Stability of Protein and Oil Content in Soybean across Dry and Normal Environments—A Case Study in Croatia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Description of Field Trials
2.3. Data Collection
2.4. Statistical Analysis
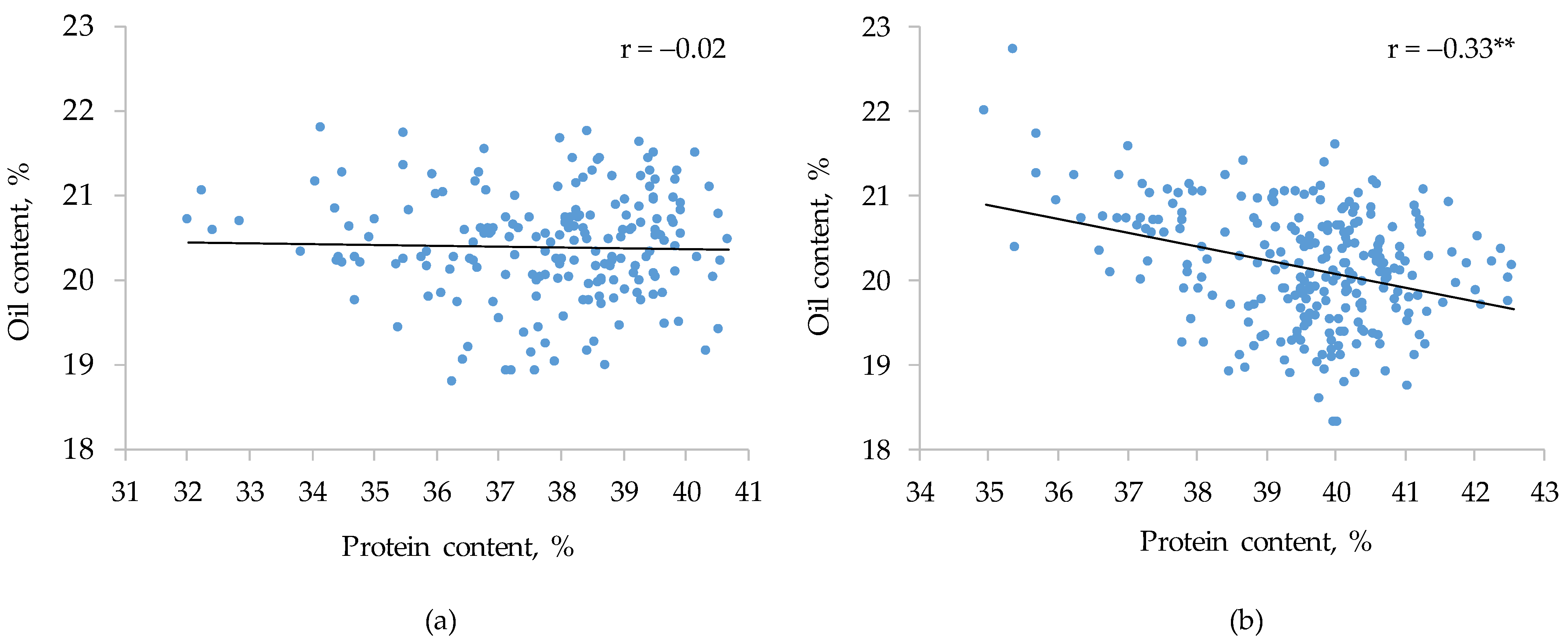
3. Results and Discussion
3.1. Weather Conditions
3.2. Analysis of Variance
3.3. Main Effects of Environments and Genotypes
