3.2. Plant Density
In 2018, two insecticides were applied as a soil treatment: the chemical insecticide Force 1.5 G, the prevailing standard in Serbia, and the biological insecticide ATTRACAP®.
For the plant density, repeated measures ANOVA showed that both main effects, treatment, and plant growth stage, were significant, with high effect sizes, while their interaction was not significant (F (2,57) = 2.84,
p = 0.067, η2
p = 0.091;
Figure 1). As the main effect of the treatment was significant (F (2,57) = 5.97 **,
p = 0.004, η2
p = 0.173), a post hoc Bonferroni pairwise comparison was used to analyse the overall differences (regardless of the plant growth stage) between treatments. Significant differences in plant density were recorded between Force 1.5 G and the control (
p = 0.003), whereas there was no significant difference between ATTRACAP
® and Force 1.5 G treatments (
p = 0.124) or between ATTRACAP
® and the control (
p = 0.558). Homogeneous groups are presented in
Figure 1. However, it must be mentioned, again, that RŠ T-12 had low natural wireworm abundance.
Since plant density increased over time, the effect of plant growth stage was significant (F (1,57) = 63.28 **, p < 0.001, η2p = 0.526); thus, there was a significant difference between mean densities at the BBCH 02 and BBCH 04/05 stages (overall, regardless of treatment).
When analysing the first and the second plant growth stage (BBCH stage) separately, a significant difference in plant density was only detected between the Force 1.5 G treatment and the control at both stages (p = 0.022). Furthermore, for each treatment the mean values of plant density increased significantly from the BBCH 02 to BBCH 04/05 stages (p < 0.001).
In 2019, the experiment encompassed three insecticides applied as seed treatments (Force 20 CS, Buteo Start 480 FS and Lumiposa) and two insecticides incorporated into the soil, during the sowing (Force 1.5 G and ATTRACAP®, Dassel-Markoldendorf, Germany).
The main effect of the treatment was significant (F (5,162) = 9.51 **,
p < 0.001, η2
p = 0.227). Post hoc analysis of the treatments showed that there was no statistical difference in the plant density between the chemical insecticides and ATTRACAP
® (
Figure 2). However, there was a significant difference in plant density between all insecticidal treatments and the control (
