to construct specific PCR primers [58]. More details about primer sequences for real-time quantitative PCR of fungi are shown in Table S1.
Ericoid mycorrhizal fungi were identified as species of Meliniomyces variabilis [59], Oidiodendron maius [60], and Phialocephala fortinii [61] due to their functions in promoting N uptake. Their abundances were quantified as potential drivers to detect their associations with plant variables and N utilization.
2.5. Parameter Calculation and Statistics
The soil C/N ratio was calculated as the SOC divided by SON [62]. Plant N utilization (NUI) was assessed using a method modified from previous studies on juvenile plants [40,63,64]:
where DM is the weight of dry mass in aboveground organs and %N is the percent of total N content in aboveground organs. Data were analyzed by SPSS ver. 19.0 (SPSS Institute, Chicago, USA) following a factorial design. Dominant species was one factor with two levels (V. uliginosum vs. L. palustre), and the interruption treatment (removal vs. control or co-exist) was the other. Each factor was replicated six times as repeated values from plots. Analysis of variance (ANOVA) was used to detect the effects of combined factors on plant and soil parameters. When a significant effect was detected, results were compared with a Tukey test (α = 0.05). Vector diagnosis was used to analyze the nomographic relationships among plant biomass, N content, and N concentration between interruption treatments or communities [65]. Pearson correlation was used to analyze the relationship between ericoid mycorrhiza microbial abundance and plant parameters. Finally, all plant and soil parameters were analyzed in principle component (PC) sectionalization.
3. Results
3.1. Soil Chemicophysical Properties
Soil bulk density was not affected by the interactive effects of dominant species and removal interruption (Table 1). Communities dominated by L. palustre had a higher BD (mean ± standard error, the same below; 32.45 ± 4.02 g cm−3) than those by V. uliginosum (29.60 ± 4.08 g cm−3) (Table 2). The interruption treatment also induced significant effects on BD, which was higher in plots with dominant species removal (34.80 ± 2.00 g cm−3) relative to the control (27.25 ± 2.83 g cm−3).

Table 1.
Analysis of variance (ANOVA) effect of dominant (D) species (Ledum palustre vs. Vaccinium uliginosum) of understory communities, interruption (I) treatment of removing dominant species, and their interaction (D × I) on soil properties in forests of northeast China.

Table 2.
Effects of combined dominant species (Ledum palustre vs. Vaccinium uliginosum) of understory communities and the interruption of removal on soil properties in forests of northeast China.
Factors of dominant species and interruption treatment had an interactive effect on the soil’s pH value (Table 1). Communities dominated by L. palustre had lower soil pH values in plots subjected to the removal treatment compared to those in the co-existing control and those dominated by V. uliginosum (Table 2).
Dominant species had no effect on soil phenol content; instead, the effect of the interruption treatment was significant (Table 1). Communities with co-existing V. uliginosum and L. palustre had higher soil phenol contents (43.91 ± 11.84 mg kg−1) than those receiving the removal treatment (23.81 ± 7.43 mg kg−1) (Table 2).
The factors of dominant species and interruption treatment had interactive effects on SOC and SON (Table 1). Communities dominated by V. uliginosum had lower SOC in the removal treatment compared to those in the control and others dominated by L. palustre (Table 2). Instead, communities dominated by V. uliginosum in the removal treatment had a higher SON than those in response to other combined factors. Communities dominated by L. palustre had higher SON in the removal treatment than controlled communities dominated by either species. Controlled communities dominated by L. palustre had the lowest SON. Accordingly, communities dominated by V. uliginosum in the removal treatment had the lowest soil C/N ratio among those subjected to all combined factors (Table 2).
Combined factors also generated interactive effects on microbial biomass C and N in soils (Table 1). Among all treatments of combined factors, MBC was highest in communities dominated by V. uliginosum in the control and lowest in communities dominated by L. palustre subjected to the removal treatment (Table 2). Microbial biomass N was higher in controlled communities dominated by either V. uliginosum or L. palustre relative to those in communities receiving the removal treatment (Table 2).
Net N mineralization rate was significantly responsive to combined factors of dominant species and interruption treatment (Table 1). Among all treatments, the net N mineralization rate was highest in communities dominated by L. palustre in the removal treatment and lowest in controlled communities dominated by V. uliginosum (Table 2). Soil ammonium N content was higher in communities dominated by L. palustre (29.14 ± 5.26 mg kg−1) compared to V. uliginosum (23.65 ± 3.82 mg kg−1). The removal of dominant species reduced soil ammonium N content (21.93 ± 2.57 mg kg−1) by 28.95% relative to the control (30.86 ± 3.81 mg kg−1). Soil nitrate N content was higher in communities dominated by L. palustre in the removal treatment compared to those subjected to other combined treatments.
Soil total N content was affected by either dominant species or the interruption treatment (Table 1). Soil total N content was higher in communities dominated by L. palustre (32.45 ± 4.02 mg g−1) than those by V. uliginosum (29.60 ± 4.08 mg g−1) (Table 2). The removal treatment increased soil total N content by 27.69% (removal: 34.80 ± 2.00 mg g−1; control: 27.25 ± 2.83 mg g−1). Neither soil total P nor total K contents were responsive to combined factors of dominant species and the interruption treatment (Table 1). Soil total P content ranged between 7.67 mg g−1 and 8.72 mg g−1, and soil total K content ranged from 19.76 to 24.10 mg g−1 (Table 2).
3.2. Plant Parameters
The factors of dominant species and interruption treatment had no interactive effects on plant parameters (Table 3). Instead, the main effects came from either of the factors. Communities dominated by V. uliginosum had higher levels of plant biomass (Figure 1A), NUI index (Figure 1E), and root colonization rate (Figure 1G) compared to those dominated by L. palustre. The removal of dominant species reduced levels in all plant parameters compared to the control (Figure 1B,D,F,H).

Table 3.
