Imidacloprid Disturbs the Nitrogen Metabolism and Triggers an Overall Stress Response in Maize Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions and Treatments
2.2. Measurement of Chlorophyll and the Degree of Membrane Lipid Peroxidation
2.3. Determination of Imidacloprid Content
2.4. RNA Sequencing
2.5. Differentially Expressed Gene (DEG) Analysis and Annotation
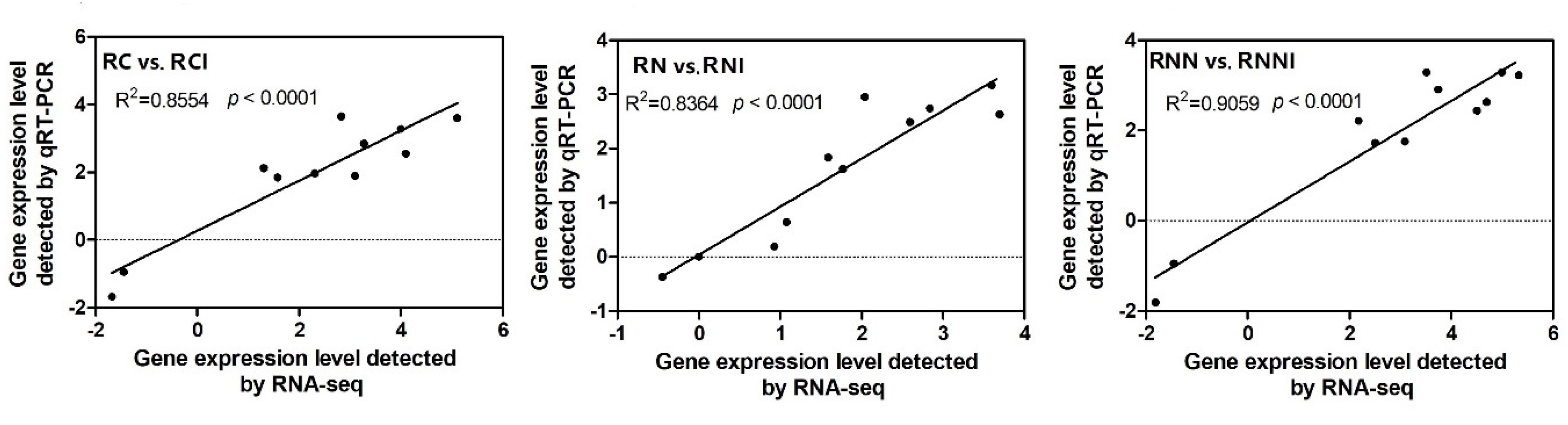
2.6. Quantitative Real-Time PCR Analysis
2.7. Nitrate Reductase, Glutamate Synthases’ Activity and Nitrate Content Assay
2.8. Statistical Analysis
3. Results
3.1. Phenotypic Analysis of Maize under Imidacloprid Treatment
3.2. Uptake and Accumulation of Imidacloprid in Maize
3.3. Identification of DEGs in Maize after Imidacloprid Treatment
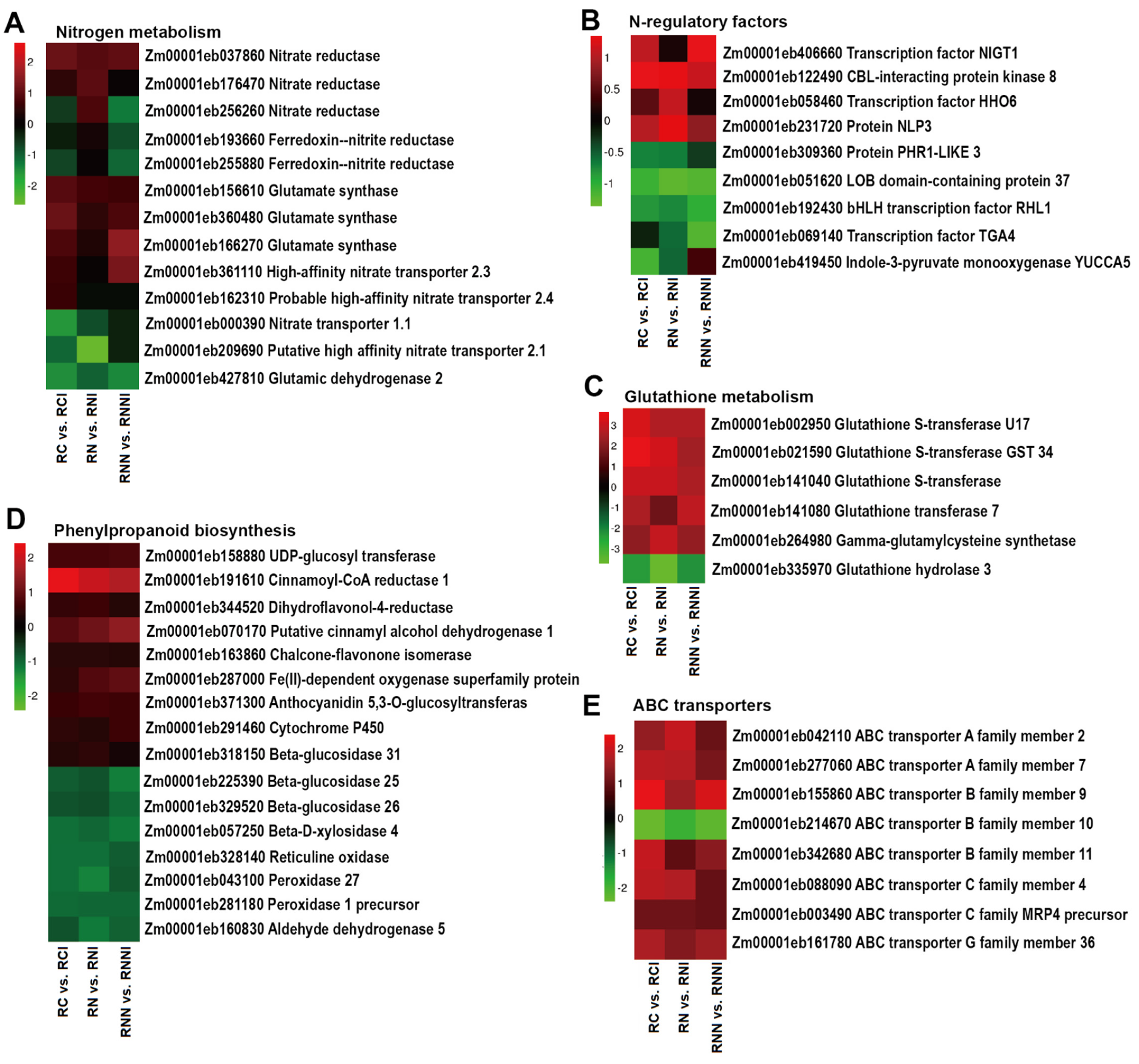
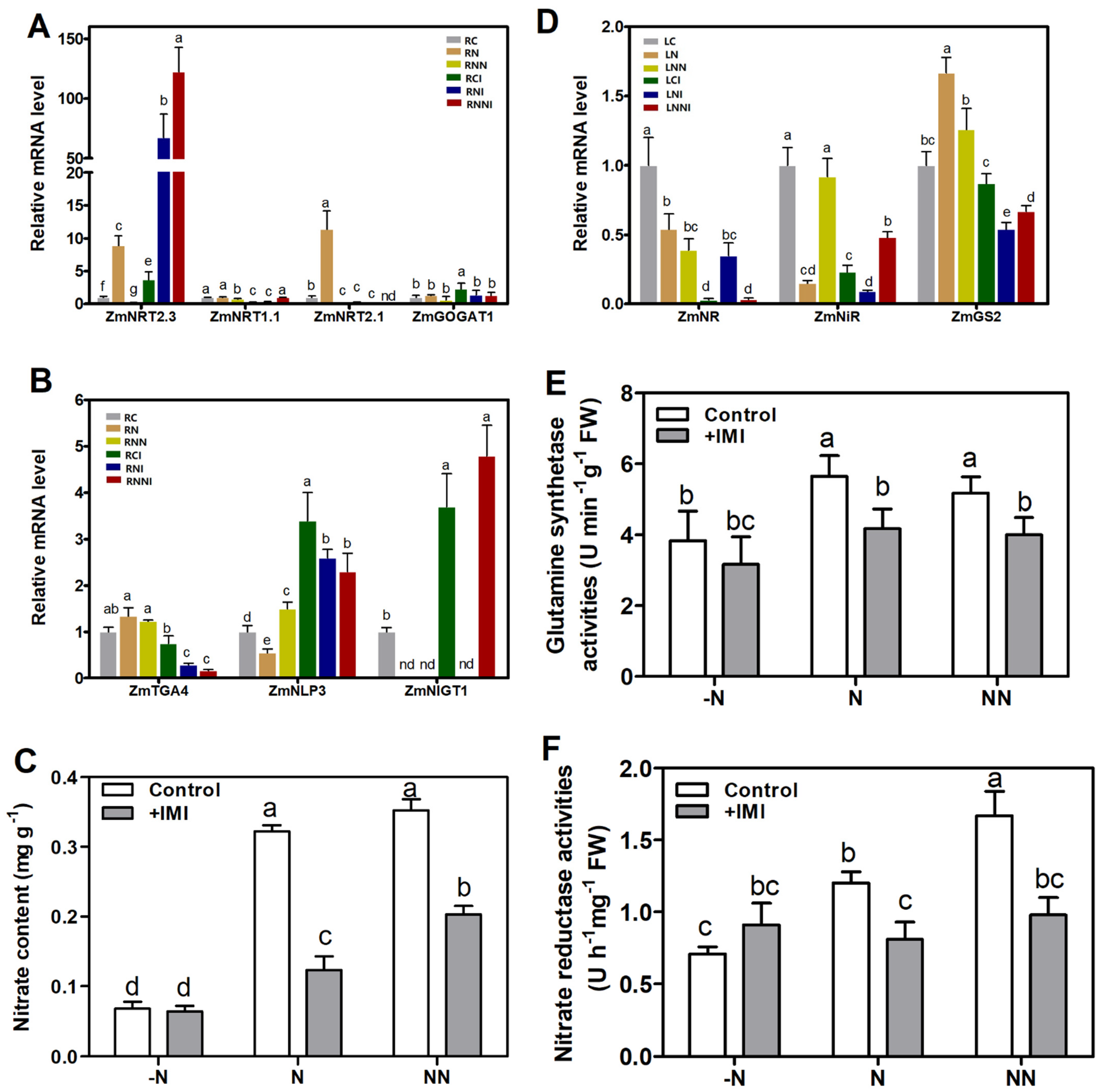
3.4. Imidacloprid Inhibited Nitrate Absorption in the Roots and Reduced Nitrogen Assimilation in the Shoots
3.5. Imidacloprid Up-Regulated Stress-Related Gene Expression
4. Discussion
4.1. The Effect of IMI Treatment on Maize Growth Varied with the Different Nitrogen Forms
4.2. NO3− Promoted the Transport of IMI from Root to Shoot in Maize
4.3. IMI Disturbed the Absorption and Assimilation of Nitrogen in Maize Roots
4.4. IMI Triggered Changes in the Expression of Stress Response Genes in Maize
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaafsma, A.; Limay-Rios, V.; Xue, Y.; Smith, J.; Baute, T. Field-scale examination of neonicotinoid insecticide persistence in soil as a result of seed treatment use in commercial maize (corn) fields in southwestern Ontario. Environ. Toxicol. Chem. 2016, 35, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Lanka, S.K.; Senthil-Nathan, S.; Blouin, D.J.; Stout, M.J. Impact of Thiamethoxam seed treatment on growth and yield of rice, Oryza sativa. J. Econ. Entomol. 2017, 110, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, T.; Paramasivam, M. Dissipation Kinetics and environmental risk assessment of thiamethoxam in the sandy clay loam soil of tropical sugarcane crop ecosystem. Bull. Environ. Contam. Toxicol. 2020, 105, 474–480. [Google Scholar] [CrossRef]
