Abstract
The fungal species belonging to the genus Trichoderma has been globally recognized as a potential candidate of biofertilizer and biocontrol agent to prevent devastating soil-borne fungal pathogens and enhance growth and productivity of agricultural crops. The antagonistic activity of Trichoderma to pathogenic fungi is attributed to several mechanisms including antibiosis and enzymatic hydrolysis, which are largely associated with a wide range of metabolites secreted by the Trichoderma species. Besides suppressing target pathogens, several metabolites produced by Trichoderma species may act against non-pathogenic beneficial soil microbial communities and perform unintended alterations within the structures and functions of microbial communities in the crop rhizosphere. Multiple microbial interactions have been shown to enhance biocontrol efficacy in many cases as compared to bioinoculant employed alone. The key advances in understanding the ecological functions of the Trichoderma species with special emphasis on their associations with plant roots and other microbes exist in the crop rhizosphere, which are briefly described here. This review focuses on the interactions of metabolites secreted by Trichoderma species and plant roots in the rhizosphere and their impacts on pathogenic and non-pathogenic soil microbial communities. The complex interactions among Trichoderma–plants–microbes that may occur in the crop rhizosphere are underlined and several prospective avenues for future research in this area are briefly explored. The data presented here will stipulate future research on sustainably maximizing the efficiency of Trichoderma inoculation and their secondary metabolites in the crop soil ecosystem.
1. Introduction
The Trichoderma species under the Hypocreaceae family has been commercially formulated as biological inoculants or biofungicides worldwide [1,2,3]. More than 50% of registered biofungicides against soil-borne pathogens are formulated based on Trichoderma [4]. Trichoderma species have a wide distribution and ecological plasticity due to their ability to generate a broad range of lytic enzymes to degrade substrates, a flexible metabolism, and high resistance to microbial inhibitors [5]. Therefore, Trichoderma is the most used bioinoculant due to its numerous beneficial characteristics, including producing several secondary metabolites, such as antibiotics, peptaibols, and other bioactive compounds with antibiosis properties for parasitizing soil pathogenic fungi [6]. As a result, mycoparasitism and antibiosis are thought to be the most important biocontrol mechanisms in Trichoderma species.
The effect of Trichoderma species as a biocontrol agent cannot be generalized since it has both harmful and beneficial impacts on pathogens and growth promotion that have been extensively studied [7,8]. Reports show that multidirectional metabolomic interactions occur in the soil ecosystem due to the interactions with introduced Trichoderma inoculants, plant root exudates, and resident microbial communities (e.g., antagonism or synergism) [9,10]. The species diversity of existing microbial communities and their richness in the root microbes results in the intra- and interspecies relationship among their members. Secondary metabolites (SMs) play a major role in executing these interactions as chemical signals [11].
In soil, how Trichoderma inoculants interact with other non-target and non-pathogenic microorganisms, which are inherently beneficial for crop productivity, is relatively lesser understood [12,13,14,15]. Several studies showed a significant increase in fungal population in rhizosphere soil upon inoculation of Trichoderma strains; consequently, this increased fungal population reduced the bacterial population [16]. In another study, Trichoderma koningii inoculation reduced the resting spore germination of arbuscular mycorrhizal (AM) fungi (e.g., Glomus spp.), which are considered key fungal communities responsible for enhancing soil biofertility and crop productivity [17,18,19]. For example, the volatile metabolites secreted by T. koningii inoculant suppressed spore germination of AM fungi and decreased the population of the beneficial Azospirillum species as well. Thus, Trichoderma application to control soil-borne pathogens and growth improvement requires comprehensive investigation considering the interactions not only with pathogens, but also with microbial communities already present in crop soils.
Overall, soil microbes contribute significantly to soil structure, fertility, and pathogens suppression [20,21]. At the same time, the release of root exudates (metabolites) influences the structure of the soil microbial population and its enzymatic activity, which provide essential nutrients to plants by decomposing and mineralizing the soil organic matters [22,23]. Furthermore, soil microbes are the primary source and mediators, such as biochemical changes during nutrients recycling, and hence play a critical role in biogeochemical process [24,25]. Research has sought to characterize these beneficial microbes from diverse agricultural ecosystems to obtain a better knowledge of the biodiversity of the soil microbial communities. However, the plant species and soil types primarily influence the composition of the soil microbial population; the interactions in the soil ecosystems are very complicated, particularly among the plants and soil microbes [26].
In this review, we categorized the effect of the metabolomic compounds secreted by different Trichoderma species on soil pathogenic (fungi), non-pathogenic (fungi and bacteria) microbial communities, and soil enzymatic activities, and outlined the ecological challenges of Trichoderma as a biofungicide/biocontrol inoculant in crop rhizosphere.
2. Trichoderma Species as a Commercial Biofungicide
Trichoderma is a filamentous fungus beneficial for its multi-prong action against numerous plant pathogens [27]. Biofungicide is an important approach against some notable plant pathogens. Several Trichoderma strains have been recognized as a potential source to formulate biofungicide because of their suitability to reduce disease incidences caused by several fungal plant pathogens [28]. Species belonging to the Trichoderma harzianum complex are mostly found in various soil habitats and on plant decay materials, and have shown parasitism to other fungi [29]. Recently, a few commercial strains, such as Trichoderma afarasin, T. afroharzianum, T. atrobrunneum, T. camerunense, T. endophyticum, T. guizhouense, T. harzianum, T. inhamatum, T. lentiforme, T. lixii, T. neotropicale, T. pyramidale, T. rifaii, and T. simmonsii, have been identified as effective biofungicide formulations [30]. T. afroharzianum is the mostly reported strain used as an active ingredient in several commercial biocontrol products [30,31]. The taxonomy of the T. harzianum complex formalized the phylogenetic progenies and opened new prospects for the revelation of biological utilities, particularly controlling the plant pathogens. For instance, newly recognized T. lentiforme and T. neotropicale showed strong antagonistic actions against the Moniliophthora roreri pathogen causing frosty pod rot disease of the cacao tree (Theobroma cacao) [32].
T. viride has also been extensively used as a well-known biofungicide that protects the plant from fungal diseases striving with systemic negative effects on foliar leaves and seedcoat. Bio-formulations based on T. viride work as potential biofungicides against seed-borne and soil-borne fungal pathogens including Armillaria, Pythium, and Rhizoctonia [33]. Moreover, Trichoderma species play a significant role against seed-borne fungi, such as Fusarium sp., M. phaseolina, and R. solani, which cause pre-harvest and post-harvest losses in cotton, cowpea, mungbean, sorghum, soybean, and tomatoes [33]. The dry powder or dust of Trichoderma is used to coat seed for seed treatment just before sowing [34,35]. T. harzianum, T. virens, and T. viride were proven as potential seed protectants against the Pythium sp. and R. solani. Incubation of Trichoderma–treated seeds under warm and humid conditions right before radical emergence, results in rapid and uniform seedling emergence [36]. Trichoderma germinates conidial masses on the seed surface and forms a layer surrounding the primed seeds. These primed seeds are capable of tolerating the adverse conditions of soil habitats, such as vegetable seedlings treated with Trichoderma spore or cell suspension showed antagonistic to damping-off disease. Trichoderma was successfully applied in aerial plant parts to control the decay fungi in wounded shrubs and trees [36]. For instance, across the globe, several Trichoderma–based commercial bioformulations are used in controlling plant pathogenic fungi are listed in Table 1.

Table 1.
