Arable Weeds at the Edges of Kettle Holes as Overwintering Habitat for Phytopathogenic Fungi
Abstract
1. Introduction
2. Materials and Methods
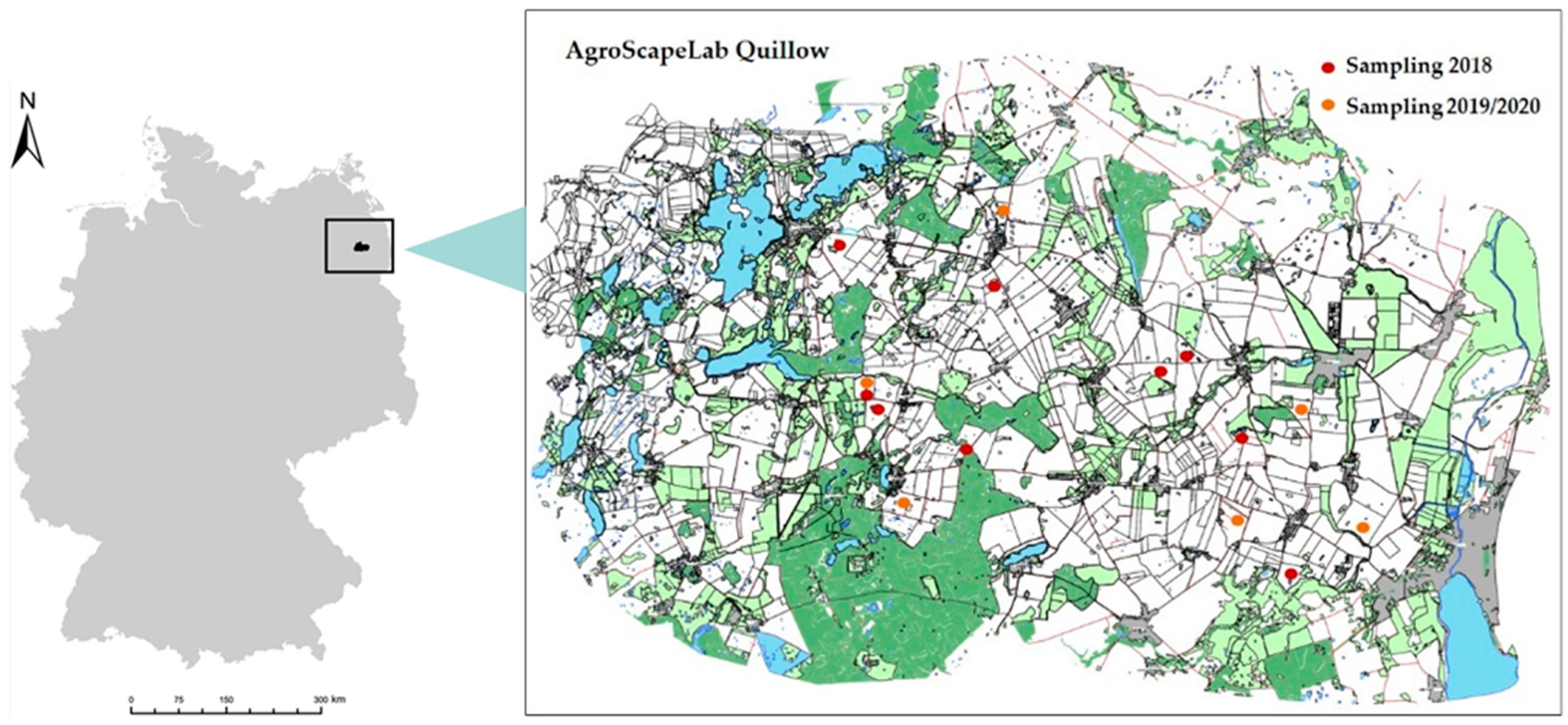
2.1. Study Site
2.2. Sampling Design
2.3. Laboratory Analyses
2.3.1. Culture-Dependent Method
2.3.2. Culture-Independent Method (qPCR Approach)
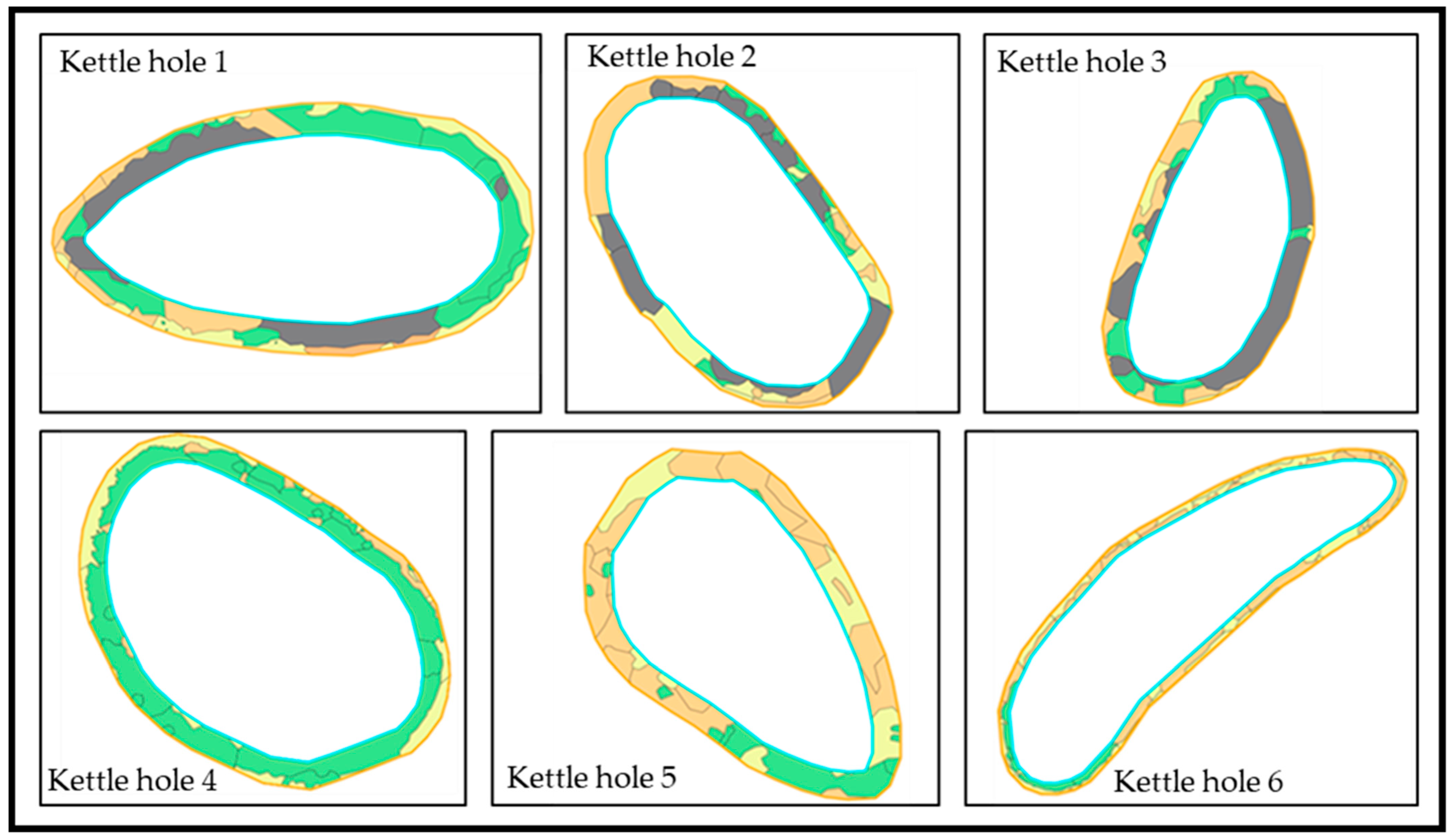
2.4. Mapping of the Kettle Hole Edge Vegetation
2.5. Statistics
3. Results
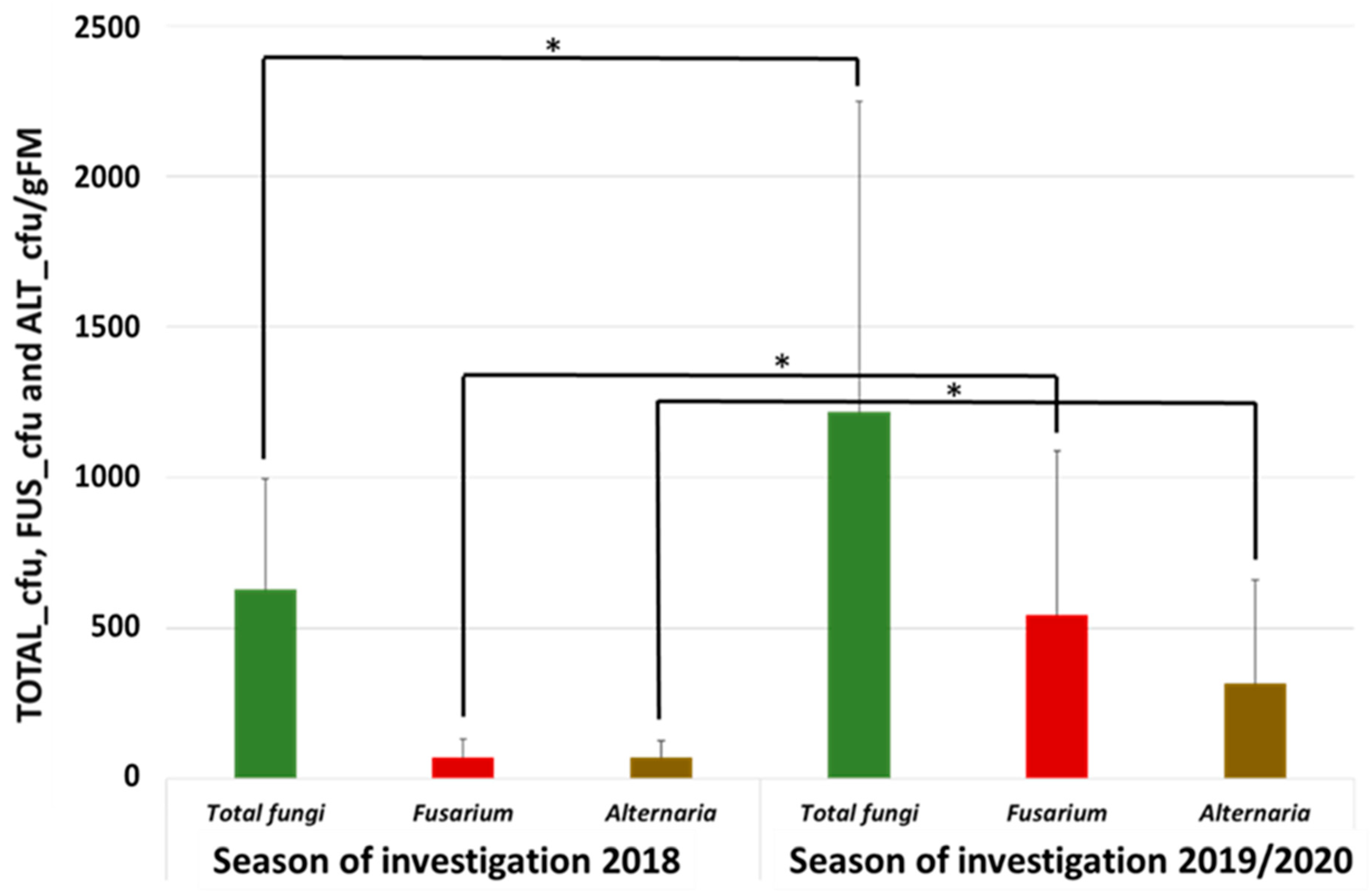
3.1. Abundance of Total Fungi, Fusarium, and Alternaria in the Autumn/Winter Months Influenced by Weather Conditions
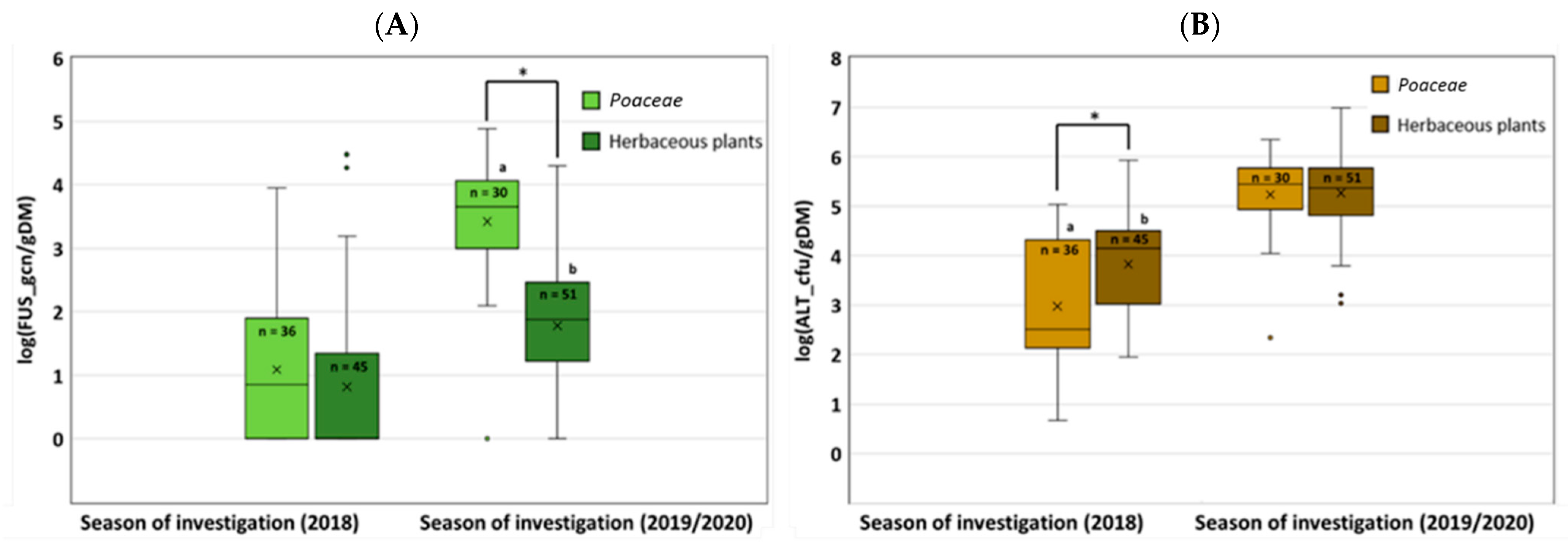
3.2. Abundance of Fusarium and Alternaria on Poaceae and Herbaceous Plants (qPCR Approach)
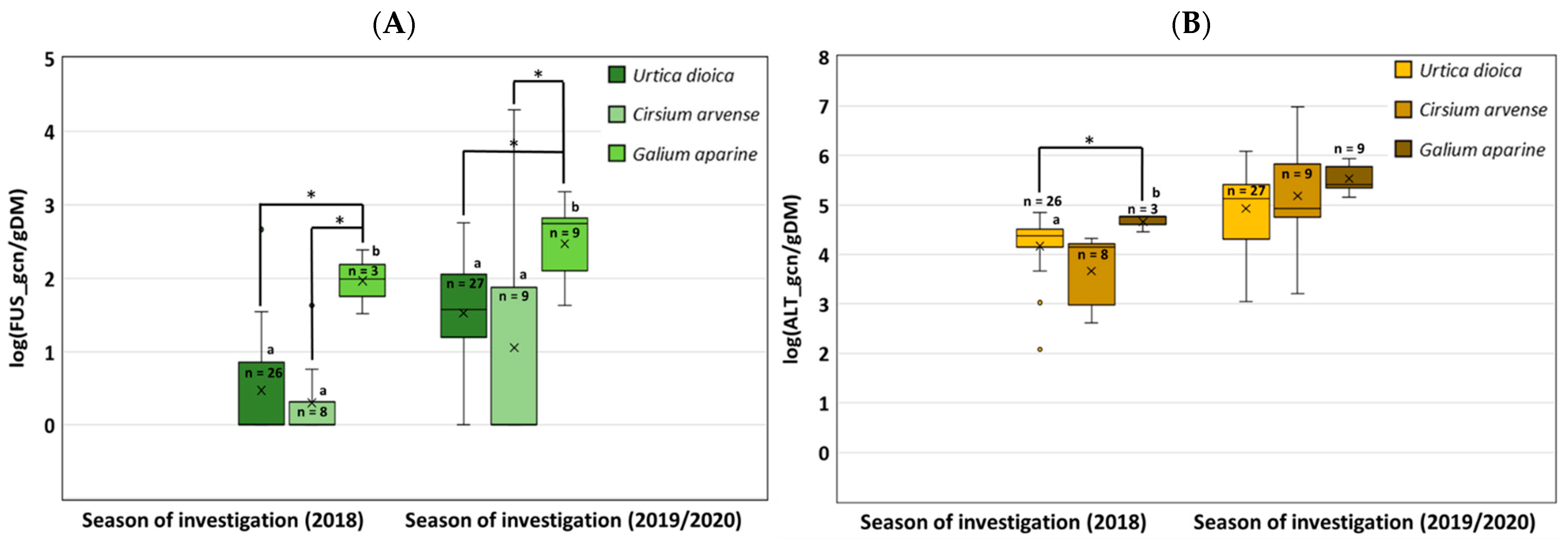
3.3. Community Composition of Different Fusarium Species Isolated from Arable Weeds (Poaceae and Herbaceous Plants)
3.4. The Abundance of Plants of the Family of Poaceae and Herbaceous Plants at the Edges of Kettle Holes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; Mcroberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases—A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Kasprzyk, I.; Sulborska, A.; Nowak, M.; Szymańska, A.; Kaczmarek, J.; Haratym, W.; Weryszko-Chmielewska, E.; Jędryczka, M. Fluctuation range of the concentration of airborne Alternaria conidiospores sampled at different geographical locations in Poland (2010–2011). Acta Agrobot. 2013, 66, 65–76. [Google Scholar] [CrossRef][Green Version]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing: Hoboken, NJ, USA, 2006; ISBN 9788578110796. [Google Scholar]
- Rampersad, S.N. Pathogenomics and management of Fusarium diseases in plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Schiro, G.; Verch, G.; Grimm, V.; Müller, M.E.H. Alternaria and Fusarium fungi: Differences in distribution and spore deposition in a topographically heterogeneous wheat field. J. Fungi 2018, 4, 63. [Google Scholar] [CrossRef]
- Buerstmayr, M.; Steiner, B.; Buerstmayr, H. Breeding for Fusarium head blight resistance in wheat—Progress and challenges. Plant Breed. 2020, 139, 429–454. [Google Scholar] [CrossRef]
- Landschoot, S.; Audenaert, K.; Waegeman, W.; Pycke, B.; Bekaert, B.; De Baets, B.; Haesaert, G. Connection between primary Fusarium inoculum on gramineous weeds, crop residues and soil samples and the final population on wheat ears in Flanders, Belgium. Crop Prot. 2011, 30, 1297–1305. [Google Scholar] [CrossRef]
- Miedaner, T.; Gwiazdowska, D.; Waśkiewicz, A. Editorial: Management of Fusarium species and their mycotoxins in cereal food and feed. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Leplat, J.; Friberg, H.; Abid, M.; Steinberg, C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron. Sustain. Dev. 2013, 33, 97–111. [Google Scholar] [CrossRef]
- Stack, R.W. A comparison of the inoculum potential of ascospores and conidia of Gibberella zeae. Can. J. Plant Pathol. 1989, 11, 137–142. [Google Scholar] [CrossRef]