- Matsuda, K.; Ihara, M.; Sattelle, D.B. Neonicotinoid Insecticides: Molecular Targets, Resistance, and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Field, L.M. Neonicotinoids. Curr. Biol. 2018, 28, R772–R773. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, E.; Huang, L.; Lu, Y.; Gao, X.; Akhtar, K.; Ye, Q.; Wang, H. Translocation and metabolism of imidacloprid in cabbage: Application of (14)C-labelling and LC-QTOF-MS. Chemosphere 2021, 263, 127928. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total. Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Easton, A.H.; Goulson, D. The neonicotinoid insecticide imidacloprid repels pollinating flies and beetles at field-realistic concentrations. PLoS ONE 2013, 8, e54819. [Google Scholar] [CrossRef]
- Leiva, J.A.; Nkedi-Kizza, P.; Borejsza-Wysocki, W.S.; Bauder, V.S.; Morgan, K.T. Imidacloprid extraction from citrus leaves and analysis by Liquid Chromatography-Mass Spectrometry (HPLC-MS/MS). Bull. Environ. Contam. Tox. 2016, 96, 671–677. [Google Scholar] [CrossRef]
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, desorption and degradation of neonicotinoids in four agricultural soils and their effects on soil microorganisms. Sci. Total. Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Giorio, C.; Safer, A.; Sánchez-Bayo, F.; Tapparo, A.; Lentola, A.; Girolami, V.; van Lexmond, M.B.; Bonmatin, J.M. An update of the worldwide integrated assessment (WIA) on systemic insecticides. Part 1: New molecules, metabolism, fate, and transport. Environ. Sci. Pollut. Res. 2021, 28, 11716–11748. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.; Mohanraj, R.; Sujata, W. Monitoring of neonicotinoid pesticides in water-soil systems along the agro-landscapes of the cauvery delta region, south India. Bull. Environ. Contam. Tox. 2021, 106, 1065–1070. [Google Scholar] [CrossRef]
