Abstract
The application of green manure to soil improves soil health by increasing biological activity. However, little attention has been paid to the effects of different green manures on the microbiological community and soil function after incorporation. Here, it was found that the green manures of Vicia villosa (leguminous) and Brassica juncea (non-leguminous) have different fungal structures, despite the soil originally being the same. Moreover, some isolated strains showed plant-growth-promoting abilities. Three strains (H1: Penicillium spp., H2: Clonostachys spp., and H3: Trichoderma spp.) from leguminous-manure-incorporated soil and four strains (B1: Purpureocillium spp., B4: Taifanglania spp., B6: Trichoderma spp., and B10: Aspergillus spp.) from non-leguminous-manure-incorporated soil showed the potential for plant growth enhancement. Plant-growth-promoting traits revealed that four strains possessed phosphate solubilization and siderophore production, although none of them showed the ability to produce indole-3-acetic acid (IAA)-like compounds with/without tryptophan. In addition, higher extracellular enzyme activities—including endoglucanase and β-glucosidase activities—were also detected in the soil-incorporated green manures. In conclusion, this study suggests that different fungal structures appeared when different green manures were applied, which promoted plant growth. This indicates the potential benefits of promoting the incorporation of green manure into the soil.
1. Introduction
The use of mineral fertilizers in agriculture increases crop yields but decreases soil quality and microbial populations [1,2]. Environmental concerns, stemming from the use of mineral fertilizers on the one hand, and the availability of these fertilizers—especially phosphorus- and nitrogen-based fertilizers—on the other hand, are vital issues in current agriculture [3]. Crop nutrition requirements can alternatively be met by organic fertilizers, including animal- or plant-based organic residues [4]. Issues with the use of organic fertilizers include slow release, bulkiness, the spread of weeds, and non-uniform composition [5]. Therefore, a sustainable solution and new alternative technologies are being developed to replace mineral and slow-release fertilizers, in order to reduce environmental problems and improve crop productivity and soil health.
The incorporation of green manure can affect soil microbial communities directly and indirectly, by providing the nutrients to the soil and plants, and also leads to changes in the soil’s nutrient status [6,7]. Green manures can influence soil microbial diversity through the release of root exudates [8]. There is a paucity of information on the effects of different green manures on microbial groups and how they increase microbial activity—especially in rice-cropping systems [8,9]. The changes in soil microbial composition following green manure amendment can provide more enzymes that regulate nutrient fluxes, leading to the promotion of plant growth [10,11]. The extent of changes may depend on the quantity and type of green manure added [10,12]. Common plant species used for green manure include Chinese milk vetch, barley, red clover, and mustard oats, and they can be leguminous or non-leguminous plants [13]. Recent studies using high-throughput sequence analysis showed that green manure could change the soil microbial community structure [9,11]. In particular, the behavior of various fungal community structures at the molecular level, affected by the introduction of leguminous and non-leguminous green manure in soil systems, leads to the promotion of plant growth. Although soil fungal communities play a crucial role in soil ecosystems’ functioning by decomposing organic matter, influencing nutrient cycling, and promoting plant growth [14], little is yet known on this subject. Therefore, monitoring of the fungal community structure after incorporation of green manure is very important. However, there is still little information on the effects of different types of green manure, such as Vicia villosa—commonly called “hairy vetch” (leguminous)—and Brassica juncea (non-leguminous), on soil fungi and soil enzyme activities.
Plant-growth-promoting microorganisms (PGPMs) are widely accepted to enhance crop production, and can be used to meet the ever-increasing food demand [15,16]. PGPMs, such as fungi and rhizobacteria, enhance plant growth and development through different functions, such as production of plant hormones, enhancing nutrient availability [17,18], and production of volatile organic compounds [19,20]. Many plant-growth-promoting fungi (PGPF) are known to produce siderophores, improving the iron nutrition of plants under an iron-depleted environment [21]. Additionally, many PGPF have been found not only to be non-pathogenic, but also to induce resistance against pathogenic infections [22]; they also help to increase nutrient uptake from the soil as needed [16]. This may directly or indirectly promote plant growth. Therefore, the consistent use of PGPMs could lead to the replacement of mineral fertilizers and pesticides. Numerous studies have explored fungal characteristics in terms of plant-growth-promoting traits [16,19,22], but the characteristics of PGPF in the soil after the incorporation of green manure have not yet been explored. In this regard, it was assumed that incorporating different green manures could lead to different fungal structures and influence plant growth through their promotion effects. Thus, keeping in mind that this study was conducted to isolate the fungal strains from the soil after incorporation of green manure (V. villosa (hairy vetch) and B. juncea), plant-growth-promoting ability was evaluated to determine their effects on the growth of two different vegetable crops: Brassica rapa var. perviridis and Lactuca sativa var. crispa—commonly called komatsuna and lettuce, respectively.
