Abstract
The performance of autumn and spring-planted strawberry cv. ‘Elsanta’ in peat and peat supplemented with fresh spent mushroom substrate (SMS) of Agaricus bisporus, Lentinus edodes, and Pleurotus ostreatus in 15% and 25% was evaluated. The morphological and yield parameters, dry matter distribution, uptake, and partitioning of macro- and micronutrients were studied. The experiment was carried out during 2020–2021 in an unheated tunnel and was laid out in a randomised complete block design in five replicates. The study aimed to deliver greater insight into utilising fresh SMS as a sustainable substitute to peat. The shoot and root length, leaf number, crown diameter, plant dry weight, and marketable yields were superior in SMS-based substrates in autumn planting. The nutrient uptake varied among substrates and planting seasons, where higher plant nutrient uptake was noticed in SMS-based substrates. Nutrient partitioning among leaves, crowns, and roots was dynamic. The N, P, K, and Mn were mainly recovered in leaves. While Mg was almost equally partitioned among plant organs. The higher amounts of Na, Zn, and Cu were found in crowns. The greater accumulation of Ca and Fe in roots was evident. Correlation indicated that plant macro- and micronutrients had both positive and negative interactions. Overall, the superior morphological and yield performances of cv. ‘Elsanta’ were more noticeable in SMSs than in peat in autumn-planted strawberries.
1. Introduction
Strawberry (Fragaria × ananassa Duch.) is a herbaceous, perennial plant and a member of the Rosaceae family of the genus Fragaria [1]. Strawberries are the most economically important soft fruit in the world. The global production of strawberries has increased nearly 40% in the last decade, making it the second-largest berry fruit produced after the grape (Vitis vinifera L.) [2].
In recent years, soilless strawberry production is becoming popular. Substrate culture represents a good alternative to conventional field production of strawberries, which offers many advantages to growers by eliminating the need for chemical fumigations, crop rotations, and ensuring better crop management, enabling efficient utilisation of inputs and often resulting in higher yields [3,4,5]. In addition, the short period from planting until harvest is one of the key reasons for the growing interest in soilless strawberry production [6].
Among berries, the strawberry responds quite well to soilless and/or substrate culture. Major factors that influence the performances of strawberries in soilless systems may be listed as: type and quality of the growing medium, source and type of planting material, cultivar, growing season, and efficiency of fertilisation [4,5,7,8,9]. It has been well documented that substrates greatly influence strawberry growth, development, and yield [4,5,9,10,11,12]. Reports on these areas demonstrated that substrates have a great influence on strawberry performances, including plant height, crown diameter, leaf area, root length, plant dry weights, marketable and total yields [5,8,13,14].
Strawberries are highly sensitive to an excessive or deficient amount of macro- and micronutrients, high salinity (EC), and pH [15,16]. The nutrient status of the substrate plays a critical role in plant growth and development [17,18]. The macro- and micronutrients such as nitrogen (N), phosphorus (P), potassium (K), iron (Fe), and manganese (Mn) are necessary for optimum growth and productivity of strawberries [19]. The most important macronutrients which support the optimal production of strawberries are N, P, and K [20].
Strawberry plants require a high acquisition of N and P nutrition for vegetative growth [21,22], where N is the most important nutrient for the strawberry plant growth, runner production, and bud formation [23]. The limited supply of N may have a deleterious effect on the vegetative growth and development of strawberry plants [19]. In strawberries, potassium is essential for the translocation of nutrients [19] and supporting root growth, establishment, and development [24].
In soilless strawberry production, growing media and nutrition are the most important factors which determine the overall development and performance of the crop [25]. Several factors influence the availability and uptake of nutrients, including substrate pH, EC, nutrient concentration and composition, as well as the type of fertilisers used [26]. The pH of growing media influences nutrient interaction, availability, uptake, and plants’ response to nutrients [22,27]. As pH decreases the nutrient elements like Al, Fe and Mn uptake increases and may lead to toxicity [26]. The EC of substrate with consistent N, P, and S immobilisation as well as retention of other essential elements (e.g., K and Mg) in growing media is a critical factor for strawberry plant establishment [28]. Hence, to avoid nutrient deficiency or toxicity, the pH and EC of growing media should remain in the optimum range [26,28].
Strawberry plants with unsatisfactory nutrient supply cannot develop genetically determined growth and characteristics [29]. In line with this, the need for optimising the soilless cultivation input and maximising the production, development of alternatives to peat by organic materials which can partially replace chemical nutrition and serve as an organic nutrition source is essential [30]. Further, the increasing demand for low cost and high performing soilless substrates has led to the search for alternative materials to peat as constituents of strawberry growing media [28]. As reported by Tagliavini et al. [21], the dynamics of nutrient uptake in strawberries vary in soil and soilless cultivation, and it is highly recommended to plan fertilisation, based on the knowledge of amounts of nutrients taken up by the crop, in particular, growing media. The adequate management of nutrient elements is crucial to guarantee strawberry growth, development, and fruit production [19]. In line with the abovementioned, the influence of fertilisers and nutrient solutions in strawberry cultivation has been well documented [14,31,32]. However, the chemical properties and nutrient availability in non-traditional peat alternatives, and their influence on strawberry performance, are still in their infancy [28]. Further, to date, the fertiliser absorption (uptake, storage, and partitioning) properties of strawberries in soilless cultivation remain unclear [33].
Peat (sphagnum peat) is the most popular and commercially used substrate in soilless strawberry cultivation. Its favourable physical and chemical properties, suitability for many species, and cultivation systems constitute peat as the most widely used substrate [34,35]. However, it has been reported that due to over-exploitation, the global peat resources are at the edge of depletion and their extensive utilisation has caused serious environmental issues [36,37]. Hence, considering its non-renewability, high cost, future availability, and environmental sustainability, growers around the globe need a high-quality, renewable, and sustainable substitute that can substantially replace peat either in whole or in combination [37,38,39].
In view of growing environmental concerns, the need for proper disposal and handling of accumulating agro-wastes is essential. Few reports demonstrated the possible utilisation of agro and/or agro-industrial wastes as a peat substitute in strawberry cultivation [3,4,28]. Among many agro-wastes, the possible utilisation of spent mushroom substrate (SMS) as a potential peat substitute can be beneficial [40]. This agro-waste has a high organic composition, is readily available, and the cost is negligible [41,42,43]. SMS is the residual material left over after commercial mushroom production [40]. The material obtained immediately after mushroom production is called fresh SMS or SMS and the same material after further decomposition or weathering is called weathered SMS (W-SMS) or spent mushroom compost (SMC) [44].
In the last decade, a significant increase in demand and production of mushrooms has been noticed, while white button mushroom—Agaricus bisporus (J.E. Lange), shiitake—Lentinus edodes (Berk.), and oyster mushroom—Pleurotus ostreatus (Jacq.)—are the most popular and commercially cultivated mushroom species around the world [45,46]. In 2019, the global mushroom production has passed 11 million tons [47]. Approximately, five kilograms of SMS is left off for each kilogram of fresh mushrooms produced and/or harvested [41,48]. Considering the abovementioned production values, the annual SMS generated from the global mushroom industry can be estimated to be nearly 60–65 million tons. Hence, the effective utilisation, handling, and disposal of such a large amount of material generated annually is a great challenge.
The SMS is often regarded as agro-waste or farm waste [49]. SMSs generated from mushroom enterprises in large quantities are often composted, burnt, discarded, incorporated into the soil, or simply thrown away, which is neither economical nor environmentally safe [50,51]. Moreover, due to limited re-use, the accumulation of this potential waste overtime in large quantities can cause a negative impact in terms of environmental concerns such as soil, water, and air pollution [44,52]. On this account, mushroom production can be considered a non-sustainable agriculture activity [41].
The research reports on effective utilisation of SMC for agricultural and horticultural purposes [53], as a soil conditioner [54], as a nursery, and as a soilless growing medium [55,56] are evident. On the contrary, the immediate use of fresh SMS is restricted [44,57]. High EC and unfavourable pH are the major limiting factors that restrict its immediate use [44], which can negatively influence the overall plant development [58,59]. As of today, the SMS is recommended only after a further decomposition/weathering/stabilisation process ranging from 3 to 24 months [44,60]. The stabilisation of SMS is carried out through weathering [44], where the composting process involves the succession of microorganisms load, stabilised C/N ratio, pH, and EC level [61,62]. However, the process of weathering releases leachates which can significantly alter underlying soil and water chemistry [44]. Further, the weathering process can be considered laborious, time-consuming, and non-economical, resulting in improper disposal which may lead to various environmental hazards.
Poland is one of the leading producers of mushrooms in the world with a production of 362,400 tons and is the biggest producer of white button mushrooms in the European Union [63]. At the same time, Poland alone generates around 1.8 million tonnes of SMSs annually, which ends as a waste. Further, to date, the Polish legislation categorises this potential waste as “other unspecified waste” [64], and inadequately addresses the issues of its management and does not properly specify SMS storage conditions in the field, which is neither economical nor environmentally safe [44].
Despite the great potentiality of SMS to partially replace peat in the soilless culture [40,65,66,67], the research knowledge in this regard is still in its infancy. To fulfil this research gap, the present investigation was carried out. Further, considering environmental concerns associated with extensive peat usage in horticulture, studying the nutrient dynamics, uptake, and partitioning as influenced by a non-traditional peat alternative like SMSs will be of great significance.
The present investigation was designed based on the preliminary research findings, as reported by Prasad et al. [40], where the SMSs demonstrated potentiality to be used as a peat substitute in lower concentrations of 15 and 25%, in which the morphological, physiological, and yield performances of strawberry cv. ‘Honeoye’ in spring planting was reported. The previous report was more focused on morphological and physiological measurements, and the results demonstrated that supplemented SMSs had no negative effect on plant performances and physiological processes. However, the nutrient dynamics and distribution as influenced by different planting seasons were not reported. Taking leads from previous study, in the present investigation, the performance of autumn-planted and spring-planted cv. ‘Elsanta’ grown in peat substrate supplemented with fresh SMS of A. bisporus (A-SMS), L. edodes (L-SMS), and P. ostreatus (P-SMS) in 15% and 25% were evaluated in an unheated plastic tunnel. The study aimed to deliver greater insight into morphological yield, dry matter distribution, and nutrition partitioning as influenced by SMSs and planting season, to support utilising SMS as a renewable, easily available, comparatively cheap, and sustainable alternative to a non-renewable, relatively expensive, and non-sustainable peat in soilless strawberry production. The investigation was carried out with three principal objectives: (1) to evaluate the suitability of fresh SMS as a peat substitute for strawberry cv. ‘Elsanta’, (2) to study the strawberry morphological and yield parameters as influenced by SMS-based substrates and planting seasons, and (3) to determine strawberry dry matter distribution, uptake, and partitioning of nutrients.
2. Materials and Methods
2.1. Growing Conditions, Cultivar and Planting Season
The experiments were carried out during 2020–2021 (November 2020 to June 2021) in an unheated plastic tunnel 30 × 7 m (length × width) at the Experimental Station Marcelin (52°24′20″ N and 16°51′35″ E) belonging to the Faculty of Agronomy, Horticulture, and Bioengineering, Poznan University of Life Sciences, Poland. The mean temperature and relative humidity (RH) inside the tunnel during the growing period (April 2021–June 2021) were 21 °C and 55.5%, respectively.
Following the previous research findings, reported by Prasad et al. [40], where the performance of strawberry cv. ‘Honeoye’, a June bearing dessert cultivar bred in New York and released in 1979 [68], was studied in spring planting, in the present investigation, the performance of another popular (in Europe) dessert cv. ‘Elsanta’ was investigated in autumn planting and spring planting. ‘Elsanta’ is a June-bearing cultivar developed at Plant Research International B.V., the Netherlands, and released in 1975 [69].
The strawberry morphological parameters, dry matter distribution, yield performances, uptake and partitioning of nutrients in cv. ‘Elsanta’ as influenced by seven substrates and two planting seasons (autumn planting and spring planting) were investigated. The fresh tray plants (A+ grade) were used as planting material for both autumn and spring-planting experiments. Strawberry plants were planted on 10 November 2020 for the autumn-planting study and on 1 April 2021 for the spring-planting study. Fruits were harvested from both periods of planting, from the last week of May 2021 till the end of June 2021. The experimental details are given in Table 1.

Table 1.
The experimental details.
2.2. Substrate Characteristics and Substrate Preparation
2.2.1. Spent Mushroom Substrates (SMS)
Three fresh SMSs after commercial production of A. bisporus (A-SMS), L. edodes (L-SMS), and P. ostreatus (P-SMS) were evaluated in the present investigation as peat substitutes in varying supplementation rates. The SMSs were obtained from local mushroom producers within the vicinity of the experiment station. A-SMS was obtained from Hajduk Grupa Producentow Pieczarek Sp. z o. o. (Hajduk Group of Mushroom Producers), L-SMS from Uprawa Grzybow shiitake Alicja Hamrol (Shiitake mushrooms Alicja Hamrol), and P-SMS from TYRMYCEL Wytwornia grzybow uprawowych i kostki boczniaka (TYRMYCEL unit of cultivated mushrooms and oyster mushrooms). The details about the substrate composition for mushroom cultivation and mushroom production cycle are described by Prasad et al. [40].
