Assessment of the Carbon and Nitrogen Mineralisation of Digestates Elaborated from Distinct Feedstock Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physicochemical Characterization of Products
2.2. Soil Characteristics
2.3. N Incubations
2.4. C Incubations
2.5. Soil Microbial Biomass
2.6. Calculations
2.7. Statistical Analysis
3. Results
3.1. Product Characteristics
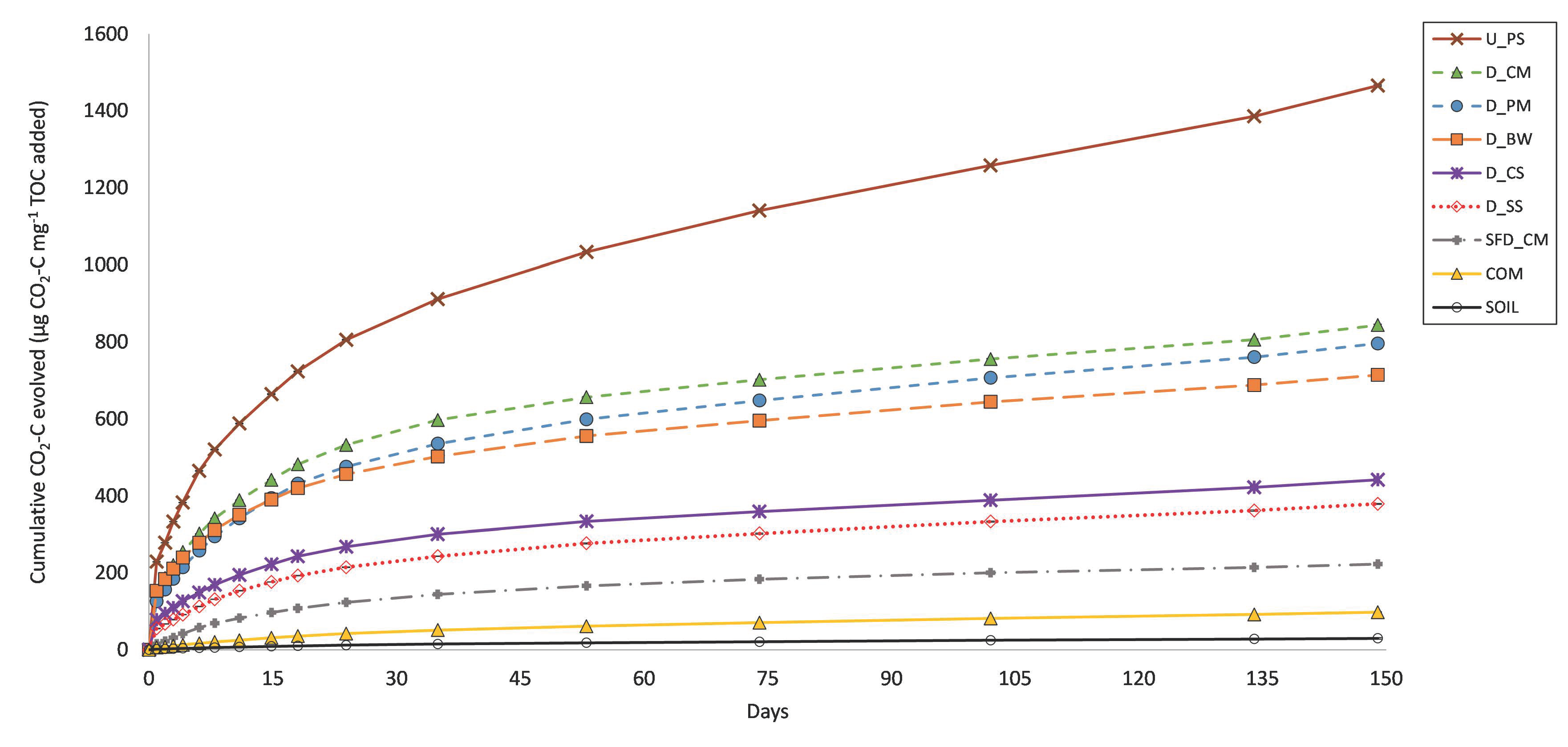
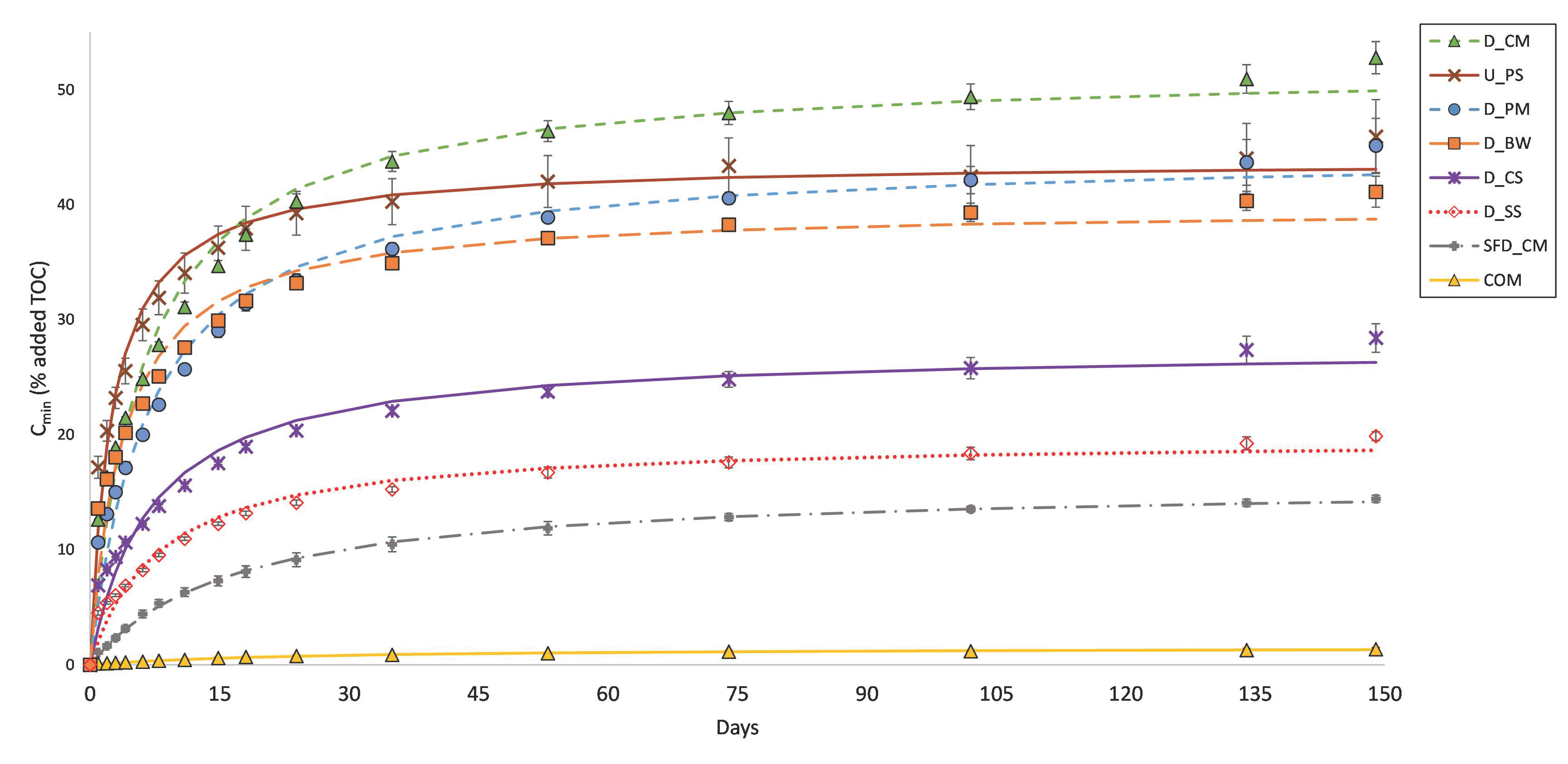
3.2. C Mineralisation
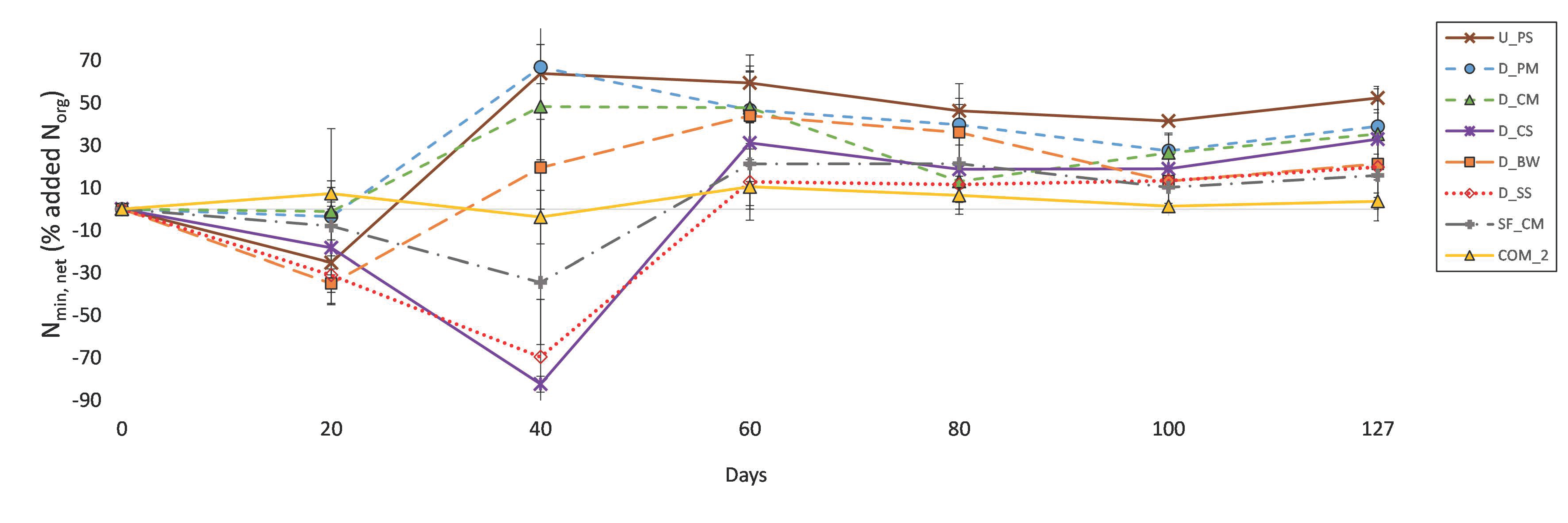
3.3. N Mineralisation
4. Discussion
4.1. C Mineralisation
4.2. C Sequestration Potential and Possible Implications for C Farming Strategies
4.3. N Mineralisation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | D_BW | D_SS | D_CS | D_PM | D_CM | COM * | U_PS | SF_CM |
|---|---|---|---|---|---|---|---|---|
| CO2-C (μg mg−1 TOC) | 714 ± 2 | 379 ± 1 | 442 ± 4 | 796 ± 4 | 843 ± 2 | 98 ± 1 | 1466 ± 2 | 223 ± 2 |
References
- Chynoweth, D.P.; Owens, J.M.; Legrand, R. Renewable Methane from Anaerobic Digestion of Biomass. Renew. Energy 2001, 22, 1–8. [Google Scholar] [CrossRef]
- EBA (European Biogas Association). EBA Statistical Report 2020; EBA: Brussels, Belgium, 2020. [Google Scholar]
- Leprich, U.; Hoffmann, P.; Luxenburger, M. Certificates in Germany’s Renewable Energy Market. In Marketing Renewable Energy; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Horschig, T.; Adams, P.W.R.; Röder, M.; Thornley, P.; Thrän, D. Reasonable Potential for GHG Savings by Anaerobic Biomethane in Germany and UK Derived from Economic and Ecological Analyses. Appl. Energy 2016, 184, 840–852. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management Strategies for Anaerobic Digestate of Organic Fraction of Municipal Solid Waste: Current Status and Future Prospects. Waste Manag. Res. 2019, 37 (Suppl. S1), 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huygens, D.; Orveillon, G.; Lugato, E.; Tavazzi, S. Technical Proposals for the Safe Use of Processed Manure above the Threshold Established for Nitrate Vulnerable Zones by the Nitrates Directive (91/676/EEC); JRC: Ispra, Italy, 2020. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valoriz. 2017, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Reuland, G.; Sigurnjak, I.; Dekker, H.; Michels, E.; Meers, E. The Potential of Digestate and the Liquid Fraction of Digestate as Chemical Fertiliser Substitutes under the RENURE Criteria. Agronomy 2021, 11, 1374. [Google Scholar] [CrossRef]
- Romero-güiza, M.S.; Mata-alvarez, J.; María, J.; Rivera, C. Nutrient Recovery Technologies for Anaerobic Digestion Systems: An Overview Tecnologías de Recuperación de Nutrientes Para Los Sistemas de Digestión Anaeróbica: Revisión Tecnologias de Recuperação de Nutrientes Para Os Sistemas de Digestão Anaeróbia: R. Bucaramanga 2015, 29, 7–26. [Google Scholar]
- Sánchez-Rodríguez, A.R.; Carswell, A.M.; Shaw, R.; Hunt, J.; Saunders, K.; Cotton, J.; Chadwick, D.R.; Jones, D.L.; Misselbrook, T.H. Advanced Processing of Food Waste Based Digestate for Mitigating Nitrogen Losses in a Winter Wheat Crop. Front. Sustain. Food Syst. 2018, 2, 1–14. [Google Scholar] [CrossRef]