- Paul, P.A.; El-Allaf, S.M.; Lipps, P.E.; Madden, L.V. Rain Splash Dispersal of Gibberella zeae Within Wheat Canopies in Ohio. Phytopathology 2004, 94, 1342–1349. [Google Scholar] [CrossRef]
- Rossi, V.; Languasco, L.; Pattori, E.; Giosuè, S. Dynamics of airborne Fusarium macroconidia in wheat fields naturally affected by head blight. J. Plant Pathol. 2002, 84, 53–64. [Google Scholar]
- Gorash, A.; Armonienė, R.; Kazan, K. Can effectoromics and loss-of-susceptibility be exploited for improving Fusarium head blight resistance in wheat? Crop J. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Pereyra, S.A.; Dill-Macky, R.; Sims, A.L. Survival and Inoculum Production of Gibberella zeae in Wheat Residue. Plant Dis. 2004, 88, 724–730. [Google Scholar] [CrossRef]
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.Y.; Leslie, J.F. Biogeography and phylogeography of Fusarium: A review. Fungal Divers. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Sneideris, D.; Ivanauskas, A.; Suproniene, S.; Kadziene, G.; Sakalauskas, S. Genetic diversity of Fusarium graminearum isolated from weeds. Eur. J. Plant Pathol. 2019, 153, 639–643. [Google Scholar] [CrossRef]
- Pereyra, S.A.; Dill-Macky, R. Colonization of the residues of diverse plant species by Gibberella zeae and their contribution to Fusarium head blight inoculum. Plant Dis. 2008, 92, 800–807. [Google Scholar] [CrossRef]
- Suproniene, S.; Kadziene, G.; Irzykowski, W.; Sneideris, D.; Ivanauskas, A.; Sakalauskas, S.; Serbiak, P.; Svegzda, P.; Auskalniene, O.; Jedryczka, M. Weed species within cereal crop rotations can serve as alternative hosts for Fusarium graminearum causing Fusarium head blight of wheat. Fungal Ecol. 2019, 37, 30–37. [Google Scholar] [CrossRef]
- Suproniene, S.; Kadziene, G.; Irzykowski, W.; Sneideris, D.; Ivanauskas, A.; Sakalauskas, S.; Serbiak, P.; Svegzda, P.; Kelpsiene, J.; Pranaitiene, S.; et al. Asymptomatic weeds are frequently colonised by pathogenic species of Fusarium in cereal-based crop rotations. Weed Res. 2019, 59, 312–323. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological responses to habitat edges: Mechanisms, models, and variability explained. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Raatz, L.; Bacchi, N.; Pirhofer Walzl, K.; Glemnitz, M.; Müller, M.E.H.; Joshi, J.; Scherber, C. How much do we really lose?—Yield losses in the proximity of natural landscape elements in agricultural landscapes. Ecol. Evol. 2019, 9, 7838–7848. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Kelly, A.C.; Clear, R.M.; O’Donnell, K.; McCormick, S.; Turkington, T.K.; Tekauz, A.; Gilbert, J.; Kistler, H.C.; Busman, M.; Ward, T.J. Diversity of Fusarium head blight populations and trichothecene toxin types reveals regional differences in pathogen composition and temporal dynamics. Fungal Genet. Biol. 2015, 82, 22–31. [Google Scholar] [CrossRef]