Analysis of variance (ANOVA) effect of dominant (D) species of understory communities, interruption (I) by removing dominant species, and their interaction (D × I) on plant parameters of Ledum palustre vs. Vaccinium uliginosum in forests of northeast China.
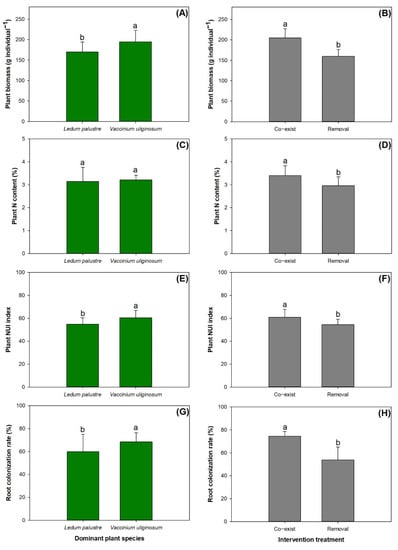
Figure 1.
Contrasting levels of plant biomass (A,B), nitrogen (N) content (C,D), N utilization index (NUI) (E,F), and root colonization rate (G,H) in communities dominated by Ledum palustre vs. Vacciniuim uliginosum (left column) or subjected to the interruption treatment of removal of dominant species vs. the control of co-existence (right column). Columns stand for means with error bars marking standard errors. Different letters label significant difference according to a Tukey test at 0.05 level.
Compared to communities dominated by L. palustre, those dominated by V. uliginosum induced an alleviation of the relative N deficiency (Figure 2A). This suggests that the V. uliginosum dominance was better acclimated to N deficiency relative to the L. palustre dominancy. The interruption treatment of removing dominant species induced an N excess state relative to the control of two co-existing species (Figure 2B). This was diagnosed as a result of antagonism induced by the removal of dominant species. When both factors were considered together, the interruption treatment of removing the dominant species still induced excessive N uptake relative to the control for both types of communities dominated by V. uliginosum and L. palustre (Figure 2C).
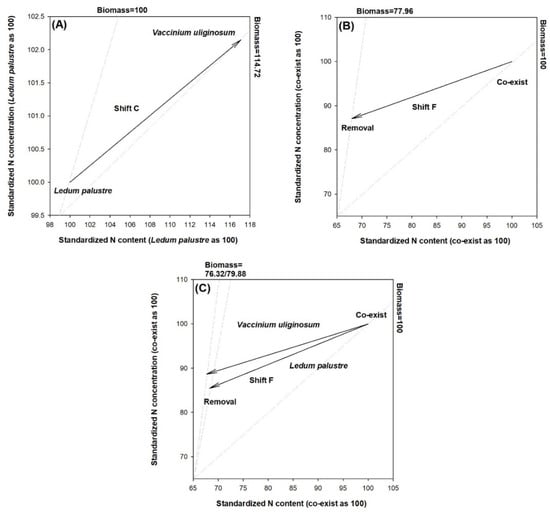
Figure 2.
Vector diagnoses of combined statuses of biomass, nitrogen (N) content, and N concentration in communities dominated by Ledum palustre and Vaccinium uliginosum (A) in the interruption treatments by removal of dominant species and control of co-existence (B) and their interactions (C). Nitrogen content and concentrations are standardized to a percent number relative to the reference (100, 100). Vector shifts mark relative N statuses with interpretations given by Salifu and Timmer [65]. Shift C stands for an alleviation of relative N deficiency by the factor level next to the arrow tip. Shift F stands for a status of excessive N status that may be caused by N antagonism.
3.3. Soil Microbial Community
Seven phyla of soil microbial communities were found by real-time quantitative PCR (Figure 3). Six microbial phyla were confirmed and classified, but there were some fungi phyla which were unclassified. Ascomycota and Basidiomycota together accounted for about 97% of the relative abundances of all soil microbial phyla, but neither was significantly responsive to factors of dominant species or the interruption treatment. Instead, the relative abundance of the Glomeromycota phylum was affected by combined dual factors (ANOVA: F value, 4.71; p value, 0.0421). The removal treatment reduced the relative abundance of this phylum in soils of communities dominated by L. palustre by 92.37% (Figure 3). In communities subjected to the interruption treatment, the V. uliginosum dominance had a higher relative abundance of the Glomeromycota phylum compared to the L. palustre dominance by a thousand times. The removal treatment also reduced the relative abundance of Rozellomycota phylum by 82.76% (Figure 3). Compared to communities dominated by L. palustre, those dominated by V. uliginosum showed a lower relative abundance of unclassified fungi phyla but a higher Zygomycota phylum abundance.
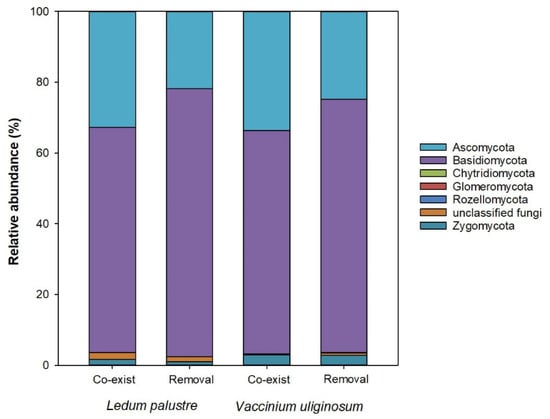
Figure 3.
Relative abundances of soil microbial phyla in communities dominated by Ledum palustre and Vaccinium uliginosum in the interruption treatment of dominant species removal and an untreated control of both species co-existing. Classifications of microbial phyla are given in the figure legend whereas some mycorrhizal fungi are unclassified.