p < 0.001). The same result was obtained when analysing the BBCH 02 and BBCH 04/05 stages separately. Furthermore, for all insecticidal treatments and the control, there was a highly significant increase in plant density from the BBCH 02 to BBCH 04/05 stages (F (1,162) = 101.82 **,
p < 0.001, η2
p = 0.386). The highest change was observed in the Force 20 CS treatment. The interaction of the main effects was not significant (F (5,162) = 2.23,
p = 0.054, η2
p = 0.067).
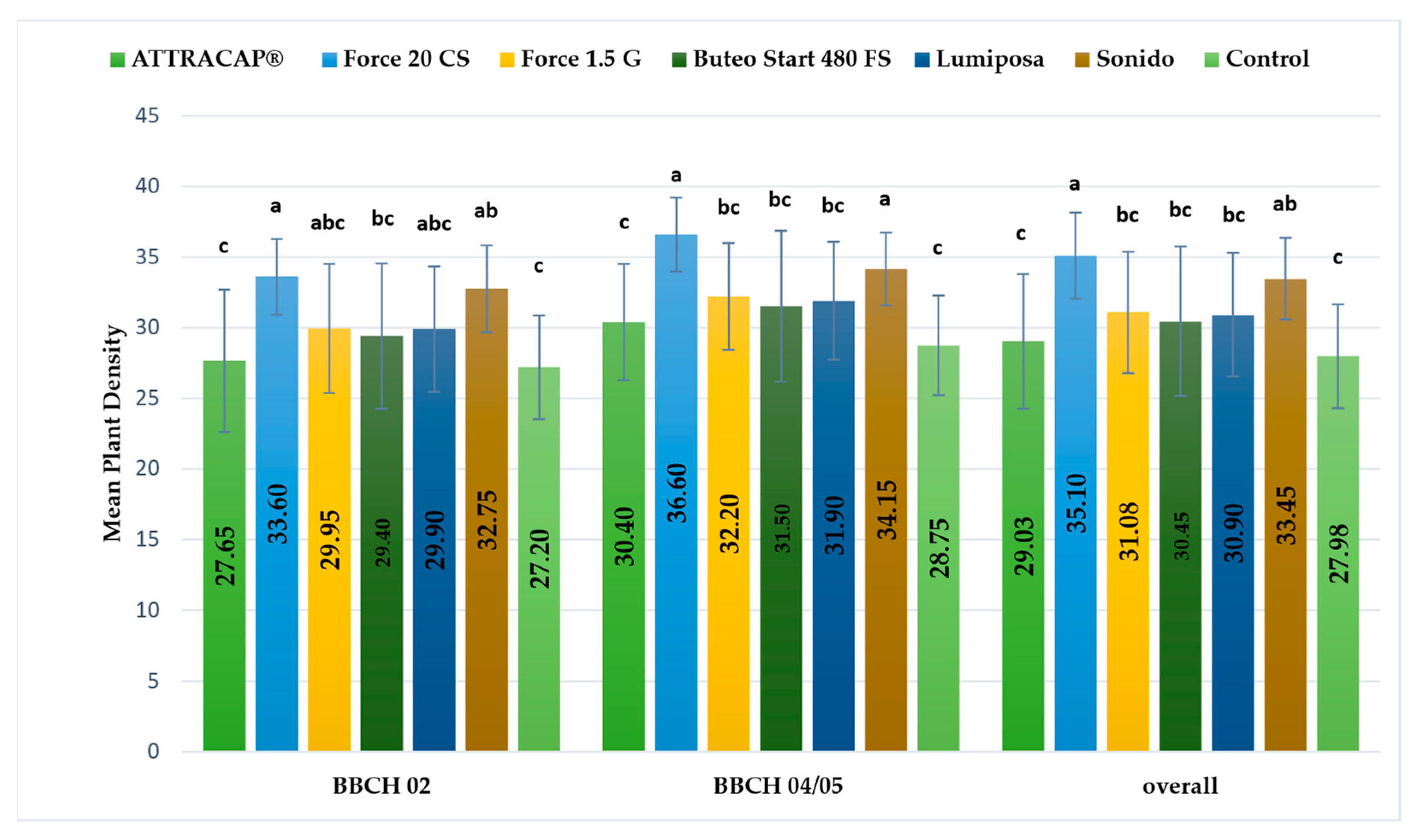
In 2021, at RŠ Field 1, the effect of the treatments was significant, (F (5,210) = 59.17 **,
p < 0.001, η2
p = 0.585), regardless of the plant growth stage, and a significant difference was found between all insecticide treatments and the control (
p < 0.001). Furthermore, the plant density significantly differed between the insecticides applied. The highest plant density was recorded in Force 20 CS and Lumiposa (no significant difference between,
p = 0.273), and these two insecticide treatments were significantly different from the other insecticide treatments, (
p < 0.001). In addition, there were no significant differences between ATTRACAP
®, Force 1.5 G and Buteo Start 480 FS (
p = 0.899). Differences were also observed in the first and the second BBCH phases, and the homogeneous groups are presented in
Figure 3.
The increase in the plant density from the BBCH 02 to BBCH 04/05 stages was significant (F (1,210) = 172.45 **, p < 0.001, η2p = 0.451) and was recorded for all insecticide and control treatments.
The interaction of the main effects was significant (F (5,210) = 3.48 **, p = 0.005, η2p = 0.076) with low effect size, implying that the increase in plant density from BBCH 02 to BBCH 04/05 was not the same for all treatments. The highest increase was recorded in Buteo Start 480 FS and Force 1.5 G (p < 0.001). In the ATTRACAP®, Lumiposa (p < 0.001) and Force 20 CS (p = 0.001) treatments, the increase was lower.
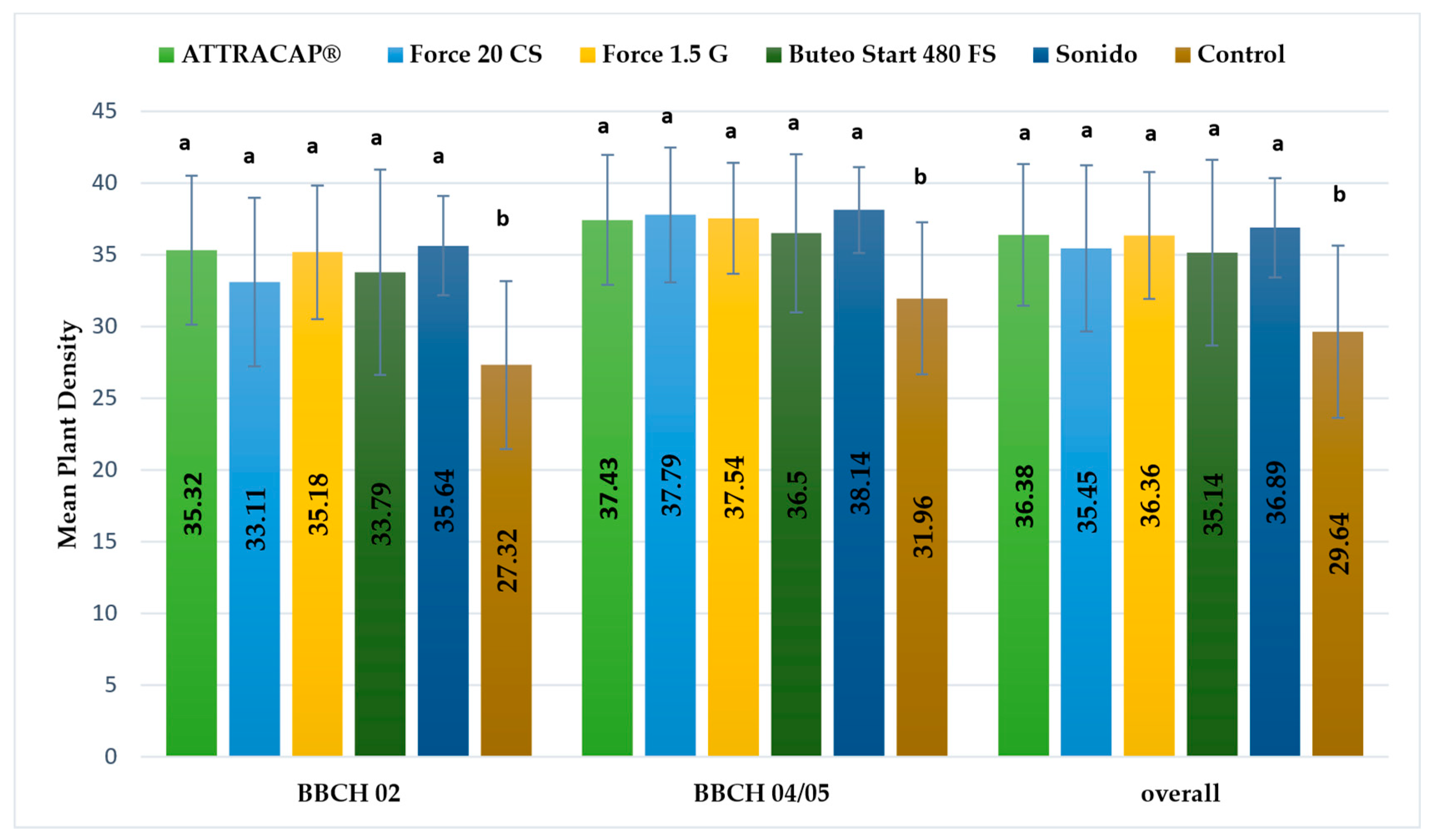
At RŠ T-12 in 2021, the main effect of the treatment was significant (F (6,133) = 8.35 **,
p < 0.001, η2
p = 0.274;
Figure 4) for plant density. The highest was recorded when Force 20 CS or Sonido were applied. Force 20 CS was significantly different compared to the ATTRACAP
® (
p < 0.001), Buteo Start (