- Tong, Z.; Duan, J.; Wu, Y.; Liu, Q.; He, Q.; Shi, Y. A survey of multiple pesticide residues in pollen and beebread collected in China. Sci. Total. Environ. 2018, 640–641, 1578–1586. [Google Scholar] [CrossRef]
- Mikolić, A.; Karačonji, I.B. Imidacloprid as reproductive toxicant and endocrine disruptor: Investigations in laboratory animals. Arh. Hig. Rada Toksikol. 2018, 69, 103–108. [Google Scholar] [CrossRef]
- Zhao, G.P.; Wang, X.Y.; Li, J.W.; Wang, R.; Ren, F.Z.; Pang, G.F.; Li, Y.X. Imidacloprid increases intestinal permeability by disrupting tight junctions. Ecotoxicol. Environ. Saf. 2021, 222, 112476. [Google Scholar] [CrossRef]
- Katić, A.; Kašuba, V.; Kopjar, N.; Lovaković, B.T.; Čermak, A.M.M.; Mendaš, G.; Micek, V.; Milić, M.; Pavičić, I.; Pizent, A.; et al. Effects of low-level imidacloprid oral exposure on cholinesterase activity, oxidative stress responses, and primary DNA damage in the blood and brain of male Wistar rats. Chem. Biol. Interact. 2021, 338, 109287. [Google Scholar] [CrossRef]
- Loser, D.; Grillberger, K.; Hinojosa, M.G.; Blum, J.; Haufe, Y.; Danker, T.; Johansson, Y.; Möller, C.; Nicke, A.; Bennekou, S.H.; et al. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 2021, 95, 3695–3716. [Google Scholar] [CrossRef]
- Abdel-Halim, K.Y.; Osman, S.R. Cytotoxicity and oxidative stress responses of imidacloprid and glyphosate in human prostate epithelial WPM-Y.1 cell line. J. Toxicol. 2020, 2020, 4364650. [Google Scholar] [CrossRef]
- USEPA. OPP Pesticide toxicity database. 2014. Available online: http://www.epa.gov/oppefed1/ecorisk_ders/aquatic_life_benchmark.htm (accessed on 25 January 2017).
