Green Corridors May Sustain Habitats for Earthworms in A Partially Converted Grassland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Mulch Materials
2.2. Tomato Production and the Control Areas
2.3. Examined Soil Parameters
2.4. Statistical Analyses
- Physical parameters
- Chemical parameters
- Biological parameters
- Plant vegetation
3. Results
3.1. Physical Parameters of the Soil
3.2. Chemical Parameters of the Soil
3.3. Biological Parameters of the Soil
3.4. Vegetation
4. Discussion
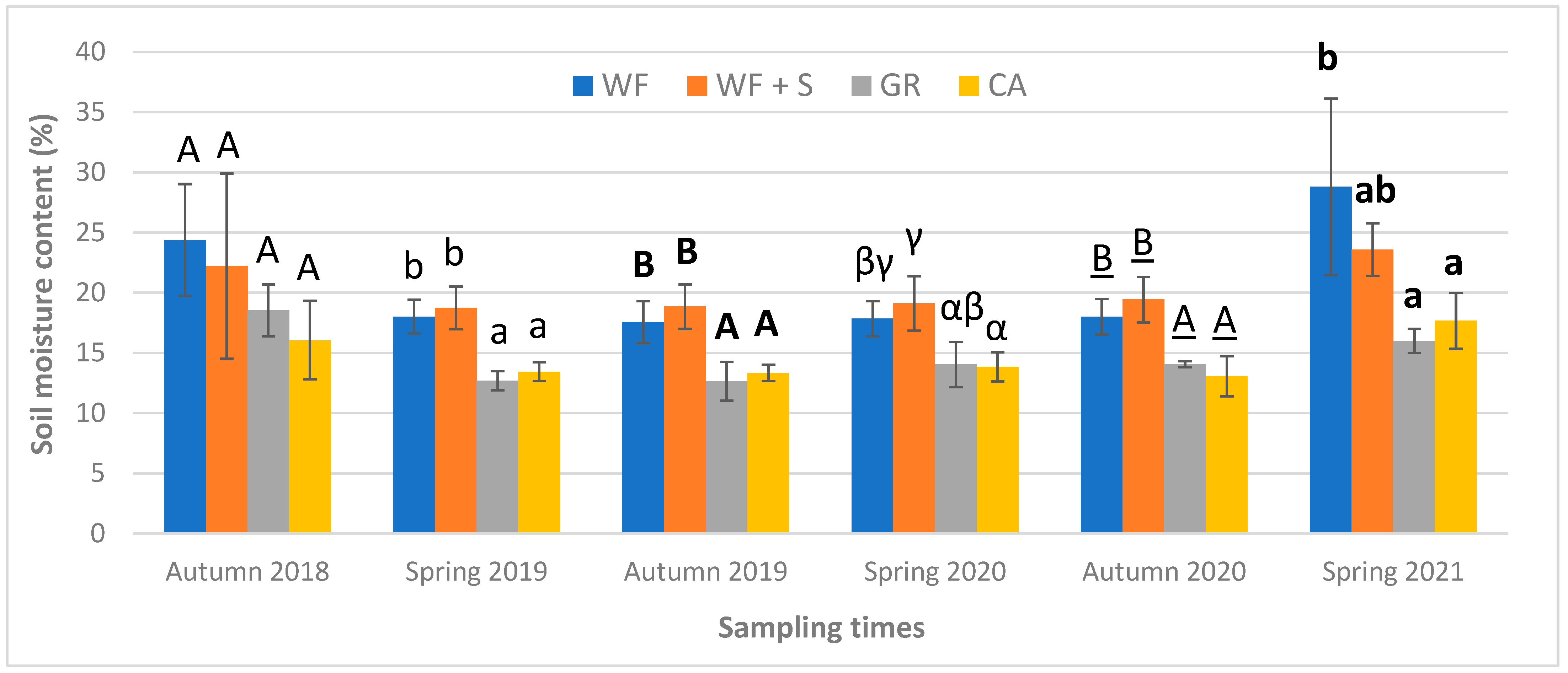