The ericoid mycorrhiza abundance of soil Odiodendron maius was higher in soils of controlled communities dominated by L. palustre than in soils subjected to other combined factors (Figure 4A). The interruption treatment showed significant effects on soil Meliniomyces varia abundance (Figure 4B). The removal treatment reduced the abundance of Meliniomyces varia (2.03 ± 1.59 thousand) relative to the control in soils by 81.60% (11.05 ± 7.58 thousand). Soil Phialocephala fort abundance in communities dominated by L. palustre (2.13 ± 1.24 thousand) was higher by 129.61% than those by V. uliginosum (0.93 ± 0.89 thousand) (Figure 4C). The removal treatment reduced soil Phialocephala fort abundance (0.71 ± 0.99 thousand) by 69.54% compared to the control (2.34 ± 0.91 thousand).
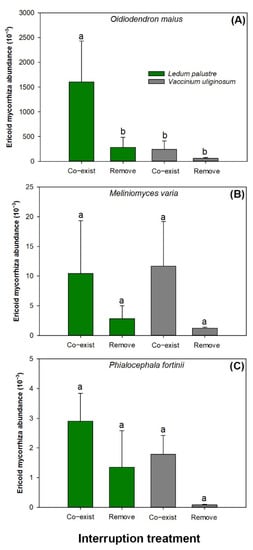
Figure 4.
Ericoid mycorrhiza abundances of Oidiodendron maius (A), Meliniomyces variabilis (B), and Phialocephala fortinii (C) in soils of communities dominated by Ledum palustre and Vaccinium uliginosum in the interruption treatment of dominant species removal and an untreated control of both species co-existing. Columns stand for means with error bars marking standard errors. Different letters label significant difference according to a Tukey test at 0.05 level.
3.4. Relationship between Soil Microbial Phyla and Plant Variables
An Ericoid mycorrhiza abundance of Oidiodendron maius had no relationship with plant biomass (Figure 5A) or root colonization rate (Figure 5B). However, it had a significantly positive relationship with plant N content (Figure 5C). Ericoid mycorrhiza abundance of Meliniomyces variabilis had positive relationships with plant biomass (Figure 5D), root colonization rate (Figure 5E), and plant N content (Figure 5F). In addition, an Ericoid mycorrhiza abundance of Phialocephala fortinii had positive relationships with plant variables (Figure 5G–I).
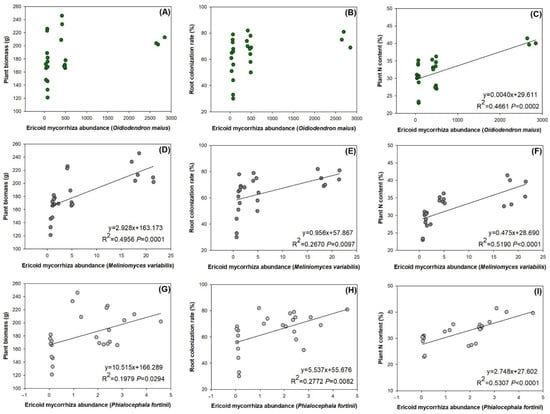
Figure 5.
Relationships between ericoid mycorrhiza abundances of Oidiodendron maius (A–C), Meliniomyces variabilis (D–F), and Phialocephala fortinii (G–I). Significant relationship is labelled as linear correlations with determinative coefficient (R2) and p values were disclosed.
3.5. Principal Component Analysis
All soil and parameters can be characterized by eigenvalues in two PCs (Figure 6). The first PC accounts for 29.99% of the variation and the second for 21.30%. Along the axis of the first PC, soil parameters of ammonium N content, MBC, phenol content and MBN had a positive relationship with plant biomass and root colonization rate, with both showing negative relationships with soil nitrate content, net N mineralization rate, bulk density, and total N content. Along the axis of the second PC, soil phyla of unclassified fungi had a negative relationship with total N content of the plant. In addition, the soil C/N ratio showed a positive relationship with the abundance of the Ascomycota phylum in soil, with both being negatively related to the abundance of the Basidiomycota phylum in soil.
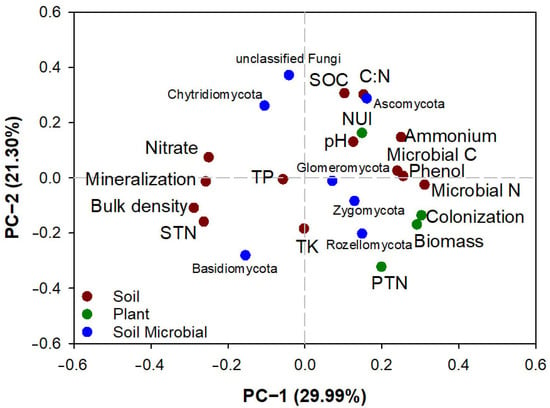
Figure 6.
Principal component (PC) analysis of soil and plant parameters in communities dominated by Ledum palustre and Vaccinium uliginosum in the interruption treatment of dominant species removal and an untreated control of both species co-existing. Parameters enclosed in red circles indicate negative relationships and those in cyan circles show positive. Spots in dark red mark soil physicochemical properties: Nitrate, soil nitrate N content; Mineralization, net mineralization rate of mycorrhiza fungi; STN, soil total N content; Ammonium, soil ammonium N content; Microbial C, MBC; Microbial N, MBN; Phenol, soil phenol content; TP, soil total phosphorus content; TK, soil total potassium content; C/N, ratio of soil SOC to SON contents. Spots in dark green mark plant parameters: PTN, plant total N content; Colonization, root colonization rate. Spots in blue mark soil ericoid mycorrhiza abundances of microbial phyla.
4. Discussion
4.1. N Availabilities in Two Communities with Contrasting Soil Chemicophysical Properties
As local dwellers in boreal forested boglands, V. uliginosum and L. palustre had natural differences in N absorption in their dominant communities. In the context of global warming, V. uliginosum presented a lower foliar N concentration than L. palustre in controlled ambient stands [16]. However, in contrast, in highly controlled environments where air temperature was caused to rise to 1.3 °C, foliar N concentration was higher in V. uliginosum than in L. palustre [66]. In our study, the two species did not show a distinct difference in plant N content when both were subjected to the removal treatment. Therefore, temperature was unlikely the factor driving the distinct responses of the two species. This can be caused by the fact that stands where two types of communities were distributed had a similar level of soil N availability. Even so, their soil characteristics were highly distinct from each other.