- CCME. Canadian water quality guidelines for the protection of aquatic life: Imidacloprid. In Scientific Supporting Document; Canadian Council of Ministers of the Environment: Winnipeg, MS, Canada, 2007. [Google Scholar]
- RIVM. Water Quality Standards for Imidacloprid: Proposal for an Update according to the Water Framework Directive; Dutch National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2014.
- Li, Y.; Long, L.; Ge, J.; Li, H.; Zhang, M.; Wan, Q.; Yu, X. Effect of imidacloprid uptake from contaminated soils on vegetable growth. J. Agr. Food Chem. 2019, 67, 7232–7242. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Zhang, F.; Xiao, C.; Tang, R.; Liu, Z.; Bai, S.; Wang, Z. Toxicity of chemical pesticides commonly used in maize to Trichogramma ostriniae (Hymenoptera: Trichogrammatidae), an egg parasitoid of Asian corn borer. Ecotoxicol. Environ. Saf. 2022, 241, 113802. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, H.; Zhang, Z.; Lin, J.; Liu, F.; Mu, W. Thiamethoxam, clothianidin, and imidacloprid seed treatments effectively control thrips on corn under field conditions. J. Insect. Sci. 2018, 18, 19. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal leaf positions for SPAD meter measurement in rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.; Gao, S.; Yang, Z.M.; Song, J.B. The F-box E3 ubiquitin ligase AtSDR is involved in salt and drought stress responses in Arabidopsis. Gene 2022, 809, 146011. [Google Scholar] [CrossRef] [PubMed]
- Collimore, W.A.; Bent, G.A. A newly modified QuEChERS method for the analysis of organochlorine and organophosphate pesticide residues in fruits and vegetables. Environ. Monit. Assess. 2020, 192, 128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Tang, L.; Peng, Y.; Qian, M.; Guo, Y.; Rui, H.; Zhang, F.; Hu, Z.; Chen, Y.; et al. The root iron transporter 1 governs cadmium uptake in Vicia sativa roots. J. Hazard. Mater. 2020, 398, 122873. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Florea, L.; Song, L.; Salzberg, S.L. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000Research 2 2013, 2, 188. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome. Biolo. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y. KEGG mapper for inferring cellular functions from protein sequences. Protein. Sci. 2020, 29, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Pirooznia, M.; Yang, J.Y.; Yang, M.Q.; Deng, Y.J.B.G. A comparative study of different machine learning methods on microarray gene expression data. BMC. Genom. 2008, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, L.; Zeng, H.Q.; Zhou, Z.S.; Yang, Z.M.; Li, H.; Sun, D.; Xie, F.; Zhang, B. A cotton miRNA is involved in regulation of plant response to salt stress. Sci. Rep. 2016, 6, 19736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y. Nitrate assay for plant tissues. Bio. Protoc. 2017, 7, e2029. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, L.; Shen, C.; Ji, Z.; Zhang, H.; Zhang, T.; Li, Y.; Yu, J.; Yang, N.; He, Y.; et al. Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. Plant Cell. 2021, 33, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Sun, L.; Sun, Z.; Li, D. Screening of a Chlorella-bacteria consortium and research on piggery wastewater purification. Algal. Res. 2020, 47, 101840. [Google Scholar] [CrossRef]