2. Materials and Methods
2.1. Isolation of Fungi
Hairy vetch (Vicia villosa) and Brassica juncea were selected based on commonly cultivated green manures in japan. Brassica juncea (TAKII Co., Ltd., Kyoto, Japan) and hairy vetch ((SNOW BRAND SEED Co., Ltd., Hokkaido, Japan) seeds were obtained commercially. Vicia villosa (leguminous) and Brassica juncea (non-leguminous) as green manures were grown in 300 g of soil contained in Neubauer pots (100 cm2 volume; Fujiwara Seisakusho, Ltd. Tokyo, Japan) for five weeks (flowering stage) under controlled climate conditions (temperature: 25 °C, 18 h day, 6 h night). Five replicated pots were established for each treatment, and no organic or chemical fertilizers were added to the green manure treatment pots. After that, 15 g of Vicia villosa and 15 g of Brassica juncea green manures were incorporated into the soil, and soil samples were collected after two months of the incorporation of green manures. Samples were carefully shaken to separate the plant roots and stones, and were stored at 4 °C until fungal isolation.
Fungi were isolated from both samples (0.5 g) by serial dilution plating on Martin agar plates [23]. Soil (0.5 g) was added to 5 mL of sterile distilled water (SDW), mixed using a vortex mixer, and serially diluted. Then, 50 µL was taken from each suspension (10−2, 10−3, and 10−4), spread onto Martin agar plates, and incubated for one week at 25 °C. After the appearance of the fungal colonies, each colony was selected based on visual morphology and re-streaked on potato dextrose agar plates until a single pure colony type per plate was achieved.
2.2. DNA Extraction and PCR Amplification for Isolated Fungi
DNA was extracted from the isolated fungal strains using the ZR bacterial/fungal DNA MiniPrep KitTM (Zymo Research Corp., Irvine, CA, USA). The internal transcribed spacer (ITS) region of rDNA was used to identify isolated strains. DNA extracted from the isolated strains was mixed before PCR amplification. The universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) were used to amplify the ITS region on a TaKaRa PCR Thermal Cycler Dice® Series Gradient (Takara, Shiga, Japan). The PCR amplification conditions were as follows: 95 °C for 5 min, followed by 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, followed by a final extension at 72 °C for 7 min. The amplification mixture for PCR (total volume: 25 µL) contained 12.5 µL of GoTaq Green Master Mix (Promega, Madison, WI, USA), 9.5 µL of sterilized distilled water, 1 µL of DNA template, and 1 µL of each primer. The amplification products (5 µL) were subjected to electrophoresis on a 1% (w/v) agarose gel in Tris-acetate-ethylenediaminetetraacetic acid buffer at 100 V for 25 min and visualized by GelRed™ staining (1:20,000 dilution; Biotium, Fremont, CA, USA) [4]. The obtained DNA sequences were compared with those previously reported in the DNA Data Bank of Japan (http://blast.ddbj.nig.ac.jp/, accessed on 5 May 2021), and closely related species were noted. Molecular Evolutionary Genetics Analysis (MEGA) software was used for phylogenetic analysis [24]. The sequences corresponding to the identified isolates were then submitted to the DNA Data Bank of Japan (DDBJ) for accession numbers.
2.3. Screening of Plant-Growth-Promoting Fungi (PGPF)
2.3.1. Microcosm Experiment under Axenic Conditions
For screening PGPF, 22 out of 42 fungal strains detected in soil incorporated with hairy vetch and B. juncea were isolated based on visual morphology and ITS region sequencing. Brassica rapa var. perviridis and L. sativa var. crispa (lettuce) were grown in lidded polypropylene tubes (exterior dimensions: 4 cm × 11 cm, volume: 120 mL) filled with 100 g (dry weight) of sterilized soil (1 h autoclave at 121 °C). Soil moisture was maintained at 60% water-holding capacity. Mycelia of each fungal strain, grown in 5 mL of potato dextrose broth (PDB) media under incubation at 25 °C for 7 days, were transferred to the pots. No fungal mycelium was added to the control pot (CK). Pre-germinated seeds of B. rapa and L. sativa were transferred to pots, which were placed in a growth chamber (temperature: 25 °C; photoperiod: 16 h day, 8 h night). The experiment continued for five weeks, and plant shoot and root biomass were measured. Seven strains (H1, H2, and H3 from hairy vetch- and B1, B4, B6, and B10 from B. juncea-incorporated soil) were selected for further analysis based on fresh weights of roots and shoots. This experiment was conducted with one replication because of pre-screening for further experiments.