p = 0.003), Force 1.5 G (
p = 0.022) and Lumiposa (
p = 0.13) treatments and the control (
p < 0.001), while Sonido was significantly different from ATTRACAP
® (
p = 0.007) and the control (
p < 0.001). Similar differences were observed in both growth stages and homogeneous groups are presented in
Figure 4.
Significant differences were recorded between plant growth stages (F (1,133) = 94.89 **, p < 0.001, η2p = 0.416) with a high effect size. A significant increase in plant density from BBCH 02 to BBCH 04/05 was recorded for all insecticides: ATTRACAP®, Buteo Start 480 FS, Force 1.5 G, Force 20 CS (p < 0.001), Lumiposa (p = 0.001), Sonido (p = 0.18) and the control (p = 0.009). The highest increase was found in the Force 20 CS, ATTRACAP® and Force 1.5 G treatments, respectively. The interaction of the main effects was not significantly different (F (6,133) = 0.994, p = 0.430, η2p = 0.043).
3.3. Plant Damage
Repeated measures ANOVA was used to analyse plant damage, with two factors: the treatment and plant growth stage. For all years and fields, the interaction between two main effects was significant, implying that the increase in plant damage from BBCH 02 to BBCH 04/05 was not the same for all treatments and depended on the treatment applied. Moreover, the main effect of the plant growth stage was always significant, as the plant damage did increase over time.
In 2018, the main effect of treatment was not significant, (F (2,12) = 1.62,
p = 0.238, η2
p = 0.213), as the percentage of damaged plants did not significantly differ between treatments. The same was observed in each of the BBCH stages (
Table 2). However, the percentage of damaged plants between BBCH 02 and BBCH 04/05 differed significantly, indicating a significant effect of the plant growth stage (F (1,12) = 52.81 **,
p < 0.001, η2
p = 0.815). The interaction between the treatments and the BBCH stages was also significant (F (2,12) = 4.68 *,
p = 0.031, η2
p = 0.438), indicating that the increase in the percentage of damaged plants from the first to the second BBCH stage was not the same for all treatments. The highest increase was recorded in the control (
p < 0.001) and Force 1.5 G (
p = 0.001), the lowest in the ATTRACAP
® treatment (
p = 0.060), indicating a continuous protective action of this insecticide.