4.1. Physical Parameters: Soil Moisture Content (SMC)
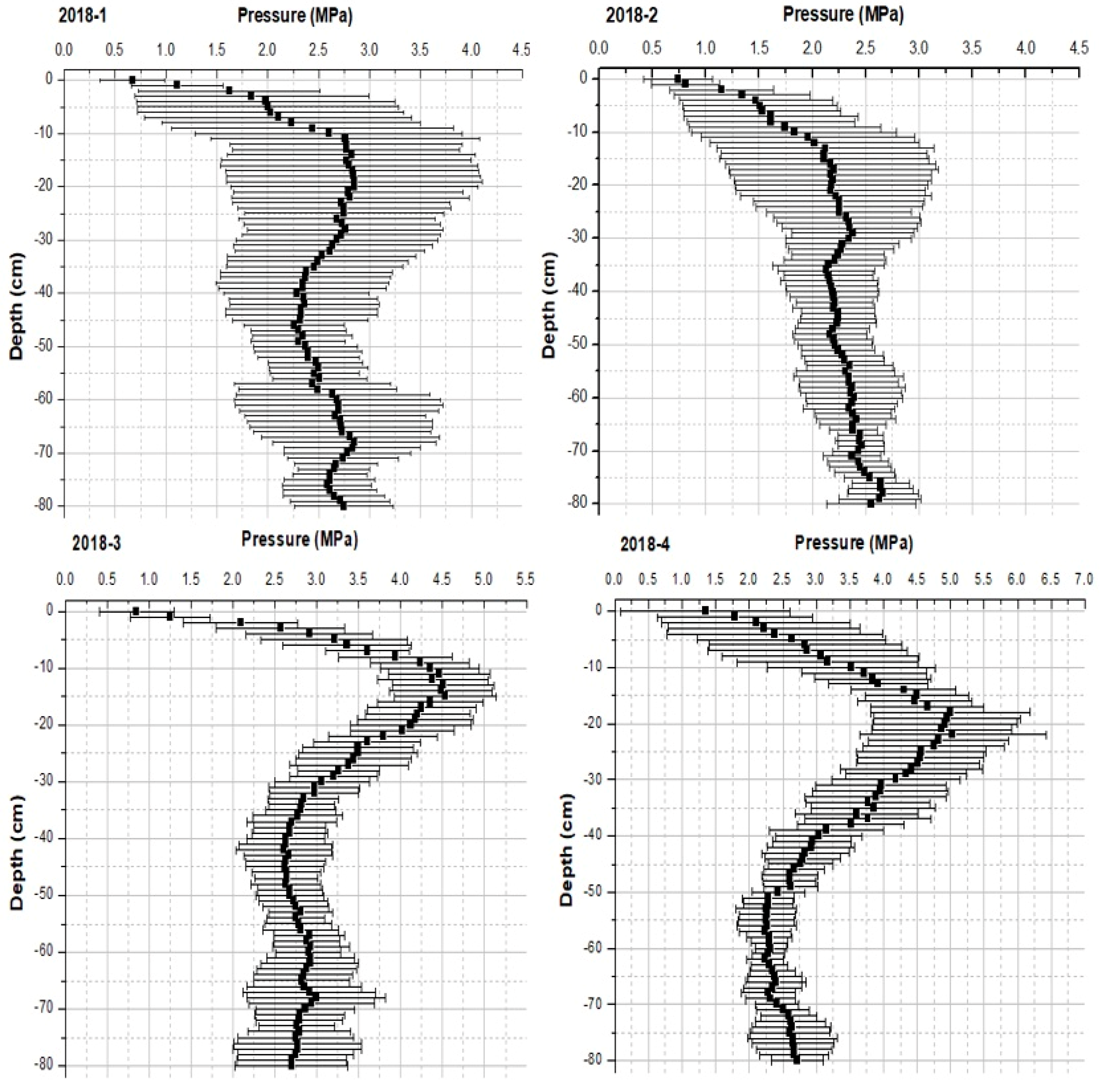
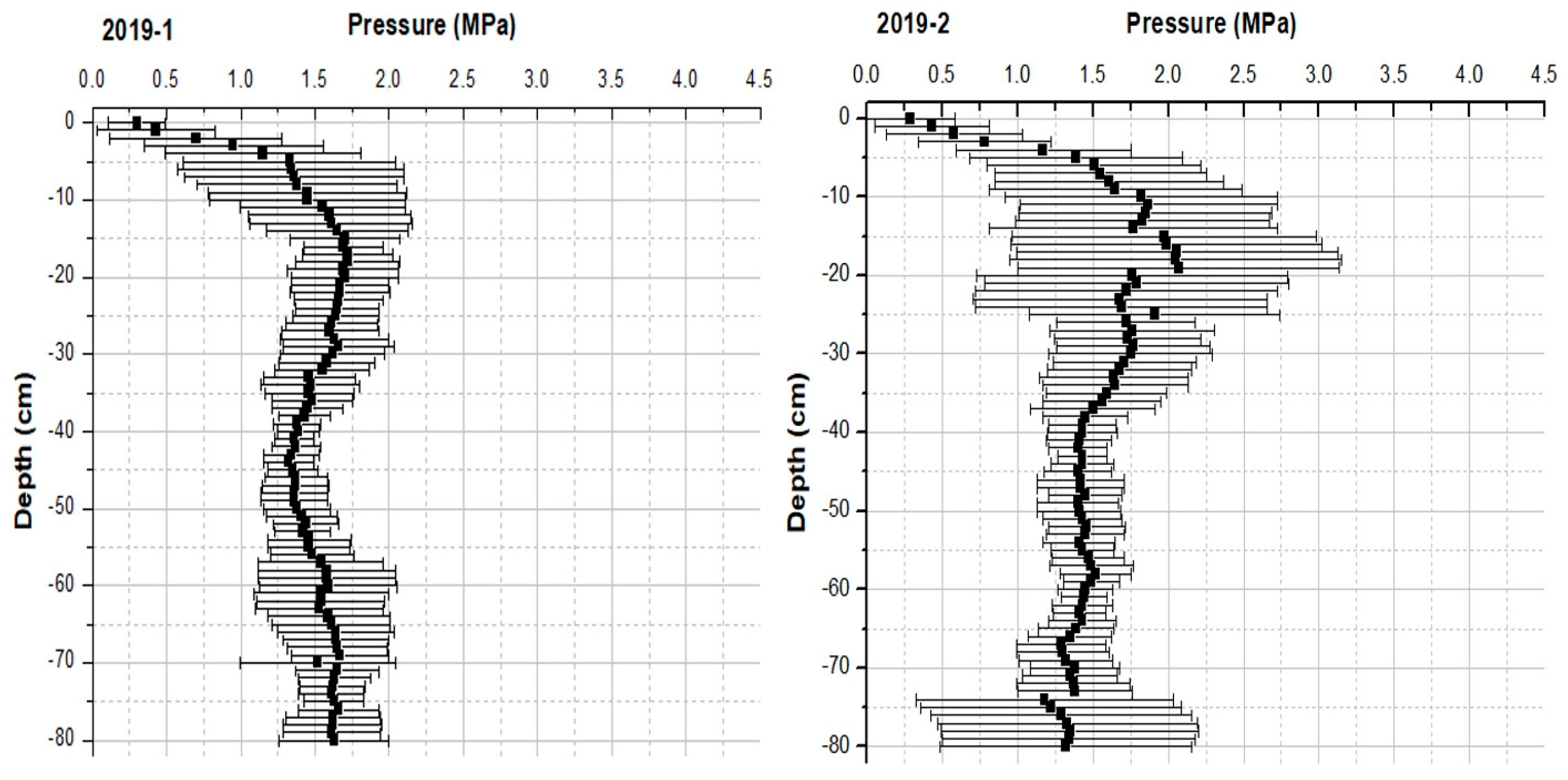
4.2. Physical Parameters: Soil Compaction
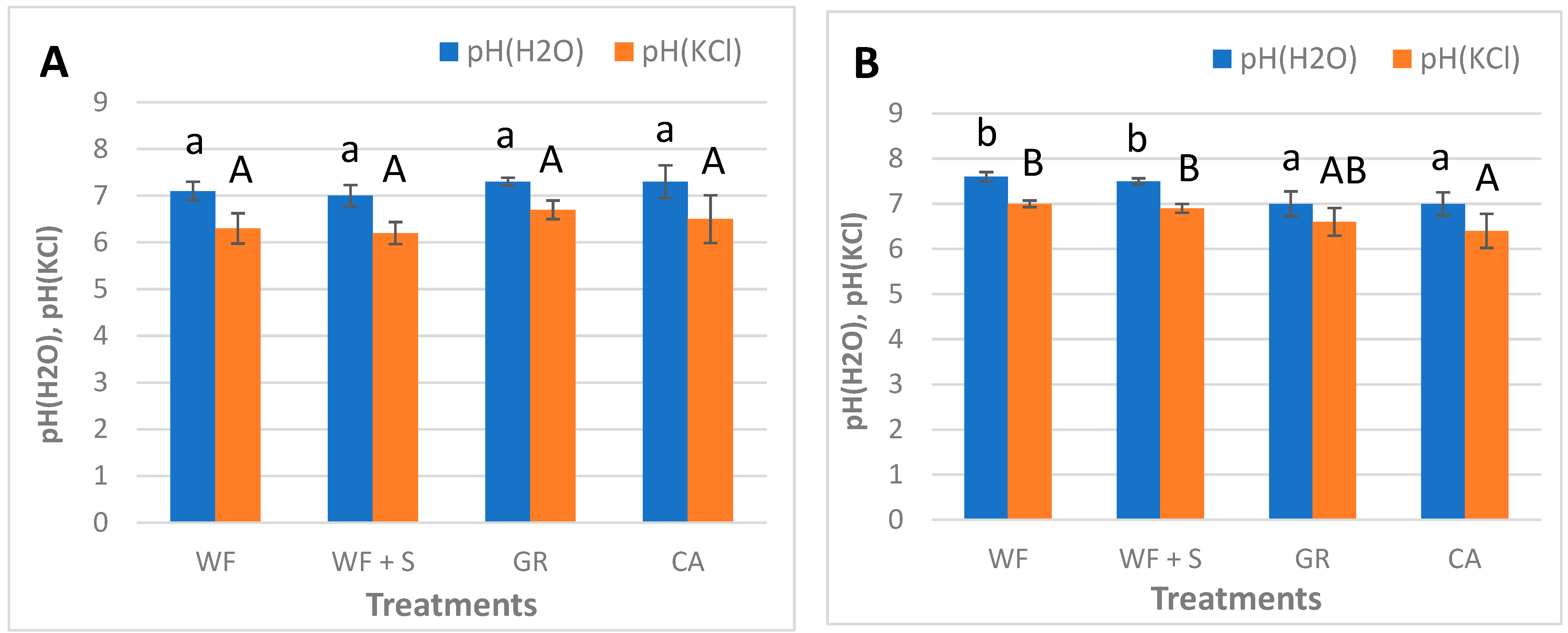
4.3. Chemical Parameters: pH
4.4. Chemical Parameters: Ca