- Insam, H.; Gómez-Brandón, M.; Ascher, J. Manure-Based Biogas Fermentation Residues - Friend or Foe of Soil Fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Amalevičiūtė-Volungė, K.; Volungevičius, J.; Šlepetys, J. The Effect of Digestate Fertilisation on Grass Biogas Yield and Soil Properties in Field-Biomass-Biogas-Field Renewable Energy Production Approach in Lithuania. Biomass Bioenergy 2021, 153, 106211. [Google Scholar] [CrossRef]
- Haraldsen, T.K.; Andersen, U.; Krogstad, T.; Sørheim, R. Liquid Digestate from Anaerobic Treatment of Source-Separated Household Waste as Fertilizer to Barley. Waste Manag. Res. 2011, 29, 1271–1276. [Google Scholar] [CrossRef]
- Schwager, E.A.; VanderZaag, A.C.; Wagner-Riddle, C.; Crolla, A.; Kinsley, C.; Gregorich, E. Field Nitrogen Losses Induced by Application Timing of Digestate from Dairy Manure Biogas Production. J. Environ. Qual. 2016, 45, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, F.; Bhogal, A.; Cardenas, L.; Chadwick, D.; Misselbrook, T.; Rollett, A.; Taylor, M.; Thorman, R.; Williams, J. Nitrogen Losses to the Environment Following Food-Based Digestate and Compost Applications to Agricultural Land. Environ. Pollut. 2017, 228, 504–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdi, L.; Kuikman, P.J.; Orlandini, S.; Mancini, M.; Napoli, M.; Dalla Marta, A. Does the Use of Digestate to Replace Mineral Fertilizers Have Less Emissions of N2O and NH3? Agric. For. Meteorol. 2019, 269–270, 112–118. [Google Scholar] [CrossRef]
- Pezzolla, D.; Bol, R.; Gigliotti, G.; Sawamoto, T.; López, A.L.; Cardenas, L.; Chadwick, D. Greenhouse Gas (GHG) Emissions from Soils Amended with Digestate Derived from Anaerobic Treatment of Food Waste. Rapid Commun. Mass Spectrom. 2012, 26, 2422–2430. [Google Scholar] [CrossRef] [PubMed]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of Digestate from Anaerobically Digested Cattle Slurry and Plant Materials on Soil Microbial Community and Emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar] [CrossRef]
- Dietrich, M.; Fongen, M.; Foereid, B. Greenhouse Gas Emissions from Digestate in Soil. Int. J. Recycl. Org. Waste Agric. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Sharifi, M.; Baker, S.; Hojabri, L.; Hajiaghaei-kamrani, M. Short-Term Nitrogen Dynamics in a Soil Amended with Anaerobic Digestate. Can. J. Soil Sci. 2019, 99, 173–181. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen Mineralization from Organic Residues. J. Environ. Qual. 2005, 34, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Alburquerque, J.A.; de la Fuente, C.; Bernal, M.P. Chemical Properties of Anaerobic Digestates Affecting C and N Dynamics in Amended Soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Tambone, F.; Adani, F. Nitrogen Mineralization from Digestate in Comparison to Sewage Sludge, Compost and Urea in a Laboratory Incubated Soil Experiment. Z. Pflanzenernahr. Bodenkd. 2017, 180, 355–365. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, C.; Alburquerque, J.A.; Clemente, R.; Bernal, M.P. Soil C and N Mineralisation and Agricultural Value of the Products of an Anaerobic Digestion System. Biol. Fertil. Soils 2013, 49, 313–322. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Liang, G.; Du, Z.; Zhou, J.; Zhu, C.; Huang, K.; Zhou, X.; Luo, Y.; Yan, L.; et al. Global Variation of Soil Microbial Carbon-Use Efficiency in Relation to Growth Temperature and Substrate Supply. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial Diversity Drives Carbon Use Efficiency in a Model Soil. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Simon, E.; Canarini, A.; Martin, V.; Séneca, J.; Böckle, T.; Reinthaler, D.; Pötsch, E.M.; Piepho, H.P.; Bahn, M.; Wanek, W.; et al. Microbial Growth and Carbon Use Efficiency Show Seasonal Responses in a Multifactorial Climate Change Experiment. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Wallenstein, M.D.; Schipanksi, M.E.; Stuart Grandy, A. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattin, M.; Semple, K.T.; Stutter, M.; Romano, G.; Lag-Brotons, A.J.; Parry, C.; Surridge, B.W. Changes in Microbial Utilization and Fate of Soil Carbon Following the Addition of Different Fractions of Anaerobic Digestate to Soils. Eur. J. Soil Sci. 2021, 2020, 1–16. [Google Scholar] [CrossRef]
- Macherey-Nagel GmbH & Co. KG. REF 985 825, Test 8-25, 12.16, BOD5-TT. Available online: https://vendart.com.au/app/uploads/2019/10/985825-INSTRUCTIONS.pdf (accessed on 4 February 2022).
- Macherey-Nagel GmbH & Co. KG. REF 985 093, Test 0-93 08.16, Total Organic Carbon. Available online: https://vendart.com.au/app/uploads/2019/10/985093-INSTRUCTIONS.pdf. (accessed on 4 February 2022).
- IUSS Working Group WRB. World Reference Base for Soil Resources. In 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 4 February 2022).
- VITO. Bodem-Bepaling van Snel Vrijkomende Organische Stikstof. Available online: https://esites.vito.be/sites/reflabos/2010/Onlinedocumenten/BAM_deel1_12.pdf (accessed on 20 December 2021).
- OVAM. Oriënterend Onderzoek Naar de Invulling van de Begrippen Mineralenrijk-Mineralenarm, Humusrijk; D/2002/5024/06; OVAM: Mechelen, Belgium, 2002. [Google Scholar]
- Anderson, J.P.E. Soil Respiration. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2, 2nd ed.; Page, A.L., Ed.; John Wiley & Sons: New York, NY, USA, 1982; Volume 9, pp. 831–871. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkison, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 1–5. [Google Scholar] [CrossRef]
- Sigurnjak, I.; De Waele, J.; Michels, E.; Tack, F.M.; Meers, E.; De Neve, S. Nitrogen Release and Mineralization Potential of Derivatives from Nutrient Recovery Processes as Substitutes for Fossil Fuel-Based Nitrogen Fertilizers. Soil Use Manag. 2017, 33, 437–446. [Google Scholar] [CrossRef]
- Sleutel, S.; De Neve, S.; Prat Roibás, M.R.; Hofman, G. The Influence of Model Type and Incubation Time on the Estimation of Stable Organic Carbon in Organic Materials. Eur. J. Soil Sci. 2005, 56, 505–514. [Google Scholar] [CrossRef]
