Effect of Alfalfa-Derived Biochar on Anaerobic Digestion of Dairy Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Preparation and Characterization of BC
2.3. Anaerobic Digestion Experiments
2.4. Analytical Methods
2.5. Modified Gompertz Model
3. Results and Discussion
3.1. Effects of AF-BC Addition on Methane and Biogas Production
3.2. Potential Roles of AF-BC in AD
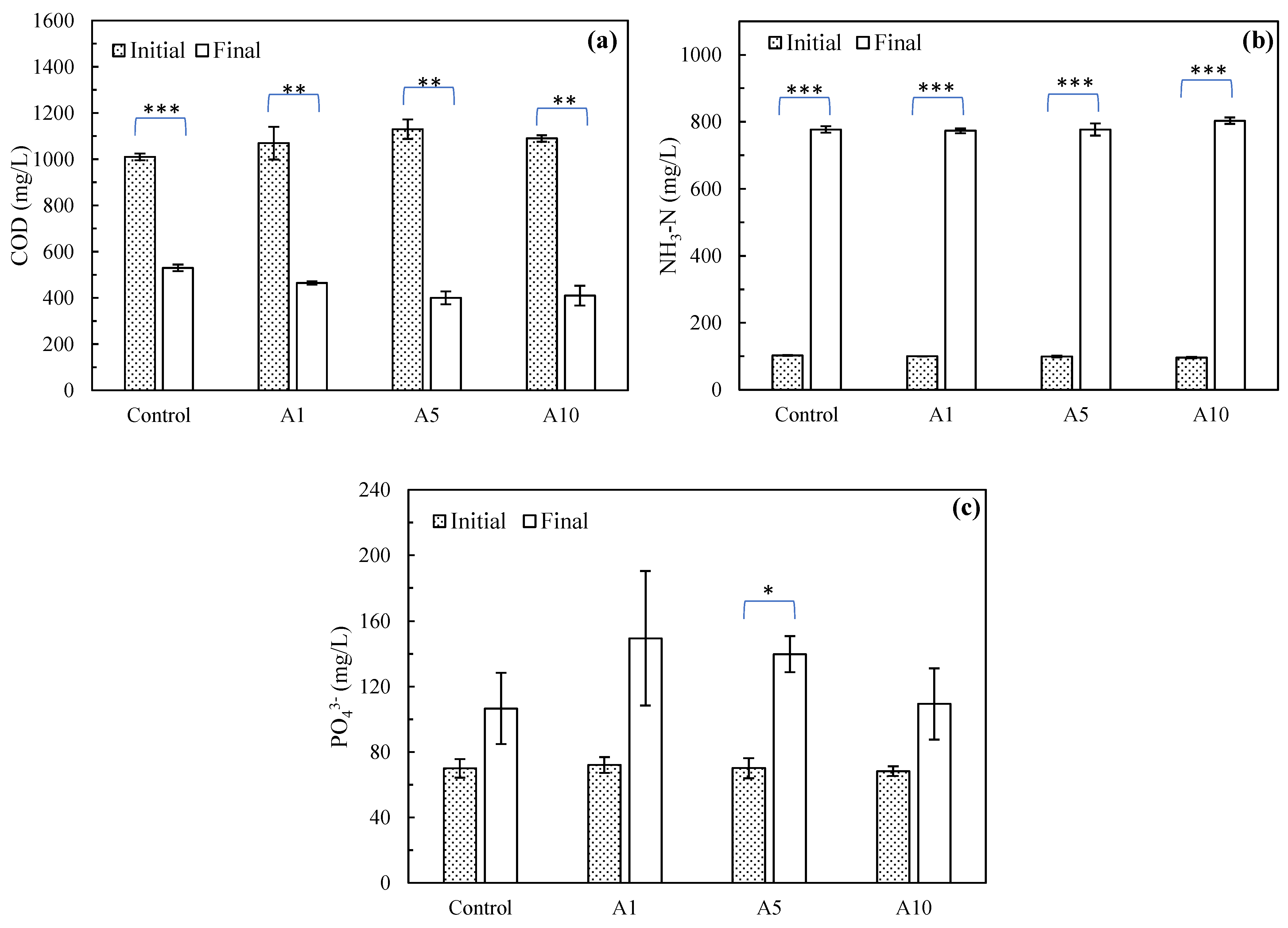
3.2.1. COD, Ammonia, and Phosphate
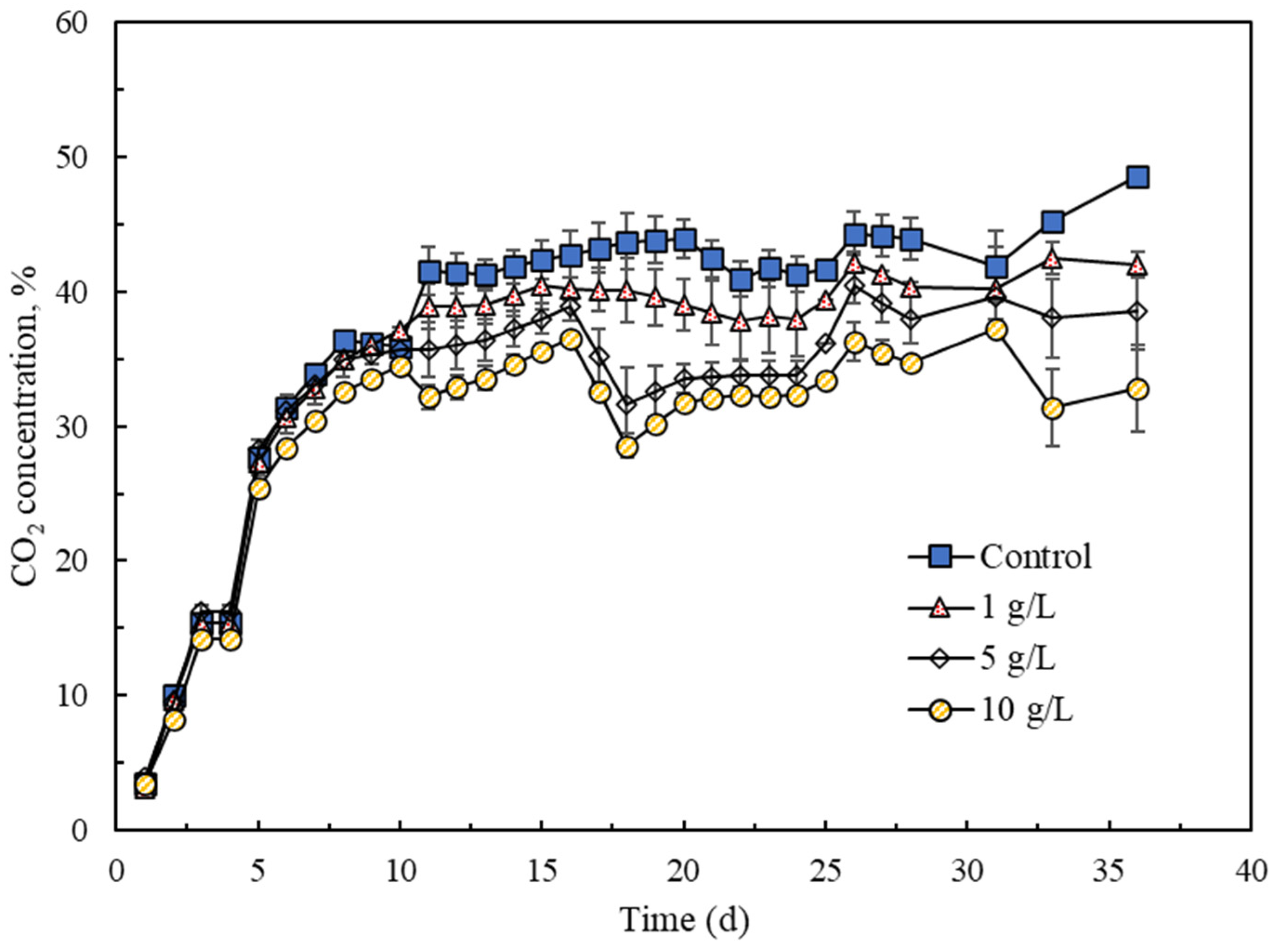
3.2.2. CO2 Content
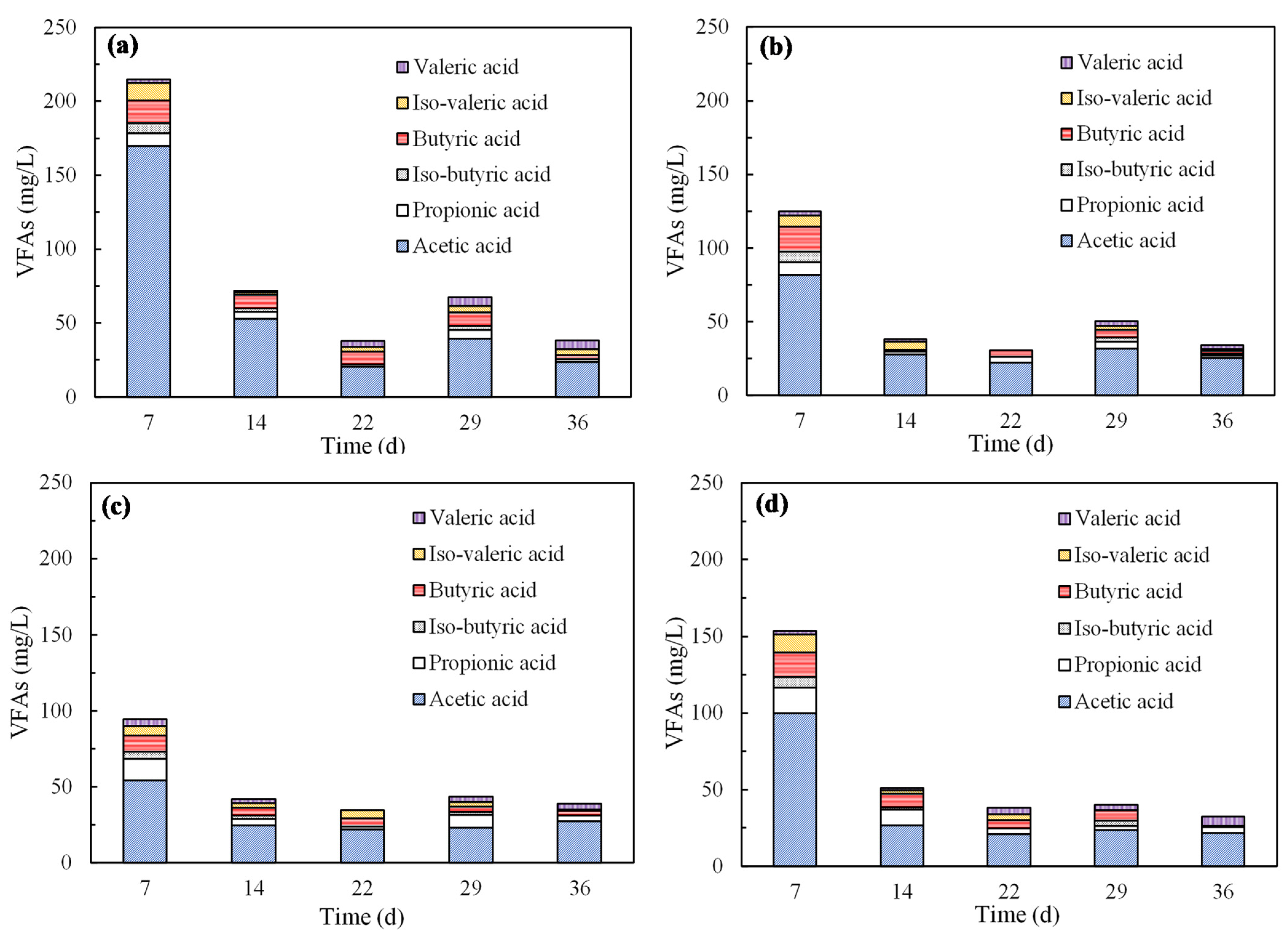
3.2.3. VFAs Analysis
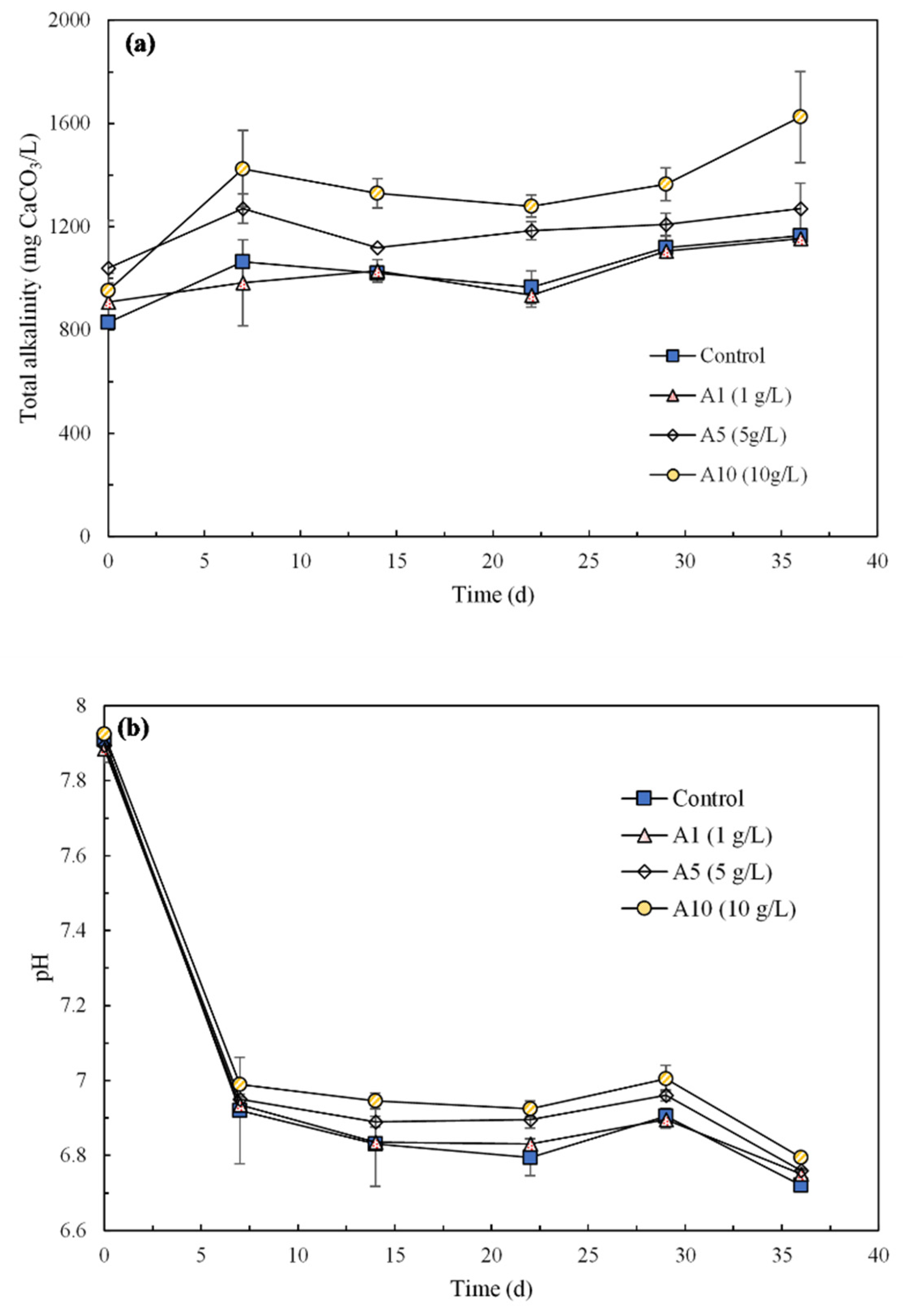
3.2.4. Total Alkalinity and pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Jing, Y.; Feng, J.; Luo, J.; Yu, J.; Zhao, L. Performance of enhanced anaerobic digestion with different pyrolysis biochars and microbial communities. Bioresour. Technol. 2020, 296, 122354. [Google Scholar] [CrossRef] [PubMed]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Liu, X.; Zhai, L.; Ouyang, X.; Liu, H. Effects of different types of biochar on the anaerobic digestion of chicken manure. Bioresour. Technol. 