2.3.2. Microcosm Experiment under Non-Axenic Conditions
Another plant growth experiment was conducted on a larger scale to further assess the effects of the seven selected strains after PGPF screening. A total of 80 Neubauer pots were filled with 300 g of air-dried soil (containing 190 mg kg−1 available phosphate and 23.1 mg kg−1 nitrate-N; not sterilized) and placed in a climate-controlled room (temperature: 25 °C; photoperiod: 18 h day, 6 h night). A suspension of selected strain media, with conditions similar to those followed while making axenic cultures, was transferred to the pots. The same volume (5 mL) of fungal-free PDB medium was used as the control (CK). The pots were covered with aluminum foil for one week to stabilize microbial activity. Pre-germinated seeds of B. rapa and L. sativa were transplanted to the pots, and plants were grown for 35 days with 5 replicates per treatment. Soil moisture was maintained at 60% water-holding capacity and monitored daily. B. rapa and L. sativa were harvested after 35 days, and shoot and root biomass were determined.
2.4. Characterization of Selected Strains
2.4.1. Plant-Growth-Promoting Traits of Isolated Fungi
To investigate the ability of P solubilization in the seven selected strains, we used the medium described by Pikovskaya [25]. Each strain was grown on the medium in triplicate and incubated at 25 °C for 7 days. Clear zones around the selected strain colonies were proof of P solubilization, and the results were compiled using the following index: (+) clear zone, (−) no clear zone, and (±) detectable but not apparent zone.
The determination of siderophore production by selected strains was carried out using a slightly modified chrome azurol S (CAS) method [26,27]. Selected strains were grown on potato dextrose agar medium in triplicate and incubated for 24 h, and then covered with 10 mL of CAS medium containing the following elements: 6.04 mg of CAS, 7.3 mg of hexadecyltrimethylammonium bromide, 3.04 g of piperazine-1,4-bis(2-ethanesulfonic acid), and 1 mL of 1 mM FeCl3·6 H2O. After pouring the medium, the plates were incubated at 25 °C for 7 days. The color of the CAS medium changed from blue to yellow or orange, indicating siderophore production. The results were compiled using the following index: (+) color change, (−) no color change, and (++) color change throughout the medium.
Isolated strains were tested for indole-3-acetic acid (IAA)-like compound production. PDB medium (20 mL) was added to 50 mL falcon tubes. Each strain was grown in the medium in triplicate and incubated at 25 °C for 7 days with and without 1 mM and 60 mM (final concentration) tryptophan. After 7 days of incubation, the medium was collected and centrifuged at 10,000× g for 10 min. Next, 1.2 mL of Salkowski reagent [28,29] was mixed with 300 µL of supernatant. A spectrophotometer was used to assess the presence or absence of a pink color at 535 nm.
2.4.2. Extracellular Enzyme Activities of Isolates
A carboxymethyl cellulose plate assay was performed to detect endoglucanase activity [30]. Briefly, the assay used 1% CMC mixture, 15 g L−1 agar, 2.0 g L−1 K2HPO4, 7.0 g L−1 KH2PO4, 10 g L−1 (NH4)2 SO4, 0.1 g L−1 MgSO4 7 H2O, 0.6 g L−1 yeast extract, and 10 g L−1 microcrystalline cellulose. The plates were filled with medium, and isolated strains were grown in triplicate; they were incubated for 4 days at 30 °C, and growth was monitored. Next, 10 mL of 0.1% Congo red dye was flooded on the medium and stained for 30 min. The medium’s surface was then washed with 5 mL of 0.5 M NaCl for 10 min, and the results were compiled by the appearance of a pale yellow halo zone surrounding the isolated strain colonies. The results were compiled using the following index: (+) clear zone, (−) no clear zone detectable.
β-Glucosidase activity was estimated using a pure culture of isolated strains in triplicate [31]. Samples (0.5 mL each) were taken from each isolated strain, centrifuged at 10,000× g for 10 min, and transferred into 15 mL conical tubes; 1.5 mL of buffer solution (containing 0.2 M sodium hydrogen phosphate and 0.1 M citric acid, pH 4.9, for β-glucosidase), 0.9 mL of distilled water, 0.1 mL of toluene, and 0.6 mL of 50 mM p-nitrophenyl-β-D-glucopyranoside monohydrate solution were then added into the tubes. The tubes were shaken vigorously for 30 s and incubated at 35 °C for 1 h. After incubation, 8 mL of ethanol was added to each tube and shaken again. Next, 2 mL of 2 M Tris buffer was mixed with 1 mL of suspension, and absorbance was assessed using a spectrophotometer at 400 nm. A standard curve was prepared with concentrations of 0, 20, 50, and 200 µM of p-nitrophenol solutions. A similar procedure was carried out to prepare the control tubes to compensate for any background unrelated to p-nitrophenol released by β-glucosidase activity.