Compared to soils in communities dominated by V. uliginosum, those by L. palustre showed higher mineral N availability at higher levels in mineralized N rate and ammonium and nitrate N contents. In addition, soil total N content was also higher in soils of L. palustre communities, where organic N was lower in response to higher BD. These findings together suggest a cooccurring process where a large proportion of soluble organic N was immobilized in soils inhabited by L. palustre. This speculation can be seen in another study conducted in a hardwood forest watershed in Alaska, USA, where L. palustre enhanced inorganic N immobilization [67]. Therein, the authors surmised that it was the high concentrations of phenolic compounds in soils that immobilized mineral N according to combined findings of low net mineralization but high soil C/N. In our study, soluble phenol content in soils did not differ between the two types of communities, suggesting that rainfall leachates from leaves and decomposing litter resulted in soluble phenolic compounds to a similar level between the two communities. Immobilized N was largely fixed in the form of soil microbial retention in communities of L. palustre, which can be confirmed by higher levels of the soil’s C/N ratio and microbial C with a low soil pH [55,68]. The immobilization of mineral N was mainly achieved by soil microbial activities.
The Glomeromycota phylum showed different responses of abundances in soils between the two types of communities, which can be an indicator of N addition to the wetlands of northeast China [69]. However, in our study, the change of Glomeromycota phylum abundance had a rare relationship with N uptake. Compared to communities dominated by L. palustre, a lower relative abundance of unclassified fungi phyla and higher Zygomycota phylum abundance together suggest a force acting to increase plant N content in V. uliginosum. As discussed above, microbial activity contributed to the N immobilization in L. palustre communities and benefited N mobilization in V. uliginosum communities. This can also be supported by our results about the root colonization rate, which was higher in V. uliginosum. This force was not effective enough to cause a significant difference in N uptake between the two plant species. In addition, Oidiodendron maius and Phialocephala fortinii are two ericoid mycorrhizal fungi that can benefit N uptake of the host plants through inoculation [61]. We also confirmed their contributions to leaf N concentration and higher abundances in soils of L. palustre communities. Again, these changes did not lead to different leaf N concentrations between the two species due to the immobilization effect by the microbial community.
4.2. Removal of Dominant Species Modifies N Availability
Compared to controlled communities with co-existing V. uliginosum and L. palustre, those receiving the removal treatment had lower plant N concentrations. The removal treatment increased mineralized N, soil organic N, microbial N, and total N contents in soils, but the N availability was still lower. This was explained by the removal treatment impacting soils and inducing higher BD. Compared to soils with a good state of aeration, compacted soils are more likely to limit root growth and N foraging [70]. We do not consider soil compaction resulting from the process of plant removal because we conducted a long-term experiment and sub-plots were reconstructed every year. Given that both plant species are prone to colonization by ectomycorrhiza [71,72], the removal treatment interrupted N acquirement through interplay with ericoid mycorrhiza, further reducing the root colonization rate. For example, abundances of ericoid mycorrhiza Meliniomyces varia and Phialocephala fort were largely reduced by the removal treatment, which was both negatively related to plant N content. In addition, removal decreased relative abundances of Rozellomycota phylum, which was also negatively related to N uptake. The change of microbial community structure will result in N mineralization and mycorrhizal infection, which together limit the rate of N uptake [73].
4.3. N Utilization and Driving Forces
It has been reported several times that NUI is assessed by a quotient of “the amount of biomass per unit amount of nutrient present in the biomass” [40,63,74,75]. The biomass production for assessing NUI was frequently adapted as the whole-plant scale in these studies. These types of studies cultured juvenile plant crops in highly manipulated conditions where the nutrient loss was controlled to an extremely low level and can even be omitted. However, it was indicated that this “perfect” condition belongs to a situation (a) of “biomass increasing proportionately more than nutrients—in which the quotient may indicate an increased efficiency of utilization” [76]. However, the actual conditions of N uptake in plants are much more complicated. At least two conditions can be characterized as other types of situations. For example, Siddiqi and Glass also put forward another situation of net negative productivity caused by nutrient loss [76]. This has a practical meaning because nutrients can be lost through (b) defoliation or (c) leaks. Therefore, the biomass production at the cost of N utilization should be taken as part of, or a group of, organisms of plants when facing complicated situations (a) to (c). For example, An et al. calculated NUI by leaf biomass divided by N concentration in fruiting Rubus idaeus, because the whole plant biomass was produced by the N cost of total uptake minus leaching loss [64]. The authors did not use biomass of above-ground organs because parts of their N reserves were probably translocated to support flowering and fruiting at the reproduction stage [77]. In our study, we focused on objects at the community level instead of an individual level, which resulted in plants of a sub-plot experiencing biomass production at N cost of loss through removal. Our plants also experienced N loss through leaching because their communities inhabited bogs and peatlands of boreal forests [33]. Therefore, we chose to use biomass production of above-ground organs to calculate the NUI quotient because there were residual plants over from the removal treatment and their above-ground organs used N as a difference of N uptake minus possible leaking loss. Our NUI has the theoretical rationality to assess N utilization by berry communities of boreal boglands.
Unfortunately, we cannot accept our first hypothesis. In our study, V. uliginosum showed a higher NUI than L. palustre, which resulted from greater dry mass production at a similar cost of plant N concentration. According to investigations in Middle Siberia [26], V. uliginosum is a dwarf shrub that dominates the layer over herbs with large canopies, and L. palustre is a tall shrub that has long stems but a small canopy. In their communities, V. uliginosum exists in a low frequency but a large vegetative growth; in contrast, L. palustre usually exists in a high frequency with small individual growth, especially in canopy size. In a tundra at Eagle Creek, Alaska, USA [27], V. uliginous was also found to have greater biomass than L. palustre in both current and old organs in stands subjected to uncontrolled soil N availability. Higher NUI can also be interpreted by vector diagnosis, which indicated a greater increment of biomass in V. uliginosum when N content and N concentrations both increased in synchronization.