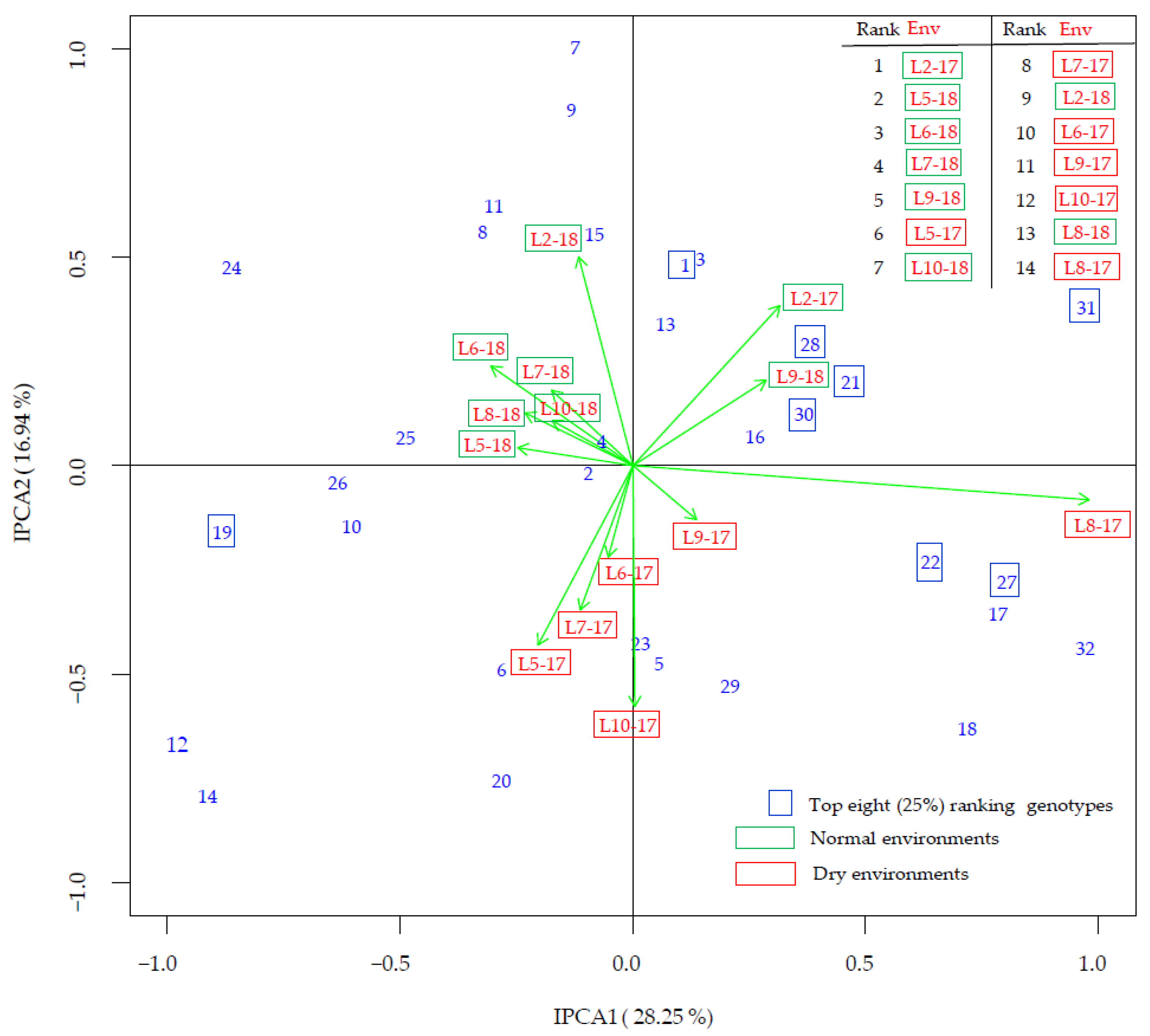
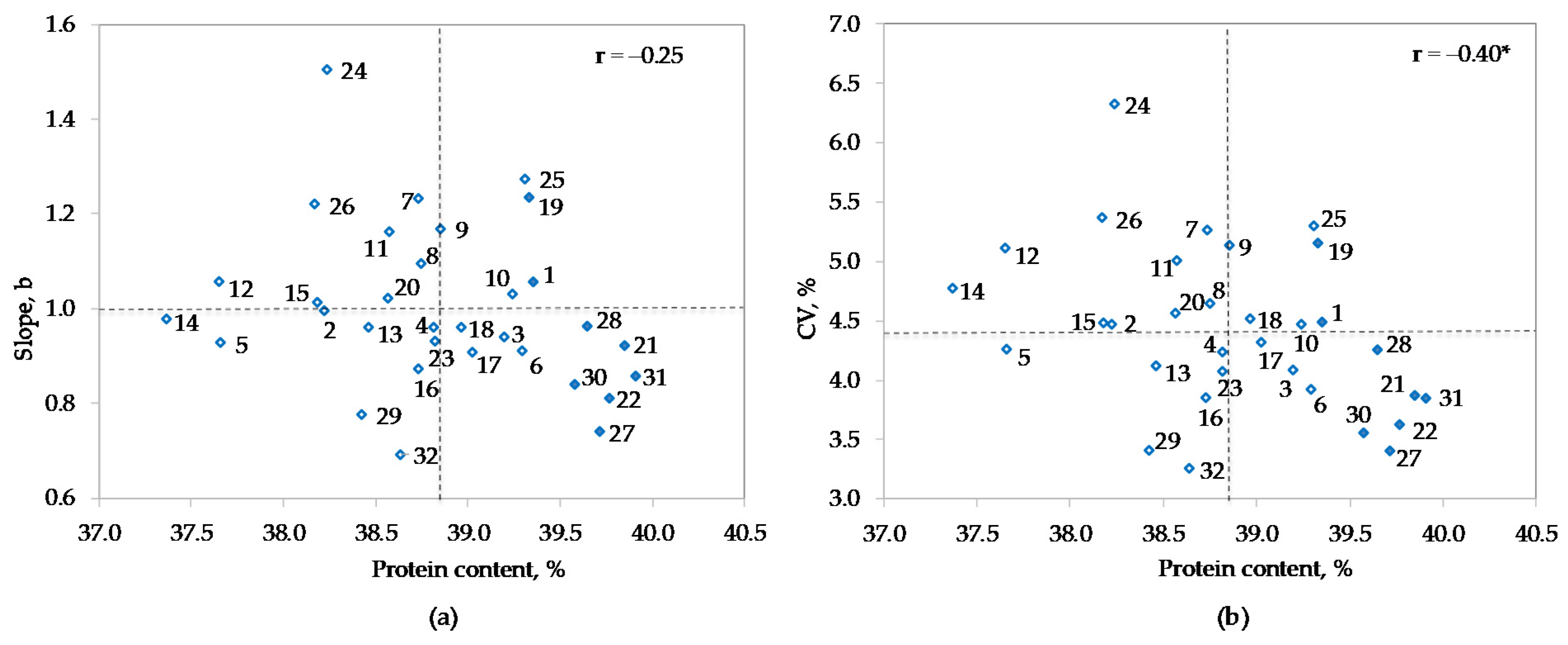
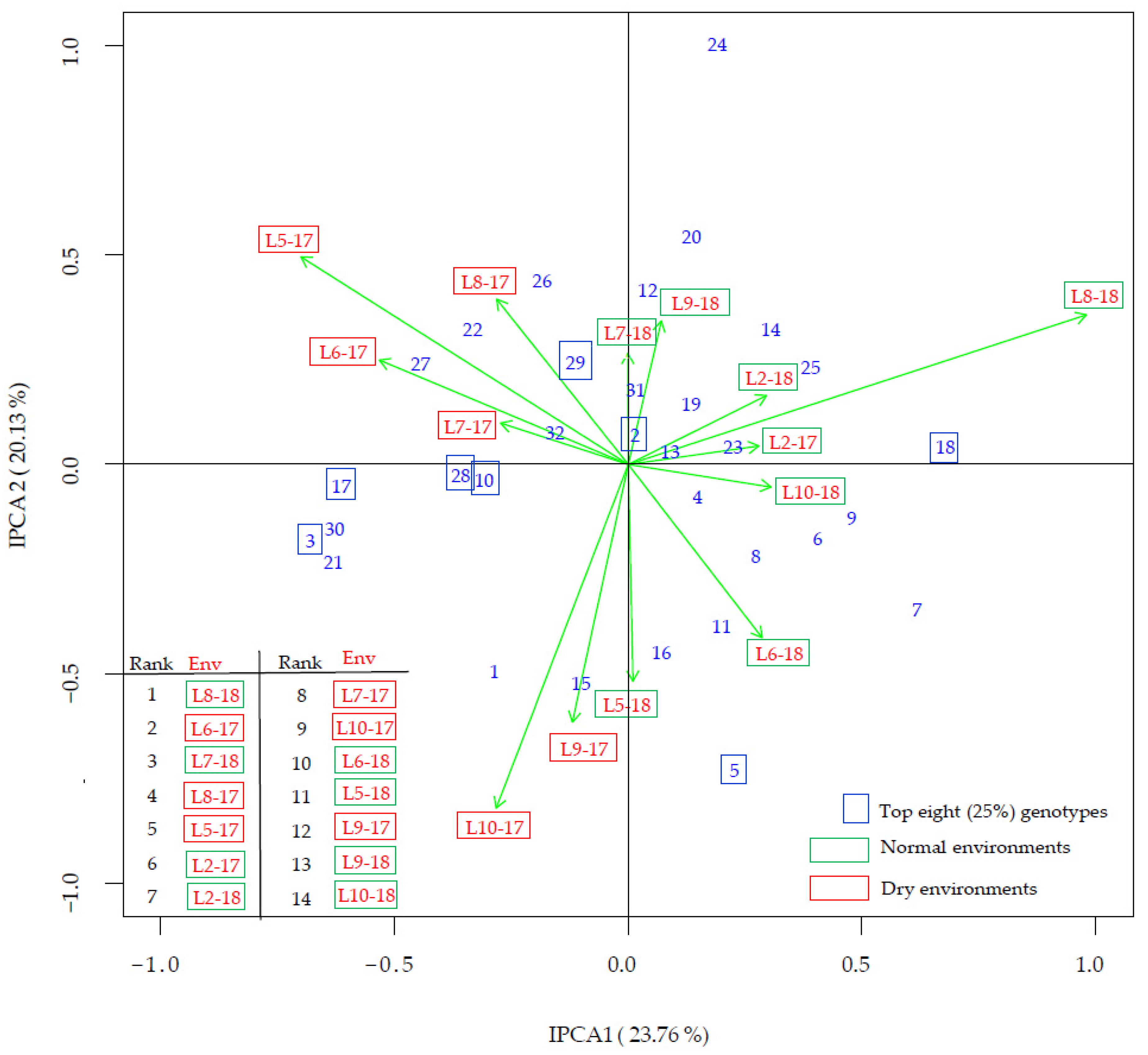
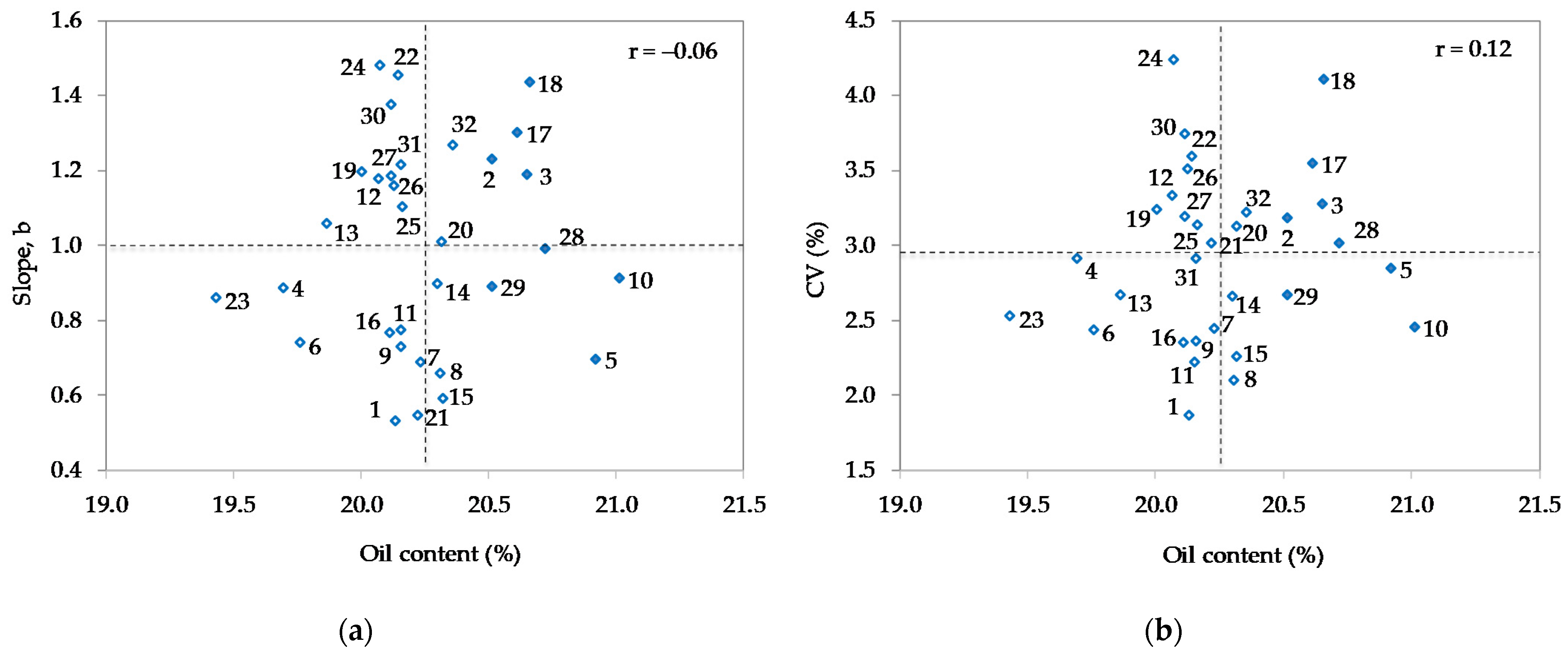
3.4. G × E Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. FAOSTAT Stratistical Database Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 1997. [Google Scholar]
- Wang, S.; Liu, S.; Wang, J.; Yokosho, K.; Zhou, B.; Yu, Y.-C.; Liu, Z.; Frommer, W.B.; Ma, J.F.; Chen, L.-Q.; et al. Simultaneous changes in seed size, oil content and protein content driven by selection of SWEET homologues during soybean domestication. Natl. Sci. Rev. 2020, 7, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- USDA National Nutrient Database for Standard Reference, Release 28. Available online: https://www.ars.usda.gov/Services/docs.htm?docid=8964 (accessed on 13 May 2021).