2019, 275, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, R.; Sun, C. Comparison of anaerobic digestion characteristics and kinetics of four livestock manures with different substrate concentrations. Bioresour. Technol. 2015, 198, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Dolliver, H.; Kumar, K.; Gupta, S. Sulfamethazine uptake by plants from manure-amended soil. J. Environ. Qual. 2007, 36, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.M.; O’Donoghue, E.J.; McBride, W.; Nehring, R.; Sandretto, C.L.; Mosheim, R. Profits, Costs, and the Challenging Structure of Dairy Farming/ERR-47; USDA ARS Rep; USDA ERS: Washington, DC, USA, 2018. [Google Scholar]
- Hill, D.; Morra, M.J.; Stalder, T.; Jechalke, S.; Top, E.; Pollard, A.T.; Popova, I. Dairy manure as a potential source of crop nutrients and environmental contaminants. J. Environ. Sci. 2021, 100, 117–130. [Google Scholar] [CrossRef]
- Flores-Orozco, D.; Patidar, R.; Levin, D.B.; Sparling, R.; Kumar, A.; Cicek, N. Effect of mesophilic anaerobic digestion on the resistome profile of dairy manure. Bioresour. Technol. 2020, 315, 123889. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl. Energy 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Zeng, S.; Jang, H.M.; Park, S.; Park, S.; Kan, E. Effects of mechanical refining on anaerobic digestion of dairy manure. ACS Omega 2021, 6, 16934–16942. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent achievements in enhancing anaerobic digestion with carbon- based functional materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Tong, Z.H.; Fang, C.Y.; Chu, J.; Yu, H.Q. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015, 70, 1–8. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Chen, H.; Zhao, Z.; Zhang, Y.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Qiu, L.; Deng, Y.F.; Wang, F.; Davaritouchaee, M.; Yao, Y.Q. A review on biochar-mediated anaerobic digestion with enhanced methane recovery. Renew. Sustain. Energy Rev. 2019, 115, 109373. [Google Scholar] [CrossRef]
- Zeng, S.; Kan, E. Chemical activation of forage grass-derived biochar for treatment of aqueous antibiotic sulfamethoxazole. ACS Omega 2020, 5, 13793–13801. [Google Scholar] [CrossRef]
