New Alternative to Control Stenoma impressella (Lepidoptera: Elachistidae) Using Bacillus thuringiensis Commercial Formulations in Oil Palm Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of Bacillus thuringiensis Formulations
2.2. Formulation Comparison
2.3. Dose Evaluation
2.4. Sprayed Commercial Formulations under Field Conditions
2.5. Statistical Analysis
3. Results
3.1. Evaluation of Bacillus thuringiensis Formulations
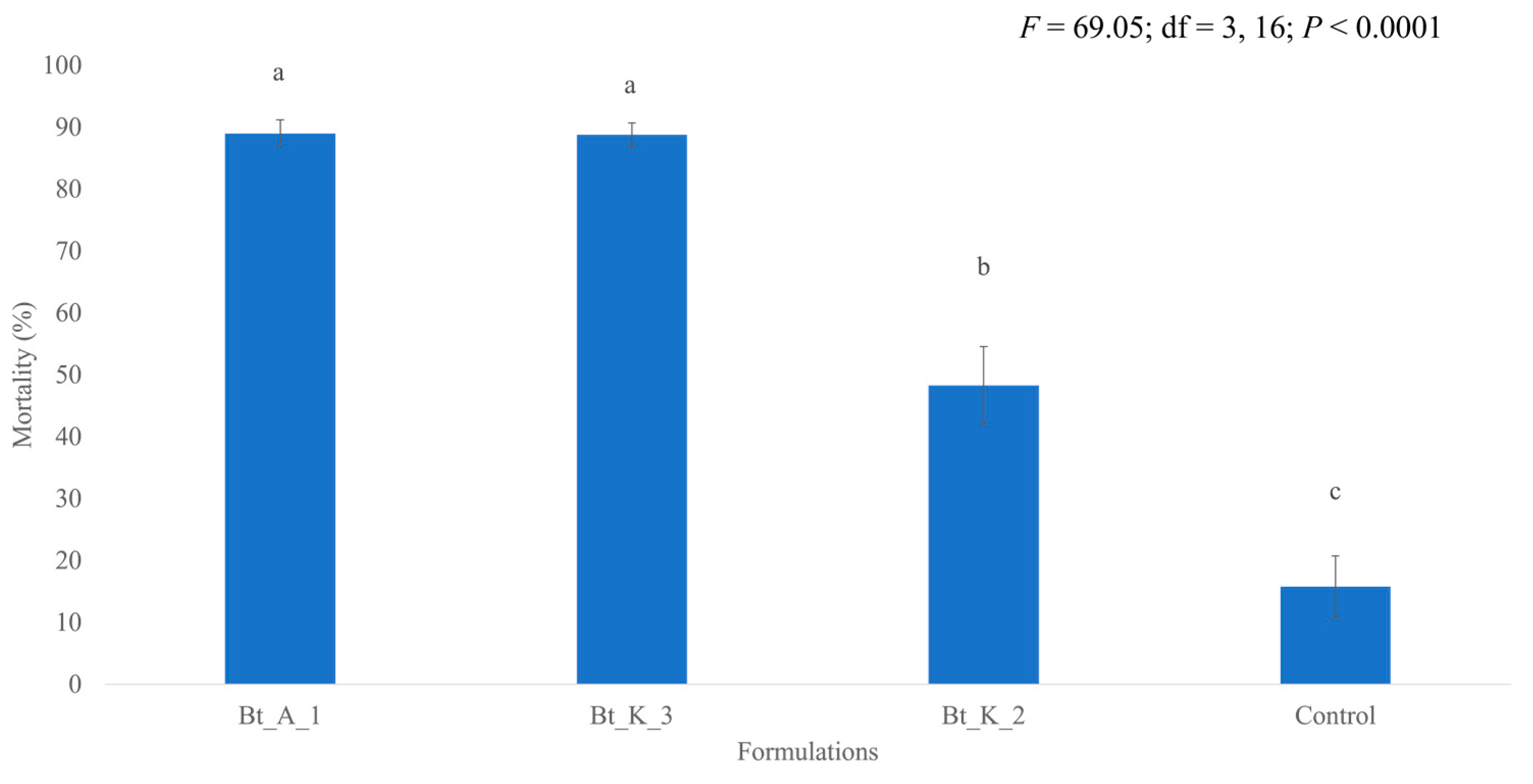
3.2. Formulation Comparison
3.3. Dose Evaluation
3.4. Sprayed Commercial Formulations under Field Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genty, P. Morphology and biology of a defoliating Lepidoptera of the oil palm in Latin America: Stenoma cecropia. Oleagineux 1978, 33, 421–427. [Google Scholar]
- Mexzon, R.; Chinchilla, C. Enemigos naturales de los artrópodos perjudiciales a la palma aceitera (Elaeis guineensis Jacq.) en América tropical. ASD Oil Palm Pap. 1996, 13, 9–33. [Google Scholar]
- Aldana-De La Torre, R.C.; Montes-Bazurto, L.G.; Barrios, C.; Matabanchoy, J.; Beltran, I.J.; Rosero, M.; Pardey, A.E.B. Guía de Bolsillo para el Reconocimiento de Las plagas más Frecuentes en la Palma de Aceite; Cenipalma: Bogota, Colombia, 2017; 59p. [Google Scholar]
- Aldana-De La Torre, R.C.; Aldana, J.; Calvache, H.; Franco, P. Manual de Plagas de la Palma de Aceite en Colombia, 4th ed.; Cenipalma: Bogota, Colombia, 2010; 198p. [Google Scholar]
- Calvache, H. El control microbiano, en el manejo de las plagas de la palma de aceite. Palmas 1993, 14, 13–21. [Google Scholar]
- Barrios, C.; Aldana-De La Torre, R.C.; Bustillo-Pardey, A.E. Biología del defoliador de la palma de aceite, Stenoma cecropia Meyrick (Lepidoptera: Elachistidae). Palmas 2013, 34, 13–19. [Google Scholar]
- Bustillo-Pardey, A.E. Manejo de insectos-plaga de la palma de aceite con énfasis en el control biológico y su relación con el cambio climático. Palmas 2014, 35, 66–77. [Google Scholar]
- Castillo, S.; Aldana, J.; Calvache, H.; Grijalva, O. Evaluación de técnicas de liberación de Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) para el manejo de Stenoma cecropia Meyrick (Lepidoptera: Stenomidae) en el cultivo de palma de aceite (Elaeis guineensis Jacq. Palmas 2000, 2, 203–211. [Google Scholar]
- Sendoya-Corrales, C.A.; Bustillo-Pardey, A.E. Enemigos naturales de Stenoma cecropia (Lepidoptera: Elachistidae) en palma de aceite, en el suroccidente de Colombia. Rev. Colomb. Entomol. 2016, 42, 146–154. [Google Scholar] [CrossRef]
- Aldana, J.; Calvache, H.; Escobar, B.; Castro, H. Las plantas arvenses benéficas dentro de un programa de manejo integrado de Stenoma cecropia meyrick, en palma de aceite. Palmas 1997, 18, 11–21. [Google Scholar]
- Mariau, D. La Fauna de la Palma de Aceite y del Cocotero. Los Insectos y Ácaros Plagas y sus Enemigos Naturales; Cirad: Montpellier, France, 2001; 265p. [Google Scholar]
- Montes-Bazurto, L.G.; Bustillo-Pardey, A.E.; Medina-Cárdenas, H.C. Cordyceps cateniannulata, a novel entomopathogenic fungus to control Stenoma impressella Busck (Lepidoptera: Elachistidae) in Colombia. J. Appl. Entomol. 2020, 144, 788–796. [Google Scholar] [CrossRef]
- Delvarf, G.; Genty, P. Interés de las plantas atractivas para la entomofauna benéfica de las plantaciones de palma, en América tropical. Palmas 1992, 13, 23–33. [Google Scholar]
- Zenner de Polanía, I.; Posada, F. Manejo de Insectos Plaga y Benéficos de la Palma Africana; Manual de Asistencia Técnica; Instituto Colombiano Agropecuario—ICA: Bogota, Colombia, 1992; Volume 54, 124p. [Google Scholar]
- Valencia, C. Patogenicidad de hongos entomopatógenos del género Beauveria sp. sobre larvas de Stenoma cecropia (Lepidoptera: Elachistidae), en condiciones de laboratorio. Ceniavance 2007, 147, 1–4. [Google Scholar]
- Calvache, H. Manejo integrado de plagas de la palma de aceite. Palmas 1995, 16, 255–264. [Google Scholar]
- Rosas-García, N.M. Avances en el desarrollo de formulaciones insecticidas a base de Bacillus thuringiensis Advances in developing Bacillus thuringiensis-based insecticde formulations. Rev. Colomb. Biotecnol. 2008, 10, 49–63. [Google Scholar]
- Corley, R.H.V.; Tinker, P.B. The Oil Palm, 5th ed.; Wiley Blackwell: Chichester, UK, 2016; 655p. [Google Scholar]
- Cotes, A.M. Control Biológico de Fitopatógenos, Insectos y Ácaros. Volumen 1: Agentes de Control Biologico; Cotes, A.M., Ed.; Agrosavia: Mosquera, Colombia, 2018; pp. 1–566. [Google Scholar]
- Jurat-Fuentes, J.L.; Jackson, T. Bacterial entomopathogens. In Insect Pathology, 2nd ed.; Elsevier: Oxford, UK, 2012; pp. 265–349. [Google Scholar]
- Bravo, A.; Pacheco, S.; Gómez, I.; Garcia-Gómez, B.; Onofre, J.; Soberón, M. Insecticidal proteins from Bacillus thuringiensis and their mechanism of action. In Bacillus Thuringiensis and Lysinibacillus Sphaericus: Characterization and Use in the Field of Biocontrol; Fiuza, L., Polanczyk, R., Crickmore, N., Eds.; Springer: New York, NY, USA, 2017; pp. 53–66. [Google Scholar]
- Osman, G.E.H.; Already, R.; Assaeedi, A.S.A.; Organji, S.R.; El-Ghareeb, D.; Abulreesh, H.H.; Althubiani, A.S. Bioinsecticide Bacillus thuringiensis a comprehensive review. Egypt. J. Biol. Pest Control 2015, 25, 271–288. [Google Scholar]
- Soberón, M.; Bravo, A. Las toxinas Cry de Bacillus thuringiensis: Modo de acción y consecuencias de su aplicación. Biotecnologia 2007, 14, 303–314. [Google Scholar]
- Portela-Dussán, D.; Chaparro-Giraldo, A.; López-Pazos, S.A. La biotecnología de Bacillus thuringiensis en la agricultura: Una revisión necesaria. Nova 2013, 11, 87–96. [Google Scholar] [CrossRef]
- López-Pazos, S.A.; Cerón, J. Proteínas Cry de Bacillus thuringiensis y su interacción con coleópteros. Nova 2010, 8, 183–194. [Google Scholar] [CrossRef]
- Ossa-o, G.A.; Bustillo-Pardey, A.E.; Valencia-jiménez, A. Determinación del pH en el fluido digestivo de larvas y adultos de Hypothenemus hampei (Coleoptera: Scolytidae). Cenicafé 2000, 51, 97–101. [Google Scholar]
- Ramírez, L.; Ramírez, N.; Fuentes, L.S.; Jiménez, J. Estandarización de un bioensayo y evaluación preliminar de tres formulaciones comerciales de Bacillus thuringiensis sobre Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Rev. Colomb. Biotecnol. 2010, 12, 12–21. [Google Scholar]
- Schneider-Orelli, O. Entomologisches Praktikum: Einführung in die Land-und Forstwirtschaftliche Insektenkunde; H.R. Sauerländer & Co.,: Aarau, Switzerland, 1947; 237p. [Google Scholar]
- Cossentine, J.; Robertson, M.; Xu, D. Biological activity of Bacillus thuringiensis in Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2016, 109, 1071–1078. [Google Scholar] [CrossRef]
- Izhar, Y.; Wysoki, M.; Gur, L. The effectivesness of Bacillus thuringiensis Berliner on Boarmia (Ascotis) selenaria Schiff (Lepidoptera, Geometridae) in laboratory test and field trials. Phytoparasitica 1979, 7, 65–77. [Google Scholar] [CrossRef]
- Bernardi, O.; Bernardi, D.; Ribeiro, R.S.; Okuma, D.M.; Salmeron, E.; Fatoretto, J.; Meddeiros, F.C.L.; Burd, T.; Omoto, C. Frequency of resistance to Vip3Aa20 toxin from Bacillus thuringiensis in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Crop Prot. 2015, 76, 7–14. [Google Scholar] [CrossRef]
- Sayed, S.M.; Elsayed, G.; Mahmoud, S.F.; Elzahrany, O.M. Efficacy of Bacillus thuringiensis and Indigenous Trichogramma turkistanica for Controlling Lepidopterous Pests on Taify Pomegranate Fruits. Afr. Entomol. 2015, 23, 443–450. [Google Scholar] [CrossRef]
- Roush, R.T. Managing Pests and Their Resistance to Bacillus thuringiensis: Can Transgenic Crops Be Better than Sprays? Biocontrol. Sci. Technol. 1994, 4, 501–516. [Google Scholar] [CrossRef]
- Roush, R.T. Resistance management for agricultural pest. In Entomopathogenic Bacteria: From Laboratory to Field Application; Delécluses, A., Roux, C.N., Eds.; Kluwer Academic Publishers—KAP (acronym): Amsterdam, The Netherlands, 2000; pp. 399–417. [Google Scholar]
| Commercial Name | Code Formulation | Composition | Dose (g/Ha) | Manufacturer’s Headquarters |
|---|---|---|---|---|
| Bacillus Agrogen WP | Bt_K_1 | Bacillus thuringiensis var. kurstaki | 1000 | Yáser S.A.S., Cali, Colombia |
| BT-Biox WP | Bt_K_2 | Bacillus thuringiensis var. kurstaki | 500 | Semillas Valle S.A., Yumbo, Colombia |
| Bassar WP | Bb_Bt_1 | Beauveria bassiana and Bacillus thuringiensis | 1000 | Natural Control, Antioquia, Colombia |
| Xentari WDG | Bt_A_1 | Bacillus thuringiensis var. Aizawai | 500 | Valent BioSciences, Libertyville, IL |
| Dipel WP | Bt_K_3 | Bacillus thuringiensis var. kurstaki | 500 | Bayer AG, Leverkusen, Germany |
| Code Formulation | Composition | Dose (g/Ha) | Mortality (%) | Standard Error | Corrected Mortality (%) |
|---|---|---|---|---|---|
| Bt_K_3 | Bacillus thuringiensis var. kurstaki | 500 | 100 | - | 100.0 |
| Bt_A_1 | Bacillus thuringiensis var. aizawai | 500 | 94 a* | 2.4 | 93.9 |
| Bt_K_2 | Bacillus thuringiensis var. kurstaki | 500 | 84 a | 6.8 | 83.7 |
| Bb_Bt_1 | Beauveria bassiana and Bacillus thuringiensis | 1000 | 52 b | 7.3 | 51.0 |
| Bt_K_1 | Bacillus thuringiensis var. kurstaki | 1000 | 22 c | 7.3 | 20.4 |
| Control | - | - | 2 c | 2.0 | - |
| Code Formulation | Composition | Dose (g/Ha) | Mortality (%) | Standard Error | Corrected Mortality (%) |
|---|---|---|---|---|---|
| Bioassay 1 (27.8 ± 4.2 °C and 83.9 ± 19.7% RH) | |||||
| Bt_K_3 | Bacillus thuringiensis var. kurstaki | 250 | 51.9 a* | 8.6 | 43.3 |
| 500 | 56.2 a | 4.0 | 48.3 | ||
| 750 | 70.8 a | 9.0 | 65.6 | ||
| 1000 | 69.8 a | 8.3 | 64.4 | ||
| Control | - | - | 15.2 b | 7.9 | - |
| Bioassay 2 (30.5 ± 6.3 °C and 80.2 ± 22.7% RH) | |||||
| Bt_A_1 | Bacillus thuringiensis var. aizawai | 250 | 93.4 a | 1.1 | 92.8 |
| 500 | 96.7 a | 1.2 | 96.4 | ||
| 750 | 93.3 a | 2.6 | 92.7 | ||
| 1000 | 96.3 a | 1.9 | 96.0 | ||
| Control | - | - | 8.3 b | 3.8 | - |
| Code Formulation | Composition | Dose (g/Ha) | No. | Larvae before Spraying (#) | Standard Error | Larvae 7 Days after Spraying (#) | Standard Error | Larvae Reduction (%) |
|---|---|---|---|---|---|---|---|---|
| Bt_K_3 | Bacillus thuringiensis var. kurstaki | 500 | 17 | 7.5 | 1.6 | 1.1 | 0.5 | 85.3 |
| Bt_A_1 | Bacillus thuringiensis var. aizawai | 500 | 11 | 15.5 | 3.9 | 3.5 | 1.5 | 77.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Bazurto, L.G.; Bustillo-Pardey, A.E.; Morales, A. New Alternative to Control Stenoma impressella (Lepidoptera: Elachistidae) Using Bacillus thuringiensis Commercial Formulations in Oil Palm Crops. Agronomy 2022, 12, 883. https://doi.org/10.3390/agronomy12040883
Montes-Bazurto LG, Bustillo-Pardey AE, Morales A. New Alternative to Control Stenoma impressella (Lepidoptera: Elachistidae) Using Bacillus thuringiensis Commercial Formulations in Oil Palm Crops. Agronomy. 2022; 12(4):883. https://doi.org/10.3390/agronomy12040883
Chicago/Turabian StyleMontes-Bazurto, Luis Guillermo, Alex Enrique Bustillo-Pardey, and Anuar Morales. 2022. "New Alternative to Control Stenoma impressella (Lepidoptera: Elachistidae) Using Bacillus thuringiensis Commercial Formulations in Oil Palm Crops" Agronomy 12, no. 4: 883. https://doi.org/10.3390/agronomy12040883
APA StyleMontes-Bazurto, L. G., Bustillo-Pardey, A. E., & Morales, A. (2022). New Alternative to Control Stenoma impressella (Lepidoptera: Elachistidae) Using Bacillus thuringiensis Commercial Formulations in Oil Palm Crops. Agronomy, 12(4), 883. https://doi.org/10.3390/agronomy12040883