- De Neve, S.; Pannier, J.; Hofman, G. Temperature Effects on C- and N-Mineralization from Vegetable Crop Residues. Plant Soil 1996, 181, 25–30. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Changes in Variability of Soil Moisture Alter Microbial Community C and N Resource Use. Soil Biol. Biochem. 2011, 43, 1837–1847. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wu, J.; Brookes, P.C. Measuring Soil Microbial Biomass Using an Automated Procedure. Soil Biol. Biochem. 2011, 43, 873–876. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Brookes, P.C.; Powlson, D.S. Measuring Soil Microbial Biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Sawada, K.; Toyota, K. Effects of the Application of Digestates from Wet and Dry Anaerobic Fermentation to Japanese Paddy and Upland Soils on Short-Term Nitrification. Microbes Environ. 2015, 30, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Stöver, D.S.; Sun, G.; Kroff, P.; Thomsen, S.T.; Hauggaard-Nielsen, H. Anaerobic Co-Digestion of Perennials: Methane Potential and Digestate Nitrogen Fertilizer Value. J. Plant Nutr. Soil Sci. 2016, 179, 696–704. [Google Scholar] [CrossRef]
- Möller, K. Effects of Anaerobic Digestion on Soil Carbon and Nitrogen Turnover, N Emissions, and Soil Biological Activity. A Review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Zmora-Nahum, S.; Markovitch, O.; Tarchitzky, J.; Chen, Y. Dissolved Organic Carbon (DOC) as a Parameter of Compost Maturity. Soil Biol. Biochem. 2005, 37, 2109–2116. [Google Scholar] [CrossRef]
- Akratos, C.S.; Tekerlekopoulou, A.G.; Vasiliadou, I.A.; Vayenas, D.V. Cocomposting of Olive Mill Waste for the Production of Soil Amendments; Elsevier Inc.: Philadelphia, PA, USA, 2017. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. The Anaerobic Digestion Process Capability to Produce Biostimulant: The Case Study of the Dissolved Organic Matter (DOM) vs. Auxin-like Property. Sci. Total Environ. 2017, 589, 36–45. [Google Scholar] [CrossRef]
- Cavalli, D.; Corti, M.; Baronchelli, D.; Bechini, L.; Marino Gallina, P. CO2 Emissions and Mineral Nitrogen Dynamics Following Application to Soil of Undigested Liquid Cattle Manure and Digestates. Geoderma 2017, 308, 26–35. [Google Scholar] [CrossRef]
- Egene, C.E.; Sigurnjak, I.; Regelink, I.C.; Schoumans, O.F.; Adani, F.; Michels, E.; Sleutel, S.; Tack, F.M.G.; Meers, E. Solid Fraction of Separated Digestate as Soil Improver: Implications for Soil Fertility and Carbon Sequestration. J. Soils Sedim. 2020, 21, 678–688. [Google Scholar] [CrossRef]
- De Neve, S.; Sleutel, S.; Hofman, G. Carbon Mineralization from Composts and Food Industry Wastes Added to Soil. Nutr. Cycl. Agroecosyst. 2003, 67, 13–20. [Google Scholar] [CrossRef]