Trichoderma–based commercial bioformulations controlling plant pathogenic fungi.
3. Effects of Trichoderma Metabolites on Plant Root Exudates
The signaling between Trichoderma and plant roots is often performed with root-derived chemicals (Table 2). Plant roots exude various organic compounds into the rhizosphere, which create and promote contact with Trichoderma [37]. Sucrose is a key molecule in carbohydrate-mediated plant signaling. Plant cells degrades sucrose to provide a carbon source for Trichoderma during Trichoderma–plant interactions [37]. T. virens intracellular invertase (TvInv) is responsible to hydrolyze sucrose and production of normal T. virens in the presence of sucrose. A plant-like sucrose transporter (TvSut) carries sucrose from the plant to Trichoderma during their beneficial interactions [38]. The ThPTR2 gene encodes the PTR family di/tripeptide transporter, which is found in T. harzianum. The secreted proteins that are found in plant–pathogen and plant–mycorrhizal interactions, also play a significant role in Trichoderma–plant interactions. Trichoderma species produce and regulate hormonal signals that help to colonize in plant roots [3]. Auxin-induced root formations (e.g., increased number of root hairs) increase the total area of the absorptive surface in the root zones, making nutrient absorption easier and resulting in increased plant growth [39].
The exchange of root exudates and other signaling molecules between Trichoderma and plants is complex and not well characterized [40]. Thus, several antibiotics, toxins, and plant antimicrobial agents affect the Trichoderma species in the crop rhizosphere. For example, benzoic acid, cinnamic acid, ferulic acid, phenolic acids, vanillic acid, 3-phenyl propionic acid, and 4-hydroxybenzoic acid can inhibit the growth of Trichoderma [41]. However, some Trichoderma species induce root branching and increase shoot biomass by the presence of auxin-like compounds, which help to exchange these root exudates and signaling molecules between Trichoderma and plants in crop rhizosphere [40]. The ATP cassette-binding cell membrane pump of Trichoderma species is an important part of a comprehensive, potent cell detox system that explains the ability of Trichoderma to cope with various chemical stresses. In addition to co-inoculating other useful organisms such as the AM fungi, the Trichoderma species appears to have a role to play in attenuating plant hormone reactions to the root colonization process [42]. The effective colonization of the Trichoderma species on the roots of their hosts implies a reprogramming of the plant, with improved growth, yield, and pathogen resistance [43].
4. Effects of Trichoderma Metabolites on Soil and Root Pathogens
Trichoderma species are commonly found on plant root surfaces in various soil habitats where they control the soil-borne pathogens causing plant root diseases [44]. The most versatile strains from the Trichoderma genus, including Trichoderma arundinaceum, T. asperellum, T. atroviride, T. citrinoviride, T. cremeum, T. crissum, T. gamsii, T. hamatum, T. harzianum, T. pseudo-koningii, T. koningii, T. koningiopsis, T. longibrachiatum, T. longipile, T. ovalisporum, T. polysporum, T. reesei, T. saturnisporum, T. spirale, T. virens, and T. viride, secrete diverse chemical compounds [45,46,47] (Table 2). A large number of soil-borne fungi are capable of generating chemicals that are recognized for their antifungal efficiency. Trichoderma species possess the fungicidal and fungistatic characteristics as they generate various cell wall-degrading enzymes and secondary metabolites (SMs) [48,49]. These metabolites enhance the plant defense response when attacked by phytopathogens. Secreted antimicrobial compounds during the Trichoderma–mediated defense response pathways are often associated with the barriers of pathogen entry into the plant cells [43]. For example, the accumulation of secondary phenolic metabolites plays a crucial role in plant defense mechanisms against various pathogens. Trichoderma produces various peptides, proteins, and low molecular weight compounds, which are involved in biochemical resistance to pathogens and induce resistance in plants [50].
Various groups of compounds are secreted by the Trichoderma species trigger to induce the defense reactions in plants. Celluloses produced by T. harzianum have been proven to act as an elicitor for systemic acquired resistance (SAR) by causing peroxides and chitinase activity. Systemic plant reactions occur via the JA/ethylene signaling pathway (Figure 1). Trichoderma has been shown to release these enzymes or otherwise functioning proteins, avirulence gene (Avr) encoded homologous proteins, oligosaccharides, and other low molecular weight compounds [51]. The chitinase enzymes are commonly known as plant gene-encoding enzymes, which degrade cell walls, and are used to induce plant resistance against phytopathogens. In terms of antifungal efficiency, the chitinase genes from Trichoderma showed dominant expression over the corresponding plant genes resulting in improved pathogenic resistance [52]. Therefore, it is expected that the transgenes inserted in the plant-host increase the resistance level against a variety of plant pathogens [53]. The Trichoderma gene chit42 encodes a powerful endochitinase enzyme that exhibits strong antifungal activity against a broader range of plant pathogens as compared to other chitinolytic enzymes. The constitutive expressions of Trichoderma genes in plants have shown higher levels and improved resistance against soil-borne plant pathogens [54].
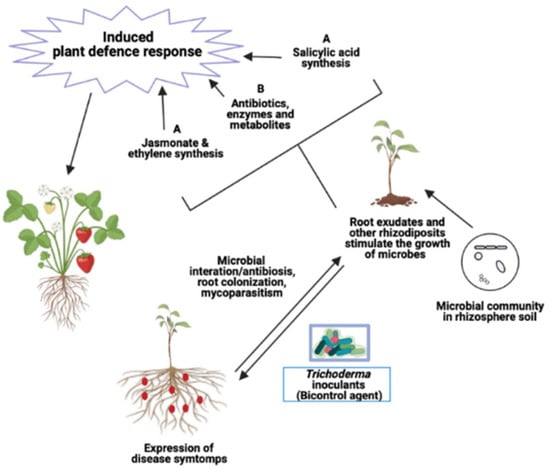
Figure 1.
Metabolites (antibiotics and enzymes) produced by Trichoderma induce plant defense responses against the pathogens: (A) Trichoderma released antibiotics, enzymes, and secondary metabolites (SMs) through metabolic pathways leading to antagonize the phytopathogens. (B) Signals involved in Trichoderma–plant interaction enhanced the plant defense responses.

Table 2.
A list of metabolites and chemical compounds secreted by Trichoderma species affects soil pathogenic fungi.
Table 2.
A list of metabolites and chemical compounds secreted by Trichoderma species affects soil pathogenic fungi.