- Ju, C.; Li, X.; He, S.; Shi, L.; Yu, S.; Wang, F.; Xu, S.; Cao, D.; Fang, H.; Yu, Y. Root uptake of imidacloprid and propiconazole is affected by root composition and soil characteristics. J. Agr. Food Chem. 2020, 68, 15381–15389. [Google Scholar] [CrossRef] [PubMed]
- Sur, R.; Stork, A. Uptake, translocation and metabolism of imidacloprid in plants. Bull. Insectol. 2003, 56, 35–40. [Google Scholar]
- Ristova, D.; Carré, C.; Pervent, M.; Medici, A.; Kim, G.J.; Scalia, D.; Ruffel, S.; Birnbaum, K.D.; Lacombe, B.; Busch, W.; et al. Combinatorial interaction network of transcriptomic and phenotypic responses to nitrogen and hormones in the Arabidopsis thaliana root. Sci. Signal. 2016, 9, 451. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Uchida, T.; Yoshida, K. Prediction of soil asorption coefficient in pesticides using physicochemical properties and molecular descriptors by machine learning models. Environ. Toxicol. Chem. 2020, 39, 1451–1459. [Google Scholar] [CrossRef]
- Dettenmaier, E.M.; Doucette, W.J.; Bugbee, B. Chemical hydrophobicity and uptake by plant roots. Environ. Sci. Technol. 2009, 43, 324–329. [Google Scholar] [CrossRef]
- Collins, C.D.; Martin, I.; Doucette, W. Plant Uptake of Xenobiotics. In Organic Xenobiotics and Plants; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Wan, W.; Huang, H.; Lv, J.; Han, R.; Zhang, S. Uptake, translocation, and biotransformation of organophosphorus esters in wheat (Triticum aestivum L.). Environ. Sci. Technol. 2017, 51, 13649–13658. [Google Scholar] [CrossRef] [PubMed]
- Naku, M.; Kambizi, L.; Matimati, I. Functional roles of ammonium (NH4+) and nitrate (NO3-) in regulation of day- and night-time transpiration in Phaseolus vulgaris. Funct. Plant Biol. 2019, 46, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Espen, L. Time-course of metabolic and proteomic responses to different nitrate/ammonium availabilities in roots and leaves of maize. Int. J. Mol. Sci. 2018, 19, 2202. [Google Scholar] [CrossRef]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty years from transport to signaling networks. Plant Cell. 2020, 32, 2094–2119. [Google Scholar] [CrossRef]
- Guan, M.; Chen, M.; Cao, Z. NRT2.1, a major contributor to cadmium uptake controlled by high-affinity nitrate transporters. Ecotoxicol. Environ. Saf. 2021, 218, 112269. [Google Scholar] [CrossRef]
- Lejay, L.; Wirth, J.; Pervent, M.; Cross, J.M.; Tillard, P.; Gojon, A. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol. 2008, 146, 2036–2053. [Google Scholar] [CrossRef]