In 2019, the main effect of the treatment was significant (F (5,36) = 3.78 **,
p = 0.007, η2
p = 0.344). Post hoc pairwise comparison revealed that the highest percentage of the damaged plants was recorded in the control and was significantly higher than in Force 20 CS and Force 1.5 G treatments (
p = 0.018;
Table 3). We analysed the first and the second BBCH stages separately, and the homogeneous groups are presented in
Table 3. The main effect of the BBCH stage was also significant (F (1,36) = 21.15 **,
p < 0.001, η2
p = 0.370).
The interaction between the treatments and the growth stages, however, did again significantly differ (F (5,36) = 3.06 *, p = 0.021, η2p = 0.298). A significant increase was observed in the control (p < 0.001) and the Buteo Start 480 FS (p = 0.008) treatment, while in Sonido (p = 0.845), ATTRACAP® (p = 0.073), Force 1.5 G (p = 0.446) and Force 20 CS (p = 0.464), there was no significant increase in the percent of damaged plants.
In 2021, at RŠ Field 1, the main effect of the treatment was significant (F (5,48) = 9.37 **,
p < 0.001, η2
p = 0.494 (
Table 4). A significant difference in the percentage of damaged plants was recorded between the control and all insecticidal treatments (
p < 0.001). However, there was neither a significant difference (
p > 0.05) between insecticidal treatments overall nor in either of the plant growth stages observed separately. The main effect of the BBCH stage was significant (F (1,48) = 28.35 **,
p < 0.001, η2
p = 0.371).
A significant (F (5,48) = 6.86 **, p < 0.001, η2p = 0.417) interaction between the treatment and the growth stage was recorded. The increase in the percentage of damaged plants from the BBCH 02 to the BBCH 04/05 stages was not the same for all treatments). The highest increase was recorded for the control treatment (p < 0.001). The increase in the percentage of damaged plants from the first to the second growth stage was not statistically significant for any of the used treatments: ATTRACAP® (p = 0.122), Buteo Start (p = 0.067), Force 1.5 G (p = 0.440), Force 20 CS (p = 0.473) or Lumiposa (p = 0.504).
In 2021, at RŠ T-12 (low wireworm abundance), the main effect of treatment was significant (F (6,28) = 3.17 **,
p = 0.017, η2
p = 0.404). Homogeneous groups in both BBCH stages and overall are presented in
Table 5. The main effect of the BBCH stage was significant (F (1,28) = 5.68 **,
p = 0.024, η2
p = 0.169).
Repeated measures ANOVA showed that the interaction between the main effects was marginally significant (F (6,28) = 2.344, p = 0.058, η2p = 0.334). For all insecticidal treatments, the percentage of damaged plants in the insecticidal treatments did not significantly differ between the BBCH 02 and BBCH 04/05 stages (p > 0.05), whereas in the control treatment, a significant increase in the percentage of damaged plants was recorded (p < 0.001).
3.4. Plant Damage Rating
3.4.1. Damage Level Depending on the Treatments Applied
Damage level was analysed using binomial regression. The percentage of undamaged and damaged plants is presented for both experimental fields and localities (
Figure 5 and
Figure 6).
At RŠ Field 1 (high wireworm abundance), the highest percentage of damaged plants was recorded in the control (90%). In the ATTRACAP
® treatment (50%), the percentage of damage was equivalent to treatments Sonido and Lumiposa (50% and 48%, respectively), while in the Force 20 CS treatment, it was the lowest (26%;
Figure 5).