- Prasad, P.; Anjali, P.; Sreedhar, R.V. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit. Rev. Food Sci. Nutr. 2020, 61, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2020, 242, 126640. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- UN Sustainable Developments Goals. 17 Goals to Transform Our World. Goal 13: Take Urgent Action to Combat Climate Change and Its Impacts. Available online: http://www.un.org/sustainabledevelopment/climate-change-2/ (accessed on 11 February 2021).
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [Green Version]
- Specht, J.E.; Hume, D.J.; Kumudini, S.V. Soybean Yield Potential-A Genetic and Physiological Perspective. Crop Sci. 1999, 39, 1560–1570. [Google Scholar] [CrossRef]
- Oya, T.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Tobita, S.; Ito, O. Drought Tolerance Characteristics of Brazilian Soybean Cultivars—Evaluation and characterization of drought tolerance of various Brazilian soybean cultivars in the field—. Plant Prod. Sci. 2004, 7, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Djanaguiraman, M.; Prasad, P.V.V.; Schapaugh, W.T. High Day- or Nighttime Temperature Alters Leaf Assimilation, Reproductive Success, and Phosphatidic Acid of Pollen Grain in Soybean [Glycine max (L.) Merr.]. Crop Sci. 2013, 53, 1594–1604. [Google Scholar] [CrossRef]
- Bhatia, V.S.; Jumrani, K. A maximin-minimax approach for classifying soybean genotypes for drought tolerance based on yield potential and loss. Plant Breed. 2016, 135, 691–700. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, S.; Jin, J.; Ning, S.; Feng, P. Quantitative assessment of soybean drought loss sensitivity at different growth stages based on S-shaped damage curve. Agric. Water Manag. 2019, 213, 821–832. [Google Scholar] [CrossRef]
- Yan, C.; Song, S.; Wang, W.; Wang, C.; Li, H.; Wang, F.; Li, S.; Sun, X. Screening diverse soybean genotypes for drought tolerance by membership function value based on multiple traits and drought-tolerant coefficient of yield. BMC Plant Biol. 2020, 20, 321. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, L.; Thomison, P. High Temperature Effects on Corn and Soybean; The Ohio State University: Columbus, OH, USA, 2012. [Google Scholar]
- Piper, E.L.; Boote, K.J. Temperature and cultivar effects on soybean seed oil and protein concentrations. J. Am. Oil Chem. Soc. 1999, 76, 1233–1241. [Google Scholar] [CrossRef]
- Carrera, C.; Martínez, M.J.; Dardanelli, J.; Balzarini, M. Water Deficit Effect on the Relationship between Temperature during the Seed Fill Period and Soybean Seed Oil and Protein Concentrations. Crop Sci. 2009, 49, 990–998. [Google Scholar] [CrossRef]
- Mourtzinis, S.; Gaspar, A.P.; Naeve, S.L.; Conley, S.P. Planting Date, Maturity, and Temperature Effects on Soybean Seed Yield and Composition. Agron. J. 2017, 109, 2040–2049. [Google Scholar] [CrossRef] [Green Version]
- Dornbos, D.L.; Mullen, R.E. Soybean seed protein and oil contents and fatty acid composition adjustments by drought and temperature. J. Am. Oil Chem. Soc. 1992, 69, 228–231. [Google Scholar] [CrossRef]
- Vollmann, J.; Fritz, C.N.; Wagentristl, H.; Ruckenbauer, P. Environmental and Genetic Variation of Soybean Seed Protein Content under Central European Growing Conditions. J. Sci. Food Agric. 2000, 80, 1300–1306. [Google Scholar] [CrossRef]
- Kumar, V.; Rani, A.; Solanki, S.; Hussain, S.M. Influence of growing environment on the biochemical composition and physical characteristics of soybean seed. J. Food Compos. Anal. 2006, 19, 188–195. [Google Scholar] [CrossRef]
- Maestri, D.M.; Labuckas, D.O.; Meriles, J.M.; Lamarque, A.L.; Zygadlo, J.A.; Guzmán, C.A. Seed composition of soybean cultivars evaluated in different environmental regions. J. Sci. Food Agric. 1998, 77, 494–498. [Google Scholar] [CrossRef]
- Ren, C.; Bilyeu, K.D.; Beuselinck, P.R. Composition, Vigor, and Proteome of Mature Soybean Seeds Developed under High Temperature. Crop Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Naeve, S.L.; Huerd, S.C. Year, Region, and Temperature Effects on the Quality of Minnesota’s Soybean Crop. Agron. J. 2008, 100, 690–695. [Google Scholar] [CrossRef]
- Specht, J.; Chase, K.; Macrander, M.; Graef, G.; Chung, J.; Markwell, J.; Germann, M.; Orf, J.; Lark, K. Soybean Response to Water: A QTL Analysis of Drought Tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Yaklich, R.W.; Vinyard, B.T. A method to estimate soybean seed protein and oil concentration before harvest. J. Am. Oil Chem. Soc. 2004, 81, 1021–1027. [Google Scholar] [CrossRef]