- de Jong, F.; Thodey, K.; Lejay, L.V.; Bevan, M.W. Glucose elevates NITRATE TRANSPORTER2.1 protein levels and nitrate transport activity independently of its HEXOKINASE1-mediated stimulation of NITRATE TRANSPORTER2.1 expression. Plant Physiol. 2014, 164, 308–320. [Google Scholar] [CrossRef]
- Lejay, L.; Tillard, P.; Lepetit, M.; Olive, F.; Filleur, S.; Daniel-Vedele, F.; Gojon, A. Molecular and functional regulation of two NO3- uptake systems by N- and C-status of Arabidopsis plants. Plant J. 1999, 18, 509–519. [Google Scholar] [CrossRef]
- Zhuo, D.; Okamoto, M.; Vidmar, J.J.; Glass, A.D. Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J. 1999, 17, 563–568. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Riveras, E.; Vidal, E.A.; Gras, D.E.; Contreras-López, O.; Tamayo, K.P.; Aceituno, F.; Gómez, I.; Ruffel, S.; Lejay, L.; et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. 2014, 80, 12618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Sun, C.; Gu, R.; Mi, G.; Yuan, L. Phylogenetic, expression and functional characterizations of the maize NLP transcription factor family reveal a role in nitrate assimilation and signaling. Physiol. Plant. 2018, 163, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.F.; Chen, Y.; Sun, M.M.; Wang, Y.; Chen, Y.F. The transcription factor NIGT1.2 modulates both phosphate uptake and nitrate influx during phosphate starvation in Arabidopsis and Maize. Plant Cell 2020, 32, 3519–3534. [Google Scholar] [CrossRef]
- Konishi, M.; Okitsu, T.; Yanagisawa, S. Nitrate-responsive NIN-like protein transcription factors perform unique and redundant roles in Arabidopsis. J. Exp. Bot. 2021, 72, 5735–5750. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.D.; Cirrone, J.; Pasquino, A.V.; Alvarez, J.M.; Swift, J.; Mittal, S.; Juang, C.-L.; Varala, K.; Gutiérrez, R.A.; Krouk, G.; et al. Network Walking charts transcriptional dynamics of nitrogen signaling by integrating validated and predicted genome-wide interactions. Nat. Commun. 2019, 10, 1569. [Google Scholar] [CrossRef] [PubMed]
- Seifrtova, M.; Halesova, T.; Sulcova, K.; Riddellova, K.; Erban, T. Distributions of imidacloprid, imidacloprid-olefin and imidacloprid-urea in green plant tissues and roots of rapeseed (Brassica napus) from artificially contaminated potting soil. Pest Manag. Sci. 2017, 73, 1010–1016. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Leng, R.; Zhang, J.; Zhou, Y.; Zhang, Y.; Yang, S.; He, K.; Huang, B. Mechanism study of the beneficial effect of sodium selenite on metabolic disorders in imidacloprid-treated garlic plants. Ecotox. Environ. Safe. 2020, 200, 110736. [Google Scholar] [CrossRef]
- Balotf, S.; Kavoosi, G.; Kholdebarin, B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings. Biotechnol. Appl. Biochem. 2016, 63, 220–229. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-Transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant. Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, X.; Yu, N.; Gao, H.; Zhang, Y.; Liu, W.; Liu, Z. Contribution of glutathione S-transferases to Iimidacloprid resistance in Nilaparvata lugens. J. Agr. Food Chem. 2020, 68, 15403–15408. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, J.; Lv, J.; Yu, J.; Xie, J.; Wu, Y.; Tang, Z. Melatonin alleviates imidacloprid phytotoxicity to cucumber (Cucumis sativus L.) through modulating redox homeostasis in plants and promoting its metabolism by enhancing glutathione dependent detoxification. Ecotox. Environ. Safe. 2021, 217, 112248. [Google Scholar] [CrossRef]