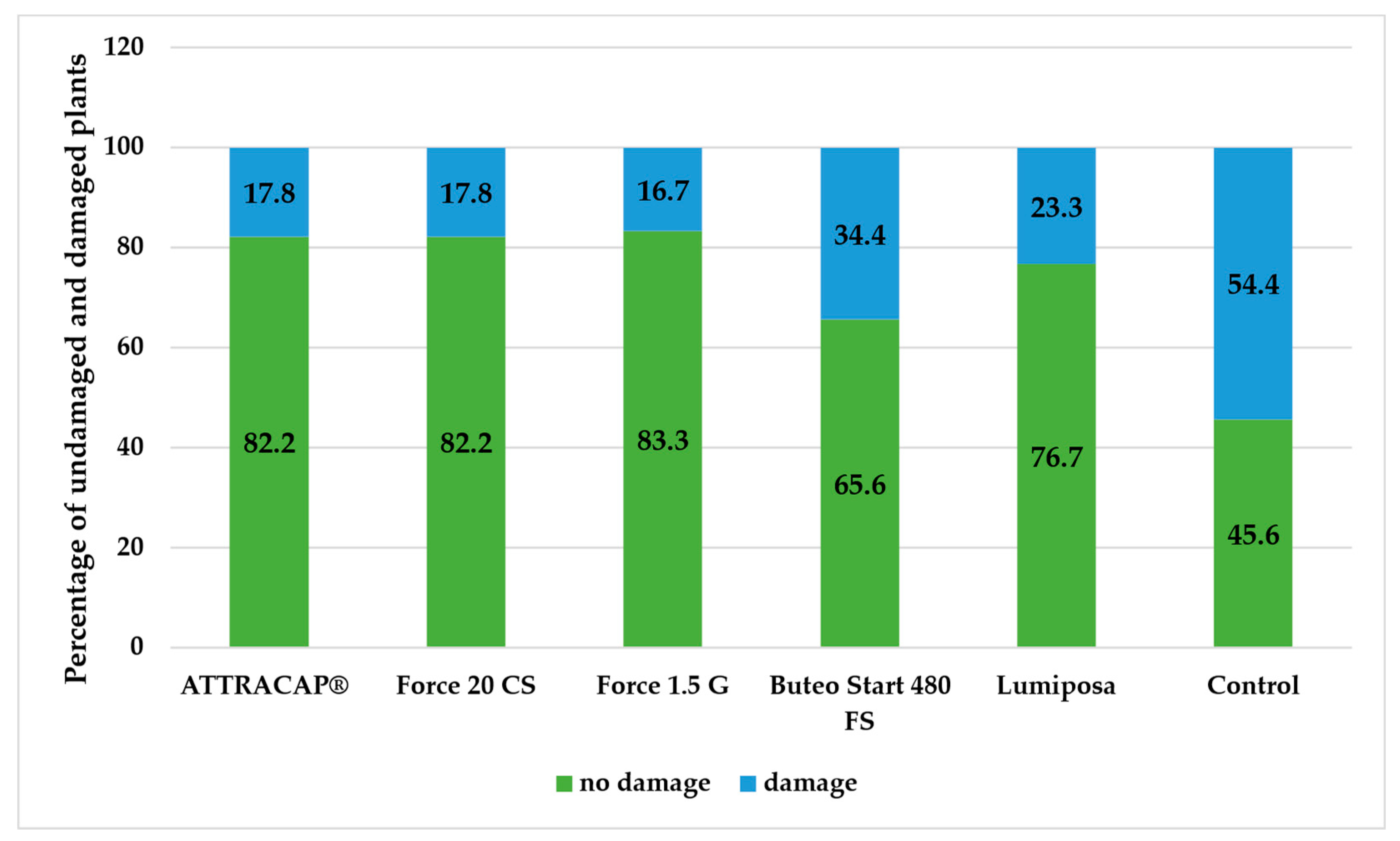
At RŠ T-12, the highest percentage of damaged plants was, again, recorded in the control (54.4%), whereas the percentages in the ATTRACAP
® treatment (17.8%) and the chemical treatments (16.7–23.3%) were at the same level, except in Buteo Start 480 FS (34.4%), which had the highest damage level (
Figure 6).
The percentage of the five damage levels is presented for both experimental fields: RŠ Field 1 and RŠ T-12 (
Figure 7 and
Figure 8).
At RŠ Field 1, having high natural wireworm abundance, the highest percentage of undamaged plants (damage level 0) was recorded in the treatment with Force 20 CS (28%) and Force 1.5 G (24%), followed by Buteo Start 480 FS (14%), Lumiposa (10%), Sonido (8%) and ATTRACAP
® (4%). The percentage of plant damage rated as 1 ranged from 34% to 46% in all treatments, while in the control, it was the lowest, 8%. When specifically considering the percentage of damage rated as level 2, the ATTRACAP
® (32%) provided the same level of protection as the chemical treatments (22–42%). The percentage of damage level 3 was higher in ATTRACAP
® (16%) compared to the chemical insecticides (4–8%) but lower compared to the control (44%). The highest percentage of damage rated as level 4/5 was recorded in the control treatment (16%), followed by Lumiposa and ATTRACAP
® (6% and 2%, respectively) (
Figure 7). The abovementioned implies that regardless of the treatment, damage occurred and neither biological nor chemical insecticides can provide absolute protection from wireworms.