- Song, W.; Yang, R.; Wu, T.; Wu, C.; Sun, S.; Zhang, S.; Jiang, B.; Tian, S.; Liu, X.; Han, T. Analyzing the Effects of Climate Factors on Soybean Protein, Oil Contents, and Composition by Extensive and High-Density Sampling in China. J. Agric. Food Chem. 2016, 64, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.; Purcell, L.C.; Salmeron, M.; Naeve, S.; Casteel, S.N.; Kovács, P.; Archontoulis, S.; Licht, M.; Below, F.; Kandel, H.; et al. Assessing Variation in US Soybean Seed Composition (Protein and Oil). Front. Plant Sci. 2019, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Ebellaloui, N.; Ebruns, H.A.; Abbas, H.K.; Emengistu, A.; Fisher, D.K.; Reddy, K.N. Agricultural practices altered soybean seed protein, oil, fatty acids, sugars, and minerals in the Midsouth USA. Front. Plant Sci. 2015, 6, 31. [Google Scholar] [CrossRef]
- Watanabe, I.; Nagasawa, T. Appearance and chemical composition of soybean seeds in germplasm collection of Japan. II. Correlation among protein, lipid and carbohydrate percentage. Jpn. J. Crop Sci. 1990, 59, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Rajcan, I. Biplot Analysis of Test Sites and Trait Relations of Soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef]
- Wu, T.; Yang, X.; Sun, S.; Wang, C.; Wang, Y.; Jia, H.; Man, W.; Fu, L.; Song, W.; Wu, C.; et al. Temporal-Spatial Characterization of Seed Proteins and Oil in Widely Grown Soybean Cultivars across a Century of Breeding in China. Crop Sci. 2017, 57, 748–759. [Google Scholar] [CrossRef]
- Sobko, O.; Zikeli, S.; Claupein, W.; Gruber, S. Seed Yield, Seed Protein, Oil Content, and Agronomic Characteristics of Soybean (Glycine max L. Merrill) Depending on Different Seeding Systems and Cultivars in Germany. Agronomy 2020, 10, 1020. [Google Scholar] [CrossRef]
- Qin, P.; Song, W.; Yang, X.; Sun, S.; Zhou, X.; Yang, R.; Li, N.; Hou, W.; Wu, C.; Han, T.; et al. Regional Distribution of Protein and Oil Compositions of Soybean Cultivars in China. Crop Sci. 2014, 54, 1139–1146. [Google Scholar] [CrossRef]
- Balesevic-Tubic, S.; Djordjevic, V.; Miladinovic, J.; Djukic, V.; Tatic, M. Stability of soybean seed composition. Genetika 2011, 43, 217–227. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Miller-Garvin, J.E.; Naeve, S.L. Regional and Temporal Variation in Soybean Seed Protein and Oil across the United States. Crop Sci. 2016, 56, 797–808. [Google Scholar] [CrossRef]
- Flajsman, M.; Santavec, I.; Kolmanic, A.; Kosmelj, K.; Kocjan-Acko, D. Agronomic performance and stability of seed, protein and oil yields of seven soybean cultivars determined in field experiments in Slovenia. Genetika 2019, 51, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Yaklich, R.W.; Vinyard, B.; Camp, M.; Douglass, S. Analysis of Seed Protein and Oil from Soybean Northern and Southern Region Uniform Tests. Crop Sci. 2002, 42, 1504–1515. [Google Scholar] [CrossRef]
- Dardanelli, J.L.; Balzarini, M.; Martínez, M.J.; Cuniberti, M.; Resnik, S.; Ramunda, S.F.; Herrero, R.; Baigorri, H. Soybean Maturity Groups, Environments, and Their Interaction Define Mega-environments for Seed Composition in Argentina. Crop Sci. 2006, 46, 1939–1947. [Google Scholar] [CrossRef]
- Sudarić, A.; Šimić, D.; Vratarić, M. Characterization of genotype by environment interactions in soybean breeding programmes of southeast Europe. Plant Breed. 2006, 125, 191–194. [Google Scholar] [CrossRef]
- Hampango, M.; Cheelo, P.; Lungu, M.D.; Mwala, M.; Agrama, H. AMMI Determination of Stability for Oil and Protein Content in Soybean (Glycine max L. Merrill) Seed in Zambia. J. Exp. Agric. Int. 2017, 17, 1–16. [Google Scholar] [CrossRef]
- Obua, T.; Sserumaga, J.; Awio, B.; Nganga, F.; Odong, T.; Tukamuhabwa, P.; Tusiime, G.; Mukasa, S.; Nabasirye, M. Multi-Environmental Evaluation of Protein Content and Yield Stability among Tropical Soybean Genotypes Using GGE Biplot Analysis. Agronomy 2021, 11, 1265. [Google Scholar] [CrossRef]
- Pacheco, A.; Vargas, M.; Alvarado, G.; Rodriguez, F.; Lopez, M.; Crossa, J.; Burgueno, J. GEA-R (Genotype x Environment Analysis with R for Windows) Version 4.0; International Maize and Wheat Improvement Center (CIMMYT): Texcoco, Mexico, 2016. [Google Scholar]