- Lu, Y.P.; Li, Z.S.; Rea, P.A. AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: Isolation and functional definition of a plant ATP-binding cassette transporter gene. Proc. Natl. Acad. Sci. USA 1997, 94, 8243–8248. [Google Scholar] [CrossRef]
- Gaillard, C.; Dufaud, A.; Tommasini, R.; Kreuz, K.; Amrhein, N.; Martinoia, E. A herbicide antidote (safener) induces the activity of both the herbicide detoxifying enzyme and of a vacuolar transporter for the detoxified herbicide. FEBS Lett. 1994, 352, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Nornberg, B.F.; Batista, C.R.; Almeida, D.V.; Trindade, G.S.; Marins, L.F. ABCB1 and ABCC4 efflux transporters are involved in methyl parathion detoxification in ZFL cells. Toxicol. Vitr. 2015, 29, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, L.; Huang, S.; Yang, C.; Wang, Y.; Yuan, H.; Xu, Q.; Zhang, W.; Wang, M.; Zeng, X.; et al. Drought resistance in qingke involves a reprogramming of the phenylpropanoid pathway and UDP-glucosyltransferase regulation of abiotic stress tolerance targeting flavonoid biosynthesis. J. Agric. Food Chem. 2021, 69, 992–4005. [Google Scholar] [CrossRef]
- Moura, J.C.; Bonine, C.A.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Davies, K.M.; Albert, N.W.; Zhou, Y.; Schwinn, K.E. Functions of flavonoid and betalain pigments in abiotic stress tolerance in plants. Annu. Plant Rev. 2018, 1, 604. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, C.; Wang, P.; Fu, W.; Wang, L.; Song, Z.; Xi, X.; Wu, H.; Zhang, G.; Wu, J. Sandbur drought tolerance reflects phenotypic plasticity based on the accumulation of sugars, lipids, and flavonoid intermediates and the scavenging of reactive oxygen species in the root. Int. J. Mol. Sci. 2021, 22, 12615. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef]
- de Oliveira, F.K.; Santos, L.O.; Buffon, J.G. Mechanism of action, sources, and application of peroxidases. Food Res. Int. 2021, 143, 110266. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell. Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
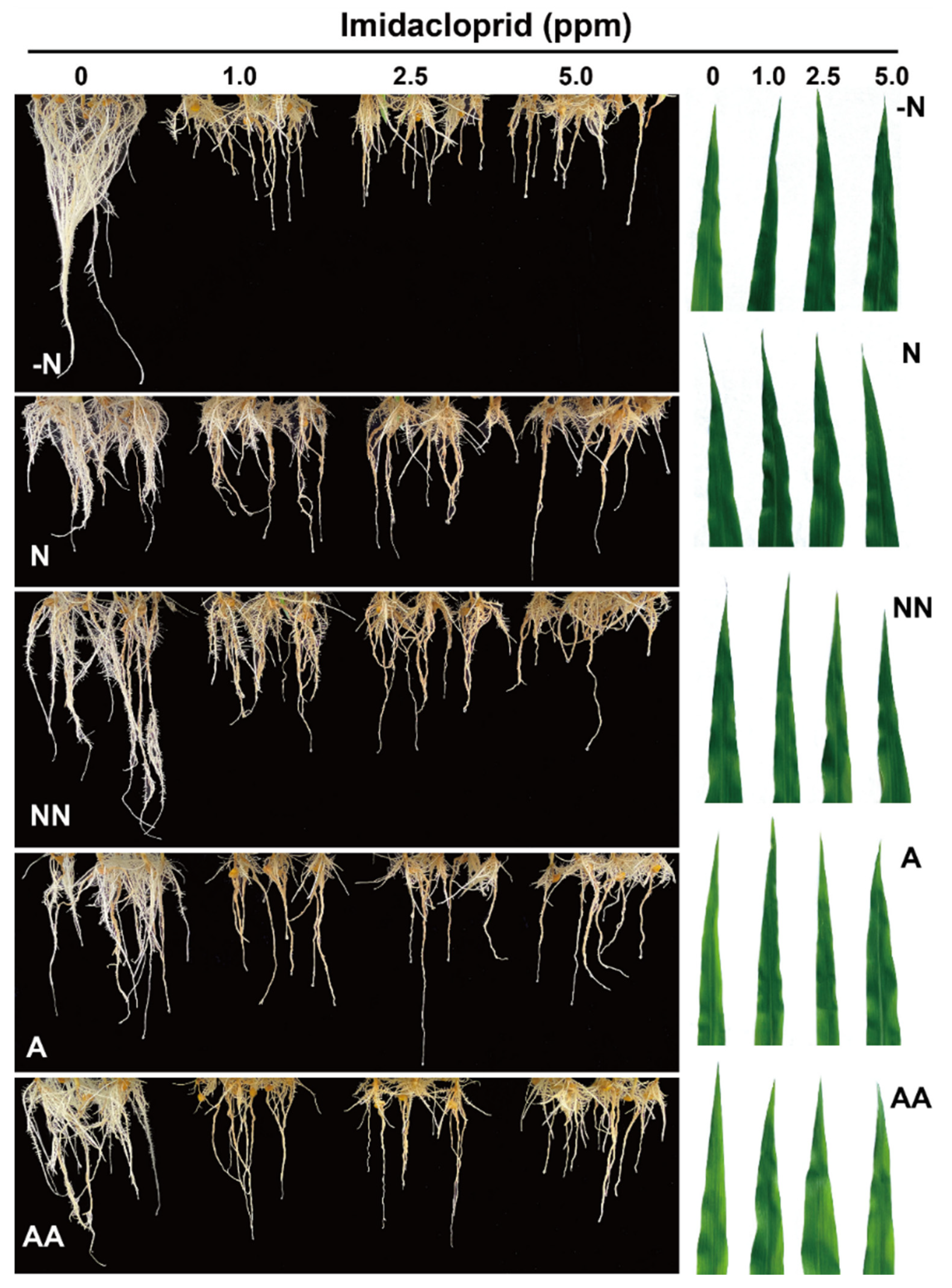
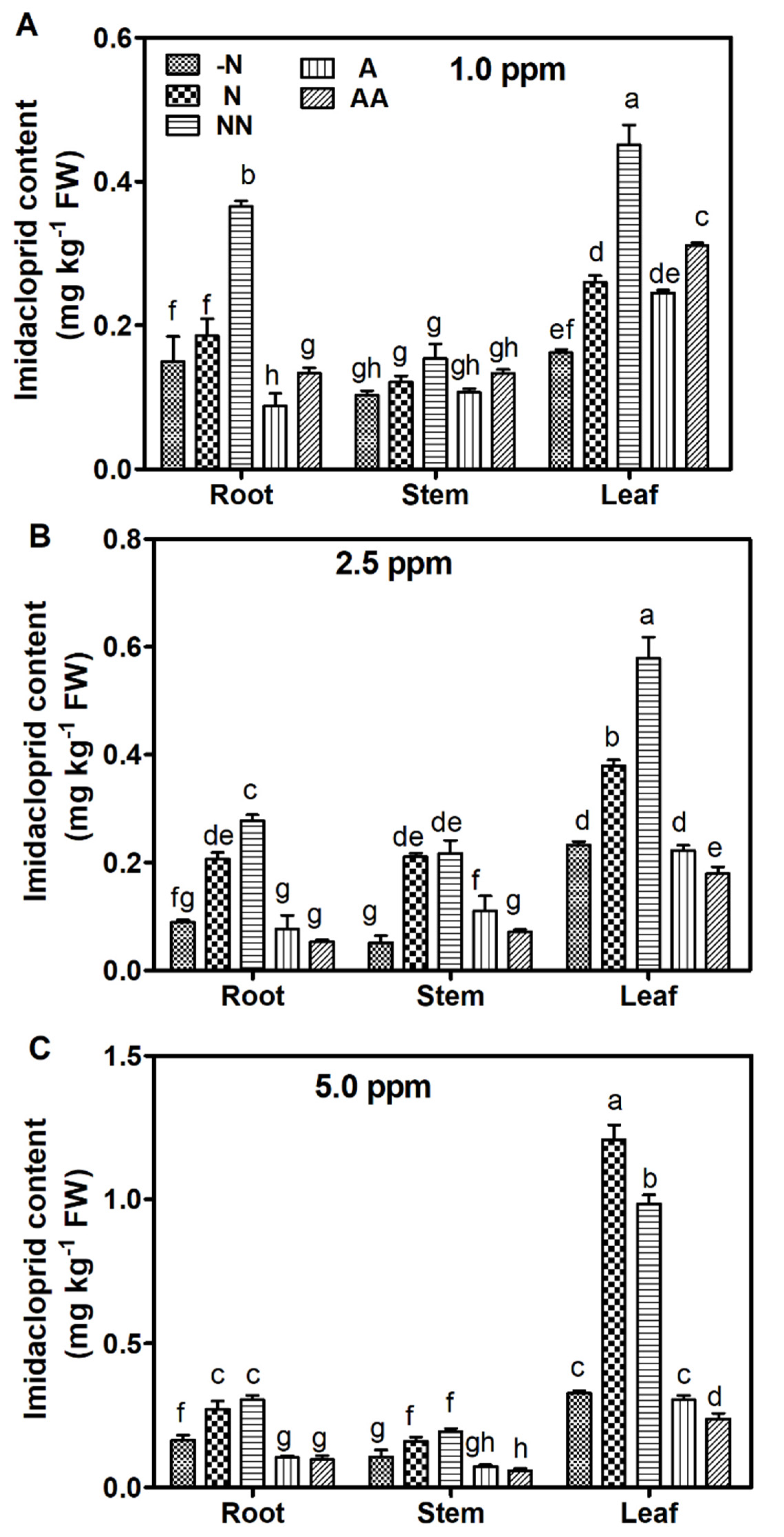



| IMI (ppm) | Groups | |||||
|---|---|---|---|---|---|---|
| -N | N | NN | A | AA | ||
| Shoots DW (mg) | 0 | 15.1 ± 3.0g | 35.1 ± 5.2b | 42.6 ± 3.2a | 35.6 ± 3.7bc | 32.5 ± 6.7bcd |
| 1.0 | 17.2 ± 3.1fg | 29.4 ± 3.3cde | 32.5 ± 5.3bcd | 28.5 ± 5.9cde | 23.5 ± 3.3def | |
| 2.5 | 17.1 ± 2.2fg | 32.2 ± 3.2bcd | 34.3 ± 2.1bcd | 26.7 ± 3.6def | 22.6 ± 5.3ef | |
| 5.0 | 16.7 ± 2.4fg | 27.5 ± 1.8cde | 28.5 ± 6.1cde | 25.6 ± 3.8def | 23.8 ± 2.2def | |
