Nitrogen Fertilizer Modulates Plant Growth, Chlorophyll Pigments and Enzymatic Activities under Different Irrigation Regimes
Abstract
:1. Introduction
2. Results
2.1. Kernel Yield and Yield Components
2.2. Plant Dry Matter
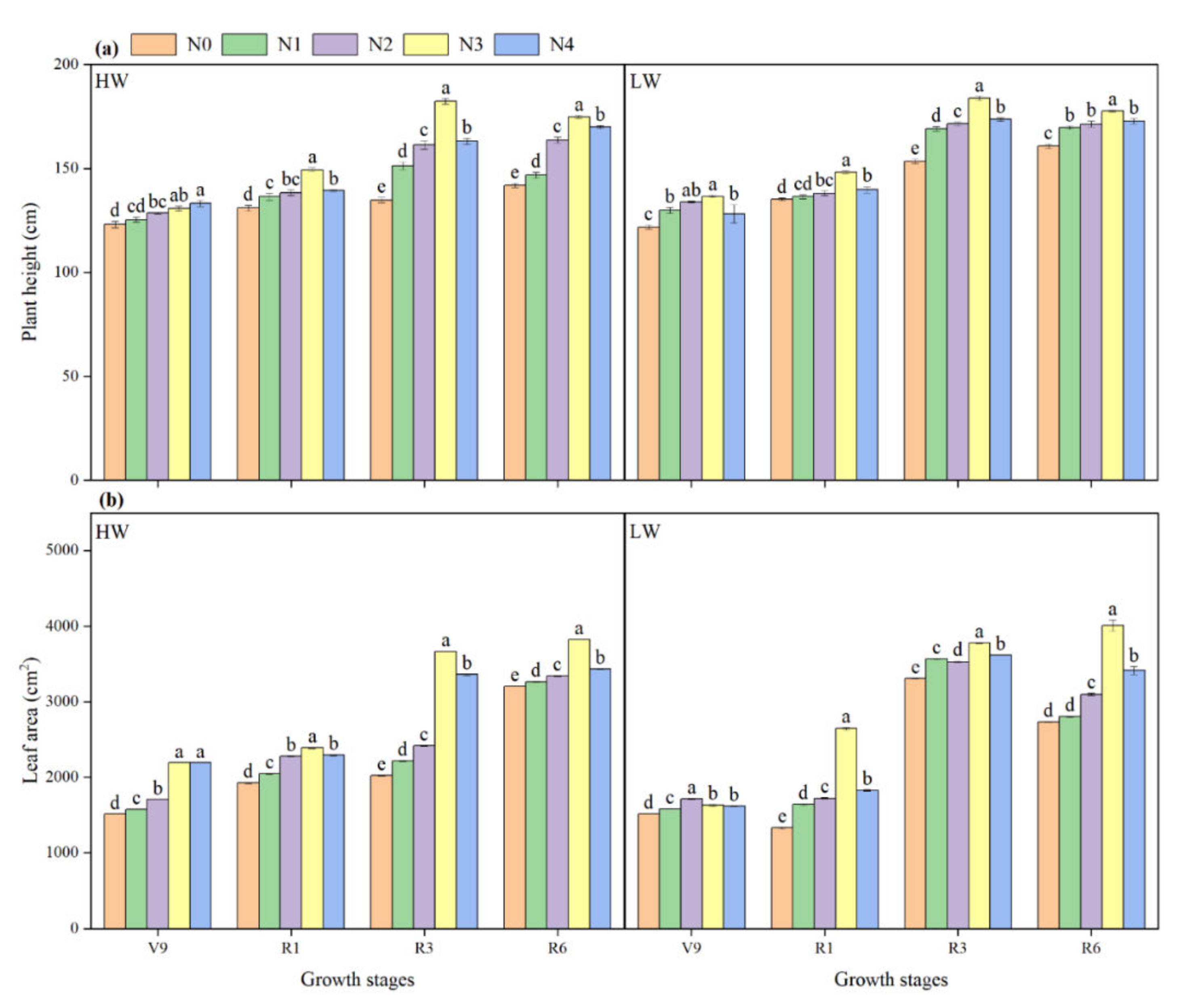
2.3. Plant Height and Leaf Area
2.4. Root Growth and Development
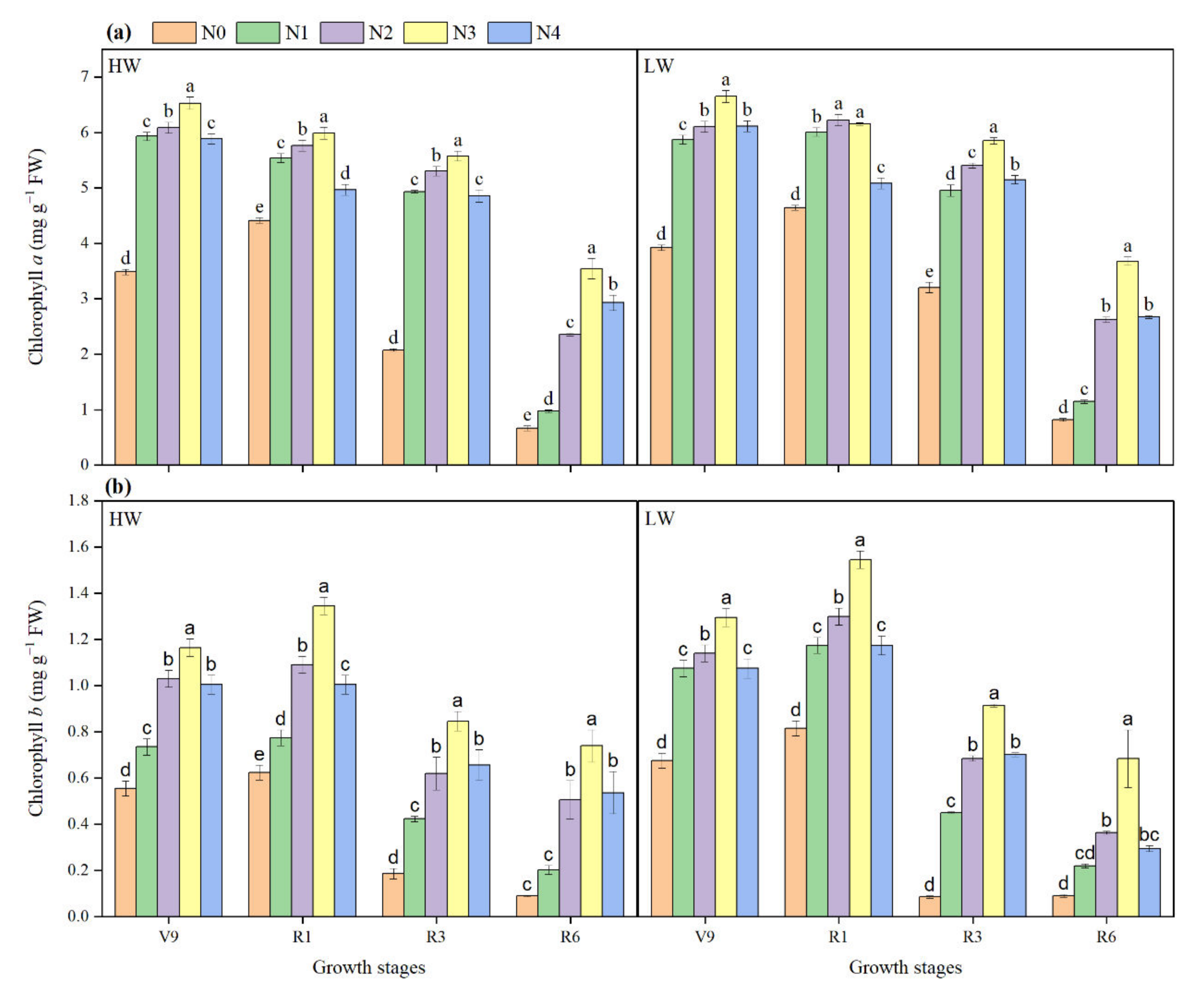
2.5. Chlorophyll a and b Contents
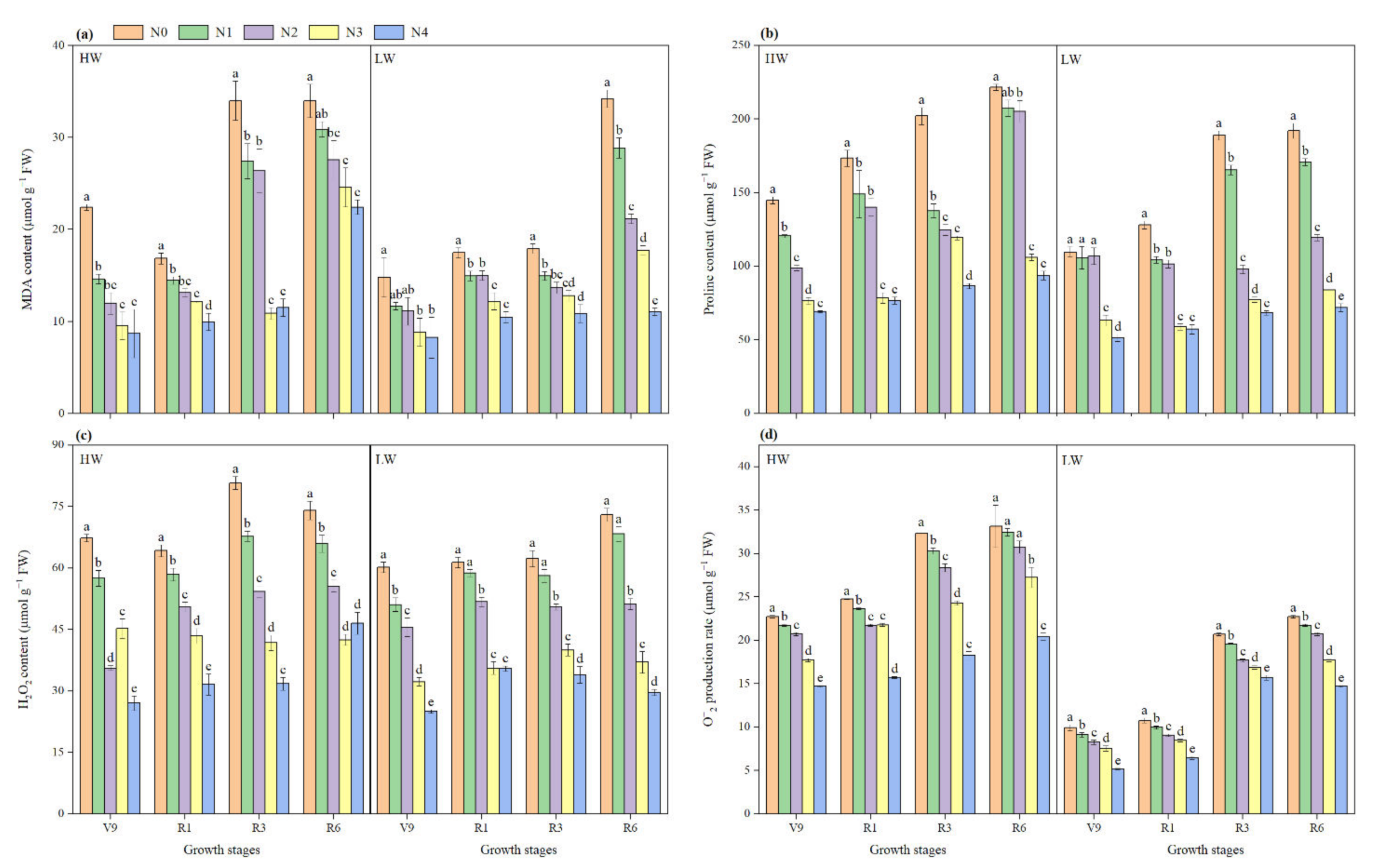
2.6. Malondialdehyde and Proline Content
2.7. Reactive Oxygen Species
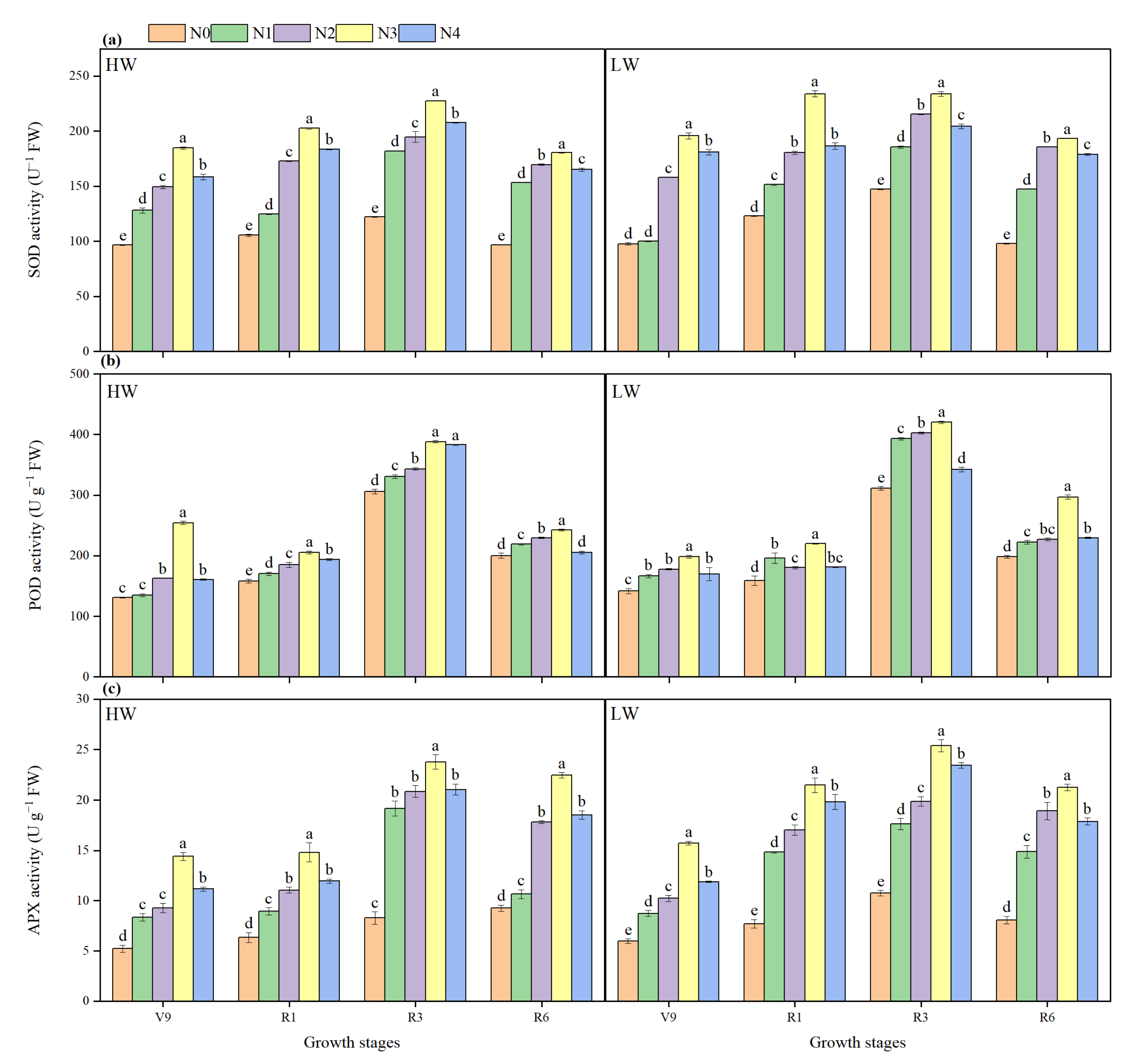
2.8. Antioxidant Enzymatic Activity
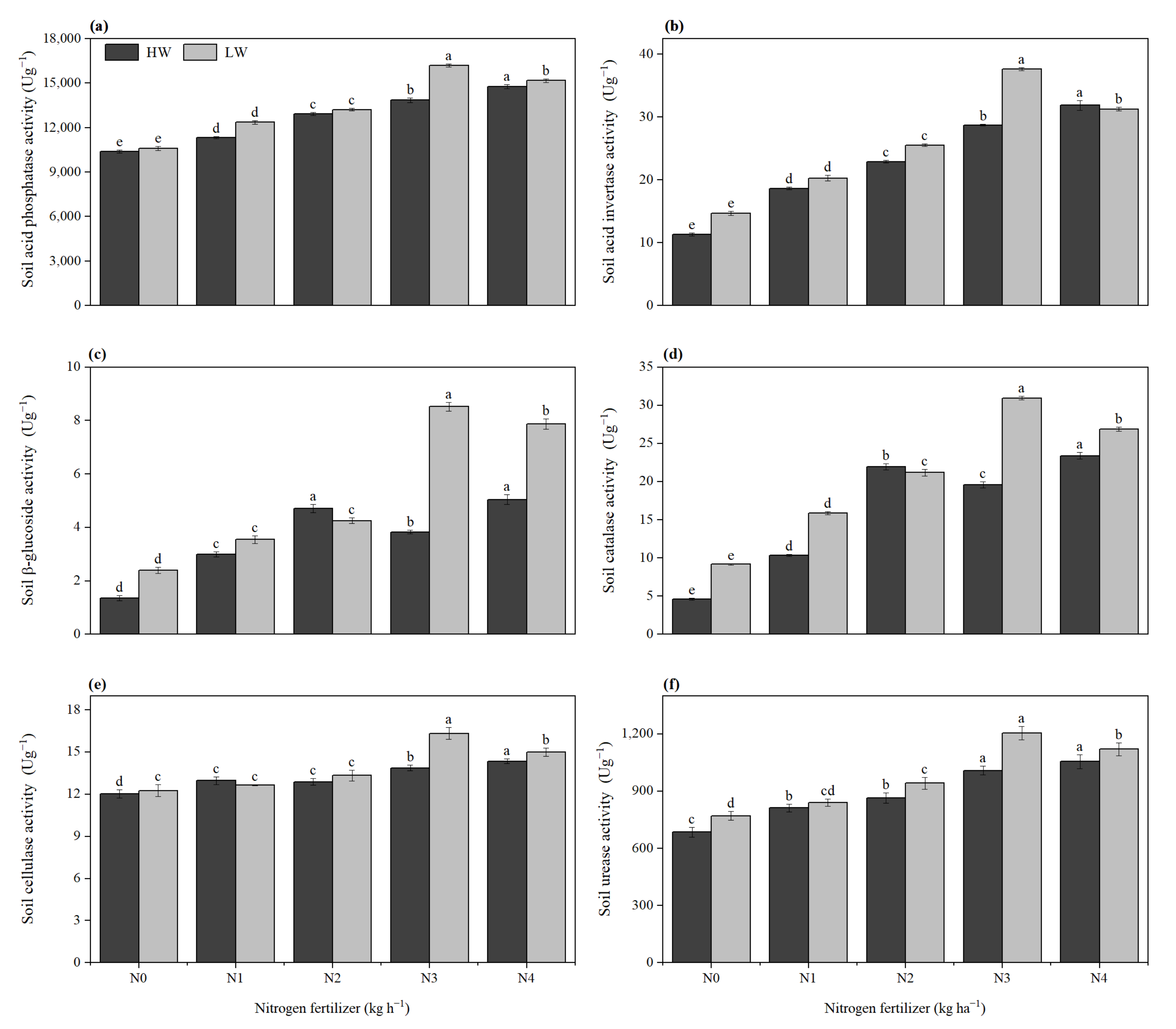
2.9. Soil Enzyme Activity
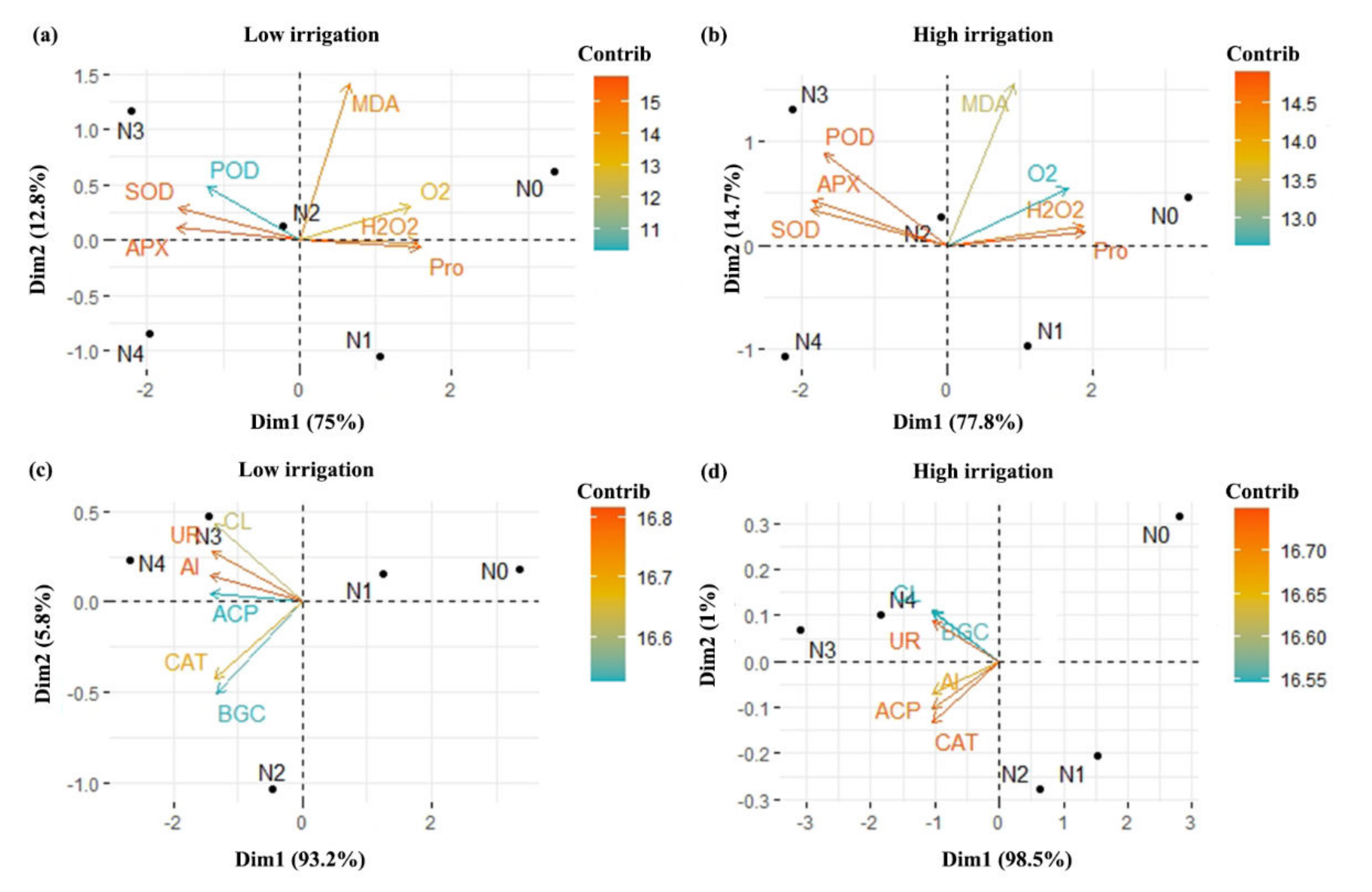
2.10. Correlation Analyses
3. Discussion
4. Materials and Methods
4.1. Experimental Site
4.2. Experimental Design and Management
4.3. Sampling and Measurements
4.3.1. Determination of Yield and Growth Attributes
4.3.2. Determination of Antioxidant Enzyme Activity
4.3.3. Chlorophyll Content
4.3.4. Determination of ROS, MDA and Proline Content
4.3.5. Soil Enzyme Activity Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chi, Y.X.; Gao, F.; Muhammad, I.; Huang, J.H.; Zhou, X.B. Effect of water conditions and nitrogen application on maize growth, carbon accumulation and metabolism of maize plant in subtropical regions. Archiv. Agron. Soil Sci. 2022, 1–15. [Google Scholar] [CrossRef]
- Khan, A.; Zahir Afridi, M.; Airf, M.; Ali, S.; Muhammad, I. A sustainable approach toward maize production: Effectiveness of farm yard manure and urea N. Ann. Biol. Sci. 2017, 5, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Hou, P.; Gao, Q.; Xie, R.; Li, S.; Meng, Q.; Kirkby, E.A.; Römheld, V.; Müller, T.; Zhang, F.; Cui, Z. Grain yields in relation to N requirement: Optimizing nitrogen management for spring maize grown in China. Field Crops Res. 2012, 129, 1–6. [Google Scholar] [CrossRef]
- Wang, G.Y.; Hu, Y.X.; Liu, Y.X.; Ahmad, S.; Zhou, X.B. Effects of supplement irrigation and nitrogen application levels on soil carbon–nitrogen content and yield of one-year double cropping maize in subtropical region. Water 2021, 13, 1180. [Google Scholar] [CrossRef]
- Jia, X.; Shao, L.; Liu, P.; Zhao, B.; Gu, L.; Dong, S.; Bing, S.H.; Zhang, J.; Zhao, B. Effect of different nitrogen and irrigation treatments on yield and nitrate leaching of summer maize (Zea mays L.) under lysimeter conditions. Agric. Water Manag. 2014, 137, 92–103. [Google Scholar] [CrossRef]
- Muhammad, I.; Khan, F.; Khan, A.; Wang, J. Soil fertility in response to urea and farmyard manure incorporation under different tillage systems in Peshawar, Pakistan. Int. J. Agric. Biol. 2018, 20, 1539–1547. [Google Scholar]