- SAS Institute Inc. The SAS System for Windows, Release 9.4; Statistical Analysis Systems Institute: Cary, NC, USA, 2013; 556p. [Google Scholar]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Francis, T.R.; Kannenberg, L.W. Yield Stability Studies in Short-Season Maize. I. A Descriptive Method for Grouping Genotypes. Can. J. Plant Sci. 1978, 58, 1029–1034. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Poczai, P.; Siddique, K. STABILITYSOFT: A new online program to calculate parametric and non-parametric stability statistics for crop traits. Appl. Plant Sci. 2019, 7, e01211. [Google Scholar] [CrossRef] [PubMed]
- Vratarić, M.; Sudarić, A. Soja Glycine Max. (L.) Merr.; Poljoprivredni institut Osijek: Osijek, Croatia, 2008. [Google Scholar]
- Kranz, W.L.; Specht, J. NebGuide G1367; University of Nebraska-Lincoln Extension: Lincoln, NE, USA, 2012. [Google Scholar]
- Ruíz-Nogueira, B.; Boote, K.; Sau, F. Calibration and use of CROPGRO-soybean model for improving soybean management under rainfed conditions. Agric. Syst. 2001, 68, 151–173. [Google Scholar] [CrossRef]
- Sentelhas, P.C.; Battisti, R.; Câmara, G.M.S.; Farias, J.R.B.; Hampf, A.C.; Nendel, C. The soybean yield gap in Brazil—magnitude, causes and possible solutions for sustainable production. J. Agric. Sci. 2015, 153, 1394–1411. [Google Scholar] [CrossRef] [Green Version]
- Jug, D.; Jug, I.; Brozović, B.; Vukadinović, V.; Stipešević, B.; Đurđević, B. The role of conservation agriculture in mitigation and adaptation to climate change. Poljoprivreda 2018, 24, 35–44. [Google Scholar] [CrossRef]
- Vučetić, V. Impact of Climate Change on Agricultural Production. In Proceedings of the Agrometeorologija u službi korisnika: “Zaštita okoliša i poljoprivreda”, Zagreb, Croatia, 14 November 2013; pp. 1–2. [Google Scholar]
- Gurmu, F.; Mohammed, H.; Alemaw, G. Genotype X environment interactions and stability of soybean for grain yield and nutrition quality. Afr. Crop Sci. J. 2010, 17. [Google Scholar] [CrossRef]
- Malosetti, M.; Ribaut, J.-M.; van Eeuwijk, F.A. The statistical analysis of multi-environment data: Modeling genotype-by-environment interaction and its genetic basis. Front. Physiol. 2013, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Rod, K.S.; Shockley, J.; Knott, C.A. Seed yield, seed quality, profitability, and risk analysis among double crop soybean maturity groups and seeding rates. Agron. J. 2021, 113, 1792–1802. [Google Scholar] [CrossRef]
- Schmitz, P.; Kandel, H. Individual and Combined Effects of Planting Date, Seeding Rate, Relative Maturity, and Row Spacing on Soybean Yield. Agronomy 2021, 11, 605. [Google Scholar] [CrossRef]
- Becker, H.C.; Leon, J. Stability Analysis in Plant Breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]

| Code | Genotype | Maturity Group | Genotype Status | Code | Genotype | Maturity Group | Genotype Status |
|---|---|---|---|---|---|---|---|
| 1 | Zlata | 0–I | cultivar | 17 | Buga | 0 | cultivar |
| 2 | Ružica | 0–I | cultivar | 18 | Gabriela | 00–0 | cultivar |
| 3 | Zagrebčanka | I | cultivar | 19 | Sanda | 0 | cultivar |
| 4 | Pedro | 0–I | cultivar | 20 | Sonja | 0 | cultivar |
| 5 | Bahia | 0–I | cultivar | 21 | Toma | 0 | cultivar |
| 6 | Ascasubi | I | cultivar | 22 | Ema | 00–0 | cultivar |
| 7 | Ika | 0–I | cultivar | 23 | Korana | 00–0 | cultivar |
| 8 | OS Zora | 0–I | cultivar | 24 | Lucija | 0 | cultivar |
| 9 | Tena | 0–I | cultivar | 25 | OS-1 | 0 | breeding line |
| 10 | Sara | 0–I | cultivar | 26 | OS-2 | 00–0 | breeding line |
| 11 | Seka | I | cultivar | 27 | OS-4 | 00–0 | breeding line |
| 12 | Tisa | I | cultivar | 28 | OS-5 | 0 | breeding line |
| 13 | OS-3 | 0–I | breeding line | 29 | OS-6 | 0 | breeding line |
| 14 | OS-8 | 0–I | breeding line | 30 | OS-7 | 00–0 | breeding line |
| 15 | DH 5170 | I | cultivar | 31 | Merkur | 0 | cultivar |
| 16 | Galina | 0 | cultivar | 32 | Xonia | 0 | cultivar |
| Location Code | Location Name | Geo-DMS North | Geo-DMS East | 2017 | 2018 | ||
|---|---|---|---|---|---|---|---|
| Sowing | Harvest | Sowing | Harvest | ||||
| L2 | Šašinovec | 45°51′09.7″ N | 16°10′49.4″ E | 2 May | 2 October | 30 April | 4 October |
| L5 | Osijek | 45°32′11.7″ N | 18°44′28.1″ E | 27 April | 30 September | 20 April | 20 September |
| L6 | Feričanci | 45°31′50.7″ N | 17°59′22.0″ E | 11 May | 3 October | 27 April | 4 October |