2.2.2. Peat
The superior quality professional peat (peat clear-class H2 according to Van Post classes) with enhanced hydrophilic capacity obtained from Hartmann Polska Sp. z o. o. (Poznan, Poland) was used as a control substrate in the experiment.
2.3. Substrate Combinations
In this study, A-SMS, P-SMS, and L-SMS were used as a substitute for peat in 15% and 25%. Seven different substrate mixes were evaluated, which included: 100% peat as a control substrate—Peat, A-SMS, P-SMS, and L-SMS substituted to peat in 15% and 25% to obtain two substrate mixes from each SMS, resulting in seven substrate combinations (Table 2). The substrate combinations were prepared based on volume (v/v).

Table 2.
The substrates evaluated in the study as growing media.
2.4. Substrate Filling, Planting, and Experimental Setup
The growing containers 90 × 13.5 × 12 cm (L × B × D) were filled with 12 L of prepared substrates as previously described in Table 2. Four strawberry plants were planted in each growing container (3 L of substrates per plant), maintaining a spacing of 20 cm between each plant. The experiment was laid out in a randomised complete block design (RCBD), in a two-factor design (with seven substrates and two planting seasons) in five replications. In the study, 40 plants (10 growing containers) were maintained in individual studied substrates and planting seasons. The growing containers were organised into 5 rows (each row as a replicate) with a spacing of 90 cm between rows and 25 cm between each growing container.
Automatic micro drip irrigation (three bent arrow emitters with a flow rate of 2 l∙h−1) was provided to individual growing containers. At the beginning of the experiment, during the vegetative phase (April), the growing containers were irrigated two times a day for 120 s (60 s in each interval) and later, during the flowering and fruiting phase (from the first week of May till the end of June), plants were irrigated three times a day for 360 s (120 s in each interval). The irrigation duration and interval were monitored by Galcon GAE2S0002U1 8006 AC station zone irrigation controller system. The average pH and EC of irrigation water during the study was 7.10 and 0.72 mS∙cm−1.
Plants were fertilised with a water-soluble nutrient solution based on Kristalon Blue (N:P:K 19:06:20 + microelements) and Calcinit (15.5% N + 26.3% CaO) from YARA (Yara Poland Sp. z o. o., Szczecin, Poland). The nutrient solution prepared from a 10% stock solution of each fertiliser was further diluted to obtain a working concentration of 0.25%. Two doses of each nutrient solution were applied alternatively to each growing container during the crop cycle at the 15th, 30th, 45th, and 60th days after planting (Table 3). The pH and EC of 10% Kristalon Blue and Calcinit ranged between 5.9–6.2 and 1.3–1.5 mS∙cm−1 and 6.0–6.2 and 1.0–1.2 mS∙cm−1, respectively.

Table 3.
A standard stock solution of nutrients at a 0.25% (v/v) dilution rate used in the study.
2.5. Evaluated Morphological Parameters
At the end of the cultivation (end of June–first week of July 2021), strawberry plants were separated from the individual growing media and prepared for measurements, i.e., carefully washed in running water till no trace of substrates was attached to the roots. Then, plant morphological parameters including shoot length, root length, number of leaves, number of crowns, as well as crown diameter were recorded. In individual substrate and planting season, five fully developed (2nd leaf from the node) strawberry leaves were scanned using the WINDIAZ leaf measurement system and the leaf area was calculated using WINDIAZ software (Delta-T Devices Ltd., Cambridge, UK), the obtained leaf area was expressed in cm2. The dry matter distribution of plant materials, i.e., leaves, crown, and root dry weights (after drying at 105 °C for 24 h) were recorded, and the total plant dry weights were calculated by adding the individual dry weights of plant materials.
2.6. Yield Parameters
The strawberry fruits were harvested from the last week of May to the end of June in both autumn planting and spring planting. The fruits from each plant in the individual substrate and planting season were harvested, and cumulative yield (g∙plant−1) per harvest was recorded. At the end of the growing season, the cumulative yields (g∙plant−1) in each substrate among autumn and spring planting were added to obtain the cumulative total yield (g∙plant−1). The total, marketable and unmarkatable yields from each substrate in individual planting seasons were determined on fruits harvested through May–June. The diseased, damaged, misshaped fruits and fruits with less than 18 mm of diameter were categorised as unmarketable fruits. Harvested fruits free from any infections and fruits with a diameter between 18–25 mm, and >25 mm were considered as marketable fruits [70].
2.7. Substrate Analysis
The analysis of pH, EC, macro- and micronutrients of all studied substrates both before and after strawberry cultivation was carried out. The individual representative substrate samples were taken before the cultivation (during substrate preparation), and at the end of the growing season (from growing containers).
The substrates were analysed at the Department of Plant Physiology, Laboratory of Nutrition, the Faculty of Agronomy, Horticulture and Bioengineering, Poznan University of Life Sciences. The substrate pH, EC, micro- and macronutrients were analysed as described by Schroeter-Zakrzewska et al. [71]. The collected substrate samples were chemically analysed by the universal method. Extraction of macronutrients (N-NH4, N-NO3, P, K, Ca, Mg, S-SO4), Cl and Na were carried out in 0.03 M acetic acid with a quantitative 1:10 proportion of substrate to extraction solution. After extraction, the following determinations were made: N-NH4, N-NO3-by micro distillation according to Bremer in Starck’s modification, P by colourimetrically with ammonium vanadomolybdate, K, Ca, and Na was determined photometrically, Mg by atomic absorption spectrometry, S-SO4-nephelometrically with BaCl2 and Cl-nephelometrically with AgNO3. Micronutrients (Fe, Mn, Zn and Cu) were extracted from the substrate with Lindsay’s Solution containing 1 dm3: 5 g EDTA (ethylenediaminetetraacetic acid), 9 cm3 of 25% NH4 solution, 4 g citric acid, 2 g Ca (CH3COO)2·2H2O. Micronutrients were determined by the ASA method. Salinity was determined conductometrically as an electrolytic conductivity (EC in mS·cm−1) (substrate:water = 1:2), and pH was determined by the potentiometric method (substrate:water = 1:2).
2.8. Plant Analysis
The dynamics of nutrient uptake and partitioning of nutrients were determined by destructive plant harvest. Plant material (leaves, crowns, and roots) were collected at the end of the experiment—the first week of July 2021. The plant material was dried at 105 °C for 24 h, then ground and stored in clean airtight containers with labels for further chemical analysis to determine the plant macro- and micronutrients.
The plant nutrient analysis was performed according to the methodology described by Schroeter-Zakrzewska et al. [71]. The assays of total N, P, K, Ca, Mg, and Na in the plant material was mineralised in concentrated sulphuric acid [72]. After mineralisation of the plant samples, chemical analyses were performed using the following methods: N-total according to Kjeldahl in a Parnas-Wagner distillation apparatus, P—by colourimetry with ammonium molybdate, and K, Ca, Mg, Na by atomic absorption spectrometry (in a Carl Zeiss Jena apparatus). For the determination of total Fe, Mg, Zn, and Cu, the plant material was mineralised in a mixture of dioxonitric and tetraoxochloric acids (3:1 v/v) [72]. After mineralisation, the Fe, Mn, Zn, and Cu contents were determined according to ASA [71]. The accuracy of the methods used for the chemical analyses and the precision of analytical measurements of nutrient levels were verified with the LGC7162 reference material, with an average nutrient recovery of 96% (N, P, K, Ca, Mg, Fe, Mn, Zn).
2.9. Statistical Analysis
The experiment was laid out in a RCBD, in a two-factor design. The evaluated and presented morphological and yield parameters are means ± SD of five replicates (n = 5), while the presented chemical nutrient analysis results (substrates and plant samples) are means ± SD of three replicates (n = 3). All recorded data were evaluated by analysis of variance (ANOVA), and the mean differences were compared by post hoc test at a p level of <0.05, according to Tukey’s HSD. Statistical analyses were performed using STATISTICA (TIBCO software Inc. Palo Alto, CA, USA). To determine the interactions among nutrients, Pearson’s correlation analysis was performed for total plant nutrients (leaves + crowns + roots) in autumn and spring-planted strawberries.
3. Results
3.1. Selected Chemical Parameters of Substrates (SMSs and Peat)
3.1.1. The pH and EC Values of 100% Peat and 100% Fresh SMSs
The pH and EC values differed among 100% peat and 100% fresh SMSs used in the study (Table 4). The pH (in H2O) of fresh SMSs ranged from 4.10 to 8.08, whereas the EC ranged from 1.15 to 8.92 mS·cm−1. The highest pH values were determined in the case of 100% A-SMS (8.08) when compared to commercial peat (6.00). Similarly, the highest EC values were also determined in 100% A-SMS (8.92 mS·cm−1) in comparison with peat (1.15 mS·cm−1). Overall, the results of chemical analysis concerning pH and EC revealed that both parameters of 100% fresh SMSs were at the levels that limit their immediate use as homogeneous media in strawberry cultivation.

Table 4.
The pH and EC (mS·cm−1) values of 100% peat and 100% fresh SMSs used in the study.
3.1.2. The pH and EC Values of Different Substrate Combinations
The pH values varied among the prepared substrate combinations both before and after the cultivation (Figure 1A,B). In autumn and spring planting, the initial value of pH in substrate combinations (A-15-P-25) ranged from 6.06–6.37 and 6.07–6.38, respectively, when compared to peat values of 6.00. The chemical analysis concerning initial pH revealed that after mixing 100% SMSs with peat in various combinations, the pH value was almost neutralised and was nearly comparable to the peat values. It was found that there was a general tendency of increasing pH of all substrates after cultivation in relation to before (except for P-15) in autumn planting, while the pH was noticed to be in decreasing trends in all substrates (except for Peat and L-25) at the end of strawberry cultivation in relation to the initial substrate pH in the spring planting.
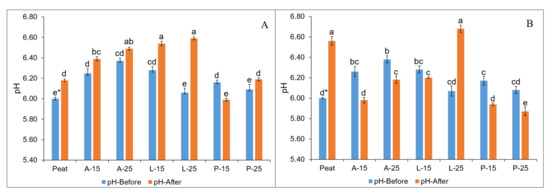
Figure 1.
Substrate pH before and after strawberry cultivation in autumn (A) and spring (B) planting as influenced by substrates, * means followed by the same letters are not significantly different at p < 0.05 according to Tukey’s HSD (n = 3); the scale on the y-axis ranges from 5.4–6.80.
Similarly, the EC values of substrate combinations varied among autumn and spring planting as well as before and after the strawberry cultivation (Figure 2A,B). In both autumn and spring planting, the initial EC value of SMS-based substrates ranged from 0.66–2.99 mS·cm−1 when compared to peat values of 1.17 mS·cm−1 and 1.14 mS·cm−1, respectively. The higher substrate EC values were noticed for A-15 and A-25 at the beginning of cultivation in both autumn and spring planting.
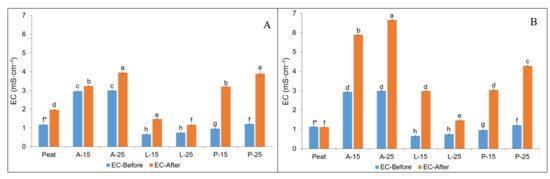
Figure 2.
Substrate EC before and after strawberry cultivation in autumn (A) and spring (B) planting as influenced by substrates; * means followed by the same letters are not significantly different at p < 0.05 according to Tukey’s HSD (n = 3).
In general, the EC values were noticed to be increasing in all studied substrates in both autumn and spring planting. Further, the results of chemical analysis revealed that after mixing 100% SMSs with peat in various combinations, the higher EC values were almost neutralised and nearly comparable to the commercial peat values, except for A-15 and A-25 (Figure 2A,B). Even though the higher EC values of 100% A-SMS (8.08 mS·cm−1) were lowered to 2.96 mS·cm−1 and 2.99 mS·cm−1 in autumn planting and in spring planting to 2.94 mS·cm−1 and 2.99 mS·cm−1 in A-15 and A-25, respectively, these EC values were still relatively higher in comparison with peat values. In this account, among the studied substrates, the higher EC values both before and after the strawberry cultivation were noticed in A-15 and A-25 substrates. The substrate EC of A-15 and A-25 was nearly three times higher in comparison with peat in autumn planting and increased in a similar trend with peat EC at the end of cultivation, while the substrate EC of A-15 and A-25 after the cultivation increased nearly two folds to the initial values and were nearly five times higher when compared to the peat EC values.