- de la Fuente, C.; Clemente, R.; Martinez, J.; Pilar Bernal, M. Optimization of Pig Slurry Application to Heavy Metal Polluted Soils Monitoring Nitrification Processes. Chemosphere 2010, 81, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and Defixation of Ammonium in Soils: A Review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Brandón, M.; Juárez, M.F.D.; Zangerle, M.; Insam, H. Effects of Digestate on Soil Chemical and Microbiological Properties: A Comparative Study with Compost and Vermicompost. J. Hazard. Mater. 2016, 302, 267–274. [Google Scholar] [CrossRef]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; García, C.; Insam, H. Pathogenic Bacteria and Mineral N in Soils Following the Land Spreading of Biogas Digestates and Fresh Manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Vaneeckhaute, C.; Michels, E.; Ryckaert, B.; Ghekiere, G.; Tack, F.M.G.; Meers, E. Fertilizer Performance of Liquid Fraction of Digestate as Synthetic Nitrogen Substitute in Silage Maize Cultivation for Three Consecutive Years. Sci. Total Environ. 2017, 599–600, 1885–1894. [Google Scholar] [CrossRef]
- Fouda, S.; Von Tucher, S.; Lichti, F.; Schmidhalter, U. Nitrogen Availability of Various Biogas Residues Applied to Ryegrass. J. Plant Nutr. Soil Sci. 2013, 176, 572–584. [Google Scholar] [CrossRef]
- Brust, G.E. Management Strategies for Organic Vegetable Fertility; Elsevier Inc.: Philadelphia, PA, USA, 2019. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing Soil Fertility in Organic Farming Systems. Soil Use Manag. 2002, 18, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Gutser, R.; Ebertseder, T.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-Term and Residual Availability of Nitrogen after Long-Term Application of Organic Fertilizers on Arable Land. J. Plant Nutr. Soil Sci. 2005, 168, 439–446. [Google Scholar] [CrossRef]
- Barduca, L.; Wentzel, S.; Schmidt, R.; Malagoli, M.; Joergensen, R.G. Mineralisation of Distinct Biogas Digestate Qualities Directly after Application to Soil. Biol. Fertil. Soils 2021, 57, 235–243. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N Availability Determine the Priming Effect: Microbial N Mining and Stoichiometric Decomposition Theories. Glob. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.C.; Meir, P.; Nottingham, A.T.; Reay, D.S.; Stott, A.W.; Salinas, N.; Whitaker, J. Carbon and Nitrogen Inputs Differentially Affect Priming of Soil Organic Matter in Tropical Lowland and Montane Soils. Soil Biol. Biochem. 2019, 129, 212–222. [Google Scholar] [CrossRef]
- Riffaldi, R.; Saviozzi, A.; Levi-Minzi, R. Carbon Mineralization Kinetics as Influenced by Soil Properties. Biol. Fertil. Soils 1996, 22, 293–298. [Google Scholar] [CrossRef]
- Calderón, F.J.; McCarty, G.W.; Reeves, J.B. Analysis of Manure and Soil Nitrogen Mineralization during Incubation. Biol. Fertil. Soils 2005, 41, 328–336. [Google Scholar] [CrossRef]
- Silveira, M.L.A. Dissolved Organic Carbon and Bioavailability of N and P as Indicators of Soil Quality. Sci. Agric. 2005, 62, 502–508. [Google Scholar] [CrossRef]
- Kirchmann, H.; Bernal, M.P. Organic Waste Treatment and C Stabilization Efficiency. Soil Biol. Biochem. 1997, 29, 1747–1753. [Google Scholar] [CrossRef]