- Fuller, R.J.; Gregory, R.D.; Gibbons, D.W.; Marchant, J.H.; Wilson, J.D.; Baillie, S.R.; Carter, N. Population Declines and Range Contractions Among Lowland Farmland Birds in Britain. Conserv. Biol. 1995, 9, 1425–1441. [Google Scholar] [CrossRef]
- Staley, J.T.; Sparks, T.H.; Croxton, P.J.; Baldock, K.C.R.; Heard, M.S.; Hulmes, S.; Hulmes, L.; Peyton, J.; Amy, S.R.; Pywell, R.F. Long-term effects of hedgerow management policies on resource provision for wildlife. Biol. Conserv. 2012, 145, 24–29. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; McCracken, M.; Chapman, R.E.; Ball, S.L.; Edwards, M.E.; Nowakowski, M.; Pywell, R.F. Spill-over of pest control and pollination services into arable crops. Agric. Ecosyst. Environ. 2016, 231, 15–23. [Google Scholar] [CrossRef]
- Lozada-Gobilard, S.; Landivar Albis, C.M.; Rupik, K.B.; Pätzig, M.; Hausmann, S.; Tiedemann, R.; Joshi, J. Habitat quality and connectivity in kettle holes enhance bee diversity in agricultural landscapes. Agric. Ecosyst. Environ. 2021, 319, 107525. [Google Scholar] [CrossRef]
- Kalettka, T.; Rudat, C. Hydrogeomorphic types of glacially created kettle holes in North-East Germany. Limnologica 2006, 36, 54–64. [Google Scholar] [CrossRef]
- Pätzig, M.; Kalettka, T.; Glemnitz, M.; Berger, G. What governs macrophyte species richness in kettle hole types? A case study from Northeast Germany. Limnologica 2012, 42, 340–354. [Google Scholar] [CrossRef]
- Platen, R.; Kalettka, T.; Ulrichs, C. Kettle holes in the agrarian landscape: Isolated and ecological unique habitats for carabid beetles (col.: Carabidae) and spiders (arach.: Araneae). J. Landsc. Ecol. 2016, 9, 29–30. [Google Scholar] [CrossRef]
- Raatz, L.; Pirhofer Walzl, K.; Müller, M.E.H.; Scherber, C.; Joshi, J. Who is the culprit: Is pest infestation responsible for crop yield losses close to semi-natural habitats? Ecol. Evol. 2021, 11, 13232–13246. [Google Scholar] [CrossRef]
- Brandenburgisches Naturschutzgesetz-BbgNatSchGM; § 32 Schutz Bestimmter Biotope. Available online: https://bravors.brandenburg.de/de/gesetze-214595#32 (accessed on 13 October 2021).
- Nitzsche, K.N.; Kalettka, T.; Premke, K.; Lischeid, G.; Gessler, A.; Kayler, Z.E. Land-use and hydroperiod affect kettle hole sediment carbon and nitrogen biogeochemistry. Sci. Total Environ. 2017, 574, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Nitzsche, K.N.; Kaiser, M.; Premke, K.; Gessler, A.; Ellerbrock, R.H.; Hoffmann, C.; Kleeberg, K.; Kayler, Z.E. Organic matter distribution and retention along transects from hilltop to kettle hole within an agricultural landscape. Biogeochemistry 2017, 136, 47–70. [Google Scholar] [CrossRef]
- Nierenberg, H. Untersuchungen über die Morphologische und Biologische Differenzierung in der Fusarium-Sektion Liseola. In Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem-169; Paul Parey: Berlin, Germany, 1976. [Google Scholar]
- Müller, T.; Ruppel, S.; Behrendt, U.; Lentzsch, P.; Müller, M.E.H. Antagonistic Potential of Fluorescent Pseudomonads Colonizing Wheat Heads Against Mycotoxin Producing Alternaria and Fusaria. Front. Microbiol. 2018, 9, 2124. [Google Scholar] [CrossRef]