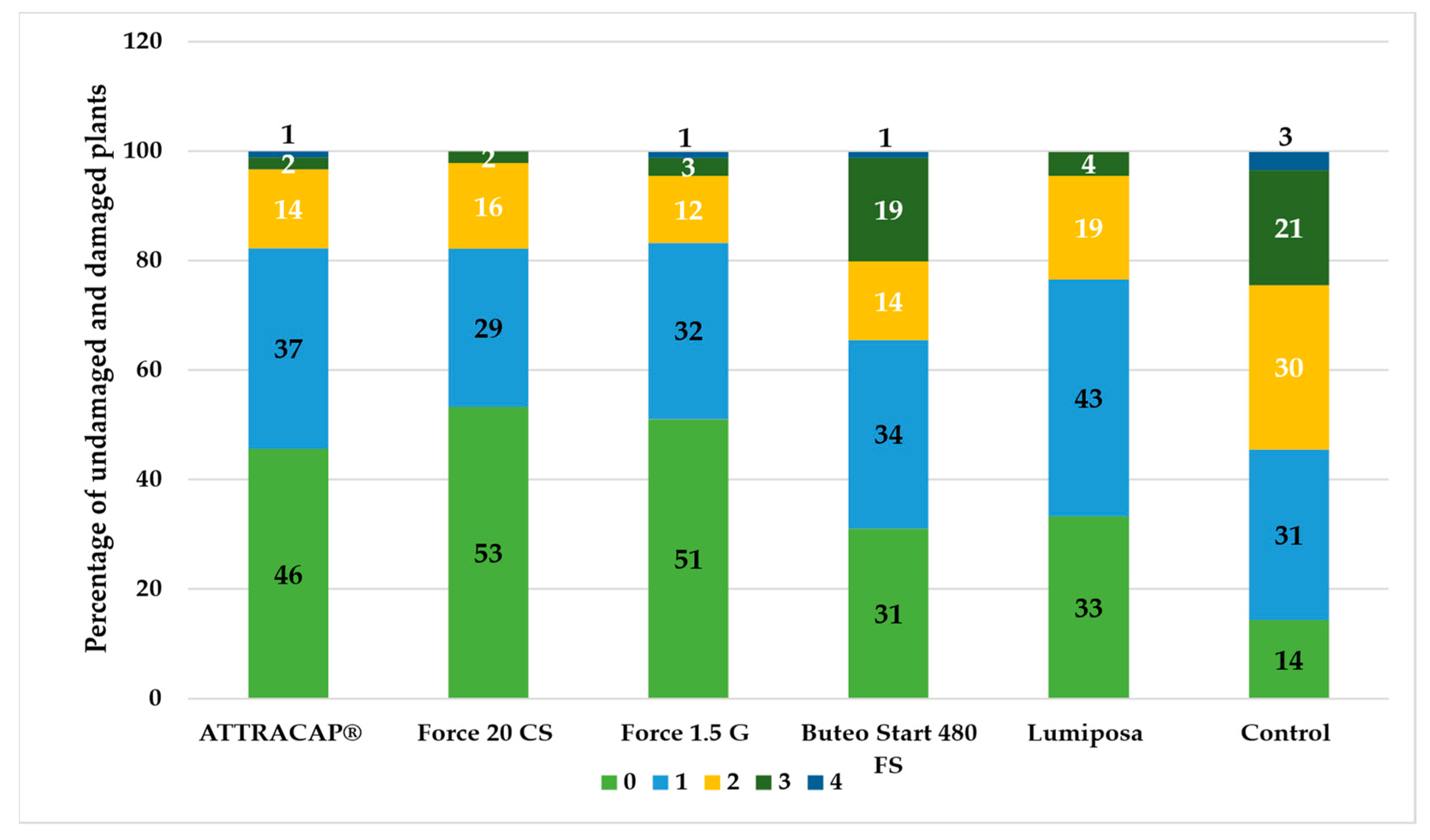
At RŠ T-12, the locality with low natural infestation, the situation was slightly different (
Figure 8). The best protection, based on the percentage of plants with no damage (damage level 0), was provided by the Force 20 CS (53%), Force 1.5 G (51%) and ATTRACAP
® (46%), followed by Lumiposa (33%) and Buteo Start 480 FS (31%). In the group rated as damage level 1, the treatments were (sorted from highest to lowest): Lumiposa (43%) > ATTRACAP
® (37%) > Buteo Start 480 FS (34%) > Force 1.5 G (32%) > control (31%) > Force 20 CS (29%). The percentage of plant damage rated as 2 was the highest in the control (30%) followed by other treatments, sorted from highest to lowest: Lumiposa (19%) > Force 20 CS (16%) > ATTRACAP
®/Buteo Start 480 FS (14%) > Force 1.5 G (12%). The percentage of plants rated as damage level 3 was the highest in the control (21%) followed by the Buteo Start 480 FS (19%), while in the ATTRACAP
® treatment and others (2–8%). The highest relative percentage of the plant damage rated as 4 was found in the control (3%), followed by the ATTRACAP
®, Force 1.5 G and Buteo Start 480 FS (2% in all three treatments). The occurrence of higher damage levels (3 and 4/5) in the ATTRACAP
® treatment was the same as in the Force 20 SC and Force 1.5 G treatments, implying a protective role against wireworms.
3.4.2. Binary Logistic Regression
A binary logistic regression was performed for modelling the occurrence of damage as a dependent variable with independent variables being locality and treatment (
Table 6). For this purpose, the damage level ratings were simplified and divided in two groups: undamaged (including levels 0–1) and damaged (including levels 2–4) plants.