- Zhu, X.; Jackson, R.D.; DeLucia, E.H.; Tiedje, J.M.; Liang, C. The Soil Microbial Carbon Pump: From Conceptual Insights to Empirical Assessments. Glob. Chang. Biol. 2020, 26, 6032–6039. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, X. The Soil Microbial Carbon Pump as a New Concept for Terrestrial Carbon Sequestration. Sci. China Earth Sci. 2021, 64, 545–558. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The Importance of Anabolism in Microbial Control over Soil Carbon Storage. Nat. Publ. Gr. 2017, 2, 1–6. [Google Scholar] [CrossRef]
- Chevallier, T.; Blanchart, E.; Albrecht, A.; Feller, C. The Physical Protection of Soil Organic Carbon in Aggregates: A Mechanism of Carbon Storage in a Vertisol under Pasture and Market Gardening (Martinique, West Indies). Agric. Ecosyst. Environ. 2004, 103, 375–387. [Google Scholar] [CrossRef]
- Luo, Z. Modelling the Dynamic Physical Protection of Soil Organic Carbon: Insights Modelling the Dynamic Physical Protection of Soil Organic Carbon: Insights into Carbon Predictions and Explanation of the Priming Effect. Glob. Chang. Biol. 2017, 23, 5273–5283. [Google Scholar] [CrossRef]
- Solomon, D.; Lehmann, J.; Harden, J.; Wang, J.; Kinyangi, J.; Heymann, K.; Karunakaran, C.; Lu, Y.; Wirick, S.; Jacobsen, C. Micro- and Nano-Environments of Carbon Sequestration: Multi-Element STXM–NEXAFS Spectromicroscopy Assessment of Microbial Carbon and Mineral Associations. Chem. Geol. 2012, 329, 53–73. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L. A Theoretical Model of Litter Decay and Microbial Interaction. Ecol. Monogr. 2006, 76, 151–174. [Google Scholar] [CrossRef]
- Makara, A.; Kowalski, Z.; Saeid, A. Properties of the Filtrate from Treatment of Pig Manure by Filtration Method. Open Chem. 2017, 15, 19–27. [Google Scholar] [CrossRef]
- Sommer, S.G.; Mathanpaal, G.; Dass, G.T. A Simple Biofilter for Treatment of Pig Slurry in Malaysia. Environ. Technol. 2005, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Brookman, S.K.E. Estimation of Biochemical Oxygen Demand in Slurry and Effluents Using Ultra-Violet Spectrophotometry. Water Res. 1997, 31, 372–374. [Google Scholar] [CrossRef] [Green Version]

| Product | Facility | Yearly Ratio of Feedstock Composition (%) | |
|---|---|---|---|
| Acronym | Description | ||
| D_BW | Digestate | Am-Power (BE) | Biowaste (food): 69 |
| Food industry sludge: 11 | |||
| Animal manure: 7 | |||
| Glycerine: 6 | |||
| Other substrates: 4 | |||
| Manure solid fraction: 3 | |||
| D_SS | Digestate | Acqua & Sole (IT) | Sewage sludge: 85 |
| Biowaste (food): 9 | |||
| Digestate from biowaste: 7 | |||
| D_CS | Digestate | BENAS-GNS (DE) | Corn silage: 44 |
| Rye silage: 31 | |||
| Chicken manure: 14 | |||
| Grass: 5 | |||
| Corn grain: 4 | |||
| Other solids: 1 | |||
| Millet: <1 | |||
| D_PM | Digestate | Groot Zevert Vergisting (NL) | Pig manure: 67 |
| By-products from dairy and feed industry: 16 | |||
| Dairy by-products: 11 | |||
| Slaughterhouse manure: 9 | |||
| Dairy cattle manure: 4 | |||
| Glycerine: 4 | |||
| D_CM | Digestate | Anonymous (UK) | Chicken manure: 100 |