- Pätzig, M.; Düker, E. Dynamic of dominant plant communities in kettle holes (northeast Germany) during a five-year period of extreme weather conditions. Water 2021, 13, 688. [Google Scholar] [CrossRef]
- Anwar, S.; Javed, N.; Shakeel, Q. Weeds as reservoir of nematodes. Pak. J. Nematol. 2009, 27, 145–153. [Google Scholar]
- Kumar, S.; Bhowmick, M.K.; Ray, P. Weeds as alternate and alternative hosts of crop pests. Indian J. Weed Sci. 2021, 53, 14–29. [Google Scholar] [CrossRef]
- Dinoor, A. Role of wild and cultivated plants in the epidemiology of plant diseases in israel. Annu. Rev. Phytopathol. 1974, 12, 413–436. [Google Scholar] [CrossRef]
- Wisler, G.C.; Norris, R.F. Interactions between Weeds and Cultivated Plants as Related to Management of Plant Pathogens. Weeds Sci. 2005, 53, 914–917. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, A.B.; Rai, M.K.; Kamal, S.; Ballabh, S.; Patel, B. Status, constraints and strategies of integrated pest management in vegetable crops. Progress. Hortic. 2009, 41, 46–53. [Google Scholar]
- Postic, J.; Cosic, J.; Vrandecic, K.; Jurkovic, D.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium species isolated from weeds and plant debris in Croatia. J. Phytopathol. 2012, 160, 76–81. [Google Scholar] [CrossRef]
- Mourelos, C.A.; Malbrán, I.; Balatti, P.A.; Ghiringhelli, P.D.; Lori, G.A. Gramineous and non-gramineous weed species as alternative hosts of Fusarium graminearum, causal agent of Fusarium head blight of wheat, in Argentina. Crop Prot. 2014, 65, 100–104. [Google Scholar] [CrossRef]
- Lofgren, L.A.; Leblanc, N.R.; Certano, A.K.; Nachtigall, J.; Labine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2018, 217, 1203–1212. [Google Scholar] [CrossRef]
- Inch, S.; Gilbert, J. The incidence of Fusarium species recovered from inflorescences of wild grasses in southern Manitoba. Can. J. Plant Pathol. 2003, 25, 379–383. [Google Scholar] [CrossRef]
- Levins, R.; Miranda, I. Mathematical Models In Crop Protection. Rev. Protección Veg. 2007, 22, 1–17. [Google Scholar]
- Billeter, R.; Liira, J.; Bailey, D.; Bugter, R.; Arens, P.; Augenstein, I.; Aviron, S.; Baudry, J.; Bukacek, R.; Burel, F.; et al. Indicators for biodiversity in agricultural landscapes: A pan-European study. J. Appl. Ecol. 2008, 45, 141–150. [Google Scholar] [CrossRef]
- Balmford, B.; Green, R.E.; Onial, M.; Phalan, B.; Balmford, A. How imperfect can land sparing be before land sharing is more favourable for wild species? J. Appl. Ecol. 2019, 56, 73–84. [Google Scholar] [CrossRef]
- Dong, F.; Xu, J.; Zhang, X.; Wang, S.; Xing, Y.; Mokoena, M.P.; Olaniran, A.O.; Shi, J. Gramineous weeds near paddy fields are alternative hosts for the Fusarium graminearum species complex that causes fusarium head blight in rice. Plant Pathol. 2020, 69, 433–441. [Google Scholar] [CrossRef]