- Zhou, X.B.; Yang, L.; Wang, G.Y.; Zhao, Y.X.; Wu, H.Y. Effect of deficit irrigation scheduling and planting pattern on leaf water status and radiation use efficiency of winter wheat. J. Agron. Crop. Sci. 2021, 207, 437–449. [Google Scholar] [CrossRef]
- Mansouri-Far, C.; Sanavy, S.A.M.M.; Saberali, S.F. Maize yield response to deficit irrigation during low-sensitive growth stages and nitrogen rate under semi-arid climatic conditions. Agric. Water Manag. 2010, 97, 12–22. [Google Scholar] [CrossRef]
- Soler, C.; Hoogenboom, G.; Sentelhas, P.; Duarte, A.P. Impact of water stress on maize grown off-season in a subtropical environment. J. Agron. Crop Sci. 2007, 193, 247–261. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, X.M.; Gu, W.R.; Wei, S. Effects of a chemical plant growth regulator and planting density on the leaf senescence and yield of spring maize in northeast china. Appl. Ecol. Environ. Res. 2020, 18, 3297–3311. [Google Scholar] [CrossRef]
- Pandey, R.; Maranville, J.; Admou, A. Deficit irrigation and nitrogen effects on maize in a Sahelian environment: I. Grain yield and yield components. Agric. Water Manag. 2000, 46, 1–13. [Google Scholar] [CrossRef]
- Moser, S.B.; Feil, B.; Jampatong, S.; Stamp, P. Effects of pre-anthesis drought, nitrogen fertilizer rate, and variety on grain yield, yield components, and harvest index of tropical maize. Agric. Water Manag. 2006, 81, 41–58. [Google Scholar] [CrossRef]
- Subedi, K.; Ma, B. Nitrogen uptake and partitioning in stay-green and leafy maize hybrids. Crop Sci. 2005, 45, 740–747. [Google Scholar] [CrossRef]
- Binder, D.L.; Sander, D.H.; Walters, D.T. Maize response to time of nitrogen application as affected by level of nitrogen deficiency. Agron. J. 2000, 92, 1228–1236. [Google Scholar] [CrossRef]
- Gheysari, M.; Mirlatifi, S.M.; Homaee, M.; Asadi, M.E.; Hoogenboom, G. Nitrate leaching in a silage maize field under different irrigation and nitrogen fertilizer rates. Agric. Water Manag. 2009, 96, 946–954. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; AghaAlikhani, M.; Sanavy, S.M.; Mirlatifi, S. Interactions of irrigation, weed and nitrogen on corn yield, nitrogen use efficiency and nitrate leaching. Agric. Water Manag. 2013, 126, 9–18. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Connolly, J.; Lüscher, A. Strong mixture effects among four species in fertilized agricultural grassland led to persistent and consistent transgressive overyielding. J. Appl. Ecol. 2009, 46, 683–691. [Google Scholar] [CrossRef]
- Kenkel, P.; Fitzwater, B. Causes of Fertilizer Price Volatility. Oklahoma State University. Oklahoma Cooperative Extension Service. AGEC-261. 2009. Available online: http://articles.extension.org/pages/72692/causes-of-fertilizer-price-volatility (accessed on 27 March 2022).
- Brown, B.; Hart, J.; Horneck, D.; Moore, A. Nutrient Management for Field Corn Silage and Grain in the Inland Pacific Northwest; University of Idaho: Moscow, ID, USA, 2010; Volume 9. [Google Scholar]
- Hu, H.; Ning, T.; Li, Z.; Han, H.; Zhang, Z.; Qin, S.; Zheng, Y. Coupling effects of urea types and subsoiling on nitrogen–water use and yield of different varieties of maize in northern China. Field Crops Res. 2013, 142, 85–94. [Google Scholar] [CrossRef]
- Berenguer, P.; Santiveri, F.; Boixadera, J.; Lloveras, J. Nitrogen fertilisation of irrigated maize under Mediterranean conditions. Eur. J. Agron. 2009, 30, 163–171. [Google Scholar] [CrossRef]
- Zhou, X.B.; Wang, G.Y.; Yang, L.; Wu, H.Y. Double-double row planting mode at deficit irrigation regime increases winter wheat yield and water use efficiency in North China Plain. Agronomy 2020, 10, 1315. [Google Scholar] [CrossRef]
- Akmal, M.; Janssens, M. Productivity and light use efficiency of perennial ryegrass with contrasting water and nitrogen supplies. Field Crops Res. 2004, 88, 143–155. [Google Scholar] [CrossRef]
- Nilahyane, A.; Islam, M.A.; Mesbah, A.O.; Garcia y Garcia, A. Evaluation of silage corn yield gap: An approach for sustainable production in the semi-arid region of USA. Sustainability 2018, 10, 2523. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, C.C.; Ridoutt, B.G.; Wang, X.C.; Ren, P.A. Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J. Clean. Prod. 2017, 159, 171–179. [Google Scholar] [CrossRef]
- Wang, X.; Fan, J.; Xing, Y.; Xu, G.; Wang, H.; Deng, J.; Wang, Y.; Zhang, F.; Li, P.; Li, Z. The effects of mulch and nitrogen fertilizer on the soil environment of crop plants. Adv. Agron. 2019, 153, 121–173. [Google Scholar]
- Mack, U.D.; Feger, K.H.; Gong, Y.; Stahr, K. Soil water balance and nitrate leaching in winter wheat–summer maize double-cropping systems with different irrigation and N fertilization in the North China Plain. J. Plant Nutr. Soil Sci. 2005, 168, 454–460. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Zheng, S.; Shen, X.; Liu, D. Regulation effects of adding nitrogen on physiological properties and yield of rapeseed after waterlogging during seedling. Soil 2017, 49, 519–526. [Google Scholar]
- Chen, Z.K.; Tao, X.P.; Khan, A.; Tan, D.K.Y.; Luo, H.H. Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front. Plant Sci. 2018, 9, 00173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.; Kamran, M.; Xie, J.; Meng, X.; Han, Q.; Liu, T.; Han, J. Shoot and root traits of summer maize hybrid varieties with higher grain yields and higher nitrogen use efficiency at low nitrogen application rates. PeerJ 2019, 7, e7294. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmad, S.; Kamran, M.; Yang, X.N.; Hou, F.J.; Yang, B.P.; Ding, R.X.; Liu, T.; Han, Q.F. Uniconazole and nitrogen fertilization trigger photosynthesis and chlorophyll fluorescence, and delay leaf senescence in maize at a high population density. Photosynthetica 2021, 59, 192–202. [Google Scholar] [CrossRef]
- Liu, H.; Song, F.B.; Liu, S.Q.; Liu, F.L.; Zhu, X.C. Physiological response of maize and soybean to partial root-zone drying irrigation under N fertilization levels. Emir. J. Food Agr. 2018, 30, 364–371. [Google Scholar] [CrossRef]
- Tian, G.; Qi, D.; Zhu, J.; Xu, Y. Effects of nitrogen fertilizer rates and waterlogging on leaf physiological characteristics and grain yield of maize. Archiv. Agron. Soil Sci. 2021, 67, 863–875. [Google Scholar] [CrossRef]
- Lamptey, S.; Li, L.; Xie, J.; Zhang, R.; Yeboah, S.; Antille, D.L. Photosynthetic response of maize to nitrogen fertilization in the semiarid western loess plateau of China. Crop Sci. 2017, 57, 2739–2752. [Google Scholar] [CrossRef]
- Yu, P.; White, P.J.; Hochholdinger, F.; Li, C. Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta 2014, 240, 667–678. [Google Scholar] [CrossRef]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Botany 2013, 112, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Ahmad, S.; Ahmad, I.; Han, Q. Nitrogen fertilization affects maize grain yield through regulating nitrogen uptake, radiation and water use efficiency, photosynthesis and root distribution. PeerJ 2020, 8, e10291. [Google Scholar] [CrossRef]
- Saengwilai, P.; Nord, E.A.; Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol. 2014, 166, 726–735. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Chen, F.; Wu, Q.; Chen, Q.; Wang, J.; Yuan, L.; Mi, G. Genetic improvement of root growth increases maize yield via enhanced post-silking nitrogen uptake. Eur. J. Agron. 2015, 63, 55–61. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Chen, G.; Li, Q.; Wang, X. Effects of spraying uniconazole on leaf senescence and yield of maize at late growth stage. J. Maize Sci. 2009, 17, 86–88. [Google Scholar]
- Yong, C.W.; Wan, R.G.; Le, F.Y.; Yang, S.; Li, J.L.; He, Z.; Jing, L.; Shi, W. Physiological mechanisms of delaying leaf senescence in maize treated with compound mixtures of DCPTA and CCC. J. Northeast Agric. Univ. 2015, 22, 1–15. [Google Scholar]
- Ahmad, I.; Kamran, M.; Su, W.; Haiqi, W.; Ali, S.; Bilegjargal, B.; Ahmad, S.; Liu, T.; Cai, T.; Han, Q. Application of uniconazole improves photosynthetic efficiency of maize by enhancing the antioxidant defense mechanism and delaying leaf senescence in semiarid regions. J. Plant Growth Regul. 2019, 38, 855–869. [Google Scholar] [CrossRef]
- Ye, Y.X.; Wen, Z.R.; Huan, Y.; Lu, W.P.; Lu, D.L. Effects of post-silking water deficit on the leaf photosynthesis and senescence of waxy maize. J. Integr. Agric. 2020, 19, 2216–2228. [Google Scholar] [CrossRef]
- He, P.; Osaki, M.; Takebe, M.; Shinano, T.; Wasaki, J. Endogenous hormones and expression of senescence-related genes in different senescent types of maize. J. Exp. Bot. 2005, 56, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Cairns, J.E.; Sonder, K.; Zaidi, P.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.; Das, B.; Govaerts, B.; Vinayan, M. Maize production in a changing climate: Impacts, adaptation, and mitigation strategies. Adv. Agron. 2012, 114, 1–58. [Google Scholar]
- Ahmad, S.; Wang, G.Y.; Muhammad, I.; Chi, Y.X.; Zeeshan, M.; Nasar, J.; Zhou, X.B. Interactive effects of melatonin and nitrogen improve drought tolerance of maize seedlings by regulating growth and physiochemical attributes. Antioxidants 2022, 11, 359. [Google Scholar] [CrossRef]
- Markelz, R.C.; Strellner, R.S.; Leakey, A.D. Impairment of C4 photosynthesis by drought is exacerbated by limiting nitrogen and ameliorated by elevated [CO2] in maize. J. Exp. Botany 2011, 62, 3235–3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, Y.Z.; Shangguan, Z.P. Nitrogen deficiency limited the improvement of photosynthesis in maize by elevated CO2 under drought. J. Integrative Agric. 2014, 13, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Zhang, X.; Chen, C.J.; Zhou, M.G.; Wang, H.C. Effects of fungicides JS399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pest. Biochem. Physiol. 2010, 98, 151–157. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Khan, M.; Naeem, M. Salinity induced changes in growth, enzyme activities, photosynthesis, proline accumulation and yield in linseed genotypes. World J. Agric. Sci. 2007, 3, 685–695. [Google Scholar]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.N.; Al-Whaibi, M.H.; Bahkali, A.H. Nitrogen in relation to photosynthetic capacity and accumulation of osmoprotectant and nutrients in Brassica genotypes grown under salt stress. Agric. Sci. China 2010, 9, 671–680. [Google Scholar] [CrossRef]
- Ding, L.; Gao, C.; Li, Y.; Li, Y.; Zhu, Y.; Xu, G.; Shen, Q.; Kaldenhoff, R.; Kai, L.; Guo, S. The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP). Plant Sci. 2015, 234, 14–21. [Google Scholar] [CrossRef]
- Qiao, Y.; Ren, J.; Yin, L.; Liu, Y.; Deng, X.; Liu, P.; Wang, S. Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.X.; Zhang, W.J.; Lang, D.Y.; Zhang, X.J.; Cui, G.C.; Zhang, X.H. Interactions between endophytes and plants: Beneficial effect ofendophytes to ameliorate biotic and abiotic stresses in plants. J. Plant Biol. 2019, 62, 1–13. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Mosaad, I.S.; Al-Ghamdi, A.A.; Abbasi, A.M.; Zhou, X.B. Melatonin application alleviates stress-induced photosynthetic inhibition and oxidative damage by regulating antioxidant defense system of maize: A meta-analysis. Antioxidants 2022, 11, 512. [Google Scholar] [CrossRef]
- Gup, W.; Chen, B.; Liu, R.; Zhou, Z. Effects of nitrogen application rate on cotton leaf antioxidant enzyme activities and endogenous hormone contents under short-term waterlogging at flowering and boll-forming stage. Yingyong Shengtai Xuebao 2010, 21, 53–60. [Google Scholar]
- Zhang, L.X.; Li, S.X. Effects of nitrogen, potassium and glycinebetaine on the lipid peroxidation and protective enzyme activities in water-stressed summer maize. Acta Agron. Sin. 2007, 33, 482–490, (In Chinese with English Abstract). [Google Scholar]
- Özçubukçu, S.; Ergün, N.; Ilhan, E. Waterlogging and nitric oxide induce gene expression and increase antioxidant enzyme activity in wheat (Triticum aestivum L.). Acta Biol. Hung. 2014, 65, 47–60. [Google Scholar] [CrossRef]
- Zhou, S.M.; Zhang, M.; Zhang, K.K.; Yang, X.W.; He, D.X.; Jun, Y.; Wang, C.Y. Effects of reduced nitrogen and suitable soil moisture on wheat (Triticum aestivum L.) rhizosphere soil microbiological, biochemical properties and yield in the Huanghuai Plain, China. J. Integr. Agric. 2020, 19, 234–250. [Google Scholar] [CrossRef]
- Muhammad, I.; Yang, L.; Ahmad, S.; Zeeshan, M.; Farooq, S.; Ali, I.; Khan, A.; Zhou, X.B. Irrigation and nitrogen fertilization alter soil bacterial communities, soil enzyme activities, and nutrient availability in maize crop. Front. Microbiol. 2022, 3, 105. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielińska, E.J.; Wójcikowska-Kapusta, A.; Barbaś, P.; Skiba, D. Effect of differentiated nitrogen fertilization on the enzymatic activity of the soil for sweet potato (Ipomoea batatas L.[Lam.]) cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Pathan, S.I.; Ceccherini, M.T.; Pietramellara, G.; Puschenreiter, M.; Giagnoni, L.; Arenella, M.; Varanini, Z.; Nannpieri, P.; Renella, G. Enzyme activity and microbial community structure in the rhizosphere of two maize lines differing in N use efficiency. Plant Soil 2015, 387, 413–424. [Google Scholar] [CrossRef]
- Xing, S.; Chen, C.; Zhou, B.; Zhang, H.; Nang, Z.; Xu, Z. Soil soluble organic nitrogen and active microbial characteristics under adjacent coniferous and broadleaf plantation forests. J. Soils Sedim. 2010, 10, 748–757. [Google Scholar] [CrossRef]
- Gloser, V.; Zwieniecki, M.A.; Orians, C.M.; Holbrook, N.M. Dynamic changes in root hydraulic properties in response to nitrate availability. J. Exp. Bot. 2007, 58, 2409–2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacAdam, J.W.; Nelson, C.J.; Sharp, R.E. Peroxidase activity in the leaf elongation zone of tall fescue: I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol. 1992, 99, 872–878. [Google Scholar] [CrossRef] [Green Version]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Schneider, K.; Schlegel, H. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem. J. 1981, 193, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Ohto, M.-A.; Onai, K.; Furukawa, Y.; Aoki, E.; Araki, T.; Nakamura, K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 2001, 127, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Weisany, W.; Sohrabi, Y.; Heidari, G.; Siosemardeh, A.; Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics 2012, 5, 60. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]


| Irrigation | N-Fertilizer | Rows Number | Kernels Per Row | Kernels Per Ear | Ear Length (cm) | Ear Diameter (cm) | Kernels Yield (g Plant−1) |
|---|---|---|---|---|---|---|---|
| HW | N0 | 11.0 ± 0.6 b | 10 ± 1.0 b | 108 ± 13.6 c | 7.5 ± 0.5 b | 4.0 ± 0.2 a | 32.2 ± 0.6 d |
| N1 | 14 ± 0.5 a | 13 ± 1.3 a | 169 ± 19.3 b | 8.4 ± 0.3 b | 4.2 ± 0.1 a | 47.1 ± 2.2 c | |
| N2 | 14 ± 0.5 a | 13 ± 0.9 a | 177 ± 3.1 b | 8.6 ± 0.3 b | 4.3 ± 0.1 a | 38.5 ± 0.2 bc | |
| N3 | 14 ± 0.5 a | 14 ± 0.5 a | 199 ± 16.5 ab | 8.8 ± 0.7 b | 4.3 ± 0.1 a | 39.4 ± 2.2 ab | |
| N4 | 15 ± 0.5 a | 14 ± 0.8 a | 220 ± 17.1 a | 11.0 ± 0.7 a | 4.3 ± 0.1 a | 44.2 ± 1.3 a | |
| LW | N0 | 12 ± 1.3 c | 11 ± 1.5 b | 119 ± 11.0 b | 8.3 ± 1.0 b | 4.1 ± 0.2 c | 27.9 ± 1.7 d |
| N1 | 13 ± 0.6 bc | 15 ± 1.9 ab | 197 ± 18.5 a | 9.3 ± 0.9 b | 4.2 ± 0.3 bc | 37.6 ± 1.8 c | |
| N2 | 14 ± 1.0 abc | 17 ± 1.6 a | 222 ± 18.5 a | 10.0 ± 0.5 b | 4.3 ± 0.1 bc | 57.2 ± 1.0 b | |
| N3 | 16 ± 0.8 a | 18 ± 1.0 a | 259 ± 22.7 a | 12.3 ± 0.2 a | 4.9 ± 0.1 a | 70.0 ± 1.2 a | |
| N4 | 15 ± 1.0 ab | 17 ± 1.1 a | 234 ± 21.3 a | 10.1 ± 0.7 ab | 4.7 ± 0.1 ab | 60.1 ± 0.9 b |
| Parameters | Irrigation | Nitrogen | Stage | Irrigation × Nitrogen | Stage × Irrigation | Stage × Nitrogen | Stage × Irrigation × Nitrogen |
|---|---|---|---|---|---|---|---|
| Number of rows/ear | 0.367 | <0.001 | --- | 0.442 | --- | --- | --- |
| Kernels per row | 0.021 | <0.001 | --- | 0.775 | --- | --- | --- |
| Kernels per ear | 0.005 | <0.001 | --- | 0.578 | --- | --- | --- |
| Ear length | 0.029 | 0.002 | --- | 0.045 | --- | --- | --- |
| Ear diameter | 0.043 | 0.007 | --- | 0.248 | --- | --- | --- |
| Kernel weight | <0.001 | <0.001 | --- | <0.001 | --- | --- | --- |
| Shoot dry matter | 0.009 | <0.001 | --- | 0.035 | --- | --- | --- |
| Root dry matter | 0.002 | <0.001 | --- | 0.328 | --- | --- | --- |
| Plant height | <0.001 | <0.001 | <0.001 | <0.001 | 0.006 | <0.001 | <0.001 |
| Leaf area | 0.035 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Malondialdehyde | <0.001 | <0.001 | <0.001 | 0.009 | 0.006 | 0.002 | <0.001 |
| Hydrogen peroxide | 0.002 | <0.001 | <0.001 | 0.002 | 0.065 | 0.002 | <0.001 |
| Superoxide anion | <0.001 | <0.001 | <0.001 | <0.001 | 0.043 | <0.001 | 0.005 |
| Proline | <0.001 | <0.001 | <0.001 | 0.004 | 0.018 | <0.001 | <0.001 |
| Superoxide dismutase | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 |
| Peroxidase | 0.005 | <0.001 | <0.001 | <0.001 | 0.040 | <0.001 | <0.001 |
| Ascorbate peroxidase | <0.001 | <0.001 | <0.001 | 0.040 | 0.001 | <0.001 | <0.001 |
| Chlorophyll a | <0.001 | <0.001 | <0.001 | <0.001 | 0.024 | <0.001 | <0.001 |
| Chlorophyll b | 0.016 | <0.001 | <0.001 | <0.001 | 0.019 | 0.002 | 0.017 |
| Irrigation | N-Fertilizer | Number of Roots | Total Root Length (cm) | Root Diameter (mm) | Root Surface Area (cm2) | Root Volume (mm3) |
|---|---|---|---|---|---|---|
| HW | N0 | 1829 e | 856.69 e | 0.8 b | 162.3 d | 5092.3 c |
| N1 | 3014 d | 1267.60 d | 1.0 a | 412.0 c | 19,579.8 b | |
| N2 | 3584 c | 1539.53 c | 0.9 a | 458.9 c | 20,793.1 b | |
| N3 | 4222 b | 1855.38 b | 0.9 a | 545.9 b | 24,704.9 b | |
| N4 | 5183 a | 2359.84 a | 1.0 a | 720.1 a | 35,036.3 a | |
| LW | N0 | 2323 c | 663.45 e | 0.7 c | 160.0 c | 20,197.8 c |
| N1 | 2868 c | 1172.20 d | 1.1 a | 398.4 b | 21,697.8 c | |
| N2 | 3699 b | 1599.53 c | 0.9 b | 472.4 b | 21,571.4 c | |
| N3 | 6256 a | 3148.81 a | 0.9 b | 881.3 a | 47,005.9 a | |
| N4 | 4397 b | 2306.67 b | 1.1 a | 818.6 a | 37,606.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, I.; Yang, L.; Ahmad, S.; Farooq, S.; Al-Ghamdi, A.A.; Khan, A.; Zeeshan, M.; Elshikh, M.S.; Abbasi, A.M.; Zhou, X.-B. Nitrogen Fertilizer Modulates Plant Growth, Chlorophyll Pigments and Enzymatic Activities under Different Irrigation Regimes. Agronomy 2022, 12, 845. https://doi.org/10.3390/agronomy12040845
Muhammad I, Yang L, Ahmad S, Farooq S, Al-Ghamdi AA, Khan A, Zeeshan M, Elshikh MS, Abbasi AM, Zhou X-B. Nitrogen Fertilizer Modulates Plant Growth, Chlorophyll Pigments and Enzymatic Activities under Different Irrigation Regimes. Agronomy. 2022; 12(4):845. https://doi.org/10.3390/agronomy12040845
Chicago/Turabian StyleMuhammad, Ihsan, Li Yang, Shakeel Ahmad, Saqib Farooq, Abdullah Ahmed Al-Ghamdi, Ahmad Khan, Muhammad Zeeshan, Mohamed S. Elshikh, Arshad Mehmood Abbasi, and Xun-Bo Zhou. 2022. "Nitrogen Fertilizer Modulates Plant Growth, Chlorophyll Pigments and Enzymatic Activities under Different Irrigation Regimes" Agronomy 12, no. 4: 845. https://doi.org/10.3390/agronomy12040845
APA StyleMuhammad, I., Yang, L., Ahmad, S., Farooq, S., Al-Ghamdi, A. A., Khan, A., Zeeshan, M., Elshikh, M. S., Abbasi, A. M., & Zhou, X.-B. (2022). Nitrogen Fertilizer Modulates Plant Growth, Chlorophyll Pigments and Enzymatic Activities under Different Irrigation Regimes. Agronomy, 12(4), 845. https://doi.org/10.3390/agronomy12040845