| Metabolites | Compound | Trichoderma Species | Target Fungal Pathogens | References |
|---|---|---|---|---|
| Anthraquinones | 1,8-dihydroxy-3-methylanthraquinone, 1-hydroxy-3-methylanthraquinone and 6-methyl-1,3,8-trihydroxyanthraquinone | T. harzianum | Fusarium oxysporum, Macrophomina phaseolina, Rizoctonia solani, and Sclerotium rolfsii | [55] |
| 1,8-dihydroxy-3-methylanthraquinone and 1-hydroxy-3-methylanthraquinone | T. harzianum | Gaeumannomyces graminis var. tritici, and Pythium ultimum | [56] | |
| Azaphilones | Harziphilone, Fleephilone and T22azaphilone | T. harzianum | G. graminis var. tritici, P. ultimum, and R. solani | [57] |
| T22azaphilone | T. harzianum | Leptosphaeria maculans, and Phytophthora cinnamomi | [58] | |
| Epipolythiodio-xopiperazines | Gliotoxins | T. virens | M. phaseolina, Pythium aphanidermatum, Pythium deharyanum, Rizoctonia bataticola, R. solani, and S. rolfsii | [1] |
| Gliovirin | T. longibrachiatum | R. solani | [1] | |
| Gliovirin | T. virens | P. ultimum | [1] | |
| Koninginins | koninginins A, B, D, E, and G | T. aureoviride T. harzianum and T. koningii | G. graminis var. tritici | [59] |
| koninginins A, B, and D | T. koningiopsis | F. oxysporum, F. solani, and S. rolfsii | [59] | |
| koninginin D | T. harzianum and T. koningii | Bipolaris sorokiniana, F. oxysporum, P. cinnamomi, and Pythium middletonii | [6] | |
| Lactones | aspinolide C | T. arundinaceum | Fusarium sporotrichioides | [60] |
| Cerinolactone | T. cerinum | Rosellinia necatrix | [60] | |
| Cremenolide | T. cremeum. | F. oxysporum and R. solani | [60] | |
| Peptaibols | trichokonins VI, VII, and VIII | T. koningii | F. oxysporum, R. solani, and Verticillium dahliae | [55] |
| Trichokonin VI | T. pseudokoningii | F. oxysporum, Phytophthora parasitica, and V. dahlia | [55] | |
| Polyketides | Harzianolide and Dehydro Harzianolide | T. harzianum | F. oxysporum and R. solani | [61] |
| 6-pentyl-α-pyrone | T. harzianum T. koningii T. viride and Trichoderma spp. | R. solani | [61] | |
| 6-pent-1-enyl-α-pyrone | T. harzianum and T. viride | R. solani | [61] | |
| Massoilactone δ-decenolactone | Trichoderma spp. | R. solani and S. rolfsii | [61] | |
| Koninginia E, B, and A | T. harzianum and T. koningii | G. graminis var. tritici | [57] | |
| Koninginin D and Seco-koninginin | T. harzianum | G. graminis var. tritici | [57] | |
| Koninginin C | T. koningii | G. graminis var. tritici | [57] | |
| Pyridones | Harzianopyridone | T. harzianum | G. graminis var. tritici, L. maculans, P. cinnamomi, P. ultimum, and R. solani | [57] |
| Pyrones | 6-Pentyl pyrone (6-PP) | T. harzianum, T. koningii, and T. viride | F. oxysporum and R. solani | [62] |
| Viridepyronone | T. viride | S. rolfsii | [62] | |
| Steroids | Stigmasterol | T. harzianum and T. koningii | F. oxysporum, M. phaseolina, R. solani, and S. rolfsii, | [57] |
| Terpenes (trichothecenes) | Trichodermin | T. polysporum T. sporulosum T. reesei and T. virens | R. solani | [63] |
| Harzianum A | T. harzianum | F. oxysporum | [63] | |
| Mycotoxin T2 | T. lignorum | R. solani and S. rolfsii | [63] | |
| Terpenes (triterpenes) (sterols) | Ergokonin A | T. koningii T. longibrachiatum and T. viride | Phoma spp. | [64] |
| Viridin | T. koningii T. virens and T. viride | F. oxysporum and R. solani | [64] | |
| Trichothecenes | Trichodermin | T. brevicompactum | R. solani | [65] |
| Trichodermin | T. harzianum | F. oxysporum and R. solani | [66] |
5. Effects of Trichoderma Metabolites on Soil Non-Pathogenic Fungal Communities
Microbial community structure, biodiversity, and functions are crucial for maintaining agroecosystem sustainability and productivity [67,68]. Very little is known about how Trichoderma interacts with non-pathogenic microbial communities. Trichoderma species secrete numerous cell wall-degrading enzymes such as cellulases, chitinases, glucanases, proteinases, and xylanases, which can substantially degrade the microbial cells (including pathogens) in soil habitats to absorb nutrients and persist longer. Thus, changing or altering the structure and functions of microbial populations, particularly fungal and bacterial communities [69,70].
Secondary metabolites (SMs) are secreted by Trichoderma play significant roles in signaling, developing, and establishing interactions with plants and soil microbes (Table 3). Trichoderma produces numerous secondary metabolites, such as peptaibols, polypeptides, pyrons, siderophores, steroids, terpenes, etc. [62]. The effectiveness of using Trichoderma in agriculture depends on their metabolic activity and the type of interaction with plants and other microbes. These fungi effectively colonize the plants roots and soil rhizosphere, and produce several metabolites with anti-microbial features [27]. For instance, T. atroviride and T. harzianum have developed different major antibiotics, such as azaphilone, butenolide, harzianolide, hydrazinopyridine, 1-hydroxy-3-methyl-anthraquinone, 1,8-dihydroxy-3-methyl-anthraquinone, and 6-Pentyl pyrone (6-PP) [71,72]. This low molecular weight, non-polar, volatile compounds (i.e., 6-PP) yield a high concentration of antibiotics in the soil environments, which influence the diversity, composition, and functional attributes of the long-distanced soil microbial community. On the other hand, the polar antibiotics and peptaibols affect the production of microbial hyphae within short-distanced ranges [73]. However, the contribution of other secondary metabolites (i.e., pyrones) by Trichoderma and their synergisms with other soil-root associated chemical compounds to beneficial soil microbial communities has not yet been well understood [74,75,76].
T. koningii producing volatile compounds induced the reduction in resting spore germination of non-pathogenic AM fungi (e.g., G. mosseae) [17]. However, these volatile compounds did not affect the mycelial growth of G. mosseae, affecting the spores produced only in the resting phase. Trichoderma inoculation on mycorrhizal maize plants reduced Azospirillum populations in soil [77]. This operation was only found in natural mycorrhizal plants as compared to non-mycorrhizal control plants. The relative abundance of fungal species was also found in the soil rhizosphere of black pepper (Piper nigrum L.). T. harzianum caused considerable alterations in the metabolic profiles of the black pepper rhizosphere, resulting in a lower number of metabolized compounds; although, absorbance was considerably greater for a specific set of metabolites in which Trichoderma was applied [78].
The treatment with T. harzianum MTCC 5179 altered the structure and functions of fungal communities such as Mortierella verticillata, Oidiodendron maius, Pseudogymnoascus pannorum, Rhizophagus irregularis, Talaromyces stipitatus, and T. harzianum [78]. Significant variations were observed among the fungal communities in the soil rhizosphere due to Trichoderma inoculation. For instance, Gibberella and Phoma were found as the dominant fungal genera, whereas relative abundances of other fungi, such as Monographella, Mortierella, Penicillium, Rhizophlyctis, Sphaerosporella, and Trichoderma, were reduced. Another study showed that after inoculation of Trichoderma, the relative abundance of Trichoderma was 98.41%, including resident Trichoderma species, while the relative abundances of other genera were reduced [79]. In contrast, inoculation of T. atroviride I-1237 resulted in a significant increase in the density of the other soil fungal community [80]. Similarly, T. longibrachiatum inoculation enhanced the capacity of microbial communities utilizing the carbon source that was the highest in rhizosphere soil [81].
6. Effects of Trichoderma Metabolites on Soil Bacterial Communities
Several metabolites released by Trichoderma species have been shown to substantially inhibit the growth of diverse bacterial strains in the tomato rhizosphere. Occasionally, volatile compounds (VCs) produced by indigenous bacterial communities affected the growth of Trichoderma and their secretion of antifungal/antibacterial metabolites. This counter-secretion within the rhizosphere raised the questions of whether these Trichoderma species significantly change the rhizosphere bacterial communities during biocontrol and how the consequent alterations influence soil and plant health. Trichoderma species strongly inhibiting the bacterial population implies that VCs might be used as soil fumigants. However, 373 distinct bacterial strains have been identified in the soil rhizosphere, though the specific activity of these microbes are still unknown in many cases [6,82,83].