- Walsh, J.L.; Laurence, M.H.; Liew, E.C.Y.; Sangalang, A.E.; Burgess, L.W.; Summerell, B.A.; Petrovic, T. Fusarium: Two endophytic novel species from tropical grasses of northern Australia. Fungal Divers. 2010, 44, 149–159. [Google Scholar] [CrossRef]
- Purss, G.S. The relationship between strains of Fusarium graminearum schwabe causing crown rot of various gramineous hosts and stalk rot of maize in Queensland. Aust. J. Agric. Res. 1969, 20, 257–264. [Google Scholar] [CrossRef]
- Costa, M.M.; Melo, M.P.; Carmo, F.S.; Moreira, G.M.; Guimarães, E.A.; Rocha, F.S.; Costa, S.S.; Abreu, L.M.; Pfenning, L.H. Fusarium species from tropical grasses in Brazil and description of two new taxa. Mycol. Prog. 2021, 20, 61–72. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Fernández, M.D.; Stenglein, S.A.; Dinolfo, M.I. Fusarium species richness in mono- and dicotyledonous weeds and their ability to infect barley and wheat. Mycol. Prog. 2021, 20, 1203–1216. [Google Scholar] [CrossRef]
- Fulcher, M.R.; Garcia, J.P.; Damann, K.C.M.; Bergstrom, G.C. Variable interactions between non-cereal grasses and Fusarium graminearum. Can. J. Plant Pathol. 2019, 41, 450–456. [Google Scholar] [CrossRef]
- Keller, M.D.; Bergstrom, G.C.; Shields, E.J. The aerobiology of Fusarium graminearum. Aerobiologia 2014, 30, 123–136. [Google Scholar] [CrossRef]
- Backhouse, D.; Abubakar, A.A.; Burgess, L.W.; Dennis, J.I.; Hollaway, G.J.; Wildermuth, G.B.; Wallwork, H.; Henry, F.J. Survey of Fusarium species associated with crown rot of wheat and barley in eastern Australia. Australas. Plant Pathol. 2004, 33, 255–261. [Google Scholar] [CrossRef]
- Waggoner, P.E.; Aylor, D.E. Epidemiology: A Science of Patterns. Annu. Rev. Phytopathol. 2000, 38, 71–94. [Google Scholar] [CrossRef]
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium head blight on small-grain cereals. Int. J. Food Microbiol. 2007, 119, 103–108. [Google Scholar] [CrossRef]
- Orina, A.S.; Gavrilova, O.P.; Gogina, N.N.; Gannibal, P.B.; Gagkaeva, T.Y. Natural Occurrence of Alternaria Fungi and Associated Mycotoxins in Small-Grain Cereals from the Urals and West Siberia Regions of Russia. Toxins 2021, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.M.; Bolat, N.; Bagci, A.; Trethowan, R.T.; William, M.; Hekimhan, H.; Yildirim, A.F.; Sahin, E.; Elekcioglu, H.; Toktay, H.; et al. The International Breeding Strategy for the Incorporation of Resistance in Bread Wheat Against the Soil Borne Pathogens (Dryland Root Rot and Cyst and Lesion Cereal Nematodes) Using Conventional and Molecular Tools. In Wheat Production in Stressed Environments; Springer: Dordrecht, The Netherlands, 2007; pp. 125–137. [Google Scholar]
- Sitton, J.W.; Cook, R.J. Comparative Morphology and Survival of Chlamydospores of Fusarium roseum “Culmorum” and “Graminearum”. Phytopathology 1981, 71, 85–90. [Google Scholar] [CrossRef]
- Smiley, R.W.; Patterson, L.-M. Pathogenic Fungi Associated with Fusarium Foot Rot of Winter Wheat in the Semiarid Pacific Northwest. Plant Dis. 1996, 80, 944–949. [Google Scholar] [CrossRef]