2.5. Statistical Analysis
Dunnett’s test was conducted for multiple comparisons of plant growth parameters in the pot experiments.
3. Results
3.1. Isolation and Identification of Fungi
The abundance of total fungal biomass was assessed based on the number of colony-forming units (CFUs). The fungal biomass was higher in the hairy-vetch-incorporated soil at 2.98 × 106 CFU g−1 of soil, whereas it was 1.14 × 106 CFU g−1 in B. juncea-incorporated soil (Table S1). A total of 42 fungal strains were isolated and selected from both types of green-manure-incorporated soil, based on visual morphology and ITS region sequencing. The fungal isolates from the hairy-vetch-incorporated soil belonged to various genera, including Penicillium, Clonostachys, Trichoderma, Talaromyces, Colletotrichum, Mucor, Cunninghamella, Metarhizium, Pseudogymnoascus, Didymella, Fusarium, and Myrothecium. The genera Purpureocillium, Stachybotrys, Mucor, Taifanglania, Trichoderma, Aspergillus, Fusarium, Monocillium, and Humicola were identified in the B. juncea-incorporated soil. It was observed that the genera Penicillium, Talaromyces, and Trichoderma were the most dominant in the hairy-vetch-incorporated soil, while the genera Mucor, Taifanglania, and Trichoderma were the most dominant in the B. juncea-incorporated soil (Figure 1a,b). The accession numbers of the isolated fungal strains are listed in Table 1a,b.

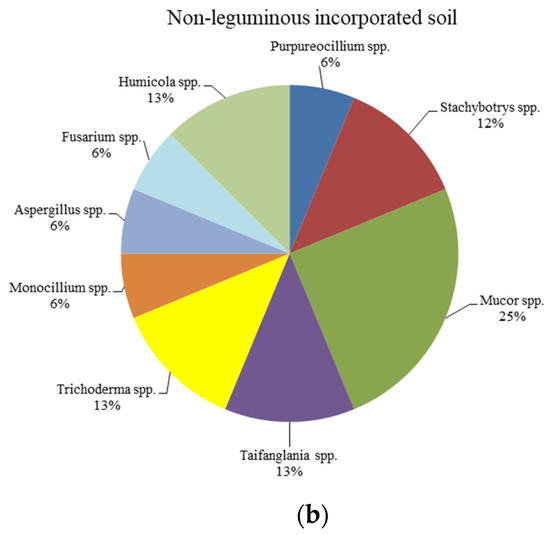
Figure 1.
(a) Twenty-five strains isolated from leguminous-manure-incorporated soil, where the dominant genera were Penicillium, Talaromyces, and Trichoderma; (b) seventeen strains isolated from non-leguminous-manure-incorporated soil, where the dominant genera were Mucor, Taifanglania, and Trichoderma.

Table 1.
(a) List of the fungi isolated from the soil incorporated with leguminous plant soil. (b) List of the fungi isolated from the soil incorporated with non-leguminous plant soil.
3.2. Screening of Plant Growth Promoting Fungi (PGPF)
3.2.1. Microcosm Experiment under Axenic Conditions
For PGPF screening, 22 isolates were tested, and fresh root and shoot biomass were measured. Three strains (H1, H2, and H3) from leguminous- and four strains (B1, B4, B6, and B10) from non-leguminous-manure-incorporated soil showed the potential for plant growth promotion (Figure S1). The seven isolates (H1, H2, H3, B1, B4, B6, and B10) were estimated to be closely related to Penicillium brasilianum (KY701767), Clonostachys rosea (KY810798), Trichoderma harzianum (KY552264), Purpureocillium lilacinum (MW113495), Taifanglania sp. (KP143091), Trichoderma harzianum (MK594269), and Aspergillus niger (MW931861), respectively. Phylogenetic analyses of PGPF screening isolates are shown in Figure S2.
3.2.2. Microcosm Experiment under Non-Axenic Conditions
For larger scale experiments under non-axenic conditions, dry weights of the shoot and root biomass of the plants were monitored, and were found to be significantly affected by different isolates. In B. rapa growth, B6, B4, B10, H3, and B1 were correlated with significant growth in terms of shoot and root biomass when compared with the control (CK) (Dunnett’s test p < 0.05). In the case of L. sativa growth, B4, B6, and B10 were correlated with significant growth in shoot and root biomass compared to CK. H1, H3, and B1 showed maximum weight of only root biomass compared to CK (Figure 2a,b).