| L7 | Đurići | 44°52′29.5″ N | 18°54′51.3″ E | 3 May | 15 September | 21 April | 19 September |
| L8 | Popovac | 45°48′09.4″ N | 18°39′08.9″ E | 27 April | 11 September | 23 April | 19 September |
| L9 | Tovarnik | 45°10′17.0″ N | 19°08′25.6″ E | 11 April | 15 September | 17 April | 20 September |
| L10 | Kutjevo | 45°24′28.0″ N | 17°53′18.1″ E | 26 April | 13 September | 19 April | 21 September |
| Source of Variation | SS | % | Cum% | DF | MS | F | PROBF |
|---|---|---|---|---|---|---|---|
| Protein content (%) | |||||||
| ENV (E) | 4197.72 | 73.31 | 73.31 | 13 | 322.90 | 310.55 | 0.00 |
| GEN (G) | 751.03 | 13.12 | 86.43 | 31 | 24.23 | 23.30 | 0.00 |
| G × E | 777.16 | 13.57 | 100.00 | 403 | 1.93 | 1.85 | 0.00 |
| IPCA1 | 219.52 | 28.25 | 28.25 | 43 | 5.11 | 4.91 | 0.00 |
| IPCA2 | 131.68 | 16.94 | 45.19 | 41 | 3.21 | 3.09 | 0.00 |
| Oil content (%) | |||||||
| ENV (E) | 360.50 | 44.44 | 44.44 | 13 | 27.73 | 76.91 | 0.00 |
| GEN (G) | 197.45 | 24.34 | 68.78 | 31 | 6.37 | 17.66 | 0.00 |
| G × E | 253.30 | 31.22 | 100.00 | 403 | 0.63 | 1.74 | 0.00 |
| IPCA1 | 60.30 | 23.81 | 23.81 | 43 | 1.40 | 3.92 | 0.00 |
| IPCA2 | 50.15 | 19.80 | 43.61 | 41 | 1.22 | 3.42 | 0.00 |
| Location | Year | Diff 1 | Diff (%) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Protein content (%) | |||||||||
| 2017 | 2018 | ||||||||
| Šašinovec | N | 40.52 | a 3 | N | 39.16 | c | 1.36 | * | 3.5 |
| Osijek | D | 39.58 | c | N | 40.22 | ab | −0.64 | * | −1.7 |
| Feričanci | D | 38.38 | d | N | 40.16 | ab | −1.78 | * | −4.7 |
| Đurići | D | 39.18 | c | N | 40.11 | ab | −0.93 | * | −2.5 |
| Popovac | D | 35.09 | g | N | 37.05 | f | −1.96 | * | −5.6 |
| Tovarnik | D | 37.74 | e | N | 40.07 | b | −2.33 | * | −6.1 |
| Kutjevo | D | 37.05 | f | N | 39.53 | c | −2.48 | * | −6.6 |
| Mean | 38.22 | 39.47 | −1.25 | * | −3.3 | ||||
| Oil content (%) | |||||||||
| 2017 | 2018 | ||||||||
| Šašinovec | N | 20.37 | cd | N | 20.36 | cd | 0.01 | ns | 0.1 |
| Osijek | D | 20.53 | bc | N | 19.84 | fg | 0.69 | * | 3.5 |
| Feričanci | D | 20.81 | a | N | 20.01 | ef | 0.80 | * | 4.0 |
| Đurići | D | 20.32 | cd | N | 20.74 | ab | −0.41 | * | −2.0 |
| Popovac | D | 20.63 | ab | N | 20.84 | a | −0.21 | ns | −1.0 |
| Tovarnik | D | 19.82 | fg | N | 19.70 | g | 0.13 | ns | 0.6 |
| Kutjevo | D | 20.22 | de | N | 19.28 | h | 0.95 | * | 4.9 |
| Mean | 20.39 | 20.11 | 0.28 | * | 1.4 | ||||
| Genotype (Code) | Maturity Group | All Env | Rank | Normal Env | Rank | Dry Env | Rank | Diff 1 | Diff (%) 2 |
|---|---|---|---|---|---|---|---|---|---|
| Merkur (31) | 0 | 39.91 | 1 | 40.68 | 2 | 38.89 | 3 | −1.79 | −4.4 |
| Toma (21) | 0 | 39.85 | 2 | 40.70 | 1 | 38.73 | 4 | −1.97 | −4.8 |
| Ema (22) | 00−0 | 39.77 | 3 | 40.24 | 5 | 39.13 | 1 | −1.11 | −2.8 |
| OS-4 (27) | 00−0 | 39.72 | 4 | 40.22 | 7 | 39.05 | 2 | −1.16 | −2.9 |
| OS-5 (28) | 0 | 39.65 | 5 | 40.59 | 3 | 38.40 | 8 | −2.19 | −5.4 |
| OS-7 (30) | 00−0 | 39.58 | 6 | 40.38 | 4 | 38.52 | 5 | −1.86 | −4.6 |
| Zlata (1) | 0−I | 39.36 | 7 | 40.24 | 6 | 38.18 | 12 | −2.06 | −5.1 |
| Sanda (19) | 0 | 39.33 | 8 | 40.17 | 9 | 38.21 | 10 | −1.96 | −4.9 |
| OS-1 (25) | 0 | 39.31 | 9 | 40.19 | 8 | 38.14 | 13 | −2.05 | −5.1 |
| Ascasubi (6) | I | 39.29 | 10 | 39.87 | 14 | 38.51 | 6 | −1.36 | −3.4 |
| Sara (10) | 0−I | 39.24 | 11 | 40.01 | 11 | 38.20 | 11 | −1.81 | −4.5 |
| Zagrepčanka (3) | I | 39.20 | 12 | 40.08 | 10 | 38.0 | 16 | −2.07 | −5.2 |
| Buga (17) | 0 | 39.03 | 13 | 39.59 | 17 | 38.28 | 9 | −1.31 | −3.3 |
| Gabriela (18) | 00−0 | 38.96 | 14 | 39.35 | 22 | 38.45 | 7 | −0.89 | −2.3 |
| Tena (9) | 0−I | 38.85 | 15 | 40.01 | 12 | 37.30 | 24 | −2.71 | −6.8 |
| Korana (23) | 00−0 | 38.82 | 16 | 39.37 | 20 | 38.08 | 15 | −1.29 | −3.3 |
| Pedro (4) | 0−I | 38.82 | 17 | 39.58 | 18 | 37.81 | 18 | −1.77 | −4.5 |
| OS Zora (8) | 0−I | 38.75 | 18 | 39.80 | 15 | 37.34 | 23 | −2.46 | −6.2 |
| Ika (7) | 0−I | 38.73 | 19 | 39.93 | 13 | 37.14 | 25 | −2.79 | −7.0 |
| Galina (16) | 0 | 38.73 | 20 | 39.35 | 21 | 37.90 | 17 | −1.46 | −3.7 |
| Xonia (32) | 0 | 38.64 | 21 | 39.04 | 26 | 38.10 | 14 | −0.95 | −2.4 |
| Seka (11) | I | 38.57 | 22 | 39.66 | 16 | 37.13 | 26 | −2.53 | −6.4 |
| Sonja (20) | 0 | 38.57 | 23 | 39.17 | 25 | 37.76 | 20 | −1.41 | −3.6 |
| OS-3 (13) | 0−I | 38.46 | 24 | 39.25 | 23 | 37.40 | 22 | −1.85 | −4.7 |
| OS-6 (29) | 0 | 38.43 | 25 | 38.92 | 28 | 37.77 | 19 | −1.14 | −2.9 |
| Lucija (24) | 0 | 38.24 | 26 | 39.43 | 19 | 36.64 | 31 | −2.79 | −7.1 |