Trichoderma has some fundamental functions to stimulate the plant beneficial bacteria to restrict pathogens through different mechanisms (Table 3) [84]. The non-target effects of T. harzianum, Bacillus megaterium, and Pseudomonas fluorescens were found on major actinomycetes and β-proteobacterial communities in soil rhizosphere [85]. T. harzianum and B. megaterium significantly increased the population of actinomycetes with greater abundance during the maturity stage of plants. Another study showed that Trichoderma inoculant reduced the total soil bacterial population of Pseudomonas fluorescens [16]. A study on biodegradation revealed, T. viride was inoculated together with a bacterial consortium of 195 strains [86]. After 12 months of observation of the biodegradation process, only 73 bacterial strains were found from the consortium population. T. viride proved to exert an antagonistic effect on the bacterial consortium; as a result, the lower relative abundance of bacterial communities was achieved, whereas higher relative abundance of bacterial communities was found in the control treatment. Gasoni et al. [87] found that the application of T. harzianum changed a particular group of compounds deferred from the uninoculated control, indicating that the inoculation of T. harzianum contributed to the growth of a distinct soil bacterial population, altering the microbial communities in the host rhizosphere.
7. Effects of Trichoderma Metabolites on Soil Enzyme Activities
The Trichoderma species applied in the crop rhizosphere affected soil enzyme activities. A variety of Trichoderma species substantially decreased the activities of β-glucosidase, chitobiosidase, and N-acetyl-β-D-glucosaminidage (NAGase) enzymes, thus inducing the plants to respond with their defense mechanisms [16]. These enzymes exhibited the alteration in microbial communities in the soil ecosystem [73] (Table 3). Enzymatic activities occurring in the soil are used to better understand the ecological functions of Trichoderma [88]. Several Trichoderma species reduced the activity of alkaline phosphatase resulting in the effective control of the soil-borne pathogen (P. ultimum). Different degrees of soil enzyme activities significantly inhibit the pathogenic effect of soil-borne fungi [16].
Trichoderma inoculation to AM fungi-colonized plants (G. deserticola) reduced phosphatase activity [73]. A significant increase in chitinase activity was found in the soil with Trichoderma inoculation of natural mycorrhizal fungi-colonized plants (121%) and non-mycorrhizal plants (151%). However, it considerably reduced the enzymatic activity of trehalase by 47%. The imbalance structure of the soil microbial community is the major reason for soil-borne diseases. Trichoderma species increase the contact area among the soil microbes and crop rhizosphere because of their strong colonization ability. Trichoderma species exhibit hyperparasitism due to their advantages of rapid growth and high vitality. The fungus secretes cell wall-degrading extracellular enzymes, such as cellulases, chitinases, glucanases, proteinases, and xylanases, which enhance the soil enzyme activity to repair soil health [89]. The inoculation of Trichoderma increases nutrient availability, nutrient recycling activity, and microbial biomass by degrading microbial cells, thus leading to the improvement of structure and function of the soil microbial community [90,91]. Trichoderma inoculation significantly enhances the nutrient contents and enzymatic activity in rhizosphere of Pinus sylvestris var. mongolica seedlings. T. virens ZT05 was proven to have a greater impact as compared to T. harzianum E15 on nutrient availability and soil enzymatic activity in the crop rhizosphere [75].

Table 3.
Rhizosphere metabolites secreted by crop roots, Trichoderma, and soil microbial communities.
Table 3.
Rhizosphere metabolites secreted by crop roots, Trichoderma, and soil microbial communities.
| Metabolites (Enzymes) | Trichoderma Strains | Bacteria | Fungi | Host Plant | References |
|---|---|---|---|---|---|
| 1,8-dihydroxy-3-methylanthraquinone | Trichoderma hamatum TR1-4 | Bacillus spp. | Gaeumannaomyces graminis var. tritici, and Rhizoctonia solani | Wheat and Eggplant | [92,93] |
| 1-hydroxy-3-methylanthraquinone | T. harzianum 2413 | Pseudomonas spp. | Phytophthora capsici | Pepper | [94] |
| 1,8-dihydroxy-3-methylanthraquinone | T. harzianum T-22 | Pseudomonas fluorescens Q8r1-96 | Gaeumannaomyces graminis var. tritici, and Pyrenophora triticis-repentis | Wheat | [95,96] |
| Harzianopyridone | T. harzianum T-1 | Pseudomonas aureofaciens AB244 | Fusarium oxysporum; Pythium ultimum, and R. solani | Bean and Tomato | [97,98] |
| 6-pentyl-α-pyrone | T. harzianum 1295-22 | Pseudomonas fluorescens VO61 | R. solani | Creeping bent grass and rice | [99,100] |
| Trichorzianin TA Trichorzianin TB | T. harzianum Th-87 | Stenotrophomonas maltophilia C3 | R. solani | Eggplant and Tall fescue | [92,101] |
| Trichodermin | T. harzianum BAFC 742 | Bacillus subtilis GB03 | Sclerotinia sclerotiorum | Soybean | [102] |
| β-1,4 endoglucanase | T. longibrachiatum CECT 2606 | Serratia plymuthica | P. ultimum | Cucumber | [101,103] |
| 6-pent-1-enyl-α-pyrone | T. viride WT-6 | Bacillus subtilis GB03 | R. solani | Eggplant | [96] |
| 3,4-dihydroxycarotane | T. virens GL-21 | Pseudomonas fluorescens VO61 | P. ultimum and R. solani | Cucumber and pea | [104] |
| 6-Pentyl pyrone (6-PP) | T. virens GL-1, GL-21, GL-23 | Bacillus subtilis BACT-D | R. solani | Eggplant | [92] |
| β-1,4 glucanase 3,4-dihydroxycarotane Viridin | T. virens GL-3 | Burkholderia cepacia A3R, B. cepacia PHQM 100, Pseudomonas aureofaciens 63-28, and P. aureofaciens AB244 | Fusarium graminearum, Pythium aphanidermatum, and P. ultimum | Barley, Cucumber, Maize, and wheat | [105,106,107,108,109] |
8. Challenges and Future Research Directions
8.1. Efficacy of Trichoderma–Based Bioformulations
The compatibility of Trichoderma–based bioformulations needs to be assessed for integrated disease control approaches. Farmers should be encouraged to use Trichoderma–based formulations for environmentally sustainable disease control. To overcome the drawbacks of biological control techniques due to adverse environmental factors, formulations based on Trichoderma strains can be developed that act employ different mechanisms under both abiotic and biotic stresses in different climate zones. The utilization of microencapsulation technology can improve the effectiveness of Trichoderma–based bioformulations, and thus help to protect the pathogens in the field [34,35]. Furthermore, encapsulation is also capable to extend the shelf life of commercial products. This new technology will allow for the development of more effective pathogen control formulations in the pre- and post-harvest periods [34,35]. It is important to highlight the optimization and adjustments of microencapsulation processes employed to produce viable Trichoderma bioformulations for field applications. In this context, the association of the nanotechnology and biologically active compounds derived from Trichoderma on the surface of the nanoparticles can promote additional benefits for the efficient management of phytopathogens. However, it is a new technology, detailed investigation should be conducted to confirm that these nanoparticles do not have adverse effects on non-target organisms or cause any environmental contamination. Moreover, to commercialize these nanotechnological products obtained by the biogenic synthesis route, it is necessary to establish protocols for the standardization of the preparation of these biocontrol agents, as well as methods for scaling up production processes. There is tremendous potential to develop and commercialize novel products for the biological control of plant pathogens based on the genus Trichoderma, especially considering their applications in sustainable agriculture. In this review, we briefly discussed recently identified few commercial strains from the T. harzianum complex, which have been used to formulate biofungicides. We suggest researchers conduct further research to confirm how these commercial strains are effective in combating phytopathogens. Nowadays, silicon–based nanoparticles are also using in controlling microbes that exist in crop rhizosphere [110]. So, to see how Trichoderma bioformulations perform compared to silicon-based nanoparticles, further investigation needs to be assessed.
8.2. Ecological Challenges of Trichoderma Inoculant in Crop Rhizosphere
Metabolites and antibiotics produced by Trichoderma inoculant might affect not only phytopathogens, but also other beneficial or neutral soil microbes. The intensity of such impacts depends upon the inoculation period of biocontrol agents and the concentration of metabolites secreted [76]. The competition for nutrients might also be responsible for the alteration of the microbial population in the soil. The soil enzymatic activities are considered as the indicator for abiotic or biotic stresses with the presence of pathogens increasing their levels. Generally, inoculation of biocontrol agents has been shown to reduce biotic stress (soil pathogens) by decreasing the level of enzyme activities [111]. However, Trichoderma inoculant has certain non-target impacts because of the increase in enzymatic activities. It is highly challenging to monitor how bioinoculants affect non-target soil microbes in the rhizosphere and to understand their functions in the soil ecosystem. Future research is also needed to analyze the ecological consequences on non-target microbes, due to the application of bioinoculants into the crop rhizosphere.
8.2.1. Survival Fitness of Microbial Communities in Crop Rhizosphere
Soil microbes have been shown to play an essential role in soil formation, pathogen suppression, and nutrient solubilization and acquisition [112]. Bioinoculation is the most efficient and successful method for manipulating soil microbial communities [113,114]. However, there is very little evidence that these microbes can compete, develop, and operate since they are not reproducible on a long-term basis in natural agricultural soil. Moreover, a wide range of bioinoculants in agricultural fields is readily attacked by many predators and face nutrient competition from native microbes. The effective bioinoculants must be capable of forming interactions with other neighboring microbes, imitating the strongly structured crosslinks found in the native soil rhizosphere. The goal behind this strategy is to introduce beneficial microbial diversity into the plants, which will enhance the plant functions and provide resistance against phytopathogens [115,116]. A systemic approach is required for the successful engineering of soil microbes in the crop rhizosphere. However, knowledge of the basic mechanism of bioinoculants regarding how they are linked with the rhizosphere is important, it and also would improve sustainable crop production simply by enhancing the beneficial symbiotic associations among the plant–soil–microbial communities [117].
8.2.2. Trichoderma Affects Chemical Signals in Crop Rhizosphere
Interaction between plants and their associated microbes occurs in the soil rhizosphere through the exchange chemicals signals secreted by inoculated Trichoderma [118]. Signaling molecules can influence the metabolomic interactions among the soil microbes in either a positive or a negative way [119]. The interchange of these signaling molecules in Trichoderma–plant relationships is complex and not well characterized. However, in recent years, increased attention has been paid to understanding the chemical composition of Trichoderma–released secondary metabolites and their impacts on plant biochemical and physiological processes with potential applications in the field. Thus, we suggest researchers investigate how these signaling molecules interchange in Trichoderma–plant complex interactions.
8.2.3. Trichoderma Challenges Abiotic and Biotic Factors in Crop Rhizosphere
So far, around 100 compounds (e.g., alcohols, alkanes, amines, arenes, esters, phenols, etc.) have been identified in the rhizosphere soil [120]. Therefore, metabolomic interactions in the microbial community imply that soil chemical ecology might play significant roles in establishing biological inoculants and agroecosystem functions under diverse abiotic and biotic environments, such as soil–plant, pathogen–pathogen, microbe–microbe, microbe–pathogen, and indigenous versus non–indigenous inoculant interactions [18,118,120], which require further investigation to ensure introduced microbial inoculants such as Trichoderma can contribute in an eco-friendly and efficient manner in an agricultural production system (see soil ecological factors in Figure 2).
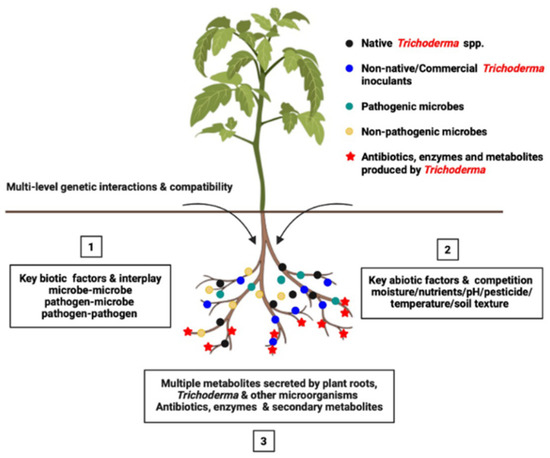
Figure 2.
Soil ecological challenges and factors (1. Biotic, 2. Abiotic, and 3. Metabolomics) of Trichoderma bioinoculants’ survival, establishment, and function in crop rhizosphere.
With the continuous growth of Trichoderma–based bioinoculants, we suggest researchers conduct further research to confirm how these commercial inoculants are effective to combat phytopathogens. To understand this multitalented biocontrol agent, further research should be conducted by which Trichoderma species can act against several lethal fungi as a potential biocontrol agent rather than minimizing the negative impact of Trichoderma on other resident microbes in the rhizosphere. To confirm an acceptable database for safe and sustainable usage of Trichoderma for better ecological balance, the extensive applications of any fungal species and their secondary metabolites for biological control management should be assessed. As a result, Trichoderma genomes can be a valuable source of candidate genes to produce transgenic or genetically modified plants resulting in improved resistance against abiotic and biotic stresses.
9. Conclusions
The successful establishment of biocontrol inoculants is reliant upon the multitrophic interactions including wide-ranging metabolites in the crop rhizosphere that play a vital role in shaping the microbial population, plant defense responses, and pathogen control. The molecular cross-talk among the contributors and understanding of these entire ecosystem processes would result not only in the safe use of biocontrol inoculants, but also expand our knowledge of the developmental process of soil and plant root diseases and their biocontrol mechanisms. The potentiality of the Trichoderma species in controlling soil-borne fungal pathogens is already renowned; however, reviewing this topic, no clear evidence of Trichoderma controlling bacterial plant pathogens in the soil rhizosphere has been found, conceivably an area that warrants further investigation. Future experiments on the mechanisms of possible synergistic actions by Trichoderma, soil microorganisms (pathogenic and non-pathogenic consortia), and environmental interactions should be performed. This could open up a new door for crop plants adapting to the Trichoderma as biological inoculants, minimizing negative impacts or unintended alterations to keystone functional soil microbiomes and crop productivity in diverse agroecosystems.
Author Contributions
M.N.I., S.A.S., C.N.W.C., M.M.I. and S.S. conceived and designed this revised version of the review article. M.N.I., S.A.S., M.A.R., P.K. and J.U. performed the investigation and metanalysis of this subject matter. M.N.I. and S.A.S. prepared the Figure 1 and Figure 2. S.A.S. wrote original draft. M.N.I., S.S. and S.A.S. wrote final draft for journal submission with contributions and reviewing support from C.N.W.C., P.K., M.A.R., M.M.I. and J.U. Funds were awarded to S.S. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledged the financial support from Universiti Malaysia Sabah Scheme UMS Great code number: GUG0276-2/2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghorbanpour, M.; Omidvari, M.; Abbaszadeh-Dahaji, P.; Omidvar, R.; Kariman, K. Mechanisms underlying the protective effects of beneficial fungi against plant diseases. Biol. Control. 2018, 117, 147–157. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to Plant Health and Productivity From Enhancing Plant Microbial Symbionts. Front. Plant Sci. 2021, 11, 2001. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.W.; Rahul, M.S.; Ambalal, N.S. Trichoderma: A significant fungus for agriculture and environment. Afric. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Jin-lian, C.; Kai, L.; Cui-ping, M.; Hui-lin, G.; Li-xing, Z.; Shi-zhong, S. Chemical Constituents with Siderophores Activities from Trichoderma koningiopsis YIM PH30002. Nat. Prod. Res. Develop. 2015, 27, 1878–1883. [Google Scholar]
- Asis, A.; Shahriar, S.A.; Naher, L.; Saallah, S.; Nur Fatihah, H.N.; Kumar, V.; Siddiquee, S. Species pattern identification of Trichoderma strains using morphological characteristics, phylogenetic analyses and lignocellulolytic activities. Mol. Biol. Rep. 2021, 48, 3285–3301. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary metabolism in Trichoderma—A genomic perspective. Microbiology 2012, 158, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Porras-Troncoso, M.D.; Olmedo-Monfil, V.; Herrera-Estrella, A. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef]
- Jangir, M.; Sharma, S.; Sharma, S. Non-target effects of Trichoderma on plants and soil microbial communities. In Plant Microbe Interface; Springer: Cham, Switzerland, 2019; pp. 239–251. [Google Scholar]
- Rout, M.E.; Southworth, D. The root microbiome influences scales from molecules to ecosystems: The unseen majority. Am. J. Bot. 2013, 100, 1689–1691. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Yong, J.W.H. Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming. Biology 2022, 11, 41. [Google Scholar] [CrossRef]
- Craney, A.; Ahmed, S.; Nodwell, J. Towards a new science of secondary metabolism. J. Antibiot. 2013, 66, 387–400. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Sharma, K.P.; Gaur, R.K. Biotechnological perspectives of microbes in agro ecosystems. Biotechnol. lett. 2011, 33, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.N.H.; Hasan, M.; Uddain, J.; Subramaniam, S. Impact of application of Trichoderma and biochar on growth, productivity and nutritional quality of tomato under reduced N-P-K fertilization. Ann. Agric. Sci. 2020, 65, 107–115. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Islam, M.N.; Uddain, J.; Chowdhury, M.S.N.; Subramaniam, S. Synergistic effect of microbial and nonmicrobial biostimulants on growth, yield, and nutritional quality of organic tomato. Crop Sci. 2020, 60, 2102–2114. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Naseby, D.C.; Pascual, J.A.; Lynch, J.M. Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum populations, soil microbial communities and soil enzyme activities. J. Appl. Microbiol. 2000, 88, 161–169. [Google Scholar] [CrossRef]
- Brimner, T.A.; Boland, G.J. A review of the non-target effects of fungi used to biologically control plant diseases. Agric. Ecosyst. Environ. 2003, 100, 3–16. [Google Scholar] [CrossRef]
- Islam, M.N.; Germida, J.J.; Walley, F.L. Survival of a commercial AM fungal inoculant and its impact on indigenous AM fungal communities in field soils. Appl. Soil Ecol. 2021, 166, 103979. [Google Scholar] [CrossRef]
- McAllister, C.B.; Garcia-Romera, I.; Godeas, A.; Ocampo, J.A. In vitro interactions between Trichoderma koningii, Fusarium solani and Glomus mosseae. Soil Biol. Biochem. 1994, 26, 1369–1374. [Google Scholar] [CrossRef]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Schlatter, D.; Kinkel, L.; Thomashow, L.; Weller, D.; Paulitz, T. Disease suppressive soils: New insights from the soil microbiome. Phytopathology 2017, 107, 1284–1297. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition—Current Knowledge and Future Directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Meng, H.; Gu, J.-D. Microbial extracellular enzymes in biogeochemical cycling of ecosystems. J. Environ. Manag. 2017, 197, 539–549. [Google Scholar] [CrossRef]
- Yang, T.; Lupway, N.; Marc, S.-A.; Siddique, K.H.M.; Bainard, L.D. Anthropogenic drivers of soil microbial communities and impacts on soil biological functions in agroecosystems. Global Ecol. Conserv. 2021, 27, e01521. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egypt. J. Biol. Pest Control 2020, 30, 133. [Google Scholar] [CrossRef]
- Chaverri, P.; Samuels, G.J. Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution 2013, 67, 2823–2837. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Jaklitsch, W.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef]
- Sudantha, I.M.; Sudirman; Ernawati, N.M.L. The effect of method and dosage application of biofungicide extract of Legundi leaf fermented with Trichoderma harzianum fungus for control of Fusarium wilt disease on shallots. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012014. [Google Scholar] [CrossRef]
- Bailey, B.A.; Bae, H.; Strem, M.D.; Crozier, J.; Thomas, S.E.; Samuels, G.J.; Vinyard, B.T.; Holmes, K.A. Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao. Biol. Control 2008, 46, 24–35. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Anani, O.A. Bio-fertilizer from Trichoderma: Boom for Agriculture Production and Management of Soil- and Root-Borne Plant Pathogens. In Innovations in Food Technology; Springer: Singapore, 2020; pp. 245–256. [Google Scholar] [CrossRef]
- Fraceto, L.F.; Maruyama, C.R.; Guilger, M.; Mishra, S.; Keswani, C.; Singh, H.B.; de Lima, R. Trichoderma harzianum-based novel formulations: Potential applications for management of Next-Gen agricultural challenges. J. Chem. Technol. Biotechnol. 2018, 93, 2056–2063. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Srivastava, M.; Kumar, V.; Shahid, M.; Pandey, S.; Singh, A. Trichoderma—A potential and effective biofungicide and alternative source against notable phytopathogens: A review. Afr. J. Agric. Res. 2016, 11, 310–316. [Google Scholar] [CrossRef]
- Guerrieri, A.; Dong, L.; Bouwmeester, H.J. Role and exploitation of underground chemical signaling in plants. Pest Manag. Sci. 2019, 75, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Vargas, W.A.; Crutcher, F.K.; Kenerley, C.M. Functional characterization of a plant-like sucrose transporter from the beneficial fungus Trichoderma virens. Regulation of the symbiotic association with plants by sucrose metabolism inside the fungal cells. New Phytol. 2011, 189, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Samolski, I.; Rincón, A.M.; Pinzón, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Raza, W.; Li, J.; Liu, Y.; Qiu, M.; Zhang, F.; Shen, Q. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Roldán, A.; Albacete, A.; Pascual, J.A. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 2011, 72, 223–229. [Google Scholar] [CrossRef]
- Chakraborty, B.N.; Chakraborty, U.; Sunar, K. Induced Immunity Developed by Trichoderma Species in Plants. In Trichoderma. Rhizosphere Biology; Sharma, A., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 125–147. [Google Scholar] [CrossRef]
- Srivastava, M.; Shahid, M.; Pandey, S.; Kumar, V.; Singh, A.; Trivedi, S.; Srivastava, Y.K.; Shivram. Trichoderma: A scientific approach against soil borne pathogens. Afr. J. Microbiol. Res. 2015, 9, 2377–2384. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.-application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A.; et al. Evolution and comparative genomics of the most common Trichoderma species. BMC Genom. 2019, 20, 485. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Schmoll, M. Biology and biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A. Trichoderma species opportunistic, a virulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a model to study effector-like molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases—Potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 2018, 8, 88. [Google Scholar] [CrossRef]
- Esse, H.P.; Reuber, T.L.; Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef]
- Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi. 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma Secondary Metabolites Active on Plants and Fungal Pathogens. Open Mycol. J. 2014, 8, 127–139. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; Hermosa, R.; García-Estrada, C.; Caradus, J.; He, Y.W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Vinale, F.; et al. Antimicrobial secondary metabolites from agriculturally important fungi as next biocontrol agents. Appl. Microbiol. Biotechnol. 2019, 103, 9287–9303. [Google Scholar] [CrossRef] [PubMed]
- Daguerre, Y.; Siegel, K.; Edel-Hermann, V.; Steinberg, C. Fungal proteins and genes associated with biocontrol mechanisms of soil-borne pathogens: A review. Fungal. Biol. Rev. 2014, 28, 97–125. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal. Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Vargas, W.A.; Mukherjee, P.K.; Laughlin, D.; Wiest, A.; Moran-Diez, M.E.; Kenerley, C.M. Role of gliotoxin in the symbiotic and pathogenic interactions of Trichoderma virens. Microbiology 2014, 160, 2319–2330. [Google Scholar] [CrossRef]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef]
- Degenkolb, T.; Fog Nielsen, K.; Dieckmann, R.; Branco-Rocha, F.; Chaverri, P.; Samuels, G.J.; Thrane, U.; von Döhren, H.; Vilcinskas, A.; Brückner, H. Peptaibol, Secondary-Metabolite, and Hydrophobin Pattern of Commercial Biocontrol Agents Formulated with Species of the Trichoderma harzianum Complex. Chem. Biodivers. 2015, 12, 662–684. [Google Scholar] [CrossRef]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites–Strategies to activate silent gene clusters. Fungal. Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol Methods 2010, 81, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A.; Wisecaver, J.H.; Lind, A.L. The birth, evolution and death of metabolic gene clusters in fungi. Nat. Rev. Microbiol. 2018, 16, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Newman, J.A.; Silverman, B.W.; Turner, S.L.; Lilley, A.K. The contribution of species richness and composition to bacterial services. Nature 2005, 436, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Assigbetsé, K.; Ciss, I.; Bakhoum, N.; Dieng, L. Effect of inoculation of Acacia senegal mature trees with mycorrhiza and rhizobia on soil properties and microbial community structure. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 22–27 April 2012; pp. 2012–8004. [Google Scholar]
- Zhang, F.G. The Affects and Mechanisms of Puta Five Trichoredma harzianum Mutant and ITS Bio-Organic Fertilizer on Growth of Cucumber; Nanjing Agricultural University: Nanjing, China, 2015; pp. 15–18. [Google Scholar]
- Degenkolb, T.; Aghcheh, K.R.; Dieckmann, R.; Neuhof, T.; Baker, S.E.; Druzhinina, I.S.; Kubicek, C.P.; Brückner, H.; Von Döhren, H. The production of multiple small peptaibol families by single 14-module peptide synthetases in Trichoderma/hypocrea. Chem. Biodivers 2012, 9, 499–535. [Google Scholar] [CrossRef]
- Abegaz, B.M.; Kinfe, H.H. Secondary metabolites, their structural diversity, bioactivity, and ecological functions: An overview. Physic. Sci. Rev. 2019, 4, 4. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Baazeem, A.; Almanea, A.; Manikandan, P.; Alorabi, M.; Vijayaraghavan, P.; Abdel-Hadi, A. In Vitro Antibacterial, Antifungal, Nematocidal and Growth Promoting Activities of Trichoderma hamatum FB10 and Its Secondary Metabolites. J. Fungi 2021, 7, 331. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Interactions of Trichoderma with Plants, Insects, and Plant Pathogen Microorganisms: Chemical and Molecular Bases. In Co-Evolution of Secondary Metabolites; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 263–290. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Umadevi, P.; Anandaraj, M.; Srivastav, V.; Benjamin, S. Trichoderma harzianum MTCC 5179 impacts the population and functional dynamics of microbial community in the rhizosphere of black pepper (Piper nigrum L.). Braz. J. Microbiol. 2018, 49, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of Two Trichoderma Strains on Plant Growth, Rhizosphere Soil Nutrients, and Fungal Community of Pinus sylvestris var. mongolica Annual Seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef]
- Cordier, C.; Alabouvette, C. Effects of the introduction of a biocontrol strain of Trichoderma atroviride on non-target soil microorganisms. Eur. J. Soil Biol. 2009, 7, 267–274. [Google Scholar] [CrossRef]
- Li, Q.R.; Tan, P.; Jiang, Y.L.; Hyde, K.D.; Mckenzie, E.H.C.; Bahkali, A.H.; Kang, J.C.; Wang, Y. A novel Trichoderma species isolated from soil in Guizhou, T. guizhouense. Mycol. Prog. 2013, 12, 167–172. [Google Scholar] [CrossRef]
- Crutcher, F.K.; Parich, A.; Schuhmacher, R.; Mukherjee, P.K.; Zeilinger, S.; Kenerley, C.M. A putative terpene cyclase, vir4, is responsible for the biosynthesis of volatile terpene compounds in the biocontrol fungus Trichoderma virens. Fungal. Genet. Biol. 2013, 56, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Reino, J.L.; Guerrero, R.F.; Hernandez-Galan, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.Q.; Wang, M.; Sun, J.; Gao, J.X.; Chen, J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium stalk rot. Sci. Rep. 2017, 7, 1771–1783. [Google Scholar] [CrossRef]
- Gupta, R.; Mathimaran, N.; Wiemken, A.; Boller, T.; Bisaria, V.S.; Sharma, S. Non-target effects of bioinoculants on rhizospheric microbial communities of Cajanus cajan. Appl. Soil Ecol. 2014, 76, 26–33. [Google Scholar] [CrossRef]