| COM_1 | Commercial compost | / | Source-separated waste from households and gardens |
| COM_2 | Commercial compost | / | Source-separated waste from gardens and parks |
| U_PS | Undigested pig slurry | Anonymous (BE) | Pig slurry: 100 |
| SF_CM | Solid fraction digestate | Rika Biofuels (UK) | Chicken manure: 100 |
| Parameter | |
|---|---|
| pH KCl | 5.3 ± 0.1 |
| EC (mS cm−1) | 0.2 ± 0.0 |
| DW (g kg−1 FW) | 991 ± 1.0 |
| OM (g kg−1) | 42 ± 1.1 |
| TC (g kg−1) | 18 ± 0.7 |
| TOC (g kg−1) | 18 ± 0.1 |
| TN (g kg−1) | 1.63 ± 0.21 |
| NH4+-N (mg kg−1) | 11.87 ± 0.48 |
| NO3−-N (mg kg−1) | 28.35 ± 0.48 |
| P (g kg−1) | 0.78 ± 0.02 |
| K (g kg−1) | 2.15 ± 0.08 |
| Ca (g kg−1) | 2.07 ± 0.03 |
| Mg (g kg−1) | 2.00 ± 0.04 |
| S (g kg−1) | 0.28 ± 0.01 |
| Unit | TN Product Application Rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N incubation | D_BW | D_SS | D_CS | D_PM | D_CM | COM * | U_PS | SF_CM | |
| mg TN 100 g−1 DM soil | 9.8 | 7.0 | 7.9 | 10.1 | 10.0 | 7.3 | 11.4 | 6.0 | |
| kg TN ha−1 (equivalent) | 275 | 197 | 223 | 283 | 281 | 204 | 318 | 167 | |
| C incubation | TOC Product Application Rate | ||||||||
| mg TOC 100 g−1 DM soil | 180 | 303 | 346 | 158 | 173 | 647 | 54 | 694 | |
| kg TOC ha−1 (equivalent) | 2519 | 4236 | 4843 | 2216 | 2418 | 9051 | 758 | 9721 | |
| Parameter | D_BW | D_SS | D_CS | D_PM | D_CM | COM_1 | COM_2 | U_PS | SF_CM |
|---|---|---|---|---|---|---|---|---|---|
| pH KCl | 8.7 ± 0.0 | 8.5 ± 0.0 | 8.5 ± 0.1 | 8.5 ± 0.0 | 8.4 ± 0.0 | 8.0 ± 0.7 | 5.1 ± 0.1 | 8.3 ± 0.0 | 8.3 ± 0.0 |
| pH H2O | 8.2 ± 0.0 | 8.3 ± 0.0 | 8.2 ± 0.0 | 8.2 ± 0.0 | 8.2 ± 0.0 | / | 5.9 ± 0.1 | 8.3 ± 0.0 | / |
| EC (mS/cm) | 1.9 ± 0.1 | 0.9 ± 0.0 | 1.8 ± 0.0 | 2.0 ± 0.1 | 2.1 ± 0.1 | 0.7 ± 0.0 | 1.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| DM (g kg−1 FM) | 85 ± 5.9 | 110 ± 1.6 | 107 ± 5.6 | 78 ± 7.5 | 80 ± 0.9 | 531 ± 7.1 | 349 ± 9.0 | 29 ± 0.3 | 812 ± 0.3 |
| OM (g kg−1) | 601 ± 2.2 | 644 ± 2.8 | 749 ± 3.8 | 732 ± 3.4 | 748 ± 2.4 | 298 ± 0.8 | 839 ± 9.9 | 569 ± 3.0 | 398 ± 1.4 |
| TC (g kg−1) | 259 ± 6.8 | 307 ± 6.1 | 369 ± 11.1 | 256 ± 5.3 | 272 ± 1.4 | 153 ± 1.7 | 467 ± 0.0 | 277 ± 2.7 | 194 ± 0.2 |
| TOC (g kg−1) | 227 ± 22.6 | 285 ± 15.4 | 336 ± 23.8 | 215 ± 29.0 | 232 ± 28.2 | 151 ± 1.2 | 435 ± 0.0 | 214 ± 44.6 | 181 ± 0.0 |
| DOC (g kg−1) | 101.1 | 60.7 | 122.4 | 106.4 | 97.2 | / | 4.9 | 63.0 | 11.1 |
| TN (g kg−1) | 85.2 ± 0.2 | 71.3 ± 0.4 | 82.8 ± 1.4 | 98.7 ± 2.2 | 96.0 ± 0.4 | 14.3 ± 0.4 | 17.3 ± 0.0 | 139.0 ± 0.0 | 34.6 ± 0.4 |
| P (g kg−1) | 25.4 ± 0.2 | 35.5 ± 0.1 | 17.3 ± 0.6 | 22.7 ± 0.8 | 10.4 ± 2.3 | 3.2 ± 0.4 | 1.1 ± 0.1 | 15.4 ± 0.7 | 5.4 ± 0.2 |
| NH4+-N (g kg−1) | 55.2 ± 5.2 | 38.0 ± 1.1 | 44.1 ± 2.2 | 63.0 ± 1.0 | 67.5 ± 7.8 | 0.0 ± 0.0 | / | 100.5 ± 2.7 | 13.1 ± 0.0 |
| NO3−-N (g kg−1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.0 | / | 0.0 ± 0.0 | 0.0 ± 0.0 |
| TC:TN | 3.04 | 4.31 | 4.46 | 2.60 | 2.83 | 10.68 | 26.97 | 1.99 | 5.60 |
| TOC:TC | 0.88 | 0.93 | 0.91 | 0.84 | 0.85 | 0.99 | 0.93 | 0.77 | 0.94 |
| TC:Norg | 8.64 | 9.22 | 9.54 | 7.18 | 9.53 | 11.23 | / | 7.19 | 9.0 |
| TOC:TN | 2.67 | 4.00 | 4.05 | 2.18 | 2.41 | 10.59 | 25.15 | 1.54 | 13.9 |
| NH4+-N:TN | 0.65 | 0.53 | 0.53 | 0.64 | 0.70 | 0.00 | / | 0.72 | 0.38 |
| Norg:TN | 0.35 | 0.47 | 0.47 | 0.36 | 0.30 | 0.95 | / | 0.28 | 0.61 |
| DOC:TOC | 0.44 | 0.21 | 0.36 | 0.49 | 0.42 | / | 0.01 | 0.30 | 0.06 |
| Product | CA (%) | k2a(1 − a) | R² | EOC (%) | CUE |
|---|---|---|---|---|---|
| D_BW | 39.7 | 0.0132 | 0.967 | 61.2 | 16.2 ± 1.5 |
| D_SS | 19.6 | 0.0129 | 0.976 | 81.3 | 13.7 ± 0.8 |
| D_CS | 27.5 | 0.0103 | 0.968 | 73.7 | 19.5 ± 7.2 |
| D_PM | 44.6 | 0.0065 | 0.978 | 57.3 | 13.3 ± 10.3 |
| D_CM | 52.0 | 0.0063 | 0.979 | 50.0 | 13.3 ± 8.7 |
| COM_2 | 1.5 | 0.0533 | 0.995 | 98.7 | 40.9 ± 10.7 |
| U_PS | 43.8 | 0.0181 | 0.978 | 56.9 | 2.3 ± 3.4 |
| SF_CM | 15.8 | 0.0076 | 0.999 | 85.8 | 40.9 ± 3.3 |
| Parameter | D_BW | D_SS | D_CS | D_PM | D_CM | COM * | U_PS | SF_CM |
|---|---|---|---|---|---|---|---|---|
| Nrel (%TN) | 72.2 ± 4.8 cd | 62.6 ± 2.8 c | 68.6 ± 8.4 cd | 77.9 ± 2.3 de | 80.8 ± 6.3 de | 8.7 ± 1.7 a | 86.8 ± 1.5 e | 47.7 ± 4.3 b |
| Cmin (%TOC) | 41.1 ± 1.4 e | 19.9 ± 0.4 c | 28.4 ± 1.2 d | 45.1 ± 2.4 ef | 52.8 ± 1.4 g | 1.4 ± 0.2 a | 45.9 ± 3.2 f | 14.4 ± 0.3 b |
| CUE | EOC | Cmin | CO2-C | Nrel | Nmin,net | NH4+-N:TN | TC:TN | DOC | DOC:TOC | |
|---|---|---|---|---|---|---|---|---|---|---|
| CUE | 1 | |||||||||
| EOC | 0.82 * | 1 | ||||||||
| Cmin | −0.82 * | -1.00 ** | 1 | |||||||
| CO2-C | −0.87 ** | −0.84 ** | 0.84 ** | 1 | ||||||
| Nrel | −0.90 ** | −0.93 ** | 0.93 ** | 0.91 ** | 1 | |||||
| Nmin,net | −0.74 | −0.7 | 0.71 | 0.88 ** | 0.87 ** | 1 | ||||
| NH4+-N:TN | −0.82 * | −0.93 ** | 0.94 ** | 0.81 * | 0.99 ** | 0.83 * | 1 | |||
| TC:TN | 0.77 * | 0.92 ** | −0.92 ** | −0.79 * | −0.99 ** | −0.83 * | −0.99 ** | 1 | ||
| DOC | −0.75 * | −0.75 * | 0.75 * | 0.56 | 0.53 | 0.36 | 0.76 * | −0.72 * | 1 | |
| DOC:TOC | −0.80 * | −0.88 ** | 0.89 ** | 0.68 * | 0.72 | 0.46 | 0.81 * | −0.78 * | 0.95 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reuland, G.; Sigurnjak, I.; Dekker, H.; Sleutel, S.; Meers, E. Assessment of the Carbon and Nitrogen Mineralisation of Digestates Elaborated from Distinct Feedstock Profiles. Agronomy 2022, 12, 456. https://doi.org/10.3390/agronomy12020456
Reuland G, Sigurnjak I, Dekker H, Sleutel S, Meers E. Assessment of the Carbon and Nitrogen Mineralisation of Digestates Elaborated from Distinct Feedstock Profiles. Agronomy. 2022; 12(2):456. https://doi.org/10.3390/agronomy12020456
Chicago/Turabian StyleReuland, Gregory, Ivona Sigurnjak, Harmen Dekker, Steven Sleutel, and Erik Meers. 2022. "Assessment of the Carbon and Nitrogen Mineralisation of Digestates Elaborated from Distinct Feedstock Profiles" Agronomy 12, no. 2: 456. https://doi.org/10.3390/agronomy12020456
APA StyleReuland, G., Sigurnjak, I., Dekker, H., Sleutel, S., & Meers, E. (2022). Assessment of the Carbon and Nitrogen Mineralisation of Digestates Elaborated from Distinct Feedstock Profiles. Agronomy, 12(2), 456. https://doi.org/10.3390/agronomy12020456