4.5. Chemical Parameters: Soil Organic Matter (SOM)
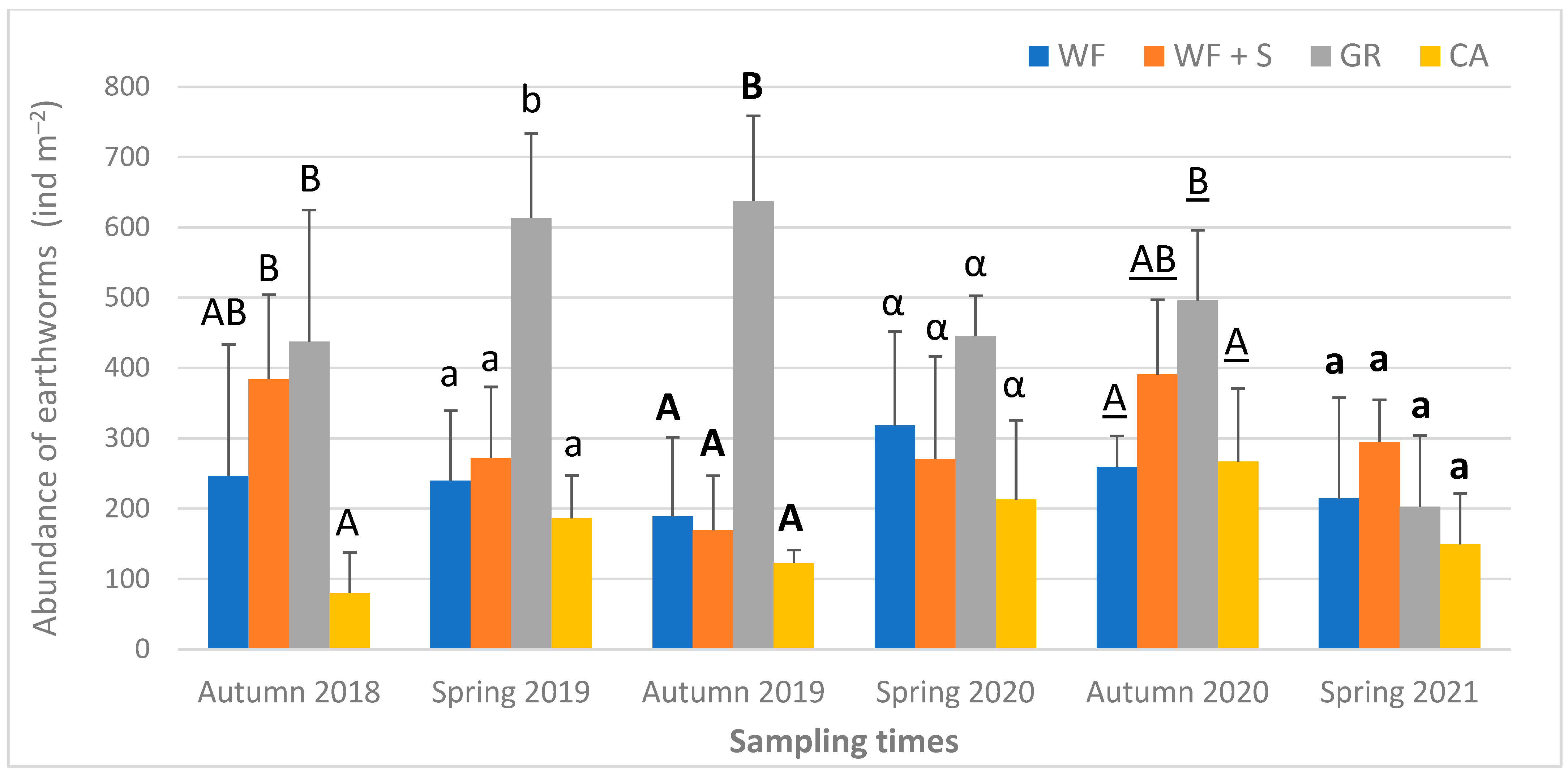
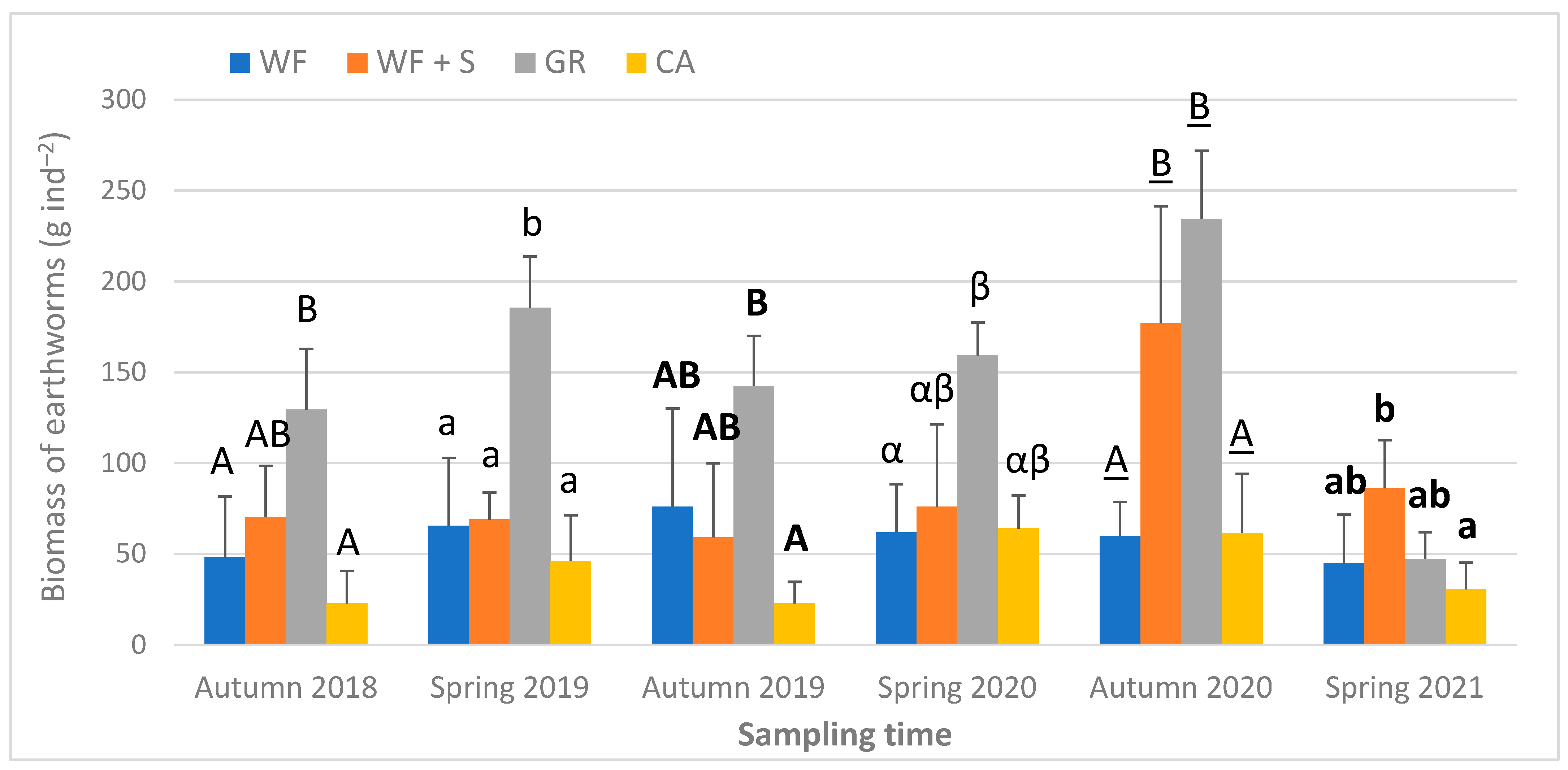
4.6. Biological Parameters: Earthworm Abundance, Biomass, Species Composition
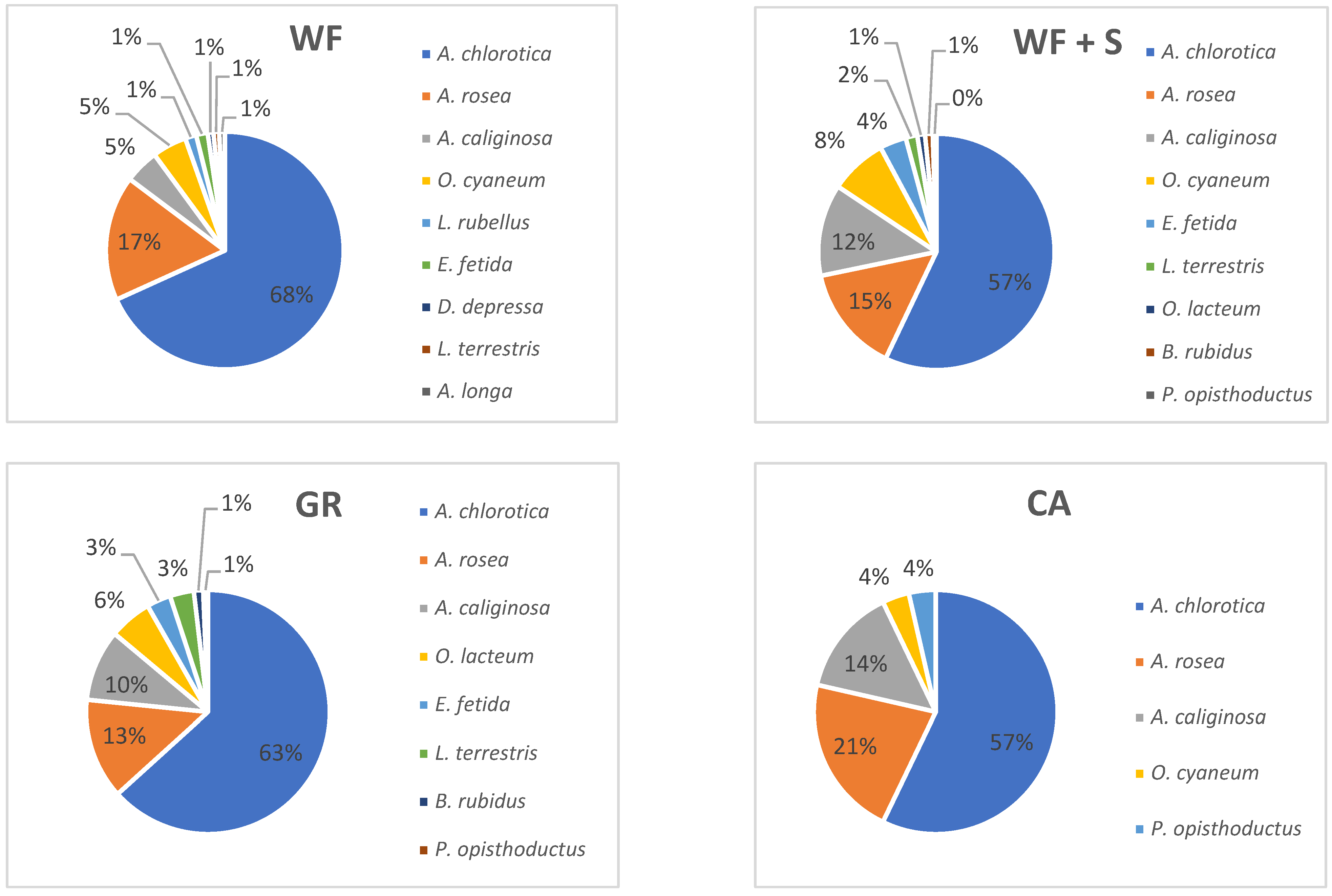