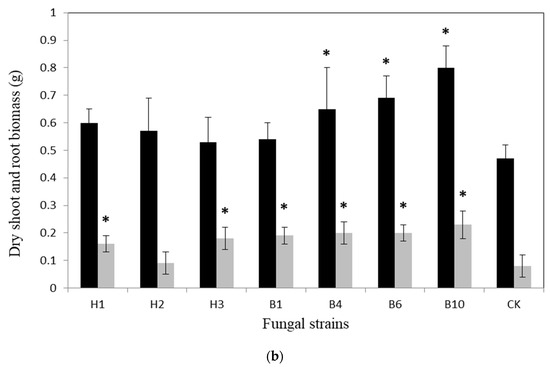
Figure 2.
Experiment with various fungal isolates: Growth response of (a) Brassica rapa and (b) Lactuca sativa in a pot. Black and grey bars show shoot and root biomass, respectively. The error bars show standard deviations between replicates (n = 5), where * indicates the statistical significance of the treatment when compared with the control (CK) using Dunnett’s test (p < 0.05).
3.3. Characterization of Selected Strains
3.3.1. Plant-Growth-Promoting Traits
Based on initial screening and the importance of PGP traits for plant growth promotion, phosphate (P) solubilization, siderophore production, and IAA were examined from isolates; the results are provided in Table 2. Strains H1, H2, B1, and B10 showed clear zones on the Pikovskaya agar plates, indicating that these strains have the ability to solubilize P. The siderophore production trend showed that H1, H3, B1, B6, and B10 produce siderophores, as confirmed by the changing color of CAS media; in contrast, none of them showed IAA-like compound production in the presence or absence of tryptophan.

Table 2.
Plant-growth-promoting traits, where + indicates possession and—indicates the lack of the trait. Each of the treatments consisted of three biological replicates.
3.3.2. Extracellular Enzyme Activities of Isolates
Enzyme activities of isolates that promote plant growth were examined directly and indirectly by providing nutrients to the soil environment. Among the isolates evaluated, H1, H2, B1, B4, and B10 showed clear halo zones around the colony growth while assessing for endoglucanase activity (Table 2). Quantification analysis was also carried out to determine the glucosidase activity of the isolates. Glucosidase activity was different among the isolates, and the highest activity was recorded for strain B10 (Table 2).
4. Discussion
The fungal structures in the soils incorporated with different green manures were poorly understood. This, to the best of our knowledge, is the first study to determine that certain fungal strains isolated from the soils incorporated with different types of green manure promote plant growth. Although our previous report showed different fungal communities between hairy-vetch- and B. juncea-incorporated soils using non-culturable methods [11], this study also showed that different green manures—such as hairy vetch (leguminous) and B. juncea (non-leguminous)—resulted in different fungal isolation even from the same soil. Notably, we found that the fungal genera Penicillium, Trichoderma, and Talaromyces were higher in abundance in leguminous-manure-incorporated soil; in contrast, Mucor, Taifanglania, and Trichoderma were more abundant in non-leguminous-manure-incorporated soil (Figure 1a,b). On the other hand, high-throughput sequencing analysis also showed that the effects of the two green manures were different, with the genera Cryptococcus, Entoloma, Olpidiaster, and Waitea being dominant in B. juncea-incorporated soils, while Coprinellus, Aspergillus, Clonostachys, and Arizonaphlyctis were dominant in hairy-vetch-incorporated soils [11]. Both culturable and non-culturable methods showed different results in our study. In addition, a total of 31.8% of strains—in which 27% (3 strains out of 11 strains) and 36% (4 strains out of 11 strains) of the strains were isolated from the soil incorporated with hairy vetch and B. juncea, respectively—isolated from both incorporated soils showed a plant-growth-promoting effect on B. rapa. In contrast, when L. sativa plants were used to evaluate plant-growth-promoting ability, fungal strains isolated from B. juncea-incorporated soil were more effective for growth promotion than those from hairy-vetch-incorporated soil (Figure 2b). Wang et al. [32] also reported that fungal communities respond differently to the strong influence of different plant species. In our findings, different plant-growth-promoting fungi contributed to the growth of B. rapa and lettuce. Since each green manure incorporation showed different fungal isolation from the soil, and PGPF also differed depending on the target plant, this indicates the importance of the selection of different types of green manure for each target plant. Generally, the effects of PGPF on plant growth promotion have been directly or indirectly linked to their PGP traits and the mineralization of nutrients, which could be their primary mechanism of action [21]. With regard to PGP traits, four out of seven strains showed P solubilization and siderophore production (Table 2). Interestingly, P solubilization and siderophore production came from common strains within the Penicillium spp. strain H1, Purpureocillium spp. strain B1, and Aspergillus spp. strain B10. In a similar study, Qarni et al. [33] also reported that inoculation with Penicillium spp. improved root growth and increased P uptake, and higher P availability was observed in post-harvest soil samples. Similarly, Aspergillus spp. have the ability to produce siderophores and solubilize P during qualitative and quantitative analyses [21]. However, to the best of our knowledge, no previous study has reported that Purpureocillium spp. produce siderophores. Generally, siderophore production chelates iron in soils with high pH to facilitate plant uptake [4,34]. In agriculture, siderophores are responsible for enhancing P availability [35], as they chelate heavy metals and solubilize iron phosphatase, enhancing nutrient uptake for plant growth [36]. Therefore, our results suggest that our isolated strains enhanced the growth of different vegetable crops via P solubilization and siderophore production in the soil.