- Poole, G.J.; Smiley, R.W.; Walker, C.; Huggins, D.; Rupp, R.; Abatzoglou, J.; Garland-Campbell, K.; Paulitz, T.C. Effect of climate on the distribution of Fusarium spp. causing crown rot of wheat in the Pacific Northwest of the United States. Phytopathology 2013, 103, 1130–1140. [Google Scholar] [CrossRef]
- Gavrilova, O.P.; Orina, A.S.; Gogina, N.N.; Gagkaeva, T.Y. Co-occurrence of the Metabolites of Alternaria and Fusarium Fungi Associated with Small-Grain Cereals. Russ. Agric. Sci. 2021, 47, 37–41. [Google Scholar] [CrossRef]
- Kosiak, B.; Torp, M.; Skjerve, E.; Andersen, B. Alternaria and Fusarium in Norwegian grains of reduced quality - A matched pair sample study. Int. J. Food Microbiol. 2004, 93, 51–62. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Darwish, A.G.; Nafady, N.A.; Ibrahim, N.A. Fusarium: Biodiversity, Ecological Significances, and Industrial Applications. In Recent Advancement in White Biotechnology Through Fungi; Springer Nature Switzerland AG: Bern, Switzerland, 2019; Volume 1, pp. 201–261. [Google Scholar]
- Phan, H.T.; Burgess, L.W.; Summerell, B.A.; Bullock, S.; Liew, E.C.Y.; Smith-White, J.L.; Clarkson, J.R. Gibberella gaditjirrii (Fusarium gaditjirrii) sp. nov., a new species from tropical grasses in Australia. Stud. Mycol. 2004, 50, 261–272. [Google Scholar]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium incarnatum-equiseti complex from China. Persoonia Mol. Phylogeny Evol. Fungi 2019, 43, 70–89. [Google Scholar] [CrossRef]
- Linde, C.C.; Smith, L.M.; Peakall, R. Weeds, as ancillary hosts, pose disproportionate risk for virulent pathogen transfer to crops. BMC Evol. Biol. 2016, 16, 101. [Google Scholar] [CrossRef]
- Köhl, J.; De Haas, B.H.; Kastelein, P.; Burgers, S.L.G.E.; Waalwijk, C. Population dynamics of Fusarium spp. and Microdochium nivale in crops and crop residues of winter wheat. Phytopathology 2007, 97, 971–978. [Google Scholar] [CrossRef]
- Paulitz, T.C. Diurnal Release of Ascospores by Gibberella zeae in Inoculated Wheat Plots. Plant Dis. 1996, 80, 674–678. [Google Scholar] [CrossRef]
- Helbig, J.B.; Carroll, R.B. Dicotyledonous Weeds as a Source of Fusarium oxysporum Pathogenic on Soybean. Plant Dis. 1984, 68, 694–696. [Google Scholar] [CrossRef]
- Goertz, A.; Zuehlke, S.; Spiteller, M.; Steiner, U.; Dehne, H.W.; Waalwijk, C.; de Vries, I.; Oerke, E.C. Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 2010, 128, 101–111. [Google Scholar] [CrossRef]
- Fallahi, M.; Saremi, H.; Javan-Nikkhah, M.; Somma, S.; Haidukowski, M.; Logrieco, A.F.; Moretti, A. Isolation, molecular identification and mycotoxin profile of fusarium species isolated from Maize Kernels in Iran. Toxins 2019, 11, 297. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in flanders’ fields: Occurrence and correlations with fusarium species in whole-plant harvested maize. Microorganisms 2019, 7, 571. [Google Scholar] [CrossRef]