| Ružica (2) | 0−I | 38.22 | 27 | 38.81 | 29 | 37.45 | 21 | −1.36 | −3.5 |
| DH 5170 (15) | I | 38.18 | 28 | 39.25 | 24 | 36.76 | 30 | −2.49 | −6.3 |
| OS-2 (26) | 00−0 | 38.17 | 29 | 38.98 | 27 | 37.08 | 27 | −1.90 | −4.9 |
| Bahia (5) | 0−I | 37.66 | 30 | 38.15 | 31 | 37.00 | 28 | −1.15 | −3.0 |
| Tisa (12) | I | 37.65 | 31 | 38.29 | 30 | 36.80 | 29 | −1.49 | −3.9 |
| OS-8 (14) | 0−I | 37.37 | 32 | 37.99 | 32 | 36.53 | 32 | −1.46 | −3.8 |
| Mean | 38.84 | 39.60 | 37.83 | −1.77 * | −4.5 |
| Genotype (Code) | Maturity Group | All Env | Rank | Normal Env | Rank | Dry Env | Rank | Diff 1 | Diff (%) 2 |
|---|---|---|---|---|---|---|---|---|---|
| Sara (10) | 0−I | 21.01 | 1 | 20.81 | 2 | 21.29 | 1 | 0.49 | 2.3 |
| Bahia (5) | 0−I | 20.92 | 2 | 20.83 | 1 | 21.05 | 4 | 0.22 | 1.0 |
| OS-5 (28) | 0 | 20.72 | 3 | 20.41 | 5 | 21.13 | 3 | 0.72 | 3.5 |
| Gabriela (18) | 00−0 | 20.66 | 4 | 20.75 | 3 | 20.54 | 10 | −0.20 | −1.0 |
| Zagrepčanka (3) | I | 20.65 | 5 | 20.26 | 13 | 21.18 | 2 | 0.91 | 4.5 |
| Buga (17) | 0 | 20.62 | 6 | 20.33 | 9 | 21.00 | 5 | 0.67 | 3.3 |
| Ružica (2) | 0−I | 20.52 | 7 | 20.33 | 8 | 20.77 | 6 | 0.43 | 2.1 |
| OS-6 (29) | 0 | 20.51 | 8 | 20.42 | 4 | 20.64 | 8 | 0.22 | 1.1 |
| Xonia (32) | 0 | 20.36 | 9 | 20.23 | 14 | 20.53 | 11 | 0.30 | 1.5 |
| DH 5170 (15) | I | 20.32 | 10 | 20.07 | 19 | 20.65 | 7 | 0.58 | 2.9 |
| Sonja (20) | 0 | 20.31 | 11 | 20.31 | 10 | 20.33 | 17 | 0.02 | 0.1 |
| OS Zora (8) | 0−I | 20.31 | 12 | 20.29 | 11 | 20.33 | 18 | 0.03 | 0.2 |
| OS-8 (14) | 0−I | 20.30 | 13 | 20.36 | 6 | 20.22 | 20 | −0.14 | −0.7 |
| Ika (7) | 0−I | 20.23 | 14 | 20.34 | 7 | 20.08 | 26 | −0.27 | −1.3 |
| Toma (21) | 0 | 20.22 | 15 | 20.05 | 20 | 20.44 | 14 | 0.39 | 1.9 |
| OS-1 (25) | 0 | 20.16 | 16 | 20.18 | 15 | 20.14 | 24 | −0.04 | −0.2 |
| Merkur (31) | 0 | 20.16 | 17 | 20.08 | 18 | 20.26 | 19 | 0.18 | 0.9 |
| Tena (9) | 0−I | 20.16 | 18 | 20.27 | 12 | 20.00 | 29 | −0.27 | −1.3 |
| Seka (11) | I | 20.16 | 19 | 20.14 | 16 | 20.18 | 21 | 0.05 | 0.2 |
| Ema (22) | 00-0 | 20.14 | 20 | 19.88 | 26 | 20.50 | 12 | 0.62 | 3.1 |
| Zlata (1) | 0−I | 20.13 | 21 | 19.96 | 23 | 20.36 | 16 | 0.40 | 2.0 |
| OS-2 (26) | 00−0 | 20.13 | 22 | 19.91 | 25 | 20.41 | 15 | 0.50 | 2.5 |
| OS-7 (30) | 00−0 | 20.12 | 23 | 19.73 | 30 | 20.63 | 9 | 0.89 | 4.5 |
| OS-4 (27) | 00−0 | 20.12 | 24 | 19.87 | 27 | 20.44 | 13 | 0.57 | 2.9 |
| Galina (16) | 0 | 20.11 | 25 | 20.08 | 17 | 20.15 | 23 | 0.06 | 0.3 |
| Lucija (24) | 0 | 20.07 | 26 | 20.04 | 21 | 20.11 | 25 | 0.08 | 0.4 |
| Tisa (12) | I | 20.07 | 27 | 19.99 | 22 | 20.17 | 22 | 0.19 | 0.9 |
| Sanda (19) | 0 | 20.00 | 28 | 19.95 | 24 | 20.08 | 27 | 0.13 | 0.6 |
| OS-3 (13) | 0−I | 19.86 | 29 | 19.74 | 29 | 20.03 | 28 | 0.29 | 1.5 |
| Ascasubi (6) | I | 19.76 | 30 | 19.77 | 28 | 19.75 | 30 | −0.02 | −0.1 |
| Pedro (4) | 0−I | 19.69 | 31 | 19.67 | 31 | 19.72 | 31 | 0.05 | 0.3 |
| Korana (23) | 00−0 | 19.43 | 32 | 19.50 | 32 | 19.33 | 32 | −0.17 | −0.9 |
| Mean | 20.25 | 20.14 | 20.39 | 0.25 * | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šarčević, H.; Iljkić, D.; Andrijanić, Z.; Sudarić, A.; Gunjača, J.; Varnica, I.; Rastija, M.; Godena, S.; Maričević, M.; Stepinac, D.; et al. Stability of Protein and Oil Content in Soybean across Dry and Normal Environments—A Case Study in Croatia. Agronomy 2022, 12, 915. https://doi.org/10.3390/agronomy12040915
Šarčević H, Iljkić D, Andrijanić Z, Sudarić A, Gunjača J, Varnica I, Rastija M, Godena S, Maričević M, Stepinac D, et al. Stability of Protein and Oil Content in Soybean across Dry and Normal Environments—A Case Study in Croatia. Agronomy. 2022; 12(4):915. https://doi.org/10.3390/agronomy12040915
Chicago/Turabian StyleŠarčević, Hrvoje, Dario Iljkić, Zoe Andrijanić, Aleksandra Sudarić, Jerko Gunjača, Ivan Varnica, Mirta Rastija, Sara Godena, Marko Maričević, Domagoj Stepinac, and et al. 2022. "Stability of Protein and Oil Content in Soybean across Dry and Normal Environments—A Case Study in Croatia" Agronomy 12, no. 4: 915. https://doi.org/10.3390/agronomy12040915
APA StyleŠarčević, H., Iljkić, D., Andrijanić, Z., Sudarić, A., Gunjača, J., Varnica, I., Rastija, M., Godena, S., Maričević, M., Stepinac, D., & Pejić, I. (2022). Stability of Protein and Oil Content in Soybean across Dry and Normal Environments—A Case Study in Croatia. Agronomy, 12(4), 915. https://doi.org/10.3390/agronomy12040915