The incorporation of different green manures influences enzyme activities produced by the isolated strain. In particular, hairy vetch green manure has a low C/N ratio (11.7) that decomposes quickly, resulting in high and rapid nutrient mineralization through microbiological activities [13]. In this study, fungal abundance was higher in hairy-vetch-incorporated soil than in B. juncea-incorporated soil, and strains H1, H3, B6, and B10 produced more β-glucosidase than other isolates (Table 2). Purahong et al. [37] and Carson et al. [38] reported that enzymes produced by fungi can decompose and mobilize plant residues, and can boost the nutrients via decomposition of soil organic matter. Our results showed that incorporating green manure increased fungal biomass and enzyme activity, meaning an increase in the release of nutrients for plants. Hairy vetch green manure, in particular, might be quite effective in promoting plant growth by providing nutrient sources and decomposition of organic matter by increasing fungal biomass. Liu et al. [39] reported that soil organic carbon and nutrients were the primary drivers of soil fungal community structure and composition. Other studies have also shown that the application of microbes increases the availability of substrates with microbial growth, leading to the enhancement of enzyme activities compared to without microbial application [40,41].
B. juncea is a typical biofumigation crop that is rich in allyl glucosiolate, much as Limnanthes alba Hartw. ex Benth produces β-glucosidase, and it has been reported to have herbicidal and fertilizer properties [42,43]. Limnanthes alba seed meal increases glucosinolate in much the same way as β-glucosidase increased when Brassica juncea was incorporated into the soil as compared to the control soil in our previously published study [11]. In this study, our isolated strains from Brassica-juncea-incorporated soil also showed the potential for production of β-glucosidase. In addition, allyl isothiocyanates also have fungicidal and nematicidal properties [44], and Hollister et al. [45] reported that the incorporation of brassicaceous and non-brassicaceous oilseed meal contributes organic matter and isothiocyanates, and land use has the potential to alter the soil microbial community structure. Allyl isothiocyanates could change the microbial community, which could suppress fungal disease [46], but these phenomena might be changed by the soil types and organic materials input into the soil. In addition, fungal sensitivity to allyl isothiocyanates should be considered; for example, Gaeumannomyces spp. were the most sensitive, Rhizoctonia spp. and Fusarium spp. were intermediate, and Bipolaris spp. and Pythium spp. were the least sensitive [47]. Yim et al. [48] also reported that biofumigation crops have more substantial effects on the soil fungal biomass/population with respect to the suppression of fungal diseases. Our results were consistent with their observation that the fungal community differed from that of hairy-vetch-incorporated soil, and fungal biomass was also lower than that of B. juncea-incorporated soil (Table S1). However, enzyme activities were maintained in isolated strains compared with those in the hairy-vetch-incorporated soil (Table 2). Since isolated strains from B. juncea-incorporated soil may be resistant to biofumigation, and could promote plant growth, it may be used in soils where soil diseases occur and it is difficult to supply organic matter.
5. Conclusions
In conclusion, the present study showed the isolation, identification, and characterization of plant-growth-promoting fungi from hairy-vetch- and B. juncea-incorporated soil. Based on our findings, the incorporation of the two green manures showed different fungal communities. The application of fungi isolated from green-manure-incorporated soils could stimulate plant growth promotion, including root growth and development. Although there are limitations to this study, our results indicate that different types of green manure are associated with varied impacts on soil fungal communities and PGPF. This indicates the potential benefits of promoting green manure incorporation in soil.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy12020323/s1: Figure S1: Pre-screening of fungal isolates: Growth response of (a) Brassica rapa with leguminous isolates, (b) Lactuca sativa with leguminous isolates, (c) Brassica rapa with non-leguminous isolates, and (d) Lactuca sativa with non-leguminous isolates. This time only one replication was used. Black and grey bars show shoot and root biomass, respectively; Figure S2: Phylogenetic tree of fungal isolates based on ITS rRNA gene sequences showing the evolutionary positions of (a) H1, (b) H2, (c) H3, (d) B1, (e) B4, (f) B6, and (g) B10. Metarhizium marquandii for B1, Diaporthe viticola for B4, Nectria eustromatica for B6, Penicillium viticola for B10, Talaromyces funiculosus for H1, Stanjemonium ochroroseum for H2, and Nectria eustromatica for H3 were used as the outgroup. The phylogenetic tree and branching pattern were generated by a neighbor-joining method through the MEGA program; Table S1: Effects of green manure treatments on fungal biomass.