- Sharma, B.; Suthar, S. Enriched biogas and biofertilizer production from Eichhornia weed biomass in cow dung biochar-amended anaerobic digestion system. Environ. Technol. Innov. 2021, 21, 101201. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Sawdust-derived biochar much mitigates VFAs accumulation and improves microbial activities to enhance methane production in thermophilic anaerobic digestion. ACS Sustain. Chem. Eng. 2018, 7, 2141–2150. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of biochar application for enhanced anaerobic digestion: A review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresour. Technol. 2017, 227, 286–296. [Google Scholar] [CrossRef]
- Jang, H.M.; Choi, Y.K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Brady, J.; Kan, E. Succession of microbial community in anaerobic digestion of dairy manure induced by manure-derived biochar. Environ. Eng. Res. 2021, 26, 138–155. [Google Scholar] [CrossRef]
- Jang, H.M.; Kan, E. Engineered biochar from agricultural waste for removal of tetracycline in water. Bioresour. Technol. 2019, 284, 437–447. [Google Scholar] [CrossRef]
- McDonald, I.; Baral, R.; Min, D. Effects of alfalfa and alfalfa-grass mixtures with nitrogen fertilization on dry matter yield and forage nutritive value. J. Anim. Sci. Technol. 2021, 63, 305–318. [Google Scholar] [CrossRef]
- Casteel, S.W. Liver disease in cattle induced by consumption of moldy hay. Vet. Hum. Toxicol. 1995, 37, 248–251. [Google Scholar]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.; Hicks, K.B.; Jung, H.-J.G.; Lamb, J.F. Production of bio-oil from alfalfa stems by fluidized-bed fast pyrolysis. Ind. Eng. Chem. Res. 2008, 47, 4115–4122. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kan, E. Effects of pyrolysis temperature on the physicochemical properties of alfalfa-derived biochar for the adsorption of bisphenol A and sulfamethoxazole in water. Chemosphere 2019, 218, 741–748. [Google Scholar] [CrossRef] [PubMed]
- ASTM D7582-12; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM: West Conshohocken, PA, USA, 2012.