- Pfordt, A.; Romero, L.R.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of environmental conditions and agronomic practices on the prevalence of fusarium species associated with ear-and stalk rot in maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of Wheat and Barley: A Re-emerging Disease of Devastating Impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef]
- Manghwar, H.; Naz, S.; Javed Chaudhary, H.; Farooq Hussain Munis, M.; Masood, S.; Bakr Umer Farooq, A. Diagnosis of Fusarium graminearum in Soil and Plant Samples of Wheat by Real-Time PCR. Rom. Biotechnol. Lett. 2018, 23, 14035. [Google Scholar] [CrossRef]
- Lori, G.A.; Sisterna, M.N.; Sarandón, S.J.; Rizzo, I.; Chidichimo, H. Fusarium head blight in wheat: Impact of tillage and other agronomic practices under natural infection. Crop Prot. 2009, 28, 495–502. [Google Scholar] [CrossRef]
- Karimi, K.; Arzanlou, M.; Pertot, I. Weeds as potential inoculum reservoir for Colletotrichum nymphaeae causing strawberry anthracnose in Iran and Rep-PCR fingerprinting as useful marker to Differentiate C. acutatum complex on strawberry. Front. Microbiol. 2019, 10, 129. [Google Scholar] [CrossRef]
- Malcolm, G.M.; Kuldau, G.A.; Gugino, B.K.; Jiménez-Gasco, M.D.M. Hidden host plant associations of soilborne fungal pathogens: An ecological perspective. Phytopathology 2013, 103, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; McAuslane, H.J.; Adkins, S.T.; Smith, H.A.; Dufault, N.; Webb, S.E. Transmission of Squash vein yellowing virus to and from Cucurbit Weeds and Effects on Sweetpotato Whitefly (Hemiptera: Aleyrodidae) Behavior. Environ. Entomol. 2016, 45, 967–973. [Google Scholar] [CrossRef] [PubMed]


| Season | Weed Species | Number of Samples |
|---|---|---|
| 2018 | Urtica dioica | 27 |
| Phragmites australis | 12 | |
| Cirsium arvense | 9 | |
| Galium aparine | 3 | |
| Sisymbrium loeselii | 3 | |
| Arctium sp. | 3 | |
| Grasses 1 | 24 | |
| 2019/2020 | Urtica dioica | 27 |
| Cirsium arvense | 9 | |
| Galium aparine | 9 | |
| Phragmites australis | 3 | |
| Rumex sp. | 3 | |
| Tanacetum vulgare | 3 | |
| Grasses 1 | 27 |
| Air Temperature (Mean in °C) | Precipitation (Sum in mm) | ||||||
|---|---|---|---|---|---|---|---|
| Month | 2018 | 2019 | 2020 | Month | 2018 | 2019 | 2020 |
| January | 2.7 | 1.1 | 4.4 | January | 70.4 | 37.5 | 23.4 |
| February | −2.2 | 4.2 | 5.6 | February | 13.6 | 15.2 | 40.8 |
| March | 0.7 | 6.6 | 4.7 | March | 39.5 | 20.4 | 24.5 |
| April | 11.9 | 9.6 | 9.1 | April | 30.5 | 6.7 | 17.5 |
| May | 16.2 | 11.7 | 12.1 | May | 14 | 47.8 | 18.3 |
| June | 18.4 | 20.9 | 18.1 | June | 15.9 | 86.6 | 44.3 |
| July | 20.8 | 18.9 | 17.7 | July | 50.3 | 51.7 | 38.4 |
| August | 20.7 | 20.1 | 20.2 | August | 21.3 | 18.7 | 65.4 |
| September | 15.7 | 14.6 | 14.7 | September | 10 | 61.4 | 59.6 |
| October | 10.4 | 10.6 | 10.9 | October | 15.5 | 47.1 | 49 |
| November | 4.6 | 5.9 | n.a. | November | 17.9 | 50.3 | n.a. |
| December | 4.0 | 3.9 | n.a. | December | 47.2 | 16.4 | n.a. |
| Annual mean | 10.4 | 10.7 | n.a. | Sum year | 346.1 | 459.8 | n.a. |