4.7. Earthworm Species Composition
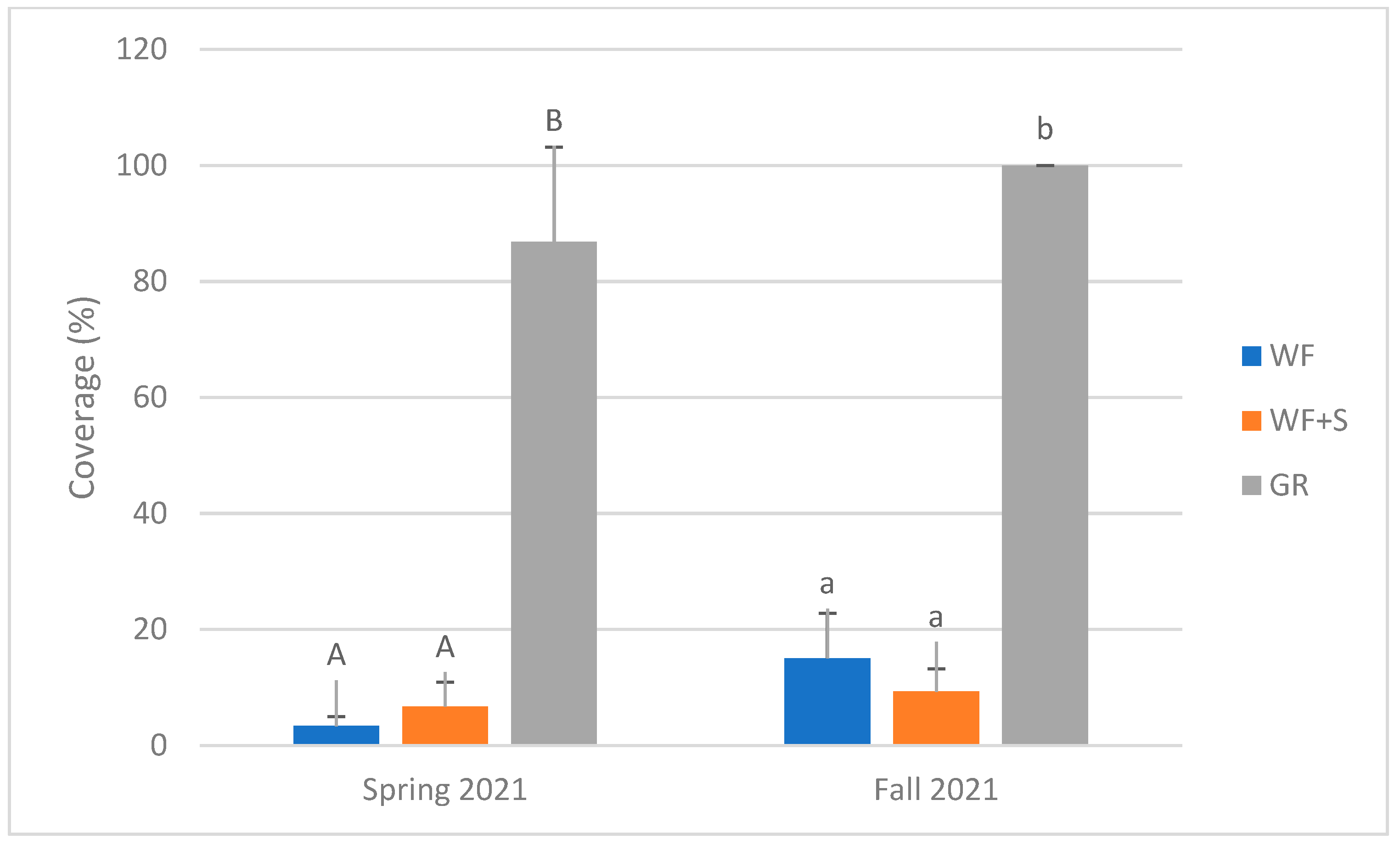
4.8. Plant Abundance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suttie, J.M.; Reynolds, S.G.; Batello, C. Grasslands of the World; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; p. 514.