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington DC, USA, 2005. [Google Scholar]
- Zeng, S.; Kan, E. Thermally enhanced adsorption and persulfate oxidation-driven regeneration on FeCl3-activated biochar for removal of microcystin-LR in water. Chemosphere 2021, 286, 131950. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Rosas, J.G.; Sotres, A.; Moran, A.; Cara, J.; Sánchez, M.E.; Gómez, X. Codigestion of sludge and citrus peel wastes: Evaluating the effect of biochar addition on microbial communities. Biochem. Eng. J. 2018, 137, 314–325. [Google Scholar] [CrossRef]
- Wei, W.; Guo, W.; Ngo, H.H.; Mannina, G.; Wang, D.; Chen, X.; Liu, Y.; Peng, L.; Ni, B.J. Enhanced high-quality biomethane production from anaerobic digestion of primary sludge by corn stover biochar. Bioresour. Technol. 2020, 306, 123159. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef] [Green Version]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of biochar addition on hydrogen and methane production in two-phase anaerobic digestion of aqueous carbohydrates food waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lu, F. Effects and optimization of the use of biochar in anaerobic digestion of food wastes. Waste Manag. Res. 2016, 34, 409–416. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Wang, P.; Peng, H.; Adhikari, S.; Higgins, B.; Roy, P.; Dai, W.; Shi, X. Enhancement of biogas production from wastewater sludge via anaerobic digestion assisted with biochar amendment. Bioresour. Technol. 2020, 309, 123368. [Google Scholar] [CrossRef]
- Shanmugam, S.R.; Adhikari, S.; Nam, H.; Sajib, S.K. Effect of bio-char on methane generation from glucose and aqueous phase of algae liquefaction using mixed anaerobic cultures. Biomass Bioenergy 2018, 108, 479–486. [Google Scholar] [CrossRef]