We can accept our second hypothesis. The removal treatment was characterized as impairing NUI as a force to induce antagonism in both plant species. This was because removal of the dominant species impaired continuous biomass production of residual species if more N was absorbed. This concurred with findings of elevated levels in soil organic and inorganic N contents but lower N uptake. In the Alaskan tussock tundra, the removal of the dominant species was found to benefit biomass accumulation in L. palustre [78]. This was because the removed species was a competitor with the remaining ones. However, in our study, V. uliginosum and L. palustre were co-existing instead of competing; the removal of dominant species may modify the community habitat and expose residual plants to more intensified sunlight [15].
4.4. Limits of This Study
Our study still has limits that can be addressed in the future. Firstly, there were some unclassified fungi phyla which prevented further detection of family and species in soil microbial communities. To our knowledge, the unclassified microbial phyla were difficult to confirm and their composition in class and species may harbor important functions benefitting soil N availability. However, for the scope of this study, it was sufficient to obtain desired results about the relative abundances of exposed soil microbial phyla with their responses to treatments and correlations with N utilization. In future works, all fungi need to be classified to identify their phyla, family, and species with a deeper scope to reveal a broader spectrum of soil microbial attributes in understory ecosystems of boreal forests. Secondly, we demonstrated the abundances of three ericoid mycorrhizae to facilitate the determination of more explanations for soil–plant interaction. More ericoid mycorrhiza should be considered in future works to verify their contributions to host plant nutrition. Finally, the removal of competitors should be considered in future studies to compare with current results. Both herbs and dwarf trees can be potential competitors and their responses to NWFP plant removals will cause more uncertainties in soils.
5. Conclusions
In a five-year study, the understory communities undergoing continuous removal of dominant shrub species resulted in a series of responses of the remaining co-existing plant communities. As a simulation of severe interruption on the understory ecosystem, the removal of dominant plants would impair N utilization of the residual co-existing plants. Soil mineralization responded to our treatments, but soil microbial communities accounted for a larger contribution to soil N utilization. Communities dominated by V. uliginosum had a higher N availability than those by L. palustre, and both obtained a higher N availability without removal interruption. Therefore, communities of boglands should be protected from further exploitation, especially those dominated by L. palustre. Strategies need to be considered to improve the function of soil microbial communities in promoting N availability in bogs and peatlands in boreal forests.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12040932/s1, Table S1. Primer sequences for real-time quantitative PCR of fungi.
Author Contributions
Conceptualization, Y.D. and F.W.; Methodology, X.Z.; Software, D.G.; Validation, D.G. and X.Z.; Formal Analysis, Y.D.; Investigation, F.W.; Resources, F.W.; Data Curation, L.Z.; Writing—Original Draft Preparation, Y.D.; Writing—Review and Editing, Y.D. and X.F.; Visualization, X.Z.; Supervision, F.W.; Project Administration, F.W.; Funding Acquisition, F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (grant number: 2018YFD1000803-06) and the Key Laboratory of Horticultural Crops Germplasm Resources Utilization, Ministry of Agriculture and Rural Affairs of the People’s Republic of China (grant number: NYZS201907).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Authors thank students for their assistances in field plant investigation and sampling. Reviewers and editors are acknowledged for their contributions to the current edition of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Unasylva. FAO Forestry: Towards a Harmonized Definition of Non-Wood Forest Products. Available online: https://www.fao.org/3/x2450e/x2450e0d.htm#fao%20forestry (accessed on 26 March 2022).
- FAO. About Non-WOOD Forest Products. Available online: https://www.fao.org/forestry/nwfp/6388/en/ (accessed on 26 March 2022).
- Calama, R.; Tome, M.; Sanchez-Gonzalez, M.; Miina, J.; Spanos, K.; Palahi, M. Modelling non-wood forest products in Europe: A review. For. Syst. 2010, 19, 69–85. [Google Scholar] [CrossRef]
- Vacik, H.; Wolfslehner, B.; Huber, P.; Ruprecht, H. Analysis of Non Wood Forest Products and Forest Services in Sustainable Forest Management. Austrian J. For. Sci. 2014, 131, 147–169. [Google Scholar]
- Wannitikul, G. Deforestation in northeast Thailand, 1975–91: Results of a general statistical model. Singap. J. Trop. Geogr. 2005, 26, 102–118. [Google Scholar] [CrossRef]
- PerezGarcia, J.; Lippke, B.; Baker, J. Trade barriers in the Pacific forest sector: Who wins and who loses. Contemp. Econ. Policy 1997, 15, 87–103. [Google Scholar] [CrossRef]
- Tumaneng-Diete, T.; Ferguson, I.S.; MacLaren, D. Log export restrictions and trade policies in the Philippines: Bane or blessing to sustainable forest management? For. Policy Econ. 2005, 7, 187–198. [Google Scholar] [CrossRef]