| Shoots length (cm) | 0 | 13.7 ± 0.3fgh | 18.3 ± 1.0b | 19.8 ± 1.0a | 16.1 ± 0.5cd | 15.7 ± 1.6cde |
| 1.0 | 14.0 ± 0.3efg | 14.7 ± 0.7def | 16.8 ± 1.2c | 13.3 ± 0.5fgh | 14.6 ± 0.7def | |
| 2.5 | 13.8 ± 0.63fgh | 13.8 ± 0.4fgh | 15.0 ± 0.5def | 13.1 ± 0.3fgh | 13.5 ± 0.4fgh | |
| 5.0 | 14.1 ± 0.4fgh | 12.9 ± 0.3ghi | 13.0 ± 0.5ghi | 11.6 ± 0.4i | 12.2 ± 0.8hi | |
| SPAD | 0 | 12.2 ± 0.4f | 20.8 ± 1.5bc | 20.6 ± 0.5bc | 18.5 ± 2.1cd | 17.4 ± 0.7cde |
| 1.0 | 18.8 ± 1.0bcd | 19.8 ± 1.0bcd | 22.2 ± 1.1ab | 20.5 ± 0.4bc | 20.5 ± 1.5bc | |
| 2.5 | 17.6 ± 1.2cde | 23.0 ± 1.5a | 21.7 ± 2.2abc | 20.9 ± 0.8abc | 19.1 ± 2.2bcd | |
| 5.0 | 18.5 ± 0.7bcd | 21.11 ± 2.2abc | 23.2 ± 1.0a | 21.5 ± 3.0abc | 19.3 ± 1.0ab | |
| TBARS (nmol g−1 FW) | 0 | 69.2 ± 4.0a | 5.6 ± 1.8h | 7.7 ± 0.7h | 31.7 ± 1.5e | 37.0 ± 0.8de |
| 1.0 | 38.7 ± 2.1de | 14.8 ± 0.4g | 18.8 ± 1.7fg | 37.4 ± 2.9de | 21.8 ± 1.1f | |
| 2.5 | 37.1 ± 4.1de | 21.5 ± 2.4f | 23.1 ± 2.8f | 39.9 ± 1.3d | 32.2 ± 2.1e | |
| 5.0 | 38.5 ± 4.0de | 36.5 ± 2.5de | 39.0 ± 2.4de | 59.0 ± 4.2b | 53.7 ± 6.0c | |
| IMI (ppm) | Groups | |||||
|---|---|---|---|---|---|---|
| -N | N | NN | A | HA | ||
| Roots DW (mg) | 0 | 8.7 ± 0.5a | 8.2 ± 1.3a | 7.8 ± 0.6a | 7.7 ± 0.7a | 8.7 ± 0.3a |
| 1.0 | 5.4 ± 0.7bcd | 6.4 ± 0.9b | 5.0 ± 0.7bcd | 5.3 ± 0.9bcd | 6.1 ± 0.7bcd | |
| 2.5 | 5.1 ± 0.8bcd | 4.6 ± 0.5bcd | 5.4 ± 0.7bcd | 5.2 ± 0.9bcd | 4.8 ± 0.9bcd | |
| 5.0 | 3.9 ± 0.5d | 4.2 ± 0.5cd | 4.3 ± 0.8bcd | 3.5 ± 0.6d | 3.9 ± 0.5d | |
| Main roots length (cm) | 0 | 32.1 ± 1.5ab | 21.4 ± 1.9bc | 23.5 ± 3.1b | 20.4 ± 1.3cd | 19.9 ± 0.5cd |
| 1.0 | 13.8 ± 1.5hij | 17.5 ± 0.7ef | 18.2 ± 0.8de | 17.8 ± 2.8ef | 17.1 ± 1.4ef | |
| 2.5 | 11.9 ± 0.1ij | 16.2 ± 0.9efg | 18.1 ± 1.1de | 16.9 ± 1.6ef | 15.3 ± 1.7fg | |
| 5.0 | 11.3 ± 0.5j | 15.8 ± 0.9fg | 15.5 ± 0.8fg | 14.7 ± 1.1gh | 13.5 ± 1.3hij | |
| TBARS (nmol g−1 FW) | 0 | 4.24 ± 0.6f | 5.2 ± 0. 8f | 5.0 ± 1.0f | 11.3 ± 1.7e | 14.4 ± 1.8cde |
| 1.0 | 13.0 ± 1.3cde | 12.9 ± 1.1cde | 12.9 ± 2.1cde | 14.1 ± 2.9cde | 16.1 ± 2.1cd | |
| 2.5 | 17.8 ± 2.18c | 19.9 ± 2.1bc | 18.8 ± 3.1bc | 23.9 ± 3.1b | 24.0 ± 3.1b | |
| 5.0 | 28.87 ± 2.88ab | 25.6 ± 4.2b | 24.5 ± 4.1b | 28.8 ± 4.1ab | 30.8 ± 2.1a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fu, H.; Wu, Q.; Chen, L.; Lu, Y.; Gao, S. Imidacloprid Disturbs the Nitrogen Metabolism and Triggers an Overall Stress Response in Maize Seedlings. Agronomy 2022, 12, 3161. https://doi.org/10.3390/agronomy12123161
Zhang X, Fu H, Wu Q, Chen L, Lu Y, Gao S. Imidacloprid Disturbs the Nitrogen Metabolism and Triggers an Overall Stress Response in Maize Seedlings. Agronomy. 2022; 12(12):3161. https://doi.org/10.3390/agronomy12123161
Chicago/Turabian StyleZhang, Xingxing, Hongkai Fu, Qihua Wu, Lijuan Chen, Yinglin Lu, and Shuai Gao. 2022. "Imidacloprid Disturbs the Nitrogen Metabolism and Triggers an Overall Stress Response in Maize Seedlings" Agronomy 12, no. 12: 3161. https://doi.org/10.3390/agronomy12123161
APA StyleZhang, X., Fu, H., Wu, