In Vitro Propagation of the Dendrobium anosmum Lindl. Collected in Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Creation of Initial Material
2.2. Propagation of Protocorm
2.3. Experimental Design and Statistical Analysis
3. Results
3.1. Observation of Protocorm Development and the Effect of Growth Regulators on the Propagation Efficiency of D. anosmum Lindl. Orchid Protocorms
3.1.1. Effect of BA on the Regeneration Efficiency of D. anosmum Lindl. Orchid Protocorms
3.1.2. Effect of Kinetin on the Regeneration Efficiency of the D. anosmum Lindl. Protocorms
3.1.3. Effect of BA and α-NAA on the Regeneration Efficiency of the D. anosmum Lindl. Protocorms
3.1.4. Effect of Kinetin and α-NAA on the Regeneration Efficiency of the D. anosmum Lindl. Protocorms
3.2. Effect of Additives on the Growth Efficiency of D. anosmum Lindl. Orchid Shoots
3.2.1. Effect of Mashed Potato on the Growth and Development of D. anosmum Lindl.
3.2.2. Effect of Mashed Banana on the Growth and Development of D. anosmum Lindl.
3.2.3. Effect of Coconut Water on the Growth and Development of D. anosmum Lindl.
3.2.4. Effect of Activated Charcoal on the Growth and Development of D. anosmum Lindl.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van, B.C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Averyanov, L.V.; Averyanova, A.L. Updated Checklist of the Orchids of Vietnam; Vietnam National University Publishing House: Hanoi, Vietnam, 2003. [Google Scholar]
- Chu, C.; Yin, H.; Xia, L.; Cheng, D.; Yan, J.; Zhu, L. Discrimination of Dendrobium officinale and Its Common Adulterants by Combination of Normal Light and Fluorescence Microscopy. Molecules 2014, 19, 3718–3730. [Google Scholar] [CrossRef] [PubMed]
- Kowitdamrong, A.; Chanvorachote, P.; Sritularak, B.; Pongrakhananon, V. Moscatilin inhibits lung cancer cell motility and invasion via suppression of endogenous reactive oxygen species. Biomed Res. Int. 2013, 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Do, T.L. Medicinal Plants and Medicines in Viet Nam; Medical Publishing House: Hanoi, Vietnam, 2004. [Google Scholar]
- Ban, N.T.; Averyanov, L.V.; Huyen, D.D. Checklist of Plant Species of Vietnam; Agriculture Publishing House: Hanoi, Vietnam, 2005; p. 3. [Google Scholar]
- Ministry of Science and Technology, Vietnam Academy of Science and Technology. Vietnam Red Data Book. Part II. Plants; Natural Science and Technology Publishing House: Hanoi, Vietnam, 2007. [Google Scholar]