3.2. Substrate Chemical Properties before and after Strawberry Cultivation
3.2.1. The Macro- and Micronutrient Concentrations at the Beginning of the Cultivation
The macro- and micronutrient concentrations among the studied substrates varied significantly at the beginning of cultivation (Table 5a,b). Among macro-nutrients, the highest amount of N-NO3 and P (207.0 mg·dm3 and 231.01 mg·dm3) was noticed in the substrate A-15 followed by A-25 (196.0 mg·dm3 and 223.0 mg·dm3). Similarly, the highest amount of K, Ca, Mg and S-SO4 were determined in A-25 followed by A-15, which were significantly superior to other substrates concentrations studied in the experiment. Among the micronutrients, the highest amount of Na was reported in A-25 followed by A-25, and similarly, the Zn was also highest in A-25 followed by A-15. The superior concentration of Cl was in A-15 followed by A-25. Differently, the highest amount of Cu was noticed in the Peat substrate. The Fe was found to be the highest in Peat and P-25 substrate, while the highest amount of Mn was determined in L-25 followed by L-15 when compared to other substrates.

Table 5.
(a) Macronutrient concentrations in different substrates before strawberry cultivation (mg·dm3). (b) Micronutrient concentrations in different substrates before strawberry cultivation (mg·dm3).
Generally, all macronutrients, as well as Na, Cl, and Zn concentrations, were observed to be highest in A-15 and A-25 substrates which are based on A-SMS, where A. bisporus SMS was substituted to peat in 15 and 25% (v/v), respectively.
3.2.2. The Macro- and Micronutrient Concentrations at the End of the Cultivation
The substrate chemical analysis at the end of the strawberry cultivation revealed that the macro- and micronutrient concentrations among the studied substrates varied significantly in autumn and spring planting (Table 6a,b). The highest residual amount of N-NH4 was observed in P-25. The N-NO3 concentration ranged between (56.8 mg·dm3–952.6 mg·dm3). Where the highest concentration at the end of cultivation was in A-15 and the lowest in Peat, both the highest and lowest amount of N-NO3 was determined in substrates from spring planting. The highest concentration of other macro-elements P, K, Ca, Mg, and S-SO4 as well as the micro-nutrient Na were determined in A-25 in spring planting. The highest substrate Cl was in A-25 (autumn planting) and A-15 (spring planting). The highest Cu was in Peat and A-15 and the Fe was highest in A-15. These highest concentrations of Cu and Fe were reported in substrates from autumn planting. The substrate Mn was noticed to be the highest in L-15 in spring planting while the substrate Zn was found to be the highest in both autumn and spring planting in L-15. In general, at the end of cultivation, the residual concentrations of macro- and micronutrients except Cu and Fe were determined in the substrates from spring planting when compared to the substrates from autumn planting.

Table 6.
(a) Macronutrient concentrations in different substrates at the end of strawberry cultivation (mg·dm3). (b) Micronutrient concentrations in different substrates at the end of strawberry cultivation (mg·dm3).
3.3. Influence of Substrates and Planting Season on Strawberry Morphological Parameters
3.3.1. Shoot Length and Root Length
In the present study, the growth parameters of strawberry plants were significantly influenced by all evaluated substrates and also differed among two planting seasons, i.e., autumn and spring planting (Table 7). Autumn-planted strawberries cultivated in A-15 and Peat achieved the highest shoot length, while the lowest shoot length was found in spring-planted plants grown in commercial peat. The mean shoot length was not significantly different among the studied substrates (28.42–30.33 cm), whereas the autumn-planted strawberry plants resulted in a superior shoot length (29.98 cm) in comparison to spring planting (28.00 cm).

Table 7.
Strawberry morphological parameters as influenced by substrates and planting seasons.
The highest root length was recorded in autumn-planted plants cultivated in A-15, whereas the lowest root length was observed in spring-planted strawberries grown in Peat. The mean root length differed significantly among the studied substrates and planting seasons. For substrates, the root lengths in A-25 (27.50 cm) and L-15 (27.17 cm) were observed to be superior, while the autumn-planted plants resulted in a superior mean root length in comparison to spring planting.
3.3.2. Leaf Number and Leaf Area
The studied factors influenced the number of leaves and leaf area (Table 8). The highest number of leaves was observed in autumn-planted strawberries grown in P-15 and P-25, while the lowest value was observed in spring-planted plants grown in P-15. The autumn-planted plants were found to be superior concerning the number of leaves (10.90) over spring-planted (8.48), while among substrates, the mean value for the number of leaves was found to be higher in L-25 and P-25.

Table 8.
The number of leaves and leaf area (cm2) as influenced by substrates and planting seasons.
Among the substrate combinations and planting season, the highest leaf area was recorded in P-15 and P-25. The lowest values, concerning leaf area, were found to be similar among both autumn-planted (195.22 cm2) and spring-planted (194.29 cm2) strawberries grown in L-25. Further, irrespective of the planting season, the reported SD values for leaf area in L-25 were smaller in comparison to other variables, indicating less variability within replicates of L-25. The mean values for leaf area among substrates were significantly superior in P-25 (270.29 cm2) and P-15 (270.05 cm2), whereas among planting seasons, no significant differences for leaf area were observed.
3.3.3. Number of Crowns and Crown Diameter
The crown numbers and crown diameter varied among the studied substrates and planting seasons (Table 9). Plants grown in A-25 and P-25 had the highest number of crowns in autumn planting while the lowest values were recorded in Peat, A-15, A-25, L-15, and L-25 in spring planting. The autumn-planted strawberries achieved a superior mean number of crowns (2.43) over spring-planted strawberries (1.43), whereas the mean values for all studied substrates were not statically different.

Table 9.
Number of crowns and crown diameter (mm) as influenced by substrates and planting seasons.
The highest crown diameter was noticed in autumn-planted strawberries cultivated in P-25 (41.42 mm) with the lowest (22.26 mm) in spring-planted strawberries grown in peat. Among the studied substrates, the mean values for crown diameter were superior in P-25 (35.31 mm), whereas among planting seasons, autumn planting resulted in the highest mean crown diameter (31.48 mm) when compared to spring planting (26.08 mm).
Overall, in the present investigation, the studied strawberry morphological performances of the cv. ‘Elsanta’ in autumn planting was found to be superior to spring planting. Among the seven substrate combinations evaluated, all the substrate mixes based on SMSs (A-15-P-25) performed significantly superior and/or equally to commercial peat.
3.4. Influence of Substrates and Planting Seasons on the Strawberry Dry Matter Distribution
The interaction among substrates and planting seasons revealed that the leaf, crown, and root dry weights were significantly different (Table 10). The autumn-planted strawberries in P-25 achieved the highest leaf dry weight (25.42 g) followed by P-15 (24.84 g), while the lowest leaf dry weight was observed in spring-planted strawberries in peat (12.45 g), P-15 (13.70 g), and L-25 (14.11 g). The mean leaf dry weight was found to be not significantly different among evaluated substrates and ranged between 16.58–20.75 g. The autumn-planted strawberries achieved superior mean leaf dry weight (21.67 g) when compared to spring planting (15.58 g).

Table 10.
Dry matter distribution (g) in strawberry plant parts as influenced by substrates and planting season.
The strawberries planted in autumn achieved the highest crown dry matter in P-25 (2.84 g). Among the studied substrates, the P-25 achieved the highest crown dry weight (2.28 g). The autumn-planted strawberries achieved superior crown dry mass (2.03 g) over spring-planted strawberries (1.50 g).
The highest root dry weight was observed in the autumn-planted plants cultivated in P-25 (13.26 g), while the lowest root dry mass (5.55 g) was noticed in spring-planted strawberry plants grown in peat. The P-15 (15.85 g) had the highest mean root dry weight followed by P-25 (12.41). The performance of autumn-planted strawberries concerning root dry matter was statistically similar to spring-planted strawberries.
The strawberry total plant dry matter was significantly influenced by both substrates and planting seasons (Figure 3). This revealed that the autumn-planted plants cultivated in P-15 had the highest total plant dry weight (46.00 g) followed by P-25 (41.52 g) and L-25 (39.98 g). The lowest total plant dry matter 19.4 g and 20.82 g was recorded in the spring-planted plants in Peat and L-25.
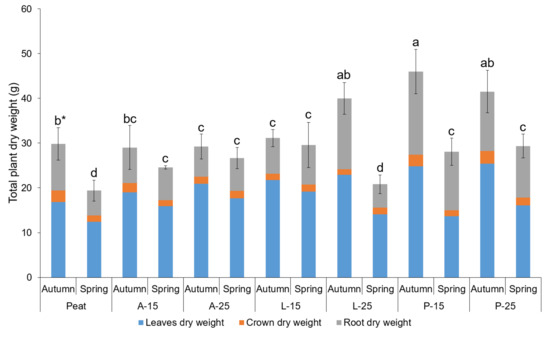
Figure 3.
Total plant dry weight of strawberry plants as influenced by substrates and planting seasons. * means followed by the same letters are not significantly different at p < 0.05 according to Tukey’s HSD (n = 5).
3.5. Influence of Substrates and Planting Season on Strawberry Yield Parameters
Cumulative Marketable, Unmarketable, and Total Yield
The yield parameters as determined by cumulative marketable, unmarketable, and total yields differed significantly among the studied substrates and planting season (Table 11 and Figure 4). The autumn-planted strawberries in P-25 and P-15 had the highest marketable yield (554.58 and 537.08 g∙plant−1). The lowest marketable yields were recorded in strawberry plants cultivated in A-25 and A-15 substrate in spring planting (96.10 and 117.86 g∙plant−1). The highest amounts of unmarketable fruits were obtained from plants in A-15 and A-25 substrates in spring planting, where more than 50% of the fruits produced were unmarketable.

Table 11.
Cumulative marketable and unmarketable yields (g∙plant−1) as influenced by substrates and planting season.
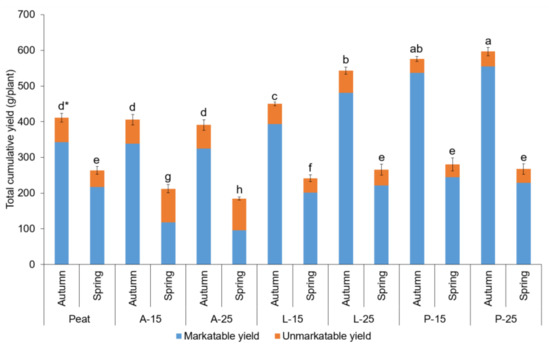
Figure 4.
Total cumulative yield (g∙plant−1) as influenced by substrates and planting seasons. * means followed by the same letter are not significantly different at p < 0.05 according to Tukey’s HSD (n = 5).
The results indicated that, in individual studied substrates, the marketable yields were superior in autumn planting. Similar to the marketable yields, among the studied substrates and planting seasons, the superior cumulative total yield was reported in strawberry plants grown in substrate P-25 followed by P-15. The lowest total yields were observed in spring-planted plants in A-15 and A-25. In general, the marketable yields and total yields among studied substrates in autumn-planted plants were superior and were nearly 2–3 folds higher in individual substrates when compared to spring-planted plants.
3.6. Influence of Substrates and Planting Season on Strawberry Nutrient Uptake and Partitioning
3.6.1. Macro- and Micronutrient Concentrations in Leaves
The leaf macro- and micronutrient concentrations were significantly influenced by the substrates and seasons of planting (Table 12a,b).

Table 12.
(a) Macronutrient concentrations in strawberry leaves (%DW). (b) Sodium (%DW) and micronutrient (mg∙kg−1 DW) concentrations in strawberry leaves.
The tendencies of macronutrient content were multidirectional, i.e., plants grown in A-25, in both autumn and spring planting, had the highest concentration of N in leaves (1.67% and 1.71% respectively), while the lowest content of N (1.13%) was determined in autumn-planted strawberries grown in peat. The highest amount of P was determined in spring-planted strawberries grown in A-15 (0.37%) while the lowest amount of P (0.17%) was in both autumn and spring-planted strawberries grown in peat. The content of K was found to be the highest in leaves of autumn planting strawberries grown in A-25 (1.75%). Similar to P, the lowest amount of K (0.74%) was recorded in peat (both autumn and spring planting). The concentration of Ca in strawberry leaves had a different trend than that of N, P, and K. The highest amount of Ca (1.72%) was noticed in peat in spring planting and the lowest (0.96%) in autumn-planted strawberries in peat. The highest and lowest concentration of Mg (0.57% and 0.25%, respectively) in strawberry leaves was in spring-planted strawberries grown in peat and P-25, respectively.
In the case of microelements generally, the highest content of Fe, Zn, and Cu (for spring planting) was determined for plants grown in A-15; meanwhile, Mn for L-25. The Na content in leaves ranged between 0.02–0.03 (%DW) and was not statistically different.
3.6.2. Macro- and Micronutrient Concentrations in Crowns
The macro- and micronutrient concentrations in strawberry crowns were significantly influenced by both studied substrates and planting seasons (Table 13a,b). The highest N in the strawberry crown was noticed in A-15 and A-25 in spring planting while the lowest was recorded in peat in both autumn and spring planting. Similarly, the highest amount of P in the crown was recorded in A-15 in spring planting and the lowest was in both autumn and spring-planted plants grown in peat. The highest amount of K was noticed in L-15, L-25, P-15, and P-25 in spring planting and the lowest was in peat in autumn planting. Ca concentration in strawberry crowns was the highest in autumn-planted plants in P-15 and the lowest in A-15 and A-25 in autumn planting. The Mg was highest in peat in autumn planting and the lowest in P-25 in spring planting. The A-25 in autumn planting had the highest amount of Na. In the present study, the strawberry crowns in spring planting had both the highest and lowest amount of Fe, Mn, Zn, and Cu (Table 12b). The highest amount of Fe was in A-25 and the lowest was in L-25, while Mn was noticed to be the highest in L-25 and the lowest in P-25. The content of Zn was the highest in A-15 and A-25. Cu was the highest in A-15; however, the lowest Cu was in L-25. A high range of Cu (22.33–499.55 mg∙kg−1 DW) accumulation in strawberry crowns among the studied substrates and planting seasons was noticed, where the highest amount of Cu was accumulated in A-15 (in spring planting) followed by P-15 (in autumn planting).

Table 13.
(a) Macronutrient concentrations in the strawberry crowns (%DW). (b) Sodium (%DW) and micronutrient (mg∙kg−1 DW) concentrations in strawberry crowns.
3.6.3. Macro- and Micronutrient Concentrations in Roots
The substrates and planting seasons had a significant influence on the chemical composition of roots (Table 14a,b). The determined tendencies were generally multidirectional. The highest amount of N in roots was observed in A-25 (spring planting). The concentration of P and K in strawberry roots followed a similar pattern, where the highest amount of P and K was noticed in A-25 in spring planting, while the lowest was in autumn planting in peat. Both the highest and lowest amount of Ca were observed in spring planting strawberries in peat and P-25. The highest concentration of Mg was in A-25 in spring planting, while the lowest was in autumn planting in P-25 and peat. The Na was observed to be in the range of (0.06–0.20%) and the highest amount of Na was in peat in spring planting.

Table 14.
(a) Macronutrient concentrations in strawberry roots (%DW). (b) Sodium (%DW) and micronutrient (mg∙kg−1 DW) concentrations in strawberry roots.
Among micronutrient concentration in strawberry roots, both the highest and the lowest amount of Fe were recorded in peat and L-25 in spring planting. The Mn content was the highest in A-25 in spring planting and L-25 in autumn planting, while the lowest was in peat in autumn planting. The Zn concentration was reported to be the highest in autumn planting in P-25 while L-15 in spring planting had the lowest Zn. The amount of Cu was found to be the highest in spring planting in peat and the lowest was in autumn planting in P-25 in spring planting.
Overall, in the present study, the range of N (1.13–1.71%DW) and P (0.17–0.37%DW) was found to be higher in strawberry leaves followed by roots and crowns, while the range of K uptake was nearly similar in leaves (0.74–1.75%DW) and crowns (0.73–1.78%DW). The Ca uptake range was higher in roots (1.25–2.54%DW) while the range of Mg in all plant organs was nearly similar and/or equally partitioned (0.25–0.58 mg∙kg−1 DW). The Na (0.07–0.22%DW) and Zn (109.35–285.50 mg∙kg−1 DW) uptake tended to be higher in strawberry crowns than that of leaves and roots, while higher uptake of Fe range was evident in roots (262.72–567.71 mg∙kg−1 DW) than that of in crowns and leaves and a higher accumulation of Cu range (22.33–499.55 mg∙kg−1 DW) was evident in crowns than in leaves and roots.
3.7. Correlation Analysis
Pearson’s correlation analysis was performed (* p < 0.05 and ** p < 0.01). This analysis reflects the degree of interaction among the nutrient elements and their total accumulation in the strawberry plant (leaves + crowns + roots) which further facilitates a better understanding of interactions between the plant nutrients influenced by planting seasons. To give a deeper insight into these correlations, the analysis was performed for autumn planting and spring planting separately.
The correlation matrix for plant nutrient concentrations varied significantly among autumn and spring planting (Figure 5A,B). According to the correlation coefficient values, statistically significant positive correlations were observed among the plant N-P, N-K, P-K, Mg-Na, Na-Zn, and Mn-Cu in autumn-planted strawberries (Figure 5A). For instance, this significant positive correlation infers that the higher the plant N concentration is, the greater will be the concentrations of P and K. Similarly, the higher/lower the nutrient concentration is, the greater/lesser will be the concentrations of the positively/negatively associated elements. On the other hand, significant negative correlations were noticed for Ca-Mg, Ca-Na, and Ca-Zn, which indicates antagonistic relations among these nutrient elements in autumn-planted plants. In spring planting, the correlation trend varied from that of autumn planting (Figure 5B). Highly significant positive correlations were noticed among N-P and positive correlations among Ca-Na, Ca-Fe, Mg-Fe, and Zn-Cu, while significant negative correlations were noticed among K and Na.
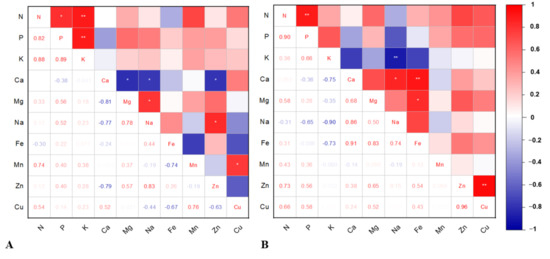
Figure 5.
Correlation matrix between total plant nutrient concentrations in autumn-planted (A) and spring-planted (B) strawberry. The size and colour intensity of squares are proportional to Pearson’s correlation coefficients. Red squares indicate positive correlations, while blue are negative correlations. In the correlogram scale from −1 to +1, Pearson’s correlation coefficient for variables is on the vertical and horizontal axis. * indicates values that are statistically different at p < 0.05 and ** at p < 0.01. The correlation matrix was generated from correlplot application in the OriginLab 2021 software for Windows (OriginLab Corp. Northampton, MA, USA).
4. Discussion
4.1. Chemical Suitability of Studied Substrates
The unfavourable pH and high EC are major factors that restrict the use of SMS in horticulture. The chemical analysis of 100% fresh SMSs (Table 4) revealed that both pH and EC were at levels that restrict their immediate use as growing media. In the present study, the EC values of 100% fresh A-SMS (8.92 mS·cm−1), L-SMS (1.46 mS·cm−1) and P-SMS (3.39 mS·cm−1) were nearly comparable to the values reported for A-SMS (0.58–10.70 mS·cm−1) by Maher et al. [73], Jordan et al. [74], and for L-SMS (1.96 mS·cm−1) and P-SMS (0.89–4.01 mS·cm−1) by Catal and Peksen [75]. Overall, the chemical analysis of SMSs in our study demonstrated that due to high EC and unfavourable pH, immediate use of SMSs in whole (100%) should be limited and/or restricted [44,57,75,76].
Interestingly, the pH and EC values of SMS-based substrates (L-SMS and P-SMS) studied in the present investigation before strawberry cultivation were lowered to nearly peat values when 100% fresh SMSs were mixed with peat in 15 and 25% (Figure 1 and Figure 2). On the other hand, the A-15 and A-25 substrates based on A-SMS still had higher EC at the beginning of the study. However, these substrate chemical parameters before the study were comparable and were within the acceptable ranges specified for growing media (pH 5.2–6.3 and EC 0.75–3.49 dS·m−1) as reported by Abad et al. [77]. Moreover, the L-SMS and P-SMS based substrate properties in the study were nearly comparable to the peat values [78]. These results revealed that mixing all studied SMSs with peat (15–25%) can benefit SMSs to achieve nearly suitable chemical properties which help to overcome the restricted usage due to unfavourable chemical properties and can be used immediately in horticulture. The obtained results are in agreement with Eudoxie and Alexander [66] and Holozlu [79], who also concluded that mixing SMS with peat in lower concentrations (<50%) can nearly neutralise the limiting nature of the SMS. On the other hand, a few reports recommend to use SMSs only after weathering (6–24 months), composting, or stabilization [44,61,62], which is neither economical nor environmentally friendly.
It has been suggested that strawberries can perform well in a slightly acidic pH level of 4.6–6.5 [80,81]. In our study, the pH value of all studied substrates based on SMSs (A-15–P-25) both in autumn and spring planting, before and after the strawberry cultivation were in this optimum range (Figure 1A,B) which demonstrated the chemical suitability of the studied SMSs as a peat substitutes. In the present investigation, the pH values of substrates before strawberry cultivation in both autumn (6.00–6.37) and spring planting (6.00–6.38) were lower than the pH values (6.28–6.75) reported by Altieri et al. [3], where olive mill waste mix (OMWM) was studied as a peat substitute (0, 25, 50, and 75% v/v); similarly to that of 7.10–7.52 as reported by Ameri et al. [4], where growing media mixes based on rice hull, sycamore pruning waste, cocopeat, perlite, and vermicompost were evaluated for strawberry cultivation. In addition, the values of pH after the strawberry cultivation in both autumn (5.99–6.59) and spring planting (5.87–6.68) observed in our study were lower than the pH values 6.96–7.37 and 7.22–7.66 reported by Altieri et al. [3] and Ameri et al. [4].
The high level of EC is one of the most important factors limiting the use of SMS as a growing medium [74,82]. Bryla and Scagel [83] reported that to achieve optimum strawberry development, the EC of the growing media should be maintained at ≤1.3 mS∙cm−1 during the early stage, and later at ≤3.4 mS∙cm−1, while Caso et al. [84] and D’Anna et al. [85] reported higher EC values of 3.49 mS·cm−1 and 2.5 mS·cm−1 of the substrates had no negative influence on the overall performance of strawberries in soilless production. In line with these findings, in our study, the EC of all SMS-based substrates before plant cultivation, both in autumn and spring planting, were in this range (0.66–2.99 mS∙cm−1), which demonstrated the suitable chemical properties of studied SMSs as peat substitutes. The substrate mixes prepared based on SMSs were reported to be highly saline [73,74,76], whereas in the present study, none of the prepared substrate combinations (A-15–P-25) based on SMSs (A-SMS, L-SMS, and P-SMS) at the beginning of cultivation were observed to be saline for strawberry cultivation. At the end of cultivation, the higher EC values were recorded in A-25 (6.66 mS∙cm−1), A-15 (5.90 mS∙cm−1), and P-25 (4.28 mS∙cm−1) in spring planting which was out of the specified optimum range of EC [77,83]. Particularly, the higher substrate EC values reported at the end of cultivation in A-15 and A-25 can be considered saline [76]. The possible reason for this could be mineralisation of organic matter. The higher substrate EC values in A-15 and A-25 in spring planting with 15% and 25% of A-SMS substitution resulted in slight marginal leaf necrosis at the end of the cultivation; similar results were reported by Altieri et al. [3], where 75% of OMWM substitution with peat resulted in strawberry leaf necrosis. That toxic effect could be the result of excessive concentrations of K and/or S-SO4. The higher EC values in substrates A-15 and A-25 at the end of the cultivation (Figure 2B) in spring planting were associated with the high level of residual nutrients (N-NO3, P, K, Ca, Mg, S-SO4, Na, and Cl) in the substrates recorded at the end of the strawberry cultivation (Table 6a,b). The higher EC and residual substrate nutrients N-NO3, P, K, Ca, Mg, S-SO4, Na, and Cl in A-15 and A-25 substrates in spring planting were associated with the use of A. bisporus SMS, which is associated with higher nutrient values [65,76] and extreme values can lead to phytoxicity [75]. Similarly, the higher percentage of OMWM added to peat increased the substrate EC values above the specified optimal range [3].
Abad et al. [77] reported that an ideal substrate for soilless cultivation should exhibit following chemical properties: N-NO3 100–199 mg mL−1, K 150–249 mg mL−1, S-SO4 < 960 mg mL−1, Na < 115 mg mL−1, and Cl < 180 mg mL−1. In our study, the concentration of N-NO3 (207.0 mg·dm3) in A-15 was noticed to be more than the above-specified range. K (1212.0 mg·dm3 and 1053.0 mg·dm3) and Na (209.0 mg·dm3 and 177.0 mg·dm3) concentrations in A-15 and A-25, respectively, were also out of the optimum values as suggested by Abad et al. [77], while the nutrient concentrations of other prepared substrates based on L-SMS and P-SMS at the beginning of cultivation for N-NO3, K, S-SO4, Na, and Cl were in the optimum range, demonstrating the chemical suitability of SMS based substrates. The high amounts of nutrients present in SMSs can serve as a source of nutrition, which is essential for plant growth at the initial stage [65,76]; on the other hand, higher amounts of nutrients in SMSs coupled with unfavourable EC can lead to phytotoxicity [75]. However, these properties may vary, as the chemical composition of SMSs largely depends on substrate composition and mushroom species cultivated and to date, limited reports are made available on SMS chemical properties. Further, there is a dire need for details reports on physical, chemical, and biological properties of different SMSs supporting potency to provide beneficial utilisation of SMSs [75].
Based on the chemical parameters achieved in the current study, the studied substrate demonstrated chemical suitability for strawberry cultivation, while plant performances varied in autumn and spring planting. At the end of cultivation, it is still possible to use the substrates after strawberry cultivation as a valuable organic-mineral fertiliser and/or organic input to soil; in particular, the substrates after autumn planting cultivation, as the residual pH, EC, and nutrients were lower than the substrates from spring-planted cultivation.
4.2. Morphological Performance
In the performed study, the substrate combinations greatly influenced the morphological performance of strawberry cv. ‘Elsanta’ as determined by shoot length, root length, leaf number, number of crowns, crown diameter, and plant dry weights. These findings are in line with Adak et al. [5], Tariq et al. [8], and Yavari et al. [14], who collectively reported that the strawberry plant height, crown diameter, leaf area, and plant dry weights were significantly influenced by the growing media. Additionally, morphological parameters (shoot and root length, number of crowns, crown diameter, number of leaves, leaf dry weight, and crown dry weight) in the present investigation were observed to be superior in autumn planting in comparison to spring planting. This demonstrated that the autumn planting facilitated strawberry plants to establish a better shoot and root system when compared to the spring-planted strawberries. Hence, based on the results, it can be inferred that, besides the growing media, the season and/or time of planting and relation with it, i.e., light conditions is also one of the key factors that influenced the better establishment of morphological traits in strawberries [85,86,87]. Spring planting has a shorter growing period and less requirement of inputs in comparison with autumn planting. However, to achieve better growth and development attributes, autumn planting is recommended which facilitates plants for the better morphological establishment and can help one to obtain higher yields [86,87].
The nutrient content in growing media plays a critical role in plant growth and development; this is partly due to nutrient availability in the substrate [17]. Previous studies by other researchers also suggest that the vegetative performance of strawberries is related to the availability of an adequate amount of N, P, Mg, Mn [58]. As reported by Tabatabaei et al. [88], the form of N can also greatly influence the growth, yield, and fruit quality. In the present investigation, the highest shoot and root length were recorded in autumn-planted strawberries grown in A-15 (Table 7), and the lowest in peat in spring planting. This superior shoot and root development in A-15 was associated with the initial high nutrient concentrations (Table 5a) in the substrate (A-15) when compared to the peat and other studied substrates. In the study by Leskovar and Othman [17], the substrate nutrient concentration also significantly influenced shoot and root growth.
Our results concerning shoot and root length indicated that substituting peat with a nutrient-rich organic matter (SMS) can increase the initial nutrient content of substrates and availability to plants. Altieri et al. [3] reported that adding OMWM to the growing media enhanced its total initial nitrogen content and overall substrate availability of nutrients which can better support plant growth. This superiority in shoot and root lengths can be also due to the better interchange of the cations which distribute water to the root growth and overall increase the plant height [89]. As reported by Linkohr et al. [90], the availability of NO3 and P in the substrate also plays a vital role in the root system architecture and development.
4.3. Dry Matter Distribution
The higher leaf, crown, and root dry weight, as well as the total plant dry weight, were determined in autumn planting strawberries cultivated in P-15 and P-25 (Table 10 and Figure 3). This superiority with total plant dry matter accumulation of plants grown in P-15 and P-25 can be related to the highest number of leaves and crown diameter recorded in these substrates (Table 8 and Table 9). The superior number of leaves, crown diameters, and plant dry weights (leaves, crowns, and roots) recorded in P-SMS substrates (P-15 and P-25) which are based on wheat straw. This superiority might be attributed due to the better nutrition retention [91] and high water holding capacity (WHC) of the substrate [4,91,92]. In line with the above mentioned, Ercisli et al. [93] reported that substrate with good aeration, low water tension, with high WHC influenced better development and growth of strawberries. These findings are supported by Peregrina et al. [94] and Kato et al. [95] where incorporation of P-SMS decreased the soil compactness and increased the soil porosity. Moreover, P-SMS was reported to be a good source of C and holds sufficient moisture [96]. Additionally, the initial and final pH and EC values of P-15 and P-25 coupled with the initial nutrient concentrations (Table 5) may have benefited the better growth, development, and accumulation of higher shoot, crown, root, and total plant dry weights.
In our study, as mentioned earlier, plants grown in the substrates based on A-SMS (A-15 and A-25) had the superior shoot length and root length (Table 7) when compared to other substrates in autumn planting, while this superior shoot and root lengths noticed in A-15 and A-25 were not reflected in higher shoot and root dry masses. The lower dry weight recorded in A-15 and A-25 (Table 10) in spring planting was associated with the highest substrate EC and NO3 concentration at the end of cultivation (Table 6a), resulting cation, and nutrient imbalance. These findings are in line with Leskovar and Othman [17], where the high levels of NO3 in growing media negatively influenced strawberry growth. These findings are supported by Pokhrel et al. [97], where organic substrate additives increased the plant dry weight, while the high substrate EC and NO3 ratios around the root zone lowered strawberry performances due to cation imbalance and nutrition deficiency.
Salinity can significantly reduce the strawberry shoot and root dry biomass [98,99]. The higher substrate EC values at the end of cultivation in A-SMS based substrates (A-15 and A-25) and P-SMS based substrates (P-15 and P-25) in autumn planting (Figure 2A) were nearly comparable to the optimum EC values reported by Bryla and Scagel [83] and the above-mentioned substrate EC values had no negative influence on the studied morphological parameters and dry matter accumulation, while the higher EC values of A-25 (6.66 mS·cm−1) and A-15 (5.90 mS·cm−1) in spring planting tend to have a reducing effect on dry matter accumulation (Table 10 and Figure 3). Keutgen and Pawelzik [100] reported that the dry weights of strawberry cv. ‘Elsanta’ were negatively affected when exposed to a high level of salinity (EC 3.9 and 7.5 mS·cm−1). Based on the chemical analysis in our study, it can be inferred that the high substrate salinity at the end of cultivation in A-SMS and P-SMS-based substrates in autumn planting had no negative impact on the overall morphological performance. These findings are in agreement with Medina et al. [65], where higher salinity of A-SMS had no negative impact on tomato seedling dry matter content. Similarly, Prasad et al. [40] noticed superior morphological and yield performance of strawberry cv. ‘Honeoye’ in SMS-based substrates with higher substrate EC (3.20 mS·cm−1 and 3.69 mS·cm−1). D’Anna et al. [85] reported higher strawberry fruit yield, better fruit quality, and higher fruit weights of cv. ‘Tulda’ at EC of 2.5 mS·cm−1 than at lower EC values under soilless conditions. The plant tolerance to the substrate salinity depends on their genotype/cultivar [101,102], while the cv. ‘Elsanta’ used in the present study is salt-sensitive [100].
In the present investigation, under the described growing and nutritional conditions, the use of SMSs as peat substitutes had a positive effect on strawberry morphological parameters and dry matter accumulation, particularly in autumn planting. The strawberry performances in our study were mainly influenced by substrate chemical properties and the planting seasons. The poor performance of strawberries cultivated in peat with no risk of unfavourable substrate pH, EC, and nutrient content was noticeable. On the other hand, the poor performance of plants in A-15 and A-25 with associated risk of higher EC and nutrient content in spring planting was evident.
4.4. Yield Performances
Several studies have demonstrated that the substrate used in soilless strawberry production influences yield parameters [4,5,8,9]. The results of the present study with respect to yield performances support these findings and suggest that substrates and their properties largely influence total yield, marketable and unmarketable yield per plant. The highest marketable and total yields recorded in plants grown in P-15 and P-25 in autumn planting were associated with the highest number of leaves, leaf area, crown number, crown diameter, and plant dry weights (leaves, crown, root, and total plant). These findings are in line with Ghazvini et al. [103], where the highest yield was associated with a higher crown number and crown diameter. Ebrahmim et al. [104] also reported higher leaf number, leaf area, root, and shoot weights resulted in the highest yield. Similarly, superior total yield was reported in olive mill waste mix when compared to peat by Altieri et al. (2010) and in aged bark substrate when compared to coconut fibre by Depardieu et al. (2016). The achieved marketable yields among the studied substrate combinations in autumn and spring plating (except for A-15 and A-25 in spring planting) were comparable to the values previously reported on agro-waste-based peat substitutes [3,28,84]. Alteiri et al. [3] recorded the highest marketable yield in 75% olive mill waste mix (224.0 g), Depardieu et al. [28] in aged bark-based substrate (342.88 g) and Caso et al. [84] in 100% rice husk (496.73 g).
The lowest cumulative marketable and total yields, and the highest unmarketable yields (>50%) reported in plants cultivated in A-15 and A-25 substrates in spring planting were associated with lower dry weights as reported earlier. Moreover, due to higher substrate EC (5.90 and 6.66 mS·cm−1) values and higher residual nutrient concentrations, these findings are in line with Pokhrel et al. [97], where higher EC, NO3, and associated nutrient imbalance resulted in lower strawberry yields. Similarly, Keutgen and Pawelzik [100] noticed that the EC of 7.5 mS·cm−1 significantly reduced the fruit yield of cv. ‘Elsanta’ up to 46%. Considering the yield performances, autumn planting is recommended to obtain higher marketable yields and the substrate EC values higher than 4.5 mS·cm−1 should be monitored, which can significantly reduce the yield performances of strawberries.
4.5. Nutrient Uptake and Partitioning
The nutritional status of strawberry plants is of major relevance for the achievement of the expected levels of productivity and overall fruit quality [21]. Leaf analysis can help assess the nutrient status (deficiency, sufficiency, and toxicity) of strawberry plants and also, more accurately determines the fertiliser requirements which guides one to develop or modify fertiliser programs [19].
In the present investigation, the statistical analyses showed clear differences among the studied substrates and planting seasons in the strawberry leaves, crowns, and roots macro- and micronutrient uptake and/or accumulation. Further, the uptake of nutrients was noticed to be dynamic among strawberry plant organs, i.e., leaves (Table 12), crowns (Table 13), and roots (Table 14).
The higher nutrient uptake in strawberry plant organs cultivated in SMS substituted substrates (A-15-P-25) in comparison with the peat was evident (Table 12, Table 13 and Table 14). Generally, higher content of N, P, and K in leaves, crowns, and roots were noticeable in SMS-based substrates (A-15-P-25) in comparison with peat in both autumn and spring planting. This demonstrated that the higher content of those nutrients’ availability found at the end of cultivation in substrates based on SMSs led to a higher uptake of these elements by plants. In particular, the higher uptake of selected nutrients was observed in both A-SMS-based substrates which can be associated with the higher residual nutrient concentration in both substrates. These results positively corresponding with earlier studies by Altieri et al. [3] in which the percentage of OMWM added to peat significantly and proportionally increased all macro- and micronutrients content in the growing media and further, the higher nutrient availability in the growing media reflected the higher nutrient content in plant parts. The SMS substituted peat substrates in our study served as a nutrient-rich reservoir and facilitated the slow release of these elements which later helped plants to uptake higher amounts of N, P, and K by different strawberry plant organs. Similarly, the OMWM amended to peat substrate led to higher uptake of nutrients by strawberry plant organs than in peat [3]. In line with the abovementioned, Medina et al. [65] and Benito et al. [105] reported that SMSs have been proven to increase the nutrient status of the growing media.
In our study, the macronutrient levels in leaves and roots (Table 12 and Table 14) were found to be within the range of sufficiency for strawberry plants [106,107]. Moreover, the determined content of nutrients in leaves and roots were nearly comparable to the values reported by Soppelsa et al. [108], where the influence of different biostimulants on strawberry performance was evaluated.
Based on the leaf analysis standards for strawberries given by Trejo-Tellez and Gomez-Merino [19], the leaf concentration of N, P, K, Ca, Mg, Na, Fe, and Mn in SMS-based substrates in the present investigation were found to be in the adequate levels; whereas in the case of plants grown in peat, the leaf nutrient content of N, P, and K both in autumn and also spring planting strawberries was found to be deficient and/or less than the adequate levels [109].
In the present study, the range of nutrient accumulation for N, P, K, Ca, Mg, and Mn was noticed to be higher in the leaves than in the roots. Similarly, in a study by Erdoğan- Bayram and Elmaci et al. [110], a higher amount of N, P, Ca, Mg, and Mn was noticed in the strawberry leaves followed by roots. The high contents of N, K, Fe, Mn, and Zn were determined in leaves and at the same time, the P concentration was noticed to be lower than sufficient levels as reported by Yavari et al. [14]; whereas our results indicated that a high level of Fe and Zn was not observed in strawberry leaves, and the P levels were noticed to be sufficient. The crowns tend to have higher Na, Zn, and Cu uptake and roots had the higher range for Ca, Mg, and Fe. According to Tagliavini et al. [21], the Ca was reported to be mainly accumulated in strawberry leaves, whereas in our study, the Ca was mainly recovered in strawberry roots. The Mg was found to be almost equally partitioned among strawberry plant organs [21]. Particularly, in our study, the higher content of Mn in leaves and Fe in roots was evident, and this trend was observed to be in line with the findings of Fattahi and Gholami [111], where higher Mn and Fe were noticed in leaves and roots of cv. ‘Selva’ and ‘Camarosa’. The higher accumulation of Cu in strawberry crowns was evident in our study, especially associated with substrates with higher EC values. These higher Cu values observed in our study were comparable to the highest Cu values in strawberry roots as reported by Soppelsa et al. [108]. However, the Cu accumulation in strawberry crowns in A-15 (spring planting) and P-15 (autumn planting) were higher than Cu values reported in strawberry roots [108]. As mentioned earlier, differently, some of the micronutrients, i.e., Mn, Fe, Zn, and Cu uptake was noticed to be higher in leaves, roots, and crowns, respectively. The higher accumulation of these microelements in different plant parts was probably a consequence of the application (mineral fertilisation) of these elements as EDTA, which is significantly available for plant uptake in varied pH conditions and is less prone to leaching by irrigation [112,113]. Additionally, the chemical suitability of studied substrate combinations with pH and EC in optimum range could have benefited better nutrient availability, mobilisation, and uptake, while higher substrate pH and EC values might cause nutrient imbalance resulting in a higher accumulation of nutrients. Lieten [26] and Depardieu et al. [28] concluded that to avoid nutrient deficiency or toxicity, the substrate pH and EC should be maintained in the optimum range.
4.6. Correlation
The macro- and micronutrients are reported to have both positive and negative influences on strawberry performances, while the relationships among these nutrient elements are not well established [19]. The plant growth and yield parameters were found to be positively correlated with the rate of nutrients employed during strawberry cultivation [21,114]. On this account, in the present study, both positive and negative correlations were observed among total plant nutrients as influenced by planting seasons. However, the correlation was variable at different planting seasons.
In our study, the positive significant correlations observed among N-P and N-K in autumn planting and N-P in spring planting suggested that interactions among these macronutrients might be essential for better strawberry development. Khayaat et al. [115,116] and Tohidloo et al. [117] reported that the relationship between N and K determines the balance between vegetative growth, fruit quality, and reproductive processes. At the same time, K plays the role of a growth regulator when the availability of N is sufficient.
According to Erdoğan-Bayram and Elmaci [110], the amount of Zn and Mn uptake by the stems were found to be non-significant, while in our study, the plant accumulation of Zn and Mn in autumn planting, as well as plant accumulation of Zn in spring planting, were statistically significant. Additionally, Zn and Mn in autumn planting were positively associated with Na and Cu and Zn with Cu in spring planting.
K is reported to have an influence on Ca and Mg uptake [19,118]. Lisjak et al. [118] reported an antagonistic relationship between K-Mg while K did not affect Ca uptake. In our study, both in autumn and spring planting, the K-Ca and K-Mg tend to have a certain dependency but it was not statistically confirmed with significant antagonism or positive relation.
Our correlation results indicated antagonism among Ca-Na, Ca-Mg, and Ca-Zn in autumn planting and K-Na in spring planting. However, to explain these relationships, the role of accompanying anions in the uptake of nutrient elements and their associations are not well established [118]; additionally, the information on nutrient concentrations in strawberry plants parts is limited [34,111].
According to Cohen [119], the correlation coefficient (r) values greater than 0.50 among variables suggest a strong relationship. Based on the ‘r’ values, besides significant differences, a strong relationship was noticed exclusively in autumn-planted strawberries among N-K (r = 0.88), where an increase in K can increase N, which can benefit the plant establishment. According to Primavesi et al. [120], the increase in K can increase the N use efficiency. Further, the ‘r’ values for Cu-N (0.54) and Cu-Ca (0.52) supports that Cu plays a role in the uptake of both N and Ca [19,30]. Similarly, the r-value for N-Mn (0.76) suggests a strong relationship. This relationship between Mn and N can support better nitrate assimilation [121]. The correlation and correlation coefficient values revealed that along with superior morphological and dry matter accumulation, the autumn-planted strawberry plants also had higher nutrient accumulation and stronger interactions than spring-planted.
The advantages of re-using neglected wastes include reduction of volume of accumulating wastes and has a high potential to use as an inexpensive and nutrient-rich substrate [105,122]. SMS is recommended to be used for horticulture purposes only after weathering (6–24 months). However, few researchers reported that the weathering alone is not sufficient to reduce salinity to a satisfactory level, and proposed further leaching as one of the possible options to reduce the salinity of SMC (weathered SMS) which include additional washing for a short period [123,124,125]; whereas our results strongly demonstrated the possibility of easy, immediate, and effective utilisation of SMS as a sustainable peat substitute without the need for weathering, decomposition, and leaching processes: which can be time-consuming, laborious, non-economical, and environmentally hazardous. Among many agro-waste-based peat substitutes, the possible utilisation of SMS can stand out, considering the easy availability [41], negligible cost [126], and considerable nutrient load in SMS after mushroom production [127]. In line with these reports and considering our results, the partial substitution of peat with the SMSs up to 25% can reduce dependency on expensive peat as a commercial substrate, and at the same time, can bring down the substituted per cent of the direct cost incurred on the substrate used in soilless strawberry cultivation.
The previous reports on the successful evaluation of agro-waste-based materials such as OMWM by Altieri et al. [3], ground reed canary grass (Phalaris arundinacea L.) by Kuisma et al. [9], and peat–sawdust mixture as well as aged bark by Depardieu et al. [28] demonstrating the possible utilisation of such agro-wastes as a promising alternative substrate to peat and coconut coir for strawberry cultivation. In line with the above reports, our research findings demonstrated the potential utilisation of SMSs (A-SMS, L-SMS, and P-SMS) which can be considered as easily available, nutrient-rich potential and sustainable peat substitutes in soilless strawberry cultivation.
5. Conclusions
In view of the growing environmental concerns associated with extensive peat utilisation, to come up with an easily available, economically feasible, and potential peat substitute will be of great significance. The results of the present investigation demonstrated that SMSs in lower concentrations of 15–25% can be considered as sustainable peat substitutes and can be recommended to be used immediately as peat-reduced growing media. Particularly, for autumn planting, all studied SMSs can be highly recommended, and for spring planting, L-SMS and P-SMS can be considered to be used in lower concentrations (<25%) along with peat. The SMS substitution with peat should be coupled with substrate moisture-based irrigation and crop-based nutrient management to achieve higher performances.
Correspondingly, to the best of our knowledge, the present investigation is the first of its kind throwing light on the influence of SMS as a non-traditional peat substitute on strawberry morphological parameters, yield performances, dry matter distribution, nutrient uptake, dynamics, and partitioning. The results of the investigation indicated that the integration of nutrients present in SMS and in applied fertilisers can significantly modify the growth performances, dry matter distribution, and better nutrient uptake by plants, facilitating higher yields. Hence, there is a dire need for further investigations optimising the studied growing media (SMSs) to better understand the nutrient uptake, utilisation, and efficiency of plant use. Our results serve as a reference for future investigations, at the same time adding scientific information on the effective use of fresh SMS in peat-reduced horticulture. Moreover, SMS can be considered as an inexpensive and nutrient-rich organic medium/additive that can reduce partial dependency on supplementary fertilisation.
Author Contributions
Conceptualization, R.P. and J.L.; methodology, R.P.; software, R.P.; validation, R.P., J.L. and T.K.; formal analysis, R.P. and T.K.; investigation, R.P. and J.L.; resources, R.P., and J.L.; data curation, R.P.; writing—original draft preparation, R.P.; writing—review and editing, R.P., J.L. and T.K.; visualization, R.P.; supervision, J.L. and T.K. All authors have read and agreed to the published version of the manuscript.
Funding
The research was financed by the Department of Vegetable Crops, Faculty of Agronomy, Horticulture, and Bioengineering, Poznan University of Life Sciences, Poland. The publication was co-financed within the framework of the Polish Ministry of Science and Higher Education’s program: “Regional Initiative Excellence” in the years 2019–2022 (No. 005/RID/2018/19)”, financing amount 12,000,000 PLN.
Data Availability Statement
All available data from the study are presented in this article.
Acknowledgments
We sincerely acknowledge Marek Siwulski for providing the spent mushroom substrates necessary for the investigations and also for other material help during the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hancock, J.F. Strawberries. Crop Production Science in Horticulture Series, No 11; CABI: Wallingford, UK, 1999; pp. 36–42. [Google Scholar]
- FAOSTAT. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 25 January 2022).
- Altieri, R.; Esposito, A.; Baruzzi, G. Use of olive mill waste mix as peat surrogate in the substrate for strawberry soilless cultivation. Int. Biodeterior. Biodegrad. 2010, 64, 670–675. [Google Scholar] [CrossRef]
- Ameri, A.; Thranifar, A.; Shoor, M.; Davarynejad, G.H. Effect of substrate and cultivar on growth characteristic of strawberry in soilless culture system. Afr. J. Biotechnol. 2012, 11, 11960–11966. [Google Scholar] [CrossRef]
- Adak, N.; Tozlu, I.; Gubbuk, H. Influence of Different Soilless Substrates to Morpho-physiological Characteristics and Yield Relations in Strawberries. Erwerbs-Obstbau 2018, 60, 341–348. [Google Scholar] [CrossRef]
- Agüero, J.J.; Kirschbaum, D.S. Response to Fertilization Associated to Leaf Mineral Content in Strawberry. J. Plant Nutr. 2015, 38, 116–126. [Google Scholar] [CrossRef]
- Jafarnia, S.; Hatamzadeh, A.; Tehranifar, A. Effect of different Substrates and Varieties on Yield and Quality of Strawberry in Soilless Culture. Adv. Environ. Biol. 2010, 4, 325–328. [Google Scholar]
- Tariq, R.; Qureshi, K.M.; Hassan, I.; Rasheed, M.; Qureshi, U.S. Effect of planting density and growing media on growth and yield of strawberry. Pak. J. Agric. Res. 2013, 26, 113–123. [Google Scholar]
- Kuisma, E.; Palonen, P.; Yli-Halla, M. Reed canary grass straw as a substrate in soilless cultivation of strawberry. Sci. Hortic. 2014, 178, 217–223. [Google Scholar] [CrossRef]
- Tomasi, N.; Pinton, R.; Costa, L.D.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New ‘solutions’ for floating cul-tivation system of ready-to-eat salad: A review. Trends Food Sci. Technol. 2015, 46, 67–276. [Google Scholar]
- Palencia, P.; Giné Bordonaba, J.; Martínez, F.; Terry, L.A. Investigating the effect of different soilless substrates on strawberry productivity and fruit composition. Sci. Hortic. 2016, 203, 12–19. [Google Scholar] [CrossRef]
- Martínez, F.; Oliveira, J.A.; Calvete, E.O.; Palencia, P. Influence of growth medium on yield, quality indexes and SPAD values in strawberry plants. Sci. Hortic. 2017, 217, 17–27. [Google Scholar] [CrossRef]
- Alsmairat, N.G.; Al-Ajlouni, M.G.; Ayad, J.Y.; Othman, Y.A.; Hilaire, R.S. Composition of soilless substrates affect the physiology and fruit quality of two strawberry (Fragaria × ananassa Duch.) cultivars. J. Plant Nutr. 2018, 41, 2356–2364. [Google Scholar] [CrossRef]
- Yavari, S.; Eshghi, S.; Tafazoli, E.; Karimian, N. Mineral elements uptake and growth of strawberry as ınfluenced by organic substrates. J. Plant Nutr. 2009, 32, 1498–1512. [Google Scholar] [CrossRef]
- Lieten, F. Effects of sodium on performance of ‘Elsanta’ strawberries grown on peat. Acta Hort. 2006, 708, 405–408. [Google Scholar] [CrossRef]
- Lieten, F. Effect of K:Ca:Mg ratio on performance of ‘Elsanta’ strawberries grown on peat. Acta Hort. 2006, 708, 397–400. [Google Scholar] [CrossRef]
- Leskovar, D.; Othman, Y. Low nitrogen fertigation promotes root development and transplant quality in globe artichoke. HortScience 2016, 51, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Madhavi, K.B.G.; Khan, F.; Bhujel, A.; Jaihuni, M.; Kim, N.E.; Moon, B.E.; Kim, H.T. Influence of different growing media on the growth and development of strawberry plants. Heliyon 2021, 7, e07170. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrient management in strawberry: Effects on yield, quality and plant health. In Strawberries: Cultivation, Antioxidant Properties and Health Benefits; Malone, N., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 239–267. [Google Scholar]
- Khan, F.; Okyere, F.G.; Basak, J.K.; Qasim, W.; Park, J.; Arulmozhi, E.; Kim, H.T. Comparison of different compost materials for growing strawberry plants. Acta Hortic. 2020, 1296, 869–876. [Google Scholar] [CrossRef]
- Tagliavini, M.; Baldi, E.; Lucchi, P.; Antonelli, M.; Sorrenti, G.; Baruzzi, G.; Faedi, W. Dynamics of nutrients uptake by strawberry plants (Fragaria × ananassa Dutch.) grown in soil and soilless culture. Eur. J. Agron. 2005, 23, 15–25. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Huang, R.; Hu, K. Ability of nitrogen and phosphorus assimilation of seven strawberry cultivars in a northern Atlantic coastal soil. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010; Paper 0739. pp. 1–4. [Google Scholar]
- Cárdenas-Navarro, R.; López-Pérez, L.; Lobit, P.; Ruiz-Corro, R.; Castellanos-Morales, V.C. Effects of nitrogen source on growth and development of strawberry plants. J. Plant Nutr. 2006, 29, 1699–1707. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Souri, M.K.; Ebrahimi, F.; Ahmadizadeh, M. Growth and yield of strawberries under different potassium concentrations of hydroponic system in three substrates. World Appl. Sci. J. 2012, 16, 1380–1386. [Google Scholar]
- Hesami, A.; Khorami, S.S.; Amini, F.; Kashkooli, A.B. Date-peat as an alternative in hydroponic strawberry production. Afr. J. Agri. Res. 2012, 7, 3453–3458. [Google Scholar] [CrossRef]
- Lieten, P. Nutrition of Protected Strawberries; International Fertiliser Society: Izmir, Turkey, 2003. [Google Scholar]
- May, G.M.; Pritts, M.P. Phosphorus, Zinc, and Boron Influence Yield Components in Earliglow ‘Strawberry. J. Am. Soc. Hortic. Sci. 1993, 118, 43–49. [Google Scholar] [CrossRef]
- Depardieu, C.; Prémont, V.; Boily, C.; Caron, J. Sawdust and bark-based substrates for soilless strawberry production: Irrigation and electrical conductivity management. PLoS ONE 2016, 11, e0154104. [Google Scholar] [CrossRef] [PubMed]
- Deák, S.M.; Mark, G.I.; Szabo, T.; Füleky, G. Spectral properties of strawberry plants. Int. J. Hortic. Sci. 2007, 13, 17–22. [Google Scholar] [CrossRef]
- Abul-Soud, M.A.; Emam, M.S.A.; Abd El-Rahman, N.G. The potential use of vermicompost in soilless culture for producing strawberry. Int. J. Plant Soil Sci. 2015, 8, 1–15. [Google Scholar] [CrossRef]
- Wang, B.; Lai, T.; Huang, Q.; Yang, X.; Shen, Q. Effect of N Fertilizers on Root Growth and Endogenous Hormones in Strawberry. Pedosphere 2009, 19, 86–95. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, J.; Zhao, R.; Dai, H.; Zhang, Z. Application of Vermicompost Improves Strawberry Growth and Quality through Increased Photosynthesis Rate, Free Radical Scavenging and Soil Enzymatic Activity. Sci. Hortic. 2018, 233, 132–140. [Google Scholar] [CrossRef]
- Ikegaya, A.; Kawata, T.; Ikari, T.; Emoto, Y.; Sato, Y.; Takeuchi, T.; Arai, E. Characteristics of fertilizer uptake and biodistriution in strawberry plants in two Japanese cultivars in hydroponic culture. Soil Sci. Plant Nutr. 2020, 66, 449–457. [Google Scholar] [CrossRef]
- Dhen, N.; Abed, S.B.; Zouba, A.; Haouala, F.; Almohandes Dridi, B. The challenge of using date branch waste as a peat substitute in container nursery production of lettuce (Lactuca sativa L.). Int. J. Recycl. Org. Waste Agric. 2018, 7, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, A.L.; Graham, L.L.B.; Putra, E.I.; Saharjo, B.H.; Applegate, G.; Grover, S.P.; Cochrane, M.A. Effects of distance from canal and degradation history on peat bulk density in a degraded tropical peatland. Sci. Total Environ. 2020, 699, 134199. [Google Scholar] [CrossRef]
- Ünal, M. The utilization of spent mushroom compost applied at different rates in tomato (Lycopersicon esculentum Mill.) seedling production. Emir. J. Food Agric. 2015, 27, 692–697. [Google Scholar] [CrossRef] [Green Version]
- Kitir, N.; Yildirim, E.; Şahin, Ü.; Turan, M.; Ekinci, M.; Ors, S.; Kul, R.; Ünlü, H. Peat use in horticulture. In Peat; Topcuoğlu, B., Turan, M., Eds.; IntechOpen: London, UK, 2018; pp. 75–90. Available online: https://www.intechopen.com/chapters/62735 (accessed on 25 January 2022).
- Drake, T.; Keating, M.; Summers, R.; Yochikawa, A.; Pitman, T.; Dodd, A.N. The cultivation of Arabidopsis for experimental research using commercially available peat-based and peat-free growing media. PLoS ONE 2016, 11, e0153625. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Prasad, R.; Lisiecka, J.; Antala, M.; Rastogi, A. Influence of Different Spent Mushroom Substrates on Yield, Morphological and Photosynthetic Parameters of Strawberry (Fragaria × ananassa Duch.). Agronomy 2021, 11, 2086. [Google Scholar] [CrossRef]
- Finney, K.N.; Ryu, C.; Sharifi, V.N.; Swithenbank, J. The reuse of spent mushroom compost and coal tailings for energy recovery: Comparison of thermal treatment technologies. Bioresour. Technol. 2009, 100, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Marín-Benito, J.M.; Sánchez-Martín, M.J.; Rodríguez-Cruz, M.S. Impact of spent mushroom substrates on the fate of pesticides in soil, and their use for preventing and/or controlling soil and water contamination: A review. Toxics 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cebula, J.; Pelczar, J.; Loska, K.; Widziewicz, K. The effect of Spent Mushroom Substrate field storage conditions on its leachate composition. Inżynieria I Ochr. Środowiska 2013, 16, 93–102. [Google Scholar]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; J. Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Ho, L.H.; Zulkifli, N.A.; Tan, T.C. Edible mushroom: Nutritional properties, potential nutraceutical values, and its utilization in food product development. In An Introduction to Mushroom; Passari, K.A., Sánchez, S., Eds.; Intech Open: London, UK, 2020; pp. 19–36. [Google Scholar]
- Zisopoulos, F.K.; Becerra Ramírez, H.A.; van der Goot, A.J.; Boom, R.M. A resource efficiency assessment of the industrial mushroom production chain: The influence of data variability. J. Clean. Prod. 2016, 126, 394–408. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Taib, S.M.; Din, M.F.M.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Phan, C.W.; Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef]
- Zhu, H.; Sheng, K.; Yan, E.; Qiao, J.; Lv, F. Extraction, purification and antibacterial activities of a polysaccharide from spent mushroom substrate. Int. J. Biol. Macromol. 2012, 50, 840–843. [Google Scholar] [CrossRef]
- Magalhães, A.C.; Moreira, B.; De, A.; Zied, D.C. Axenic cultivation of Pleurotus ostreatus var. Florida in supplemented sugarcane bagasse briquettes. Eng. Agrícola 2018, 38, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Suess, A.; Curtis, J.P. Report: Value-Added Strategies for Spent Mushroom Substrate in BC; British Columbia Mushroom Industry: Columbia, UK, 2006; 101p. [Google Scholar]
- Roy, S.; Barman, S.; Chakraborty, U.; Chakraborty, B. Evaluation of Spent Mushroom Substrate as biofertilizer for growth improvement of Capsicum annuum L. J. Appl. Biol. Biotechnol. 2015, 2, 322–327. [Google Scholar]
- Gao, W.; Liang, J.; Pizzul, L.; Feng, X.M.; Zhang, K. Evaluation of spent mushroom substrate as substitute of peat in Chinese biobeds. Int. Biodeterior. Biodegrad. 2015, 98, 107–112. [Google Scholar] [CrossRef]
- Raviv, M. The Future of Composts as Ingredients of Growing Media. Acta Hortic. 2011, 891, 19.–32. [Google Scholar] [CrossRef]
- Atikmen, N.C.; Kutuk, C.; Karahan, G. Response of Chrysanthemum (Chrysanthemum morifolium) to Fresh and Exhausted Mushroom Compost Substrates in Greenhouse Conditions. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Horticulture 2014, 71, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Roosta, H.R. Effect of Ammonium: Nitrate Ratios in the Response of Strawberry to Alkalinity in Hydroponics. J. Plant Nutr. 2014, 37, 1676–1689. [Google Scholar] [CrossRef]
- Garriga, M.; Muñoz, C.A.; Caligari, P.D.S.; Retamales, J.B. Effect of salt stress on genotypes of commercial (Fragaria × ananassa) and Chilean strawberry (F. chiloensis). Sci. Hortic. 2015, 195, 37–47. [Google Scholar] [CrossRef]
- González-Marcos, A.; Alba-Elías, F.; Martínez-De-Pisón, F.J.; Alfonso-Cendón, J.; Castejón-Limas, M. Composting of spent mushroom substrate and winery sludge. Compost. Sci. Util. 2015, 23, 58–65. [Google Scholar] [CrossRef]
- Khater, E.S.G. Some physical and chemical properties of compost. Int. J. Waste Resour. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Pascual, J.A.; Ceglie, F.; Tuzel, Y.; Koller, M.; Koren, A.; Hitchings, R.; Tittarelli, F. Organic Substrate for Transplant Production in Organic Nurseries. A Review. Agron. Sustain. 2018, 38, 1–23. [Google Scholar] [CrossRef] [Green Version]
- EU STAT- Database (2020). Available online: https://appsso.eurostat.ec.europa.eu (accessed on 29 January 2022).
- Regulation of the Minister of Climate of 2020 on the waste catalogue. Journal of Laws 2020 item 10; In Polish. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20200000010 (accessed on 10 January 2022).
- Medina, E.; Paredes, C.; Pérez-Murcia, M.D.; Bustamante, M.A.; Moral, R. Spent mushroom substrates as component of growing media for germination and growth of horticultural plants. Bioresour. Technol. 2009, 100, 4227–4232. [Google Scholar] [CrossRef] [PubMed]
- Eudoxie, G.D.; Alexander, I.A. Spent Mushroom Substrate as a Transplant Media Replacement for Commercial Peat in Tomato Seedling Production. J. Agric. Sci. 2011, 3, 41–49. [Google Scholar] [CrossRef]
- Collela, C.F.; Costa, L.M.A.S.; De Moraes, T.S.J.; Zied, D.C.; Rinker, D.L.; Dias, E.S. Potential utilization of spent Agaricus bisporus mushroom substrate for seedling production and organic fertilizer in tomato cultivation. Ciência e Agrotechnologia 2019, 43, e017119. [Google Scholar] [CrossRef]
- Anon. The Brooks and Olmo register of fruit & nut varieties. Choice Rev. Online 1997, 2, 35. [CrossRef]
- RHS. Plant Finder 2009–2010; RHS: Dorling Kindersley, London, 2009; p. 282. ISBN 978-1-4053-4176-9. [Google Scholar]
- Directorate-General for Agriculture and Rural Development (European Commission), European Commission. Commission Delegated Regulation (EU) 2019/428 of amending Implementing Regulation (EU) No 543/2011 as regards marketing standards in the fruit and vegetables sector. Off. J. Eur. Union 2019, L 75, 43–44.
- Schroeter-Zakrzewska, A.; Wolna-Maruwka, A.; Kleiber, T.; Wróblewska, H.; Głuchowska, K. Influence of Compost from Post-Consumer Wood on Development, Nutrition State of Plants, Microbiological and Biochemical Parameters of Substrates in Zonal Pelargonium (Pelargonium zonale). Agronomy 2021, 11, 994. [Google Scholar] [CrossRef]
- IUNG. Laboratory Test Methods in Chemical and Agricultural Station. Part. III. Examination of Soil, Growing Media under Vegetables and Flowers as well as Indicator Parts of Plants for Diagnostic Purposes; IUNG: Puławy, Poland, 1983; pp. 28–81. (In Polish) [Google Scholar]
- Maher, M.J.; Smyth, S.; Dodd, V.A.; McCabe, T.; Magette, W.L.; Duggan, J.; Hennerty, M.J. Managing Spent Mushroom Compost; Teagasc: Dublin, Ireland, 2000; 4p. [Google Scholar]
- Jordan, S.N.; Mullen, G.J.; Murphy, M.C. Composition variability of spent mushroom compost in Ireland. Bioresour. Technol. 2008, 99, 411–418. [Google Scholar] [CrossRef]
- Catal, S.; Peksen, A. Physical, chemical and biological properties of spent mushroom substrates of different mushroom species. Acta Hortic. 2020, 1287, 353–360. [Google Scholar] [CrossRef]
- Demir, H. The effects of spent mushroom compost on growth and nutrient contents of pepper seedlings. Mediterr. Agric. Sci. 2017, 30, 91–96. [Google Scholar]
- Abad, M.; Noguera, P.; Bures, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Vasconcelos, E.; dos Santos, J.Q. Fertilisation of potted Geranium with a municipal solid waste compost. Bioresour. Technol. 2000, 73, 247e249. [Google Scholar] [CrossRef]
- Holozlu, A. The Effect of Washed and Unwashed Waste Mushroom Compost on Some Soil Quality Parameters. Master’s Thesis, Institute of Science and Technology, Selcuk University, Selcuk, Turkey, 23 September 2013. (In Turkish). [Google Scholar]
- Niskanen, R.; Dris, R. Nutritional status of strawberry fields. Acta Hortic. 2002, 567, 439–442. [Google Scholar] [CrossRef]
- Milosevic, T.M.; Milosevic, N.T.; Glisic, I.P. Strawberry (Fragaria × ananassa Duch.) yield as affected by the soil pH. An. Da Acad. Bras. De Cienc. 2009, 81, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Chorover, J.; Rosario, R.; Fox, R.H. Leachate Chemistry of Field-Weathered Spent Mushroom Substrate. J. Environ. Qual. 2001, 30, 1699–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryla, D.R.; Scagel, C.F. Limitations of CaCl2 salinity to shoot and root growth and nutrient uptake in “Honeoye” strawberry (Fragaria × ananassa Duch.). J. Hortic. Sci. Biotechnol. 2014, 89, 458–470. [Google Scholar] [CrossRef]
- Caso, C.; Chang, M.; Rodríguez-Delfín, A. Effect of the growing media on the strawberry production in column system. Acta Hortic. 2008, 843, 373–380. [Google Scholar] [CrossRef]
- D’Anna, F.; Incalcaterra, G.; Moncada, A.; Miceli, A. Effects of different electrical conductivity levels on strawberry grown in soilless culture. Acta Hortic. 2003, 609, 355–360. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.M.; Hossain, M.M.; Khaliq, Q.A.; Moniruzzaman, M. Effect of planting time and genotypes growth, yield and quality of strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2014, 167, 56–62. [Google Scholar] [CrossRef]
- Torres-Quezada, E.A.; Zotarelli, L.; Whitaker, V.M.; Darnell, R.L.; Santos, B.M.; Morgan, K.T. Planting Dates and Transplant Establishment Methods on Early-yield Strawberry in West-central Florida. HortTechnology 2018, 28, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, S.J.; Fatemi, L.S.; Fallahi, E. Effect of ammonium: Nitrate ratio on yield, calcium concentration, and photosynthesis rate in strawberry. J. Plant Nutr. 2016, 29, 1273–1285. [Google Scholar] [CrossRef]
- Raja, W.; Mir, J.; Un nabi, S.; Lal, S.; Qureshi, I.; Kumawat, K. Effect of different substrates on growth and quality of Strawberry cv. chandler in soilless culture. Pharma Innov. J. 2018, 7, 449–453. [Google Scholar]
- Linkohr, B.; Williamson, L.; Fitter, A.; Leyser, H. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 2002, 29, 751–760. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.; Shylla, B. Effect of Different Soilless Substrates on Flowering, Yield and Fruit Quality of Strawberry (Fragaria × ananassa Duch.) cv. Chandler under Protected Conditions. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2830–2836. [Google Scholar] [CrossRef]
- Zahid, N.; Maqbool, M.; Hamid, A.; Shehzad, M.; Tahir, M.M.; Mubeen, K.; Shah, S.Z.A. Changes in Vegetative and Reproductive Growth and Quality Parameters of Strawberry (Fragaria × ananassa Duch.) cv. Chandler Grown at Different Substrates. J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Ercisli, S.; Sahin, U.; Esitken, A.; Anapali, O. Effects of some growing media on the growth of strawberry cvs. ‘Camarosa’ and ‘Fern’. Acta Agrobot. 2005, 58, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Peregrina, F.; Larrieta, C.; Colina, M.; Mariscal-Sancho, I.; Martín, I.; Martínez-Vidaurre, J.M.; García-Escudero, E. Spent mushroom substrates influence soil quality and nitrogen availability in a semiarid vineyard soil. Soil Sci. Soc. Am. J. 2012, 76, 1655–1666. [Google Scholar] [CrossRef]
- Kato, K.; Matsushima, U.; Muto, Y.; Tatsuzawa, F.; Okada, M. Effects of application of spent Shiitake substrates in farmlands damaged by the great East Japan earthquake on soil conditions and sweet corn growth and yield. Hort. J. Jpn. 2013, 12, 381–387. [Google Scholar]
- Nakatsuka, H.; Oda, M.; Hayashi, Y.; Tamura, K. Effects of fresh spent mushroom substrate of Pleurotus ostreatus on soil micromorphology in Brazil. Geoderma 2016, 269, 54–60. [Google Scholar] [CrossRef]
- Pokhrel, B.; Laursen, K.H.; Petersen, K. Yield, Quality, and Nutrient Concentrations of Strawberry (Fragaria × ananassa Duch. cv. “Sonata”) Grown with Different Organic Fertilizer Strategies. J. Agric. Food Chem. 2015, 63, 5578–5586. [Google Scholar] [CrossRef]
- Saied, A.S.; Keutgen, A.J.; Noga, G. The influence of NaCl salinity on growth, yield and fruit quality of strawberry cvs. “Elsanta” and “Korona”. Sci. Hortic. 2005, 103, 289–303. [Google Scholar] [CrossRef]
- Turhan, E.; Eris, A. Changes of growth, amino acids, and ionic composition in strawberry plants under salt stress conditions. Commun. Plant Sci. Plant Anal. 2009, 40, 3308–3322. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Cultivar-dependent cell wall modification of strawberry fruit under NaCl salinity stress. J. Agric. Food Chem. 2007, 55, 7580–7585. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, G.; Wallace, R.; Masabni, J.; Gu, M. Relative salt tolerance of seven strawberry cultivars. Horticulturae 2015, 1, 27–43. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, D.; Pudussery, M.V.; Ferreira, J.F.S.; Liu, X.; Pallete, A.; Grover, K.K.; Hummer, K. Variable salinity responses and comparative gene expression in woodland strawberry genotypes. Sci. Hortic. 2019, 254, 61–69. [Google Scholar] [CrossRef]
- Ghazvini, R.F.; Payvast, G.; Azarian, H. Effect of clinoptololitic-zeolite and perlite mixtures on the yield and quality of strawberry in soil-less culture. Int. J. Agric. Biol. 2007, 9, 885–888. [Google Scholar]
- Ebrahimi, R.; Ebrahimi, F.; Ahmadizadeh, M. Effect of Different Substrates on Herbaceous Pigments and Chlorophyll Amount of Strawberry in Hydroponic Cultivation System. AEJAES 2012, 12, 154–158. [Google Scholar]
- Benito, M.; Masaguer, A.; de Antonio, R.; Moliner, A. Use of pruning waste compost as a component in soilless growing media. Bioresour. Technol. 2005, 96, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.R.; Miner, G.S. Strawberry, annual hill culture. In Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; Southern Cooperative Series Bulletin 394; North Carolina Department of Agriculture & Consumer Services: Raleigh, NC, USA, 2000; ISBN 1-58161-394-6. [Google Scholar]
- Almaliotis, D.; Velemis, D.; Bladenopoulou, S.; Karapetsas, N. Leaf nutrient levels of strawberries (cv. Tudla) in relation to crop yield. Acta Hortic. 2002, 567, 447–450. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- SLTEC. Strawberry & Rubus Nutritional Guide. SLTEC Fertilizers. Nutrient solutions. 2014. Available online: http://www.sltec.com.au/sltec/brochures/Strawberry&RubusNutritionalGuide_2013.pdf (accessed on 15 December 2021).
- Erdoğan-Bayram, S.; Elmacı, Ö. Comparison of nutrient uptake by strawberry (Fragaria × ananassa Duch.) varieties according to phenological stages. Acta Scientiarum. Polonorum. Hortorum. Cultus. 2021, 20, 49–59. [Google Scholar] [CrossRef]
- Fattahi, M.; Gholami, M. Nutrient content changes in strawberry plant parts at different development stages. J. Appl. Hortic. 2009, 11, 150–152. [Google Scholar] [CrossRef]
- Liphadzi, M.S.; Kirkham, M.B. Availability and plant uptake of heavy metals in EDTA-assisted phytoremediation of soil and composted biosolids. South Afr. J. Bot. 2006, 72, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Bloem, E.; Haneklaus, S.; Haensch, R.; Schnug, E. EDTA application on agricultural soils affects microelement uptake of plants. Sci. Total Environ. 2017, 577, 166–173. [Google Scholar] [CrossRef]
- Shirko, R.; Nazarideljou, M.J.; Akbar, M.A.; Naser, G. Photosynthetic reaction, mineral uptake, and fruit quality of strawberry affected by different levels of macronutrients. J. Plant Nutr. 2018, 41, 1807–1820. [Google Scholar] [CrossRef]
- Khayyat, M.; Tafazoli, E.; Rajaee, S.; Vazifeshenas, M.; Mahmoodabadi, M.R.; Sajjadinia, A. Effects of NaCl and supplementary potassium on gas exchange, ionic content, and growth of salt-stressed strawberry plants. J. Plant Nutr. 2009, 32, 907–918. [Google Scholar] [CrossRef]
- Khayyat, M.; Vazifeshenas, M.R.; Rajaee, S.; Jamalian, S. Potassium effect on ion leakage, water usage, fruit yield and biomass production by strawberry plants grown under NaCl stress. J. Fruit Ornam. Plant Res. 2009, 17, 79–88. Available online: http://www.inhort.pl/files/journal_pdf/journal2009/vol17(1)2009/Full8%202009_1_pdf (accessed on 28 January 2021).
- Tohidloo, G.; Souri, M.K.; Eskandarpour, S. Growth and fruit biochemical characteristics of three strawberry genotypes under different potassium concentrations of Nutrient Solution. Open Agric. 2018, 3, 356–362. [Google Scholar] [CrossRef]
- Lisjak, M.; Stanisavljević, A.; Špoljarević, M.; Đurđević, B. Potassium rate and accompanying anions impact on potassium, calcium and magnesium uptake by strawberries in soilless culture. Cereal Res. Commun. 2008, 36, 483–486. [Google Scholar]
- Cohen, J. Set Correlation and Contingency Tables. Appl. Psychol. Meas. 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Primavesi, O.; Primavesi, A.C.; Corrêa, L.A.; Silva, A.G.; Cantarella, H. Nitrate leaching in heavily nitrogen fertilized coastcross pasture. Rev. Bras. Zootec. 2006, 35, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Domoto, P. Recognizing and Correcting Nutrient Deficiencies in Strawberries. Iowa State University-University Extension. 2011. Available online: http://www.iowaproduce.org/pages/fruit/files/strawberry/Strawberry_nutrition_guide.pdf (accessed on 25 January 2022).
- Zoes, V.; Dinel, H.; Paré, T.; Jaouich, A. Growth substrates made from duck excreta enriched wood shavings and source-separated municipal solid waste compost and separates: Physical and chemical characteristics. Bioresour. Technol. 2001, 78, 21–30. [Google Scholar] [CrossRef]
- Riahi, H.; Arab, A. Spent mushroom compost as an alternative for casing soil. Mushroom Sci. 2004, 71, 585–589. [Google Scholar]
- Riahi, H.; Azizi, A. Leached SMC as a component and replacement for peat in casing soil and increasing dry matter in mushrooms. In Proceedings of the 2nd International Spent Mushroom Substrate Symposium, Concordville, PA, USA, 17–20 September 2006; Paley, K., Ed.; The Pennsylvania State University: University Park, PA, USA, 2006; pp. 41–49. [Google Scholar]
- Gonani, Z.; Riahi, H.; Sharifi, K. Impact of using leached spent mushroom compost as a partial growing media for horticultural plants. J. Plant Nutr. 2011, 34, 337–344. [Google Scholar] [CrossRef]
- Danai, O.; Cohen, H.; Ezov, N.; Yehieli, N.; Levanon, D. Recycling of spent mushroom substrate (SMS) in avocado orchards. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products, Arcachon, France, 4–7 October 2011; Volume 4, pp. 352–360. [Google Scholar]
- Adedokun, O.M.; Orluchukwu, J.A. Pineapple: Organic Production on Soil amended with Spent Mushroom Substrate. ABJNA 2013, 4, 590–593. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).