Locality had a significant influence on the occurrence of wireworm damage on the sunflower plants, since the coefficient for the variable locality was positive and significantly different from zero (p < 0.001), with RŠ T-12 taken as the reference category. The odds ratio (OR) (RŠ Field 1) = 2.89, which implied that the odds for the occurrence of damage on RŠ Field 1 was 2.89 times higher than on field RŠ T-12. Considering that the natural wireworm abundance was much higher at RŠ Field 1, the obtained results were close to the real conditions.
The results of the binary logistic regression also confirmed that the application of all insecticides had an influence on the occurrence of damage, taking the control as the reference category. Since the coefficients for all treatments were negative and significantly different from zero (p < 0.001), all insecticides reduced the odds of plant damage occurrence compared to the control. The odds ratio for the ATTRACAP®: OR (Attracap) = 0.184, which implied that the odds for the occurrence of damage using ATTRACAP® were 81.6% (0.816 = −0.184) lower compared to the control, where no insecticide was applied. The odds of damage occurrence in the control were the highest (8.8 and 6.6 times, respectively) compared to the odds in the Force 20 SC and Force 1.5 G treatments.
The Hosmer–Lemeshow test of goodness of fit test (χ2 (8) = 9.627, p = 0.292) indicates that the obtained model adequately described the data. The model explained 18.7% (Nagelkerke R2 = 0.187) of the variance occurrence of damage and correctly classified 68.2% of cases.
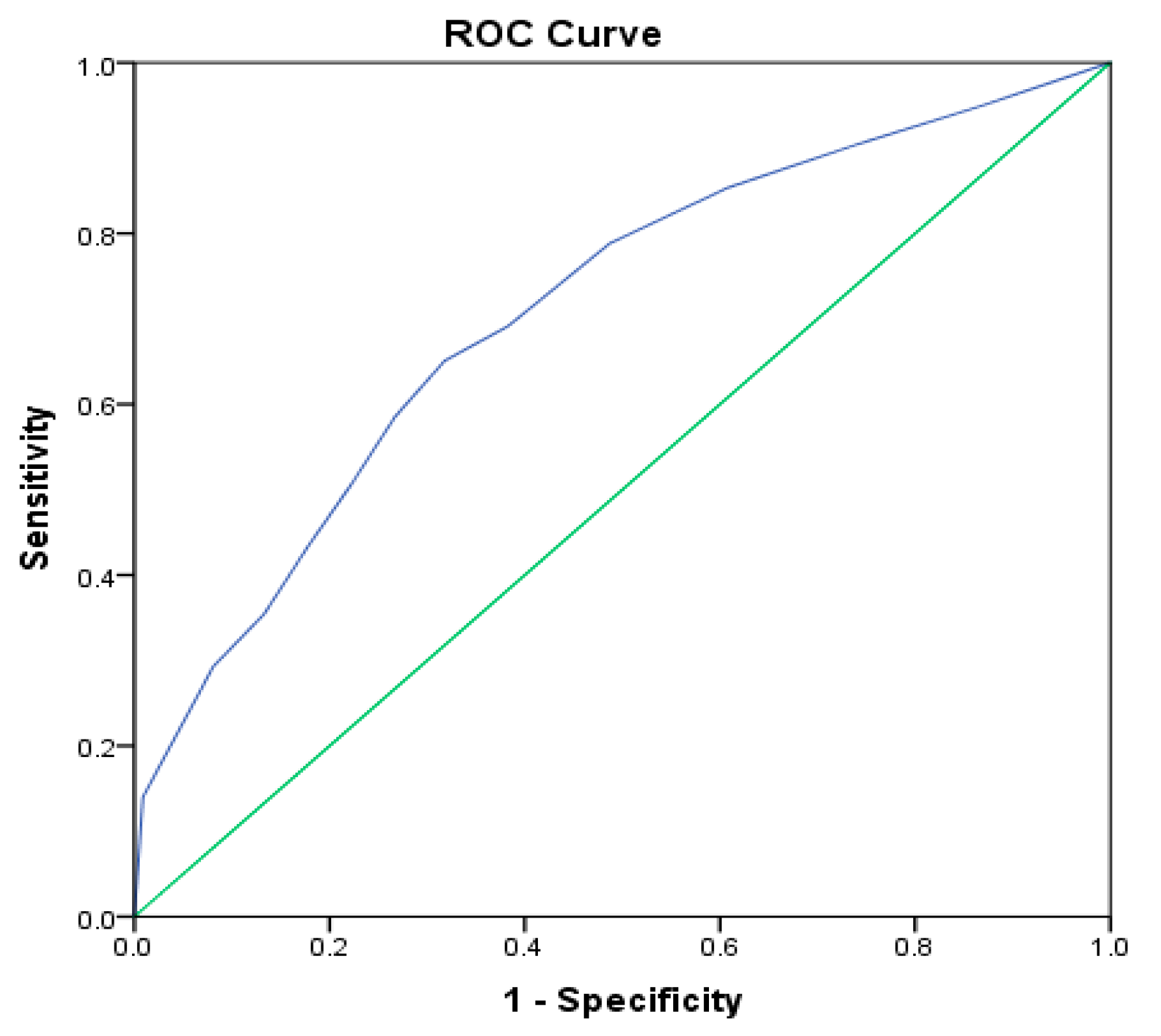
To check the model quality, a receiver operating characteristic curve (ROC curve) was constructed (
Figure 9). The area under the ROC curve was 0.711, with 95% CI (0.675, 0.746;
p < 0.001), demonstrating a 71.1% chance that the model could distinguish between damaged and undamaged plants.
3.4.3. Multinomial Logistic Regression
The multinomial logistic regression was used to model the influence of the locality and treatment on the occurrence of all five damage levels. As a reference category for dependant variable, level 0 was taken. For all damage levels, the control was used as the reference category for the variable treatment, and the field RŠ T-12 was used as the reference category for the variable locality. The coefficients for both independent variables were statistically different from zero; therefore, they had an influence on the occurrence of different damage levels (
Table 7).
Observing the influence of the locality on the plant damage level, the coefficients for all treatments were significantly different from zero and the corresponding p-values were less than 0.001, implying that the locality had an important influence on the damage levels.
When analysing locality RŠ Field 1, the odds for the occurrence of hardly visible damage on plant roots (damage level 1), compared to the undamaged plants, was 3.23 times higher when compared to the odds at field RŠ T-12, given that the odds ratio was OR = 3.23. When using the treatment as a variable, Force 20 CS and Force 1.5 G were the most effective, since the odds of the occurrence of hardly visible damages (level 1) were 3.37 and 3.30 times higher for the control. In the case of ATTRACAP
®, the odds were 1.94 higher for the control. The least effective treatment was Sonido, with the corresponding odds ratio being not significantly different from 1 (
Table 7).
The odds of the occurrence of clearly visible damage on plant roots (level 2) at RŠ Field 1 were 5.72 times higher than at RŠ T-12 (OR (Field 1) = 5.72). Force 20 CS proved to be the most effective treatment with the odds of the occurrence of clearly visible damages (level 2) for the control being 9.61 times higher (OR (Force 20 CS) = 9.61). The other insecticide treatments reduced the odds of visible damages in the following order: Force 1.5 G (7.52 times), ATTRACAP® (5.26), Buteo Start 480 FS (4.13), Lumiposa (3.45) and Sonido (2.21 times).
The odds of the occurrence of visibly damaged plants having a chance to recover (damage level 3) compared to the control group, at the locality RŠ Field 1, were 6.03 times higher than at RŠ T-12. Force 20 CS proved to be the most efficient treatment, because the odds (OR = 58.8) were 58.8 times higher compared to the control treatment. Force 1.5 G (35.7 times), Lumiposa (16.9 times), ATTRACAP® (14.9 times) and Sonido (11.6 times), respectively, were ranked thereafter. Buteo Start 480 FS had the lowest efficiency in reducing the odds of the level 3 damage (5.6 times) occurrence.
The odds of the occurrence of heavily damaged plant with no chance for recovery (damage level 4/5), at RŠ Field 1 were 14.78 times higher than at RŠ T-12. As there were no level 4 damages in the Force 20 CS treatment, a large negative coefficient was obtained. This finding implies that the occurrence of complete plant damage would be many times lower when using the Force 20 CS than in the control group. Among the other treatments, Force 1.5 G (66.67), Buteo Start 480, FS 480 FS (34.48) and ATTRACAP® (22.73 times) were the most effective when compared to the untreated plants. Lumiposa (11.63) was the least efficient.
The likelihood ratio test in the multinomial regression showed that the logistic regression model was significant (χ2 (4) = 239.1, p < 0.0005). The model explained 25.1% (Nagelkerke R2) of the variance of the damage occurrence. The percentage of correctly classified cases was 42.9%.
To check the model quality, ROC curves were constructed for each damage level (
Table 8). The model was good at predicting each damage level, since
p-values for each level were less than 0.001. The area under curve (AUC) indicated the extent at which the model would be able to distinguish the damage level from the other levels. The proposed model was able to distinguish damage level 0 from the other levels (1–4/5) in 73.7% of cases; damage level 1 from levels 0, 2, 3 and 4/5 in 58.7% of cases; level 2 from levels 0, 1, 3 and 4/5 in 63.7% of cases; level 3 from damage levels 0, 1, 2 and 4/5 in 75.7% of cases and damage level 4 from levels 0–3 in 82.6% of cases, respectively.