- Zhao, Z.F.; Guo, Y.L.; Zhu, F.X.; Jiang, Y. Prediction of the impact of climate change on fast-growing timber trees in China. For. Ecol. Manag. 2021, 501, 119653. [Google Scholar] [CrossRef]
- Liu, K.; Liang, Y.; He, H.S.; Wang, W.J.; Huang, C.; Zong, S.W.; Wang, L.; Xiao, J.T.; Du, H.B. Long-Term Impacts of China’s New Commercial Harvest Exclusion Policy on Ecosystem Services and Biodiversity in the Temperate Forests of Northeast China. Sustainability 2018, 10, 1071. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.D.; Sun, S.B.; Yeo-Chang, Y. Impact of Forest Logging Ban on the Welfare of Local Communities in Northeast China. Forests 2021, 12, 3. [Google Scholar] [CrossRef]
- Song, Y.Y.; Song, C.C.; Ren, J.S.; Tan, W.W.; Jin, S.F.; Jiang, L. Influence of nitrogen additions on litter decomposition, nutrient dynamics, and enzymatic activity of two plant species in a peatland in Northeast China. Sci. Total Environ. 2018, 625, 640–646. [Google Scholar] [CrossRef]
- Qu, H.C.; Xiang, R.; Obsie, E.Y.; Wei, D.W.; Drummond, F. Parameterization and Calibration of Wild Blueberry Machine Learning Models to Predict Fruit-Set in the Northeast China Bog Blueberry Agroecosystem. Agronomy 2021, 11, 1736. [Google Scholar] [CrossRef]
- Li, R.; Wang, P.; Guo, Q.Q.; Wang, Z.Y. Anthocyanin composition and content of the Vaccinium uliginosum berry. Food Chem. 2011, 125, 116–120. [Google Scholar] [CrossRef]
- Jiang, J.; Wei, J.; Yu, H.; He, S. The Developing Blueberry Industry in China. In The Developing Blueberry Industry in China; IntechOpen: London, UK, 2019. [Google Scholar]
- Ejankowski, W. Demographic variation of dwarf birch (Betula nana) in communities dominated by Ledum palustre and Vaccinium uliginosum. Biologia 2010, 65, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Rohrs-Richey, J.K.; Mulder, C.P.H. Effects of local changes in active layer and soil climate on seasonal foliar nitrogen concentrations of three boreal forest shrubs. Can. J. For. Res. 2007, 37, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Dampc, A.; Luczkiewicz, M. Rhododendron tomentosum (Ledum palustre). A review of traditional use based on current research. Fitoterapia 2013, 85, 130–143. [Google Scholar] [CrossRef]
- Gretsusnikova, T.; Jarvan, K.; Orav, A.; Koel, M. Comparative analysis of the composition of the essential oil from the shoots, leaves and stems the wild Ledum palustre L. from Estonia. In Proceedings of the 5th International Conference of the Nordic-Separation-Science-Society, Tallinn Univ Technol, Tallinn, Estonia, 26–29 August 2009; Tallinn University of Technology: Tallinn, Estonia, 2010; pp. 168–173. [Google Scholar]
- Jin, C.; Strembiski, W.; Kulchytska, Y.; Micetich, R.G.; Danesstalab, M. Flavonoid glycosides from Ledum palustre L. subsp. decumbens (Ait.) Hulton. DARU J. Pharm. Sci. 1999, 7, 5–8. [Google Scholar]
- Gao, C.Y.; He, J.B.; Cong, J.X.; Zhang, S.Q.; Wang, G.P. Impact of forest fires generated black carbon deposition fluxes in Great Hinggan Mountains (China). Land Degrad. Dev. 2018, 29, 2073–2081. [Google Scholar] [CrossRef]
- Qu, B.; Li, W.; Chen, Y.; Liu, J. Protection versus culture-driven exploitation of wild plant resources: The case on Changbai Mountain. Int. J. Sustain. Dev. World Ecol. 2011, 18, 404–411. [Google Scholar] [CrossRef]
- Kocianova-Mackova, D. Population structure of Ledum palustre in Klin peat bog in NW Slovakia. Biologia 1999, 54, 61–65. [Google Scholar]
- Che, L.N.; Cheng, M.Y.; Xing, L.B.; Cui, Y.F.; Wan, L.H. Effects of permafrost degradation on soil organic matter turnover and plant growth. Catena 2022, 208, 9. [Google Scholar] [CrossRef]
- Shan, W.; Xu, Z.; Guo, Y.; Zhang, C.; Hu, Z.; Wang, Y. Geological methane emissions and wildfire risk in the degraded permafrost area of the Xiao Xing’an Mountains, China. Sci. Rep. 2020, 10, 21297. [Google Scholar] [CrossRef]
- Iturrate-Garcia, M.; Heijmans, M.; Cornelissen, J.H.C.; Schweingruber, F.H.; Niklaus, P.A.; Schaepman-Strub, G. Plant trait response of tundra shrubs to permafrost thaw and nutrient addition. Biogeosciences 2020, 17, 4981–4998. [Google Scholar] [CrossRef]
- Mukhortova, L.; Krivobokov, L.; Sergeeva, O.; Meteleva, M.; Schepaschenko, D.J.B.W.C. Specifisity of phytocoenotic structure and biomass of ground cover in northern boreal forests of Middle Siberia. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2020; Volume 24, p. 57. [Google Scholar]
- Shaver, G.R.; Chapin, F.S. Effect of Fertilizer on Production and Biomass of Tussock Tundra, Alaska, U.S.A. Arct. Alp. Res. 1986, 18, 261–268. [Google Scholar] [CrossRef]
- Wei, H.X.; Zhao, H.T.; Chen, X. Foliar N:P Stoichiometry in Aralia elata Distributed on Different Slope Degrees. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.X.; Zhao, H.T.; Chen, X.; He, X.Y. Secondary metabolites, carbohydrate accumulation, and nutrient uptake in Aralia elata (Miq.) Seem seedlings exposed to shoot cutting and different LED spectra. Acta Physiol. Plant. 2020, 42, 162. [Google Scholar] [CrossRef]
- Wei, H.X.; Chen, X.; Chen, G.S.; Zhao, H.T. Foliar nutrient and carbohydrate in Aralia elata can be modified by understory light quality in forests with different structures at Northeast China. Ann. For. Res. 2019, 62, 125–137. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.X.; Xu, C.Y.; Ma, L.Y.; Wang, W.J.; Duan, J.; Jiang, L.N. Short-term Nitrogen (N)-Retranslocation within Larix olgensis Seedlings is Driven to Increase by N-deposition: Evidence from a Simulated N-15 Experiment in Northeast China. Int. J. Agric. Biol. 2014, 16, 1031–1040. [Google Scholar]
- Xing, A.J.; Xu, L.C.; Shen, H.H.; Du, E.Z.; Liu, X.Y.; Fang, J.Y. Long term effect of nitrogen addition on understory community in a Chinese boreal forest. Sci. Total Environ. 2019, 646, 989–995. [Google Scholar] [CrossRef]
- Wei, H.X.; Xu, C.Y.; Ren, J.; Ma, L.Y.; Duan, J.; Jiang, L.N. Newly transplanted Larix olgensis Henry stock with greater root biomass has higher early nitrogen flux rate. Soil Sci. Plant Nutr. 2013, 59, 740–749. [Google Scholar] [CrossRef]
- Wei, H.X.; Guo, P. Carbohydrate metabolism during new root growth in transplanted Larix olgensis seedlings: Post-transplant response to nursery-applied inorganic fertilizer and organic amendment. Iforest-Biogeosci. For. 2017, 10, 15–22. [Google Scholar] [CrossRef] [Green Version]
- He, C.X.; Gao, J.; Zhao, Y.; Liu, J. Root Foraging Precision of Pinus pumila (Pall.) Regel Subjected to Contrasting Light Spectra. Plants 2021, 10, 1482. [Google Scholar] [CrossRef]
- Tan, L.; Fan, R.F.; Sun, H.F.; Guo, S.L. Root foraging of birch and larch in heterogeneous soil nutrient patches under water deficit. PLoS ONE 2021, 16, e0255848. [Google Scholar] [CrossRef] [PubMed]
- Bonan, G.B.; Shugart, H.H. Environmental-factors and ecological processes in boreal forests. Annu. Rev. Ecol. Syst. 1989, 20, 1–28. [Google Scholar] [CrossRef]
- Xu, W.Y.; Elberling, B.; Ambus, P.L. Fire increases soil nitrogen retention and alters nitrogen uptake patterns among dominant shrub species in an Arctic dry heath tundra. Sci. Total Environ. 2022, 807, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Y.; Wei, H.X. Chitosan oligosaccharide addition affects current-year shoot of post-transplant Buddhist pine (Podocarpus macrophyllus) seedlings under contrasting photoperiods. Iforest-Biogeosci. For. 2017, 10, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Z.; Xu, S.J.; Li, Y.J.; He, C.X. Nutrient assimilation and utilization in Korean pine (Pinus koraiensis) seedlings exposed to exponential fertilization under contrasting spectra. Commun. Soil Sci. Plant Anal. 2020, 51, 2414–2428. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Wei, H.X.; Lv, J.; Chen, C.; Liu, X.Y.; Wen, Q.; Jia, L.M. Nutrient uptake and utilization in Prince Rupprecht’s larch (Larix principis-rupprechtii Mayr.) seedlings exposed to a combination of light-emitting diode spectra and exponential fertilization. Soil Sci. Plant Nutr. 2019, 65, 358–368. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Zhao, S.J.; Tang, J.Y.; Zhu, H.; Wei, H.X.; Cui, W.; Wang, M.H.; Guo, P. White-light emitting diodes’ spectrum effect on photosynthesis and nutrient use efficiency in Podocarpus macrophyllus seedlings. J. Plant Nutr. 2020, 43, 2876–2884. [Google Scholar] [CrossRef]
- Chu, X.L.; Luo, X.Y.; Zhou, Z.C. Exponential fertilization on red-seed tree (Ormosia hosiei) seedlings subjected to contrasting light conditions: Do we really need intensive nutrient loading? Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12244. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, Q.X.; Lei, H.Q.; Wang, J.W.; Yang, S.; Wei, H.X. Nutrient Uptake and Utilization by Fragrant Rosewood (Dalbergia odorifera) Seedlings Cultured with Oligosaccharide Addition under Different Lighting Spectra. Forests 2018, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.X.; Hauer, R.J.; Chen, G.S.; Chen, X.; He, X.Y. Growth, Nutrient Assimilation, and Carbohydrate Metabolism in Korean Pine (Pinus koraiensis) Seedlings in Response to Light Spectra. Forests 2020, 11, 44. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Tan, W.Z.; Li, B.; Wen, L.; Lei, G.C.; Blakeslee, A. Habitat alteration facilitates the dominance of invasive species through disrupting niche partitioning in floodplain wetlands. Divers. Distrib. 2021, 27, 1861–1871. [Google Scholar] [CrossRef]
- Feng, J.Y.; Li, Z.; Hao, Y.F.; Wang, J.; Ru, J.Y.; Song, J.; Wan, S.Q. Litter removal exerts greater effects on soil microbial community than understory removal in a subtropical-warm temperate climate transitional forest. For. Ecol. Manag. 2022, 505, 119867. [Google Scholar] [CrossRef]
- Deng, J.J.; Zhou, W.M.; Dai, L.M.; Yuan, Q.; Zhou, L.; Qi, L.; Yu, D.P. The Effects of Shrub Removal on Soil Microbial Communities in Primary Forest, Secondary Forest and Plantation Forest on Changbai Mountain. Microb. Ecol. 2022, online. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.X.; Chen, G.S.; Chen, X.; Zhao, H.T. Geographical distribution of Aralia elata characteristics correlated with topography and forest structure in Heilongjiang and Jilin Provinces, Northeast China. J. For. Res. 2021, 32, 1115–1125. [Google Scholar] [CrossRef]
- Raison, R.J.; Connell, M.J.; Khanna, P.K. Methodology for studying fluxes of soil mineral-N in situ. Soil Biol. Biochem. 1987, 19, 521–530. [Google Scholar] [CrossRef]
- Kuiters, A.T.; Sarink, H.M. Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees. Soil Biol. Biochem. 1986, 18, 475–480. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.; Mulvaney, C. Nitrogen-Total, Methods of Soil Analysis; American Society of Agronomy: Madison, MI, USA, 1982. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass-C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.K.; Liang, W.J.; Jiang, Y.; Dai, G.H.; Wang, X.G.; Han, S.J. Distribution of Soil Organic Carbon Fractions Along the Altitudinal Gradient in Changbai Mountain, China. Pedosphere 2011, 21, 615–620. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–160. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Black, T. Abundance and distribution of the multi-functional root associated fungus Meliniomyces variabilis. Master Thesis, Saint Mary’s University, Halifax, NS, Canada, 2016. [Google Scholar]
- Grelet, G.A.; Johnson, D.; Paterson, E.; Anderson, I.C.; Alexander, I.J. Reciprocal carbon and nitrogen transfer between an ericaceous dwarf shrub and fungi isolated from Piceirhiza bicolorata ectomycorrhizas. New Phytol. 2009, 182, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Lin, W.R.; Hsu, Y.C.; Pan, H.Y. Influences of Three Oidiodendron maius Isolates and Two Inorganic Nitrogen Sources on the Growth of Rhododendron kanehirae. Hortic. Sci. Technol. 2020, 38, 742–753. [Google Scholar]
- Vohnik, M.; Albrechtova, J.; Vosatka, M. The inoculation with Oidiodendron maius and Phialocephala fortinii alters phosphorus and nitrogen uptake, foliar C: N ratio and root biomass distribution in Rhododendron cv. Azurro. Symbiosis 2005, 40, 87–96. [Google Scholar]
- Ding, X.; Li, X.; Qi, Y.; Zhao, Z.; Sun, D.; Wei, H. Depth-Dependent C-N-P Stocks and Stoichiometry in Ultisols Resulting from Conversion of Secondary Forests to Plantations and Driving Forces. Forests 2021, 12, 1300. [Google Scholar] [CrossRef]
- Li, X.W.; Gao, Y.; Wei, H.X.; Xia, H.T.; Chen, Q.X. Growth, biomass accumulation and foliar nutrient status in fragrant rosewood (Dalbergia odorifera TC Chen) seedlings cultured with conventional and exponential fertilizations under different photoperiod regimes. Soil Sci. Plant Nutr. 2017, 63, 153–162. [Google Scholar] [CrossRef] [Green Version]
- An, B.Y.; Wei, H.X.; Li, L.L.; Guo, P. Nutrient Uptake and Utilization and Antioxidants of Fruits in Red Raspberry (Rubus idaeus L.) Cultivar ’Autumn Bliss’ in response to Fertilization under Extended Photoperiod. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Salifu, K.F.; Timmer, V.R. Optimizing nitrogen loading of Picea mariana seedlings during nursery culture. Can. J. For. Res. 2003, 33, 1287–1294. [Google Scholar] [CrossRef]
- Suzuki, S.; Kudo, G. Short-term effects of simulated environmental change on phenology, leaf traits, and shoot growth of alpine plants on a temperate mountain, northern Japan. Glob. Change Biol. 1997, 3, 108–115. [Google Scholar] [CrossRef]
- Castells, E.; Penuelas, J.; Valentine, D.W. Influence of the phenolic compound bearing species Ledum palustre on soil N cycling in a boreal hardwood forest. Plant Soil 2003, 251, 155–166. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, J.J.; Zhang, M.; Yan, Q.L.; Sun, O.J. Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: A comparison between natural secondary forest and larch plantation. J. Plant Ecol. 2010, 3, 175–182. [Google Scholar] [CrossRef]
- Sui, X.; Liu, Y.N.; Yang, L.B.; Li, M.S.; Zhang, S.Y.; Zhang, T.; Wang, J.F.; Cui, X.Y.; Ni, H.W. Response of Soil AMF Diversity to Nitrogen Deposition in a Calamagrostis augustifolia Wetland of Sanjiang Plain, China. Int. J. Agric. Biol. 2019, 22, 1565–1572. [Google Scholar]
- Parlak, M.; Parlak, A.O. Effect of soil compaction on root growth and nutrient uptake of forage crops. J. Food Agric. Environ. 2011, 9, 275–278. [Google Scholar]
- Makarov, M.I.; Malysheva, T.I.; Kadulin, M.S.; Verkhovtseva, N.V.; Sabirova, R.V.; Lifanova, V.O.; Zhuravleva, A.I.; Karpukhin, M.M. The Effect of Ericoid Mycorrhizal and Ectomycorrhizal Plants on Soil Properties of Grass Meadow in Tundra of the Khibiny Mountains. Eurasian Soil Sci. 2020, 53, 569–579. [Google Scholar] [CrossRef]
- Urcelay, C.; Bret-Harte, M.S.; Diaz, S.; Chapin, F.S. Mycorrhizal colonization mediated by species interactions in arctic tundra. Oecologia 2003, 137, 399–404. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, C.; Bao, J.; Zhu, H.; Chen, Y.; Luo, Y.; Zhang, L. Microbial diversity and physicochemical properties in farmland soils amended by effective microorganisms and fulvic acid for cropping Asian ginseng. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12563. [Google Scholar] [CrossRef]
- Miller, B.D.; Hawkins, B.J. Nitrogen uptake and utilization by slow- and fast-growing families of interior spruce under contrasting fertility regimes. Can. J. For. Res. -Rev. Can. Rech. For. 2003, 33, 959–966. [Google Scholar] [CrossRef]
- Hawkins, B.J. Family variation in nutritional and growth traits in Douglas-fir seedlings. Tree Physiol. 2007, 27, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, M.Y.; Glass, A.D.M. Utilization index: A modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J. Plant Nutr. 1981, 4, 289–302. [Google Scholar] [CrossRef]
- Martinez-Alcantara, B.; Quinones, A.; Primo-Millo, E.; Legaz, F. Nitrogen remobilization response to current supply in young citrus trees. Plant Soil 2011, 342, 433–443. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Shevtsova, A.; Chapin, F.S. Plant responses to species removal and experimental warming in Alaskan tussock tundra. Oikos 1999, 84, 417–434. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).