- Lemaire, G.; Hodgson, J.; Chabbi, A. Grassland Productivity and Ecosystem Services; CABI: Wallingford, UK, 2011; p. 315. [Google Scholar]
- Han, X.; Li, Y.; Du, X.; Li, Y.; Wang, Z.; Jiang, S.; Li, Q. Effect of grassland degradation on soil quality and soil biotic community in a semi-arid temperate steppe. Ecol. Process. 2020, 9, 63. [Google Scholar] [CrossRef]
- Hejcman, M.; Hejcmanová, P.; Pavlu, V.; Beneš, J. Origin and history of grasslands in Central Europe—A review. Grass Forage Sci. 2013, 68, 345–363. [Google Scholar] [CrossRef]
- Nakahama, N.; Uchida, K.; Ushimaru, A.; Yuji, I. Historical changes in grassland area determined the demography of semi-natural grassland butterflies in Japan. Heredity 2018, 121, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.C.; Dengler, J.; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M. European grassland ecosystems: Threatened hotspots of biodiversity. Biodivers. Conserv. 2013, 22, 2131–2138. [Google Scholar] [CrossRef] [Green Version]
- Lyu, X.; Li, X.; Gong, J.; Wang, H.; Dang, D.; Dou, H.; Li, S.; Liu, S. Comprehensive grassland degradation monitoring by remote sensing in Xilinhot, Inner Mongolia, China. Sustainability 2020, 12, 3682. [Google Scholar] [CrossRef]
- Sala, O.E.; Paruelo, J.M. Ecosystem services in grasslands. In Nature’s Services: Societal Dependence on Natural Ecosystems; Daily, G.C., Ed.; Island Press: Washington, DC, USA, 1997; pp. 237–251. [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; p. 155. [Google Scholar]
- Villoslada Peciña, M.; Ward, R.D.; Bunce, R.G.H.; Sepp, K.; Kuusemets, V.; Luuk, O. Country-scale mapping of ecosystem services provided by semi-natural grasslands. Sci. Total Environ. 2019, 661, 212–225. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, J.; Chen, X.; Wang, Z. Straw mulch as an alternative to plastic film mulch: Positive evidence from dryland wheat production on the Loess Plateau. Sci. Total Environ. 2019, 676, 782–791. [Google Scholar] [CrossRef]
- Werling, B.P.; Dickson, T.L.; Isaacs, R.; Gaines, H.; Gratton, C.; Gross, K.L.; Liere, H.; Malmstrom, C.M.; Meehan, T.D.; Ruan, L.; et al. Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Natl. Acad. Sci. USA 2014, 111, 1652–1657. [Google Scholar] [CrossRef] [Green Version]
- Dengler, J.; Janišová, M.; Török, P.; Wellstein, C. Biodiversity of palaearctic grasslands: A synthesis. Agric. Ecosyst. Environ. 2014, 182, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kemp, D.R.; Michalk, D.L. Towards sustainable grassland and livestock management. J. Agric. Sci. 2007, 145, 543–564. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Deng, X.; Song, W.; Li, Z.; Chen, J. What is the main cause of grassland degradation? A case study of grassland ecosystem service in the middle-south Inner Mongolia. Catena 2017, 150, 100–107. [Google Scholar] [CrossRef]
- Sollenberger, L.E.; Kohmann, M.M.; Dubeux, J.C.B.; Silveira, M.L. Grassland management affects delivery of regulating and supporting ecosystem services. Crop Sci. 2019, 59, 441–459. [Google Scholar] [CrossRef]
- Wu, T.H.; Milner, H.; Diaz-Perez, J.C.; Ji, P.S. Effects of soil management practices on soil microbial communities and development of southern blight in vegetable production. Appl. Soil Ecol. 2015, 91, 58–67. [Google Scholar] [CrossRef]
- Docherty, K.M.; Gutknecht, J.L.M. Soil microbial restoration strategies for promoting climate-ready prairie ecosystems. Ecol. Appl. 2019, 29, e01858. [Google Scholar] [CrossRef]
- Prosdocimi, M.; Tarolli, P.; Cerdà, A. Mulching practices for reducing soil water erosion: A review. Earth Sci. Rev. 2016, 161, 191–203. [Google Scholar] [CrossRef]
- Kumar, S.D.; Lal, B.R. Effect of mulching on crop production under rainfed condition: A Review. Int. J. Res. Chem. Environ. 2012, 2, 8–20. [Google Scholar]
- Blanco-Canqui, H.; Lal, R. Impacts of long-term wheat straw management on soil hydraulic properties under no-tillage. Soil Sci. Soc. Am. J. 2007, 71, 1166–1173. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wen, X.; Sun, Y.; Zhang, J.; Wu, W.; Liao, Y. Mulching practices altered soil bacterial community structure and improved orchard productivity and apple quality after five growing seasons. Sci. Hortic. 2014, 172, 248–257. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland ecosystem services: A systematic review of research advances and future directions. Landsc. Ecol. 2020, 35, 793–814. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, Z.; Chen, C. Effect of mulching on labile soil organic matter pools, microbial community functional diversity and nitrogen transformations in two hardwood plantations of subtropical Australia. Appl. Soil Ecol. 2008, 40, 229–239. [Google Scholar] [CrossRef]
- Jodaugienė, D.; Pupalienė, R.; Sinkevičienė, A.; Marcinkevičienė, A.; Žebrauskaitė, K.; Baltaduonytė, M.; Čepulienė, R. The influence of organic mulches on soil biological properties. Zemdirbyste 2010, 97, 33–40. [Google Scholar]
- Li, R.; Hou, X.; Jia, Z.; Han, Q.; Ren, X.; Yang, B. Effects on soil temperature, moisture, and maize yield of cultivation with ridge and furrow mulching in the rainfed area of the Loess Plateau, China. Agric. Water Manag. 2013, 116, 101–109. [Google Scholar] [CrossRef]
- Kahlon, M.S.; Lal, R.; Ann-Varughese, M. Twenty-two years of tillage and mulching impacts on soil physical characteristics and carbon sequestration in Central Ohio. Soil Tillage Res. 2013, 126, 151–158. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; De Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Fründ, H.C.; Graefe, U.; Tischer, S. Earthworms as bioindicators of soil quality. In Biology of Earthworms; Karaca, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 24, pp. 261–278. [Google Scholar] [CrossRef]
- D’Hose, T.; Cougnon, M.; De Vliegher, A.; Vandecasteele, B.; Viaene, N.; Cornelis, W.; Van Bockstaele, E.; Reheul, D. The positive relationship between soil quality and crop production: A case study on the effect of farm compost application. Appl. Soil Ecol. 2014, 75, 189–198. [Google Scholar] [CrossRef]
- Fusaro, S.; Gavinelli, F.; Lazzarini, F.; Paoletti, M.G. Soil Biological Quality Index based on earthworms (QBS-e). A new way to use earthworms as bioindicators in agroecosystems. Ecol. Indic. 2018, 93, 1276–1292. [Google Scholar] [CrossRef]
- Plaas, E.; Meyer-Wolfarth, F.; Banse, M.; Bengtsson, J.; Bergmann, H.; Faber, J.; Potthoff, M.; Runge, T.; Schrader, S.; Taylor, A. Towards valuation of biodiversity in agricultural soils: A case for earthworms. Ecol. Econ. 2019, 159, 291–300. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. Organic matter decomposition and the soil food web. In The Importance of Soil Organic Matter—Key to Drought-Resistant Soil and Sustained Food Production; Bot, A., Benites, J., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; pp. 5–10. [Google Scholar]
- Lavelle, P.; Martin, A. Small-scale and large-scale effects of endogeic earthworms on soil organic matter dynamics in soils of the humid tropics. Soil Biol. Biochem. 1992, 24, 1491–1498. [Google Scholar] [CrossRef]
- Brussaard, L. Biodiversity and ecosystem functioning in soil. Ambio 1997, 26, 563–570. Available online: http://www.jstor.org/stable/4314670 (accessed on 4 February 2022).
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; p. 654. [Google Scholar] [CrossRef]
- Lavelle, P.; Charpentier, F.; Villenave, C.; Rossi, J.P.; Derouard, L.; Pashanasi, B.; André, J.; Ponge, J.F.; Bernier, N. Effects of earthworms on soil organic matter and nutrient dynamics at a landscape scale over decades. In Earthworm Ecology, 2nd ed.; Edwards, C.A., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 145–160. [Google Scholar] [CrossRef] [Green Version]
- Bhadauria, T.; Saxena, K.G. Role of earthworms in soil fertility maintenance through the production of biogenic structures. Appl. Environ. Soil Sci. 2010, 41, 816073. [Google Scholar] [CrossRef]
- WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; p. 192.
- Buzás, I. Talaj—és agrokémiai vizsgálati módszerkönyv 2 [Method Book for Soil and Agricultural Physical Analyses 2]; INDA 4231 Publishing House: Budapest, Hungary, 1993; p. 357. (In Hungarian) [Google Scholar]
- Buzás, I. Talaj—és agrokémiai vizsgálati módszerek 1 [Method Book for Soil and Agricultural Chemistry Analyses 1]; Mezőgazdasági Kiadó Publishing House: Budapest, Hungary, 1988; p. 243. (In Hungarian) [Google Scholar]
- Walkley, A. A critical examination of a rapid method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Csuzdi, C.; Zicsi, A. Earthworms of Hungary, Pedozoologica Hungarica No 1; Hungary Natural History Museum; Hungary Academy of Sciences: Budapest, Hungary, 2003. [Google Scholar] [CrossRef]
- Csuzdi, C. Magyarország földigiliszta-faunájának áttekintése (Oligochaete, Lumbricidae) [A review of the Hungarian earthworm fauna]. Állattani Közlemények 2007, 92, 3–38. (In Hungarian) [Google Scholar]
- Cusser, S.; Grando, C.; Zucchi, M.I.; López-Uribe, M.M.; Pope, N.S.; Ballare, K.; Luna-Lucena, D.; Almeida, E.A.B.; Neff, J.L.; Young, K.; et al. Small but critical: Semi-natural habitat fragments promote bee abundance in cotton agroecosystems across both Brazil and the United States. Landsc. Ecol. 2019, 34, 1825–1836. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Impact of mulches on landscape plants and the environment—A review. J. Environ. Hortic. 2007, 25, 239–249. [Google Scholar] [CrossRef]
- Xiukang, W.; Zhanbin, L.; Yingying, X. Effects of mulching and nitrogen on soil temperature, water content, nitrate-N content and maize yield in the Loess Plateau of China. Agric. Water Manag. 2015, 161, 53–64. [Google Scholar] [CrossRef]
- Kader, M.A.; Senge, M.; Mojid, M.A.; Ito, K. Recent advances in mulching materials and methods for modifying soil environment. Soil Tillage Res. 2017, 68, 155–166. [Google Scholar] [CrossRef]
- Stelli, S.; Hoy, L.; Hendrick, R.; Taylor, M. Effects of different mulch types on soil moisture content in potted shrubs. Afr. J. Online 2018, 44, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.S.; Hu, X.B.; Zhang, X.X.; Li, J. Effects of plastic mulch and crop rotation on soil physical properties in rain-fed vegetable production in the mid-Yunnan plateau, China. Soil Tillage Res. 2015, 145, 111–117. [Google Scholar] [CrossRef]
- Chen, B.; Garré, S.; Liu, H.; Yan, C.; Liu, E.; Gong, D.; Mei, X. Two-dimensional monitoring of soil water content in fields with plastic mulching using electrical resistivity tomography. Comput. Electron Agric. 2019, 159, 84–91. [Google Scholar] [CrossRef]
- Jourgholami, M.; Fathi, K.; Labelle, E.R. Effects of litter and straw mulch amendments on compacted soil properties and Caucasian alder (Alnus subcordata) growth. New For. 2020, 51, 349–365. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bandopadhyay, S.; English, M.E.; Bary, A.; Liquet y González, J.E.; De Bruyn, J.M.; Schaeffer, S.M.; Miles, C.A.; Flury, M. Four years of continuous use of soil-biodegradable plastic mulch: Impact on soil and groundwater quality. Geoderma 2021, 381, 114665. [Google Scholar] [CrossRef]
- Job, M.; Bhakar, S.R.; Singh, P.K.; Tiwari, G.S.; Sharma, R.K.; Lakhawat, S.S.; Sharma, D. Evaluation of plastic mulch for changes in mechanical properties during onion cultivation. Int. J. Sci. Environ. Technol. 2016, 5, 575–584. [Google Scholar]
- Qi, Y.; Beriot, N.; Gort, G.; Lwanga, E.H.; Gooren, H.; Yang, X.; Geissen, V. Impact of plastic mulch film debris on soil physicochemical and hydrological properties. Environ. Pollut. 2020, 266, 115097. [Google Scholar] [CrossRef] [PubMed]
- Gandomkar, A. The effects of living and non-living mulches on soil chemical properties in apple tree. J. Biochem. Technol. 2018, 2, 131–135. [Google Scholar]
- Moreno, M.M.; Moreno, A. Effect of different biodegradable and polyethylene mulches on soil properties and production in a tomato crop. Sci. Hortic. 2008, 116, 256–263. [Google Scholar] [CrossRef]
- Jones, A.; Fortier, J.; Gagnon, D.; Truax, B. Trading tree growth for soil degradation: Effects at 10 years of black plastic mulch on fine roots, earthworms, organic matter and nitrate in a multi-species riparian buffer. Trees For. People 2020, 2, 100032. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, X.G.; Fu, T.; Wang, L.; Turner, N.C.; Siddique, K.H.M.; Li, F.M. Multi-site assessment of the effects of plastic-film mulch on the soil organic carbon balance in semiarid areas of China. Agric. For. Meteorol. 2016, 228–229, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Moure, N.; Gracia, R.; Bielsa, A.C.; López, M.V. Soil organic matter fractions as affected by tillage and soil texture under semiarid Mediterranean conditions. Soil Tillage Res. 2016, 155, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Thomson, L.J.; Hoffmann, A.A. Effects of ground cover (straw and compost) on the abundance of natural enemies and soil macro invertebrates in vineyards. Agric. For. Entomol. 2007, 9, 173–179. [Google Scholar] [CrossRef]
- Curry, J.P.; Schmidt, O. The feeding ecology of earthworms—A review. Pedobiologia 2007, 50, 463–477. [Google Scholar] [CrossRef]
- Joschko, M.; Fox, C.A.; Lentzsch, P.; Kiesel, J.; Hierold, W.; Krück, S.; Timmer, J. Spatial analysis of earthworm biodiversity at the regional scale. Agric. Ecosyst. Environ. 2006, 112, 367–380. [Google Scholar] [CrossRef]
- Tischer, S. Lumbricids species diversity and heavy metal amounts in lumbricids on soil monitoring sites in Saxony-Anhalt (Germany). Arch. Agron. Soil Sci. 2005, 51, 391–403. [Google Scholar] [CrossRef]
- Hoeffner, K.; Santonja, M.; Monard, C.; Barbe, L.; Le Moing, M.; Cluzeau, D. Soil properties, grassland management, and landscape diversity drive the assembly of earthworm communities in temperate grasslands. Pedosphere 2021, 31, 375–383. [Google Scholar] [CrossRef]
- Munn, D.A. Comparison of shredded newspaper and wheat straw as crop mulches. HortTechnology 1992, 2, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Crutchfield, D.A.; Wicks, G.A.; Burnside, O.C. Effect of winter wheat (Triticum aestivum) straw mulch level on weed control. Weed Sci. 1986, 34, 110–114. [Google Scholar] [CrossRef]
- Pusztai, P. Comparative Evaluation of Mulching Methods in Tomato Production. Ph.D. Thesis, Corvinus University of Budapest, Budapest, Hungary, 2010. [Google Scholar]
- Ramakrishna, A.; Tam, H.M.; Wani, S.P.; Long, T.D. Effect of mulch on soil temperature, moisture, weed infestation and yield of groundnut in northern Vietnam. Field Crop. Res. 2006, 95, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Pupalienė, R.; Sinkevičienė, A.; Jodaugienė, D.; Bajorienė, K. Weed control by organic mulch in organic farming system. In Weed Biology and Control; Pilipavicius, V., Ed.; InTech: Rijeka, Croatia, 2015; pp. 65–86. [Google Scholar] [CrossRef] [Green Version]
- Petrikovszki, R.; Zalai, M.; Tóthné-Bogdányi, F.; Tóth, F. The effect of organic mulching and irrigation on the weed species composition and the soil weed seed bank of tomato. Plants 2020, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Awodoyin, R.O.; Ogbeide, F.I.; Oluwole, O. Effects of three mulch types on the growth and yield of tomato (Lycopersicon esculentum Mill.) and weed suppression in Ibadan, rainforest-savanna transition zone of Nigeria. Trop. Agric. 2010, 10, 53–60. [Google Scholar] [CrossRef] [Green Version]



| Measured Parameters | Time of Measurements | Frequency | |
|---|---|---|---|
| I. | Physical parameters | ||
| soil texture | autumn 2018 | once | |
| soil moisture content | autumn 2018; spring and autumn 2019, 2020; spring 2021 | 6 times | |
| soil penetration resistance | autumn 2018 and 2019 | twice | |
| II. | Chemical parameters | ||
| soil pH(H2O), pH(KCl) | autumn 2018; January 2022 | twice | |
| CaCO3 content | autumn 2018; January 2022 | twice | |
| soil organic matter | autumn 2018; January 2022 | twice | |
| III. | Biological parameters | ||
| earthworm abundance, biomass, species composition | autumn 2018; spring and autumn 2019, 2020; spring 2021 | 6 times | |
| plant abundance (cover) | spring and autumn 2021 | twice |
| Habitat | Order of Dominance | Spring 2021 | Autumn 2021 |
|---|---|---|---|
| Weed control fabric | 1 | Elymus repens | Urtica dioica |
| 2 | Urtica dioica | Polygonum aviculare | |
| 3 | Taraxacum officinale | Elymus repens | |
| 4 | Veronica hederifolia | Setaria glauca | |
| 5 | Glechoma hederacea | Taraxacum officinale | |
| Total cover | 3.34% | 15.03% | |
| Weed control fabric + straw | 1 | Elymus repens | Urtica dioica |
| 2 | Stellaria media | Elymus repens | |
| 3 | Capsella bursa-pastoris | Convolvulus arvensis | |
| 4 | Taraxacum officinale | Setaria glauca | |
| 5 | Glechoma hederacea | Cichorium intybus | |
| Total cover | 6.73% | 9.29% | |
| Grass | 1 | Elymus repens | Elymus repens |
| 2 | Lolium perenne | Lolium perenne | |
| 3 | Urtica dioica | Taraxacum officinale | |
| 4 | Taraxacum officinale | Urtica dioica | |
| 5 | Lamium purpureum | Polygonum aviculare | |
| Total cover | 86.85% | 100% |
| Variable | d.f. | MANOVA | Comparison | |||
|---|---|---|---|---|---|---|
| F | p-Value | Group | Avg. Value (%) | Sign. Class | ||
| Habitat | 2 | 636.695 | <0.001 | WF | 9.18 | a |
| WF + S | 8.01 | a | ||||
| GR | 93.43 | b | ||||
| Season | 1 | 16.596 | <0.001 | spring 2021 | 32.3063 | a |
| autumn 2021 | 41.4375 | b | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, B.; Boziné Pullai, K.; Selmeczi, D.; Sebők, A.; Tóthné Bogdányi, F.; Weldmichael, T.G.; Zalai, M.; Nsima, J.P.; Tóth, F. Green Corridors May Sustain Habitats for Earthworms in A Partially Converted Grassland. Agronomy 2022, 12, 793. https://doi.org/10.3390/agronomy12040793
Simon B, Boziné Pullai K, Selmeczi D, Sebők A, Tóthné Bogdányi F, Weldmichael TG, Zalai M, Nsima JP, Tóth F. Green Corridors May Sustain Habitats for Earthworms in A Partially Converted Grassland. Agronomy. 2022; 12(4):793. https://doi.org/10.3390/agronomy12040793
Chicago/Turabian StyleSimon, Barbara, Krisztina Boziné Pullai, Dóra Selmeczi, András Sebők, Franciska Tóthné Bogdányi, Tsedekech G. Weldmichael, Mihály Zalai, Justine Phenson Nsima, and Ferenc Tóth. 2022. "Green Corridors May Sustain Habitats for Earthworms in A Partially Converted Grassland" Agronomy 12, no. 4: 793. https://doi.org/10.3390/agronomy12040793
APA StyleSimon, B., Boziné Pullai, K., Selmeczi, D., Sebők, A., Tóthné Bogdányi, F., Weldmichael, T. G., Zalai, M., Nsima, J. P., & Tóth, F. (2022). Green Corridors May Sustain Habitats for Earthworms in A Partially Converted Grassland. Agronomy, 12(4), 793. https://doi.org/10.3390/agronomy12040793