Author Contributions
Conceptualization, R.K.; methodology, R.K. and W.A.; writing—original draft preparation, R.K. and W.A.; writing—review and editing, R.K.; visualization, R.K., supervision, R.K.; project administration, R.K.; funding acquisition, R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Nippon Life Insurance Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. In addition, sequencing data were deposited to DNA Data Bank of Japan (DDBJ).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahmood, A.; Iguchi, R.; Kataoka, R. Multifunctional food waste fertilizer having the capability of Fusarium-growth inhibition and phosphate solubility: A new horizon of food waste recycle using microorganisms. Waste Manag. 2019, 94, 77–84. [Google Scholar] [CrossRef]
- Babu, A.G.; Kim, S.W.; Yadav, D.R.; Hyum, U.; Adhikari, M.; Lee, Y.S. Penicillium menonorum: A Novel Fungus to Promote Growth and Nutrient Management in Cucumber Plants. Mycobiology 2015, 43, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef] [Green Version]
- Asghar, W.; Kondo, S.; Iguchi, R.; Mahmood, A.; Kataoka, R. Agricultural Utilization of Unused Resources: Liquid Food Waste Material as a New Source of Plant Growth-Promoting Microbes. Agronomy 2020, 10, 954. [Google Scholar] [CrossRef]
- Sinha, R.K.; Herat, S.; Bharambe, G.; Patil, S.; Bapat, P.; Chauhan, K.; Valani, D. Human waste-apotential resource: Converting trash into treasure by embracing the 5 r’s philosophy for safe and sustainable waste management. Environ. Res. J. 2009, 3, 143. [Google Scholar]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Hai-Ming, T.; Xiao-Ping, X.; Wen-Guang, T.; Ye-Chun, L.; Ke, W.; Guang-Li, Y. Effects of winter cover crops residue returning on soil enzyme activities and soil microbial community in double-cropping rice fields. PLoS ONE 2014, 9, e100443. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Gao, J.; Wang, X.; Fan, F.; Ma, X.; Yin, H.; Zhang, C.; Feng, K.; Deng, Y. Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol. Biochem. 2017, 104, 208–217. [Google Scholar] [CrossRef]
- Brennan, E.B.; Acosta-Martinez, V. Cover cropping frequency is the main driver of soil microbial changes during six years of organic vegetable production. Soil Biol. Biochem. 2017, 109, 188–204. [Google Scholar] [CrossRef]
- Chavarria, D.N.; Verdenelli, R.A.; Serri, D.L.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Effect of cover crops on microbial community structure and related enzyme activities and macronutrient availability. Eur. J. Soil Biol. 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Asghar, W.; Kataoka, R. Green manure incorporation accelerates enzyme activity, plant growth, and changes in the fungal community of soil. Arch. Microbiol. 2022, 204, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Khan, M.I.; Gwon, H.S.; Alam, M.A.; Song, H.J.; Das, S.; Kim, P.J. Short term effects of different green manure amendments on the composition of main microbial groups and microbial activity of a submerged rice cropping system. Appl. Soil Ecol. 2020, 147, 103400. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Zhang, H.; Wei, G.; Li, Z. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol. 2020, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turbat, A.; Rakk, D.; Vigneshwari, A.; Kocsubé, S.; Thu, H.; Szepesi, Á.; Bakacsy, L.; Škrbić, B.D.; Jigjiddorj, E.-A.; Vágvölgyi, C. Characterization of the Plant Growth-Promoting Activities of Endophytic Fungi Isolated from Sophora flavescens. Microorganisms 2020, 8, 683. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 1–19. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F. Application and mechanisms of plant growth promoting fungi (PGPF) for phytostimulation. Org. Agric. 2020, 1–31. [Google Scholar] [CrossRef]
- Naziya, B.; Murali, M.; Amruthesh, K.N. Plant growth-promoting fungi (PGPF) instigate plant growth and induce disease resistance in Capsicum annuum L. upon infection with Colletotrichum capsici (Syd.) Butler & Bisby. Biomolecules 2020, 10, 41. [Google Scholar]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant Growth Enhancement using Rhizospheric Halotolerant Phosphate Solubilizing Bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 Isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; Franco, D.G.; Chaves, P.O.; Giannesi, G.C.; Masui, D.C.; Ruller, R.; Corrêa, B.O.; da Silva Brasil, M.; Zanoelo, F.F. Plant growth promoting potential of endophytic Aspergillus niger 9-p isolated from native forage grass in Pantanal of Nhecolândia region, Brazil. Rhizosphere 2021, 18, 100332. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.-S.; Wu, X.-Q.; Luan, F.-G.; Zhang, L.-P.; Fang, X.-M.; Wan, S.-Z.; Hu, X.-F.; Ye, J.-R. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS ONE 2018, 13, e0199625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.P. Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.; Fernández, F. O-CAS, a fast and universal method for siderophore detection. J. Microbiol. Methods 2007, 70, 127–131. [Google Scholar] [CrossRef]