| Long-time average | 8.6 | Long-time average | 563.8 | ||||
| 2018 | Grasses | Urtica dioica | Cirsium arvense | Galium aparine | Phragmites australis | Sisymbrium loeselii | Arctium sp. | Total Amount of Infected Weed Species |
|---|---|---|---|---|---|---|---|---|
| F. arthrosporioides | X | X | X | X | 4 | |||
| F. avenaceum | X | X | X | 3 | ||||
| F. cerealis | X | X | X | X | X | 5 | ||
| F. culmorum | X | X | X | 3 | ||||
| F. equiseti | X | X | X | X | 4 | |||
| F. graminearum | X | X | X | 3 | ||||
| F. oxysporum | X | 1 | ||||||
| F. poae | X | X | X | 3 | ||||
| F. sambucinum | X | X | X | X | X | 5 | ||
| F. solani | X | 1 | ||||||
| F. sporotrichioides | X | X | X | 3 | ||||
| Unidentified species | X | X | X | X | X | 5 | ||
| Total amount of detected Fusarium species | 8 | 8 | 5 | 3 | 5 | 3 | 3 |
| 2019/2020 | Grasses | Urtica dioica | Cirsium arvense | Galium aparine | Phragmites australis | Rumex sp. | Tanacetum vulgare | Total Amount of Infected Weed Species |
|---|---|---|---|---|---|---|---|---|
| F. arthrosporioides | X | 1 | ||||||
| F. avenaceum | X | X | X | X | X | X | X | 7 1 |
| F. cerealis | X | X | X | X | X | 5 | ||
| F. culmorum | X | X | 2 | |||||
| F. equiseti | X | X | X | X | X | X | X | 7 1 |
| F. graminearum | X | X | X | X | X | X | X | 7 1 |
| F. oxysporum | X | 1 | ||||||
| F. poae | X | X | X | 3 | ||||
| F. sambucinum | X | X | X | 3 | ||||
| F. sporotrichioides | X | X | X | X | X | X | X | 7 1 |
| F. tricinctum | X | X | 2 | |||||
| Unidentified species | X | X | 2 | |||||
| Total amount of detected Fusarium species | 9 | 7 | 7 | 7 | 4 | 6 | 5 |
| Kettle Hole | Poaceae (m2) | Poaceae (%) | Herbaceous Plants (m2) | Herbaceous Plants (%) | Wood (m2) | Wood (%) |
|---|---|---|---|---|---|---|
| 1 | 76 | 25 | 117 | 38 | 113 | 37 |
| 2 | 125 | 33 | 88 | 23 | 169 | 44 |
| 3 | 53 | 18 | 90 | 31 | 149 | 51 |
| 4 | 76 | 22 | 271 | 78 | 0 | 0 |
| 5 | 161 | 52 | 147 | 48 | 0 | 0 |
| 6 | 591 | 65 | 320 | 35 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerling, M.; Pätzig, M.; Hempel, L.; Büttner, C.; Müller, M.E.H. Arable Weeds at the Edges of Kettle Holes as Overwintering Habitat for Phytopathogenic Fungi. Agronomy 2022, 12, 823. https://doi.org/10.3390/agronomy12040823
Gerling M, Pätzig M, Hempel L, Büttner C, Müller MEH. Arable Weeds at the Edges of Kettle Holes as Overwintering Habitat for Phytopathogenic Fungi. Agronomy. 2022; 12(4):823. https://doi.org/10.3390/agronomy12040823
Chicago/Turabian StyleGerling, Marina, Marlene Pätzig, Lina Hempel, Carmen Büttner, and Marina E. H. Müller. 2022. "Arable Weeds at the Edges of Kettle Holes as Overwintering Habitat for Phytopathogenic Fungi" Agronomy 12, no. 4: 823. https://doi.org/10.3390/agronomy12040823
APA StyleGerling, M., Pätzig, M., Hempel, L., Büttner, C., & Müller, M. E. H. (2022). Arable Weeds at the Edges of Kettle Holes as Overwintering Habitat for Phytopathogenic Fungi. Agronomy, 12(4), 823. https://doi.org/10.3390/agronomy12040823