- Choe, U.; Mustafa, A.M.; Lin, H.; Xu, J.; Sheng, K. Effect of bamboo hydrochar on anaerobic digestion of fish processing waste for biogas production. Bioresour. Technol. 2019, 283, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; He, Y.; Tang, J.; Hecker, M.; Liu, Q.; Jones, P.D.; Codling, G.; Giesy, J.P. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ. Pollut. 2016, 218, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Hatcher, P.G.; Kumar, S.; Lee, J.W. Investigation into the sources of biochar water-soluble organic compounds and their potential toxicity on aquatic microorganisms. ACS Sustain. Chem. Eng. 2016, 4, 2550–2558. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Paliulis, D.; Kolodynskij, V. The experimental study of biogas production when digesting chicken manure with a biochar additive. Greenh. Gases Sci. Technol. 2019, 9, 837–847. [Google Scholar] [CrossRef]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.; Lee, S.; Mohamad Nor, N.; Mohamed, A.; Al-Wabel, M.; Lee, S.; Ok, Y. Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Creamer, A.E.; Gao, B.; Zhang, M. Carbon dioxide capture using biochar produced from sugarcane bagasse and hickory wood. Chem. Eng. J. 2014, 249, 174–179. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Z.; Zhang, Y. Enhancing anaerobic digestion of kitchen wastes with biochar: Link between different properties and critical mechanisms of promoting interspecies electron transfer. Renew. Energy 2021, 167, 791–799. [Google Scholar] [CrossRef]
- Ren, S.; Usman, M.; Tsang, D.C.M.; O-Thong, S.; Angelidaki, I.; Zhu, X.; Zhang, S.; Luo, G. Hydrochar-facilitated anaerobic digestion: Evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environ. Sci. Technol. 2020, 54, 5755–5766. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Delgado, A.G.; Parameswaran, P.; Fajardo-Williams, D.; Halden, R.U.; Krajmalnik-Brown, R. Role of bicarbonate as a pH buffer and electron sink in microbial dechlorination of chloroethenes. Microb. Cell Fact. 2012, 11, 128. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wang, H.; Li, X.; Cheng, J.J.; Wu, W. Improving methane yield from organic fraction of municipal solid waste (OFMSW) with magnetic rice-straw biochar. Bioresour. Technol. 2017, 245, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Yang, Q.; Yao, F.; Chen, S.; He, L.; Hou, K.; Pi, Z.; Yin, H.; Fu, J.; Wang, D.; et al. Role of bicarbonate as a pH buffer and electron sink in microbial dechlorination of chloroethenes. Microb. Cell Fact. 2012, 11, 128. [Google Scholar] [CrossRef] [Green Version]
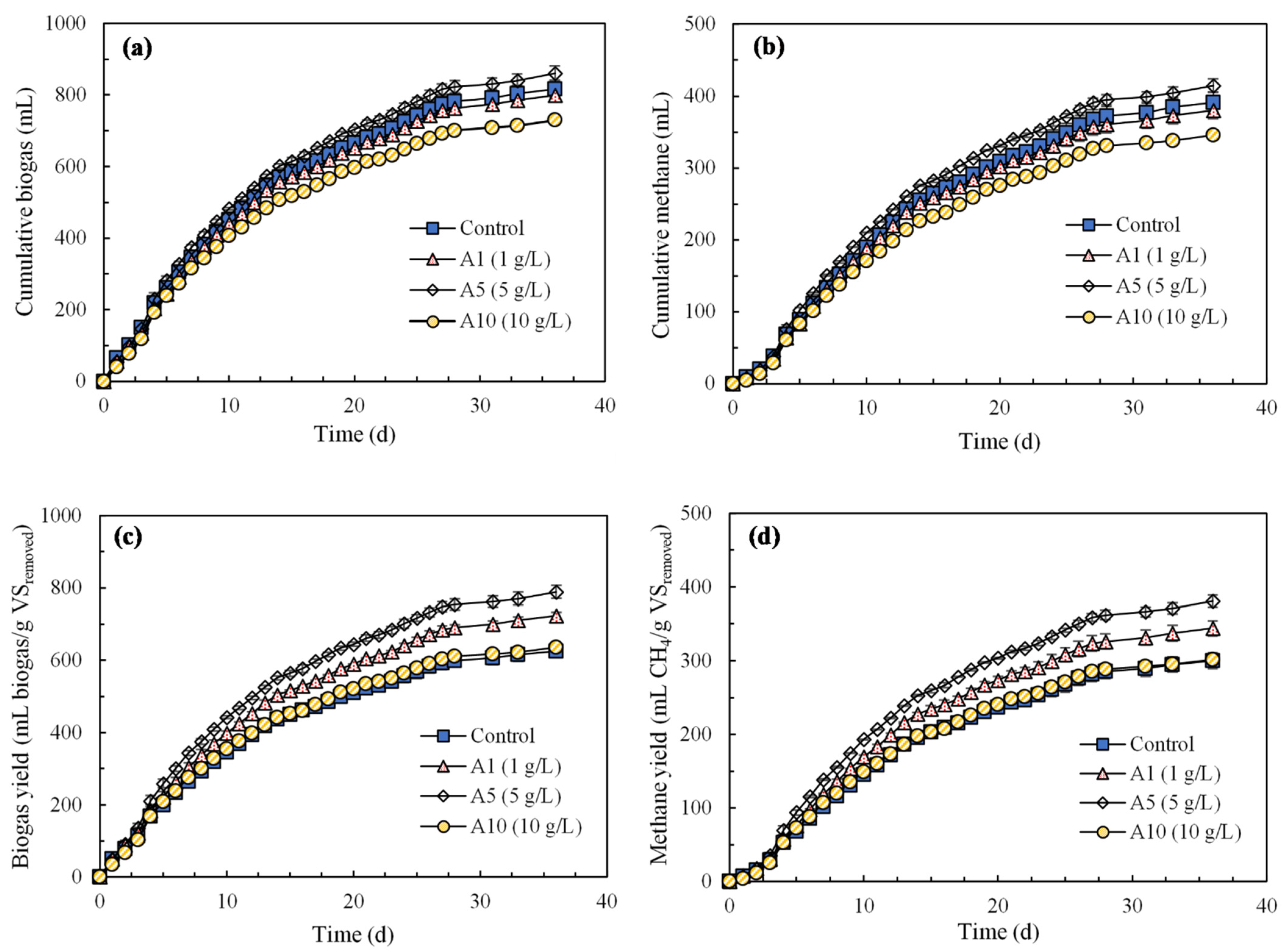
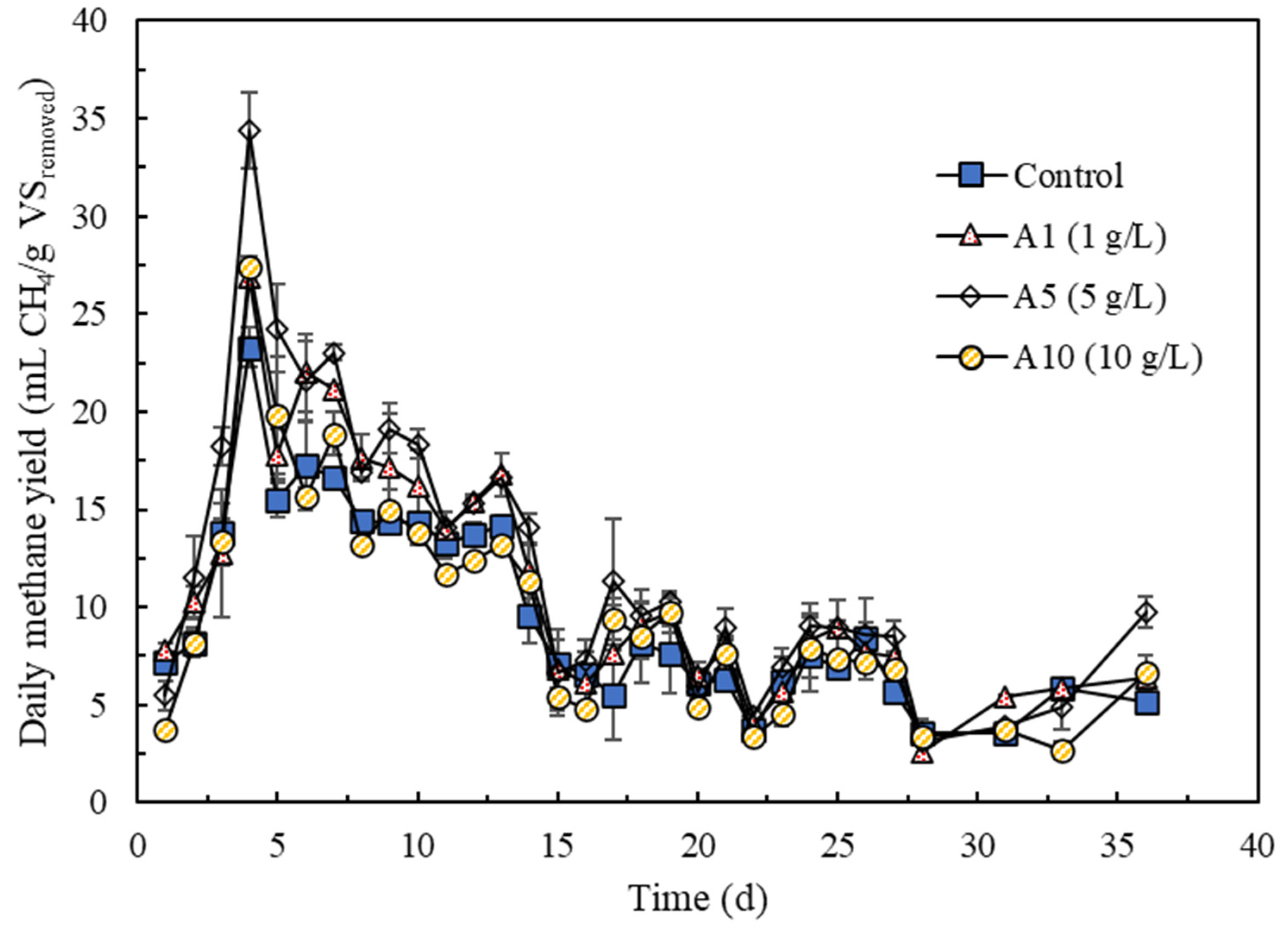
| Biochar Addition | Lag Phase, λ (d) | Rmax (mL CH4/g VSremoved·d) | P (mL CH4/g VSremoved) | R2 | p Value |
|---|---|---|---|---|---|
| Control | 0.98 ± 0.01 | 15.20 ± 0.12 | 295.78 ± 11.71 | 0.99 | <0.001 |
| A1 (1 g/L) | 0.91 ± 0.17 | 17.89 ± 0.39 | 336.85 ± 11.87 | 0.99 | <0.001 |
| A5 (5 g/L) | 0.76 ± 0.08 | 19.89 ± 0.48 | 371.51 ± 9.30 | 0.99 | <0.001 |
| A10 (10 g/L) | 0.86 ± 0.03 | 15.51 ± 0.24 | 297.47 ± 3.21 | 0.99 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Harris, R.; Kan, E. Effect of Alfalfa-Derived Biochar on Anaerobic Digestion of Dairy Manure. Agronomy 2022, 12, 911. https://doi.org/10.3390/agronomy12040911
Zeng S, Harris R, Kan E. Effect of Alfalfa-Derived Biochar on Anaerobic Digestion of Dairy Manure. Agronomy. 2022; 12(4):911. https://doi.org/10.3390/agronomy12040911
Chicago/Turabian StyleZeng, Shengquan, Riley Harris, and Eunsung Kan. 2022. "Effect of Alfalfa-Derived Biochar on Anaerobic Digestion of Dairy Manure" Agronomy 12, no. 4: 911. https://doi.org/10.3390/agronomy12040911
APA StyleZeng, S., Harris, R., & Kan, E. (2022). Effect of Alfalfa-Derived Biochar on Anaerobic Digestion of Dairy Manure. Agronomy, 12(4), 911. https://doi.org/10.3390/agronomy12040911