- Acuña, J.J.; Jorquera, M.A.; Martínez, O.A.; Menezes-Blackburn, D.; Fernández, M.T.; Marschner, P.; Greiner, R.; Mora, M. Indole acetic acid and phytase activity produced by rhizosphere bacilli as affected by pH and metals. J. Soil Sci. Plant Nutr. 2011, 11, 1–12. [Google Scholar]
- Shah, S.; Shrestha, R.; Maharjan, S.; Selosse, M.-A.; Pant, B. Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants 2019, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Colonia, B.S.O.; Junior, A.C. Screening and detection of extracellular cellulases (endo-and exo-glucanases) secreted by filamentous fungi isolated from soils using rapid tests with chromogenic dyes. Afr. J. Biotechnol. 2014, 13, 52. [Google Scholar]
- Asghar, W.; Kataoka, R. Effect of co-application of Trichoderma spp. with organic composts on plant growth enhancement, soil enzymes and fungal community in soil. Arch. Microbiol. 2021, 203, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Li, J.; Bai, S.; Tian, T. Fungal community composition and diversity in the rhizosphere soils of Argentina (syn. Potentilla) anserina, on the Qinghai Plateau. Fungal Ecol. 2021, 54, 101107. [Google Scholar] [CrossRef]
- Qarni, A.; Billah, M.; Hussain, K.; Shah, S.H.; Ahmed, W.; Alam, S.; Sheikh, A.A.; Jafri, L.; Munir, A.; Malik, K.M. Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and their Potential Effect for Sustainable Agriculture. Sustainability 2021, 13, 2151. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator–Siderophore: A review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Alleviation of mercury toxicity in wheat by the interaction of mercury-tolerant plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2016, 35, 1000–1012. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: Novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef]
- Carson, C.M.; Jumpponen, A.; Blair, J.M.; Zeglin, L.H. Soil fungal community changes in response to long-term fire cessation and N fertilization in tallgrass prairie. Fungal Ecol. 2019, 41, 45–55. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Böhme, L.; Böhme, F. Soil microbiological and biochemical properties affected by plant growth and different long-term fertilisation. Eur. J. Soil Biol. 2006, 42, 1–12. [Google Scholar] [CrossRef]
- Tang, H.-M.; Xiao, X.-P.; Tang, W.-G.; Li, C.; Wang, K.; Cheng, K.-K.; Sun, G. Dynamic change of soil enzyme activities and soil microbe during rice main growth stages in different long-term fertilizer regimes. J. Pure Appl. Microbiol. 2017, 11, 649–661. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Winkelmann, T. Biofumigation for fighting replant disease-A Review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef] [Green Version]
- Intanon, S.; Hulting, A.G.; Myrold, D.D.; Mallory-Smith, C.A. Short-term effects of soil amendment with meadowfoam seed meal on soil microbial composition and function. Appl. Soil Ecol. 2015, 89, 85–92. [Google Scholar] [CrossRef]
- Brown, P.D. Control of soil-borne plant pests using glucosinolate-containing plants. Adv. Agron. 1997, 61, 168–231. [Google Scholar]
- Hollister, E.B.; Hu, P.; Wang, A.S.; Hons, F.M.; Gentry, T.J. Differential impacts of brassicaceous and nonbrassicaceous oilseed meals on soil bacterial and fungal communities. FEMS Microbiol. Ecol. 2013, 83, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of glucosinolate-derived isothiocyanates on fungi: A comprehensive review on direct effects, mechanisms, structure-activity relationship data and possible agricultural applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.; Wong, P.; Desmarchelier, J. Biofumigation potential of brassicas. Plant Soil 1998, 201, 103–112. [Google Scholar] [CrossRef]
- Yim, B.; Hanschen, F.S.; Wrede, A.; Nitt, H.; Schreiner, M.; Smalla, K.; Winkelmann, T. Effects of biofumigation using Brassica juncea and Raphanus sativus in comparison to disinfection using Basamid on apple plant growth and soil microbial communities at three field sites with replant disease. Plant Soil 2016, 406, 389–408. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).