- Tran, H. Viet Nam Orchid; Agriculture Publishing House: Hanoi, Vietnam, 1988. [Google Scholar]
- Silva, J.A.T.; Cardoso, J.C.; Dobránszki, J.; Zeng, S. Dendrobium micropropagation: A review. Plant Cell Rep. 2015, 34, 671–704. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Tu, B.T.; Dang, T.N.; Nguyen, T.L.A.; Hoang, T.N.; Nguyen, Q.T. In vitro propagation of Dendrobium officinale Kimura et Migo. J. Sci. Dev. 2014, 12, 1274–1282. [Google Scholar]
- Nguyen, V.V. Using in vitro culture techniquie for the propagation of Dendrobium lituiflorum Lindl. J. For. Sci. Technol. 2017, 4, 39–45. [Google Scholar]
- Dang, T.T.; H’Yon, N.B.; Nguyen, T.T.H.; Dinh, V.K.; Nong, V.D.; Tran, T.V.; Quach, V.H.; Vu, K.C. Micropropagation of Dendrobium heterocarpum Lindl. J. Biotechnol. 2018, 16, 127–135. [Google Scholar]
- Millner, H.J.; Obeng, A.; McCrea, A.R.; Baldwin, T.C. Axenic Seed Germination and in vitro Seedling Development of Restrepia brachypus (Orchidaceae). J. Torrey Bot. Soc. 2008, 135, 497–505. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zahara, M.; Datta, A.; Boonkorkaew, P.; Mishra, A. The Effects of Different Media, Sucrose Concentrations and Natural Ad-ditives on Plantlet Growth of Phalaenopsis Hybrid “Pink”. Braz. Arch. Biol. Technol. 2017, 60, e17160149. [Google Scholar] [CrossRef]
- Trigiano, R.N.; Gray, D.J. Plant Tissue Culture, Development, and Biotechnology; CRC Press: Boka Raton, FL, USA, 2011; pp. 36–38. [Google Scholar]
- Eckardt, N.A. A New Classic of Cytokinin Research: Cytokinin-Deficient Arabidopsis Plants Provide New Insights into Cy-tokinin Biology. Plant Cell 2003, 15, 2489–2492. [Google Scholar] [CrossRef]
- Miller, C.; Skoog, F. Chemical regulation of growth and organ formation in plant tissues cultured. In Vitro Symp. Soc. Exp. Biol. 1957, 11, 118–131. [Google Scholar]
- Nicomrat, D.; Anantasaran, J. A Reliable Homemade Tissue Culture Protocol for Dendrobium Orchid Cultivation. Appl. Mech. Mater. 2015, 804, 227–230. [Google Scholar] [CrossRef]
- Lekharani, C. Intra and Interspecific Hybridization in Dendrobium spp. Ph.D. Thesis, Kerala Agricultural University, Thiruvananthapuram, India, 2002. [Google Scholar]
- Yam, T.W.; Ernsr, R.; Arditti, J.; Nair, H.; Weatherhead, M.A. Charcoal in orchid seed germination and tissue culture media: A review. Lindleyana 1990, 5, 256–265. [Google Scholar]
- Nongdam, P.; Tikendra, L. Establishment of an Efficient In Vitro Regeneration Protocol for Rapid and Mass Propagation of Dendrobium chrysotoxum Lindl. Using Seed Culture. Sci. World J. 2014, 2014, 740150. [Google Scholar] [CrossRef]
- Srivastava, D.; Gayatri, M.C.; Sarangi, S.K. In vitro seed germination and plant regeneration of an epiphytic orchid Aerides ringens Fischer. Indian J. Biotechnol. 2015, 14, 574–580. [Google Scholar]
- Asghar, S.; Ahmed, T.; Ahmed, H.I.; Yaseen, M. In vitro propagation of orchid (Dendrobium nobile) var. Emma white. Afr. J. Biotechnol. 2011, 10, 3097–3103. [Google Scholar]
- Kalimuthu, K.; Senthilkumar, R.; Vijayakumar, S. In vitro micropropagation of orchid, Oncidium sp. (Dancing Dolls). Afr. J. Biotechnol. 2007, 6, 1171–1174. [Google Scholar]
- Acemi, A.; Bayrak, B.; Çakır, M.; Demiryürek, E.; Gün, E.; El Gueddari, N.E.; Özen, F. Comparative analysis of the effects of chitosan and common plant growth regulators on in vitro propagation of Ipomoea purpurea (L.) Roth from nodal explants. In Vitro Cell. Dev. Biol. Plant 2018, 54, 537–544. [Google Scholar] [CrossRef]
- Reddy, J.; Niveshika; Shaju, A.; Jose, A.; Betty, A.; Yarmichon, H. Plant Growth Regulators Used for In Vitro Micropropagation of Orchids: A Research Review. Int. J. Biol. Res. 2021, 8, 37–42. [Google Scholar]
- Tikendra, L.; Amom, T.; Nongdam, P. Effect of phytohormones on rapid In vitro propagation of Dendrobium thyrsiflorum Rchb. f.: An endangered medicinal orchid. Pharmacogn. Mag. 2018, 14, 495. [Google Scholar]
- Zhou, J.; Liu, Y.; Wu, L.; Zhao, Y.; Zhang, W.; Yang, G.; Xu, Z. Effects of Plant Growth Regulators on the Rapid Propagation System of Broussonetia papyrifera L. Vent Explants. Forests 2021, 12, 874. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Phan, H.V.; Truong, T.B.P. In vitro Micropropagation of Dendrobium chrysotoxum. Hue Univ. J. Sci. 2011, 10, 24–30. [Google Scholar]
- Yao, L.; Huang, J.; Zhang, S. An Improved Protocol for Asymbiotic Seed Germination and Seedling Development of Paphio-pedilum tigrinum. Horticulturae 2021, 7, 298. [Google Scholar] [CrossRef]
- Adugna, A.Y.; Feyissa, T.; Tasew, F.S. Optimization of growth regulators on in vitro propagation of Moringa stenopetala from shoot explants. BMC Biotechnol. 2020, 20, 60. [Google Scholar] [CrossRef]
- Gnasekaran, P.; Poobathy, R.; Mahmood, M.; Samian, R.; Subramaniam, S. Effects of complex organic additives on improving the growth of PLBs of Vanda Kasem’s Delight. Aust. J. Crop Sci. 2012, 6, 1245–1248. [Google Scholar]
- David, D.; Jawan, R.; Marbawi, H.; Azlan, G.J. Organic Additives Improves the in vitro Growth of Native Orchid Vanda helvola Blume. Notulea Sci. Biol. 2015, 7, 192–197. [Google Scholar] [CrossRef][Green Version]
- An, J.; Kim, P.B.; Park, H.B.; Kim, S.; Park, H.J.; Lee, C.W.; Lee, B.-D.; Kim, N.Y.; Hwang, J.E. Effects of Different Growth Media on In Vitro Seedling Development of an Endangered Orchid Species Sedirea japonica. Plants 2021, 10, 1193. [Google Scholar] [CrossRef]
- Wu, K.; Zeng, S.; Lin, D.; da Silva, J.A.T.; Bu, Z.; Zhang, J.; Duan, J. In vitro Propagation and Reintroduction of the Endangered Renanthera imschootiana Rolfe. PLoS ONE 2014, 9, e110033. [Google Scholar] [CrossRef]
- Li, Z.Y.; Xu, L. In vitro propagation of white flower mutant of Rhynchostylis gigantea (Lindl.) Ridl. Through immature seed-derived protocorm-like bodies. J. Hortic. For. 2009, 1, 093–097. [Google Scholar]
- Murdad, R.; Latip, M.; Aziz, Z.; Ripin, R. Effects of carbon source and potato homogenate on in vitro growth and development of Sabah’s Endangered orchid: Phalaenopsis gigantea. Asia-Pac. J. Mol. Biol. Biotechnol. 2010, 18, 197–200. [Google Scholar]
- Prando, M.S.; Chiavazza, P.; Faggio, A.; Contessa, C. Effect of coconut water and growth regulator supplements on in vitro propagation of Corylus avellana L. Sci. Hortic. 2014, 171, 91–94. [Google Scholar] [CrossRef]
- Santana, J.R.F.; Paiva, R.; Souza, A.V.; Oliveira, L.M. Effect of different culture tube caps and concentrations of activated charcoal and sucrose on in vitro growth and budding induction of Annona glabra L. Ciência Agrotecnologia 2011, 35, 916–923. [Google Scholar] [CrossRef][Green Version]
- Nguyen, T.H.Y.; Tran, N.N. Study on in vitro propagation of Dendrobium nobile Lindl. TNU J. Sci. Technol. 2020, 225, 68–75. [Google Scholar]
- Islam, M.; Akter, M.; Prodhan, A.K.M.A. Effect of potato extract on in vitro seed germination and seedling growth of local Vanda roxburghii orchid. J. Bangladesh Agric. Univ. 2011, 9, 211–215. [Google Scholar] [CrossRef]
- Vilcherrez-Atoche, J.A.; Rojas-Idrogo, C.; Delgado-Paredes, G.E. Micropropagation of Cattleya maxima J. Lindley in Culture Medium with Banana Flour and Coconut Water. Int. J. Plant Anim. Environ. Sci. 2020, 10, 179–193. [Google Scholar]
- Baque, M.D.A.; Shin, Y.K.; Elshmari, T.; Lee, E.J.; Paek, K. Effect of light quality, sucrose and coconut water concentration on the micropropagation of Calanthe hybrids (“Bukduseong” × “Hyesung” and “Chunkwang” × “Hyesung”). Aust. J. Crop Sci. 2011, 5, 1247–1254. [Google Scholar]
- Sipayung, P.; Matanari, J.; Lafau, M.B.; Sulastri, Y.S.; Ginting, B.B.; Sihombing, D.R.; Pandiangan, M.; Giawa, T. The effect of activated charcoal dose and benzyl amino purine concentration on the growth of orchid plantlets in murashige and skoog media in vitro. IOP Conf. Ser. Earth Environ. Sci. 2018, 205, e012025. [Google Scholar] [CrossRef]
- Abdulwahed, M.S. Identification of the effect of different levels of activated charcoal and sucrose on multiplication shoots of date palm Phenix dactylifera L.C.v. sufedy in vitro. J. Hortic. For. 2013, 5, 139–145. [Google Scholar]
- Nguyen, T.S.; Nguyen, T.L.A.; Vu, N.L.; Tran, T.M. In vitro Micropropagation of Dendrobium fimbriatum Hook. J. Scl. Devel. 2012, 10, 263–271. [Google Scholar]
- Prizão, E.C.; Gonçalves, L.D.M.; Gutierre, M.A.M.; Mangolin, C.A.; Machado, M.D.F.P.D.S. Activated charcoal and graphite for the micropropagation of Cattleya bicolor Lindl. and a orchid double-hybrid ‘BLC Pastoral Innocence’. Acta Sci. Agron. 2012, 34, 157–161. [Google Scholar] [CrossRef][Green Version]
- Abahmane, L. Cultivar-Dependent Direct Organogenesis of Date Palm from Shoot Tip Explants. Methods Mol. Biol. 2017, 1637, 3–15. [Google Scholar] [CrossRef]
- Mittal, P.; Devi, R.; Gosal, S.S. Effect of genotypes and activated charcoal on high frequency in vitro plant regeneration in sugarcane. IJBT 2016, 15, 261–265. [Google Scholar]
- Rodrigues, F.A.; Rezende, A.L.S.R.; Pasqual, M.; Lopes, M.T.R. Solidifying agents and activated charcoal for in vitro culture of Solanum sessiliflorum. Pesqui. Agropecuária Bras. 2017, 52, 1123–1126. [Google Scholar] [CrossRef]

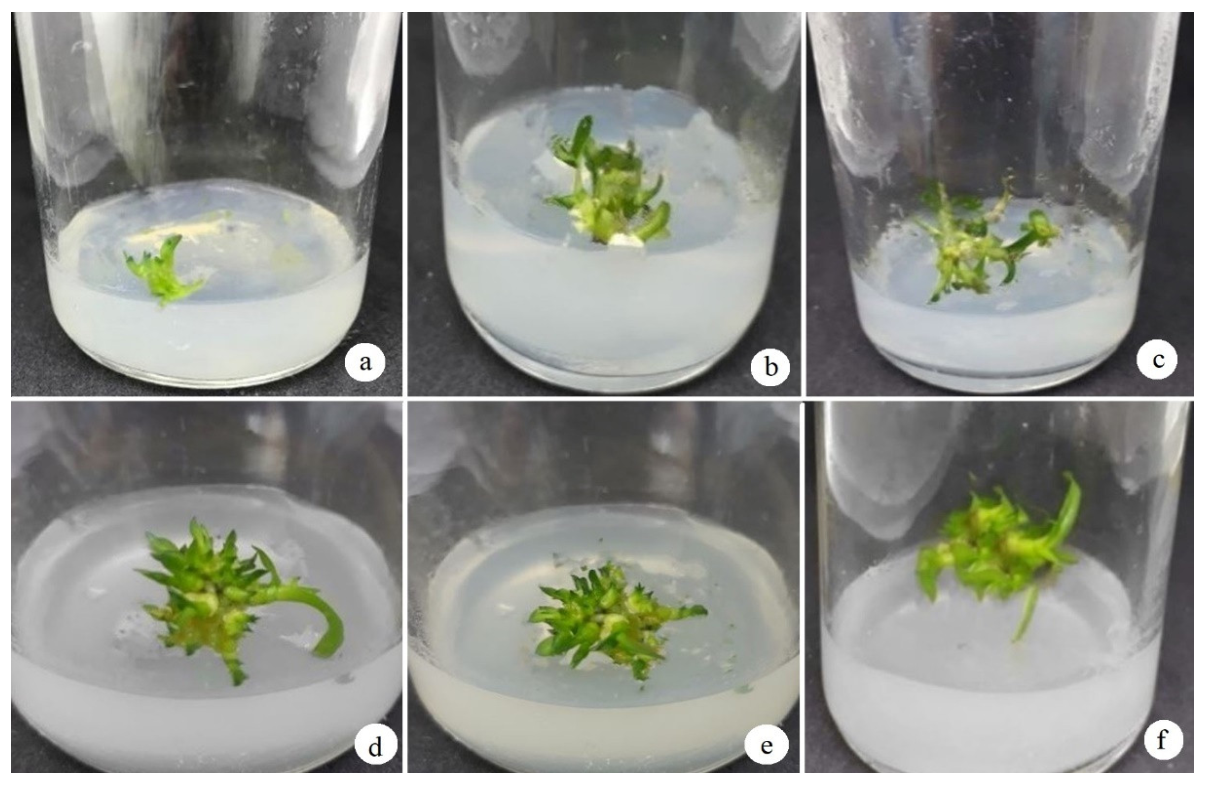

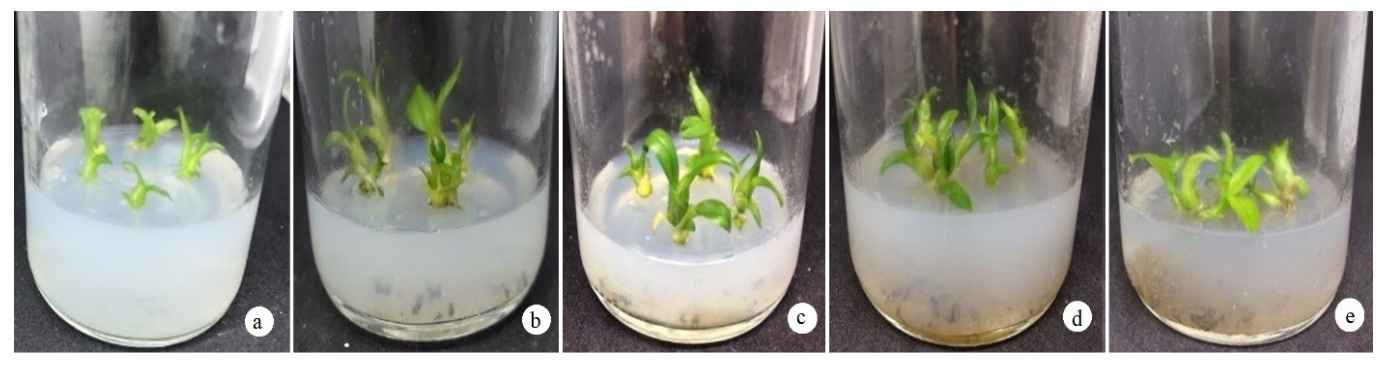
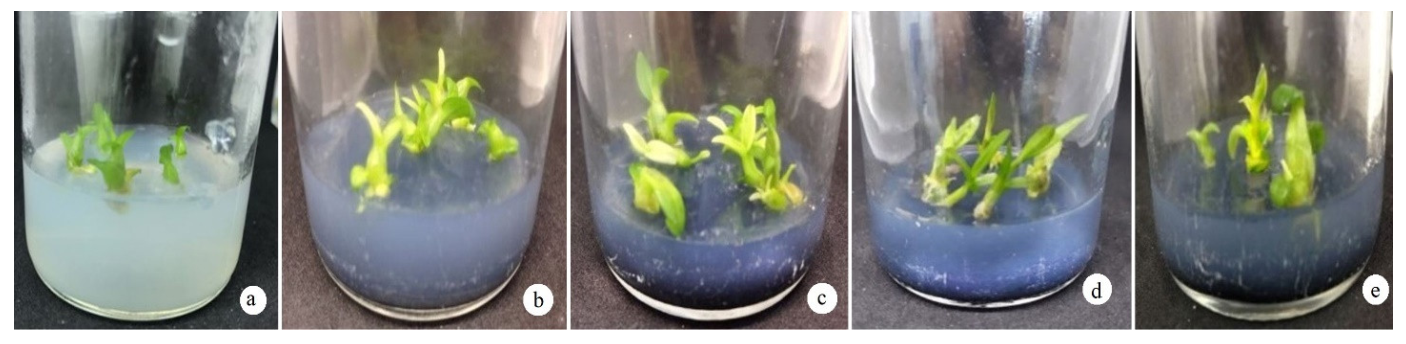
| Treatment | BA (mg/L) | Protocorm Formation Rate (%) | Shoot Length (cm) | Leaves (Leaves/Plant) | Rooting Rate (%) |
|---|---|---|---|---|---|
| T1(Control) | 0.0 | 100 | 0.33 a | 1.47 a | 53.33 |
| T2 | 0.5 | 100 | 0.51 b | 0.53 b | 20.00 |
| T3 | 1.0 | 100 | 0.69 c | 1.93 c | 45.00 |
| T4 | 1.5 | 100 | 0.49 be | 0.33 be | 33.67 |
| T5 | 2.0 | 100 | 0.37 ad | 0.33 be | 27.78 |
| T6 | 2.5 | 100 | 0.42 ab | 1.00 d | 40.00 |
| p-value (ANOVA) | p < 0.001 | p < 0.001 | |||
| LSD0.05 | 0.11 | 0.40 |
| Treatments | Kinetin (mg/L) | Protocorm Formation Rate (%) | Shoot Length (cm) | Leaves (Leaves/Plant) | Rooting Rate (%) |
|---|---|---|---|---|---|
| T1 (Control) | 0.0 | 100 | 0.33 a | 1.47 ae | 53.33 |
| T2 | 0.5 | 100 | 0.46 ab | 0.93 ab | 42.86 |
| T3 | 1.0 | 100 | 1.15 cb | 3.00 c | 75.00 |
| T4 | 1.5 | 100 | 0.50 ac | 1.83 a | 57.14 |
| T5 | 2.0 | 100 | 0.44 ac | 0.23 b | 40.00 |
| T6 | 2.5 | 100 | 0.40 ac | 0.77 be | 38.46 |
| p-value (ANOVA) | p < 0.001 | p < 0.001 | |||
| LSD0.05 | 0.81 | 0.99 |
| Treatment | BA (mg/L) | α-NAA (mg/L) | Protocorm Formation Rate (%) | Shoot Length (cm) | Leaves (Leaves/Plant) | Rooting Rate (%) |
|---|---|---|---|---|---|---|
| T1(Control) | 1.0 | 0.0 | 100 | 0.69 a | 1.93 a | 45.00 |
| T2 | 1.0 | 0.1 | 100 | 0.64 ae | 2.5 ab | 7.69 |
| T3 | 1.0 | 0.2 | 100 | 0.75 ad | 4.23 c | 40.91 |
| T4 | 1.0 | 0.3 | 100 | 0.98 b | 7.17 d | 50.00 |
| T5 | 1.0 | 0.5 | 100 | 0.52 c | 3.97 ce | 28.57 |
| p-value (ANOVA) | p < 0.001 | p < 0.001 | ||||
| LSD0.05 | 0.12 | 0.95 | ||||
| Treatment | Kinetin (mg/L) | α-NAA (mg/L) | Protocorm Formation Rate (%) | Shoot Length (cm) | Leaves (Leaves/Plant) | Rooting Rate (%) |
|---|---|---|---|---|---|---|
| T1(Control) | 1.0 | 0.0 | 100 | 1.15 a | 3.00 a | 75.00 |
| T2 | 1.0 | 0.1 | 100 | 1.28 ab | 3.97 b | 90.00 |
| T3 | 1.0 | 0.2 | 100 | 1.32 b | 4.03 bc | 100.00 |
| T4 | 1.0 | 0.3 | 100 | 1.38 bc | 4.23 be | 100.00 |
| T5 | 1.0 | 0.5 | 100 | 1.49 c | 4.40 bd | 100.00 |
| p-value (ANOVA) | p < 0.001 | 0.031 | ||||
| LSD0.05 | 0.14 | 0.92 | ||||
| Treatment | Potato (g/L) | Shoot Length (cm) | Leaves (Leaves/Plant) | Roots (Roots/Plant) | Root Length (cm) |
|---|---|---|---|---|---|
| T1 (Control) | 0 | 0.33 ab | 1.47 a | 0.93 ab | 0.36 a |
| T2 | 10 | 0.59 ac | 3.30 b | 1.33 ae | 0.45 ab |
| T3 | 20 | 1.10 c | 3.40 bc | 2.50 c | 0.57 c |
| T4 | 30 | 0.59 ac | 2.17 ad | 1.23 ad | 0.55 bc |
| T5 | 40 | 0.61 bc | 2.83 bd | 1.50 de | 0.52 bc |
| p-value (ANOVA) | p < 0.001 | p < 0.001 | p < 0.001 | 0.003 | |
| LSD0.05 | 0.77 | 0.71 | 0.48 | 0.12 |
| Treatment | Banana (g/L) | Shoot Length (cm) | Leaves (Leaves/Plant) | Roots (Roots/Plant) | Root Length (cm) |
|---|---|---|---|---|---|
| T1(Control) | 0.0 | 0.33 a | 1.47 a | 0.93 a | 0.36 a |
| T2 | 10 | 0.78 bd | 1.87 bd | 1.00 ab | 0.39 ab |
| T3 | 20 | 0.82 bc | 2.07 b | 1.40 c | 0.55 c |
| T4 | 30 | 0.72 be | 1.67 ad | 1.00 ab | 0.49 ac |
| T5 | 40 | 0.70 de | 1.63 ac | 1.00 ab | 0.49 ac |
| p-value (ANOVA) | 0.0017 | 0.024 | 0.008 | 0.017 | |
| LSD0.05 | 0.11 | 0.38 | 0.35 | 0.13 |
| Treatment | Coconut Water (mL/L) | Shoot Length (cm) | Leaves (Leaves/Plant) | Roots (Roots/Plant) | Root Length (cm) |
|---|---|---|---|---|---|
| T1 (Control) | 0 | 0.33 e | 1.47 a | 0.93 a | 0.36 a |
| T2 | 50 | 0.84 ac | 1.53 ab | 1.07 ab | 0.38 ab |
| T3 | 100 | 0.86 ab | 1.87 ac | 1.30 bc | 0.54 cd |
| T4 | 200 | 0.88 a | 2.17 c | 1.40 b | 0.56 c |
| T5 | 300 | 0.71 d | 1.80 bc | 1.03 ac | 0.44 bd |
| p-value (ANOVA) | p < 0.001 | 0.01 | 0.045 | p < 0.001 | |
| LSD0.05 | 0.13 | 0.42 | 0.34 | 0.11 |
| Treatment | Activated Charcoal (g/L) | Shoot Length (cm) | Leaves (Leaves/Plant) | Roots (Roots/Plant) | Root Length (cm) |
|---|---|---|---|---|---|
| T1(Control) | 0.0 | 0.33 a | 1.47 a | 0.93 a | 0.36 a |
| T2 | 0.5 | 0.93 bd | 5.77 bc | 3.13 b | 0.48 b |
| T3 | 1.0 | 1.09 c | 6.13 b | 3.17 bc | 0.60 cd |
| T4 | 1.5 | 0.97 b | 5.97 bd | 2.53 bd | 0.58 bc |
| T5 | 2.0 | 0.84 d | 4.23 cd | 2.47 be | 0.53 bd |
| p-value (ANOVA) | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| LSD0.05 | 0.10 | 1.86 | 1.01 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Dinh, S.T.; Ninh, T.T.; Nong, H.T.; Dang, T.T.T.; Khuat, Q.V.; Dang, A.T.P.; Ly, M.T.; Kirakosyan, R.N.; Kalashnikova, E.A. In Vitro Propagation of the Dendrobium anosmum Lindl. Collected in Vietnam. Agronomy 2022, 12, 324. https://doi.org/10.3390/agronomy12020324
Nguyen HT, Dinh ST, Ninh TT, Nong HT, Dang TTT, Khuat QV, Dang ATP, Ly MT, Kirakosyan RN, Kalashnikova EA. In Vitro Propagation of the Dendrobium anosmum Lindl. Collected in Vietnam. Agronomy. 2022; 12(2):324. https://doi.org/10.3390/agronomy12020324
Chicago/Turabian StyleNguyen, Hai T., Son T. Dinh, Thao T. Ninh, Hue T. Nong, Tam T. T. Dang, Quyet V. Khuat, Anh T. P. Dang, My T. Ly, Rima N. Kirakosyan, and Elena A. Kalashnikova. 2022. "In Vitro Propagation of the Dendrobium anosmum Lindl. Collected in Vietnam" Agronomy 12, no. 2: 324. https://doi.org/10.3390/agronomy12020324
APA StyleNguyen, H. T., Dinh, S. T., Ninh, T. T., Nong, H. T., Dang, T. T. T., Khuat, Q. V., Dang, A. T. P., Ly, M. T., Kirakosyan, R. N., & Kalashnikova, E. A. (2022). In Vitro Propagation of the Dendrobium anosmum Lindl. Collected in Vietnam. Agronomy, 12(2), 324. https://doi.org/10.3390/agronomy12020324