- Szczepaniak, Z.; Cyplik, P.; Juzwa, W.; Czarny, J.; Staninska, J.; Piotrowska-Cyplik, A. Antibacterial effect of the Trichoderma viride fungi on soil microbiome during PAH’s biodegradation. Int. Biodeterior. Biodegrad. 2015, 104, 170–177. [Google Scholar] [CrossRef]
- Gasoni, L.; Khan, N.; Yokoyama, K.; Chiessa, G.H.; Kobayashi, K. Impact of Trichoderma harzianum biocontrol agent on functional diversity of soil microbial community in tobacco monoculture in Argentina. World J. Agric. Sci. 2008, 4, 527–532. [Google Scholar]
- Naseby, D.C.; Lynch, J.M. Impact of wild-type and genetically modified Pseudomonas fluorescens on soil enzyme activities and microbial population structure in the rhizosphere of pea. Mol. Ecol. 1998, 7, 617–625. [Google Scholar] [CrossRef]
- Mbarki, S.; Cerdà, A.; Brestic, M.; Mahendra, R.; Abdelly, C.; Pascual, J.A. Vineyard compost supplemented with Trichoderma harzianum T78 improve saline soil quality. Land Degrad. Develop. 2016, 28, 1028–1037. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Del Rio, T.G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Yadav, R.L.; Shukla, S.K.; Suman, A.; Singh, P.N. Trichoderma inoculation and trash management effects on soil microbial biomass, soil respiration, nutrient uptake and yield of ratoon sugarcane under subtropical conditions. Biol. Fertil. Soils 2009, 45, 461–468. [Google Scholar] [CrossRef]
- Lewis, J.A.; Larkin, R.P. Formulation of the biocontrol fungus Cladorrhinum foecundissimum to reduce damping-off diseases caused by Rhizoctonia solani and Pythium ultimim. Biol. Control. 1998, 12, 182–190. [Google Scholar] [CrossRef]
- Ryder, M.H.; Yan, Z.; Terrace, T.E.; Rovira, A.D.; Tong, W.; Correll, R.L. Use of strains of Bacillus isolated in China to suppress take-all and Rhizoctonia root rot and promote seedling growth of glasshouse-grown wheat in Australian soils. Soil. Biol. Biochem. 1999, 31, 19–29. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Perez-Sanchez, C.; Egea, C.; Candela, M.E. Evaluation of Trichoderma harzianum for controlling root rot caused Phytophthora capsica in pepper plants. Plant. Pathol. 1999, 48, 58–65. [Google Scholar] [CrossRef]
- da Luz, W.C.; Bergstorm, G.C.; Stockwell, C.A. Seed-applied bioprotectants for control of seed-borne Pyrenophora tritici-repentis and agronomic enhancement of wheat. Can. J. Plant Pathol. 1998, 19, 384–386. [Google Scholar]
- Raaijmakers, J.M.; Weller, D.M. Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in take-all decline soils. Mol. Plant-Microbe Interact 1998, 11, 144–152. [Google Scholar] [CrossRef]
- Warren, J.E.; Bennett, M.A. Bio-osmopriming tomato (Lycopersicon esculentum Mill.) seeds for improved stand establishment. Seed Sci. Technol. 1999, 27, 489–499. [Google Scholar]
- Woo, S.L.; Donzelli, B.; Scala, F.; Mach, R.; Harman, G.E.; Kubicek, C.P.; Del Sorbe, G.; Lorito, M. Disruption of the ech42 (endochitinase-encoding) gene affects biocontrol activity in in Trichoderma harzianum P1. Mol. Plant-Microbe Interact. 1999, 12, 419–429. [Google Scholar] [CrossRef]
- Lo, C.-T.; Nelson, E.B.; Hayes, C.K.; Harman, G.E. Ecological studies of transformed Trichoderma harzianum strain 1295-22 in the rhizosphere and on the phylloplane of creeping bentgrass. Phytopathology 1998, 88, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Vidhyasekaran, P.; Muthamilan, M. Evaluation of a powder formulation of Pseudomonas fluorescens Pf1 for control of rice sheath blight. Biocontrol. Sci. Technol. 1999, 9, 67–74. [Google Scholar] [CrossRef]
- Giesler, L.J.; Yuen, G.Y. Evaluation of Stenotrophomonas maltophilia strain C3 for biocontrol of brown patch disease. Crop Prot. 1998, 17, 509–513. [Google Scholar] [CrossRef]
- Menendez, A.B.; Godeas, A. Biological control of Sclerotinia sclerotiorum attacking soybean plants. Degradation of the cell walls of this pathogen by Trichoderma harzianum (BAFC 742). Mycopathologia 1998, 143, 153–160. [Google Scholar] [CrossRef]
- Migheli, Q.; Gonzalez-Candelas, L.; Dealessi, L.; Camponogara, A.; Ramon-Vidal, D. Transformants of Trichoderma longibrachiatum overexpressing the β-1,4-endoglucanase gene egl1 show enhanced biocontrol of Pythium ultimum on cucumber. Phytopathology 1998, 88, 673–677. [Google Scholar] [CrossRef]
- Koch, E. Evaluation of commercial products for microbial control of soil-borne plant diseases. Crop Prot. 1999, 18, 119–125. [Google Scholar] [CrossRef]
- Chen, C.; Belanger, R.R.; Benhamou, N.; Paulitz, T.C. Induced systemic resistance (ISR) by Pseudomonas spp. impairs pre- and post-infection development of Pythium aphanidermatum on cucumber roots. Eur. J. Plant Pathol. 1998, 104, 877–886. [Google Scholar] [CrossRef]
- Hebbar, K.P.; Martel, M.H.; Heulin, T. Suppression of pre- and post-emergence damping-off in corn by Burkholderia cepacia. Eur. J. Plant Pathol. 1998, 104, 29–36. [Google Scholar] [CrossRef]
- Huang, Y.; Wong, P.T.W. Effect of Burkholderia (Pseudomonas) cepacia and soil type on the control of crown rot in wheat. Plant Soil 1998, 203, 103–108. [Google Scholar] [CrossRef]
- Johnsson, L.; Hokeberg, M.; Gerhardson, B. Performance of the Pseudomonas chlororaphis biocontrol agent MA 342 against cereal seed-borne diseases in field experiments. Eur. J. Plant Pathol. 1998, 104, 701–711. [Google Scholar] [CrossRef]
- Mao, W.; Lewis, J.A.; Hebbar, P.K.; Lumsden, R.D. Seed treatment with a fungal or a bacterial antagonist for reducing corn damping-off caused by species of Pythium and Fusarium. Plant Dis. 1997, 81, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Feizi, M.; Kumari, A.; Khan, M.; Mandzhieva, S.; Sushkova, S.; El-Ramady, H.; Verma, K.K.; Singh, A.; et al. Effects of Silicon and Silicon-Based Nanoparticles on Rhizosphere Microbiome, Plant Stress and Growth. Biology 2021, 10, 791. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Kour, D.; Kaur, T.; Devi, R.; Yadav, A.; Singh, M.; Joshi, D.; Singh, J.; Suyal, D.C.; Kumar, A.; Rajput, V.D.; et al. Beneficial microbiomes for bioremediation of diverse contaminated environments for environmental sustainability: Present status and future challenges. Environ. Sci. Pollut. Res. Int. 2021, 28, 24917–24939. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd_Allah, E.F. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef]
- Kaul, S.; Choudhary, M.; Gupta, S.; Dhar, M.K. Engineering Host Microbiome for Crop Improvement and Sustainable Agriculture. Front. Microbiol. 2021, 12, 635917. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Expt. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Chen, C.; Wang, M.; Zhu, J.; Tang, Y.; Zhang, H.; Zhao, Q.; Jing, M.; Chen, Y.; Xu, X.; Jiang, J.; et al. Long-term effect of epigenetic modification in plant–microbe interactions: Modification of DNA methylation induced by plant growth-promoting bacteria mediates promotion process. Microbiome 2022, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, X.; Huo, Y.; Xiao, Y. Trichoderma–Inoculation and Mowing Synergistically Altered Soil Available Nutrients, Rhizosphere Chemical Compounds and Soil Microbial Community, Potentially Driving Alfalfa Growth. Front. Microbiol. 2019, 7, 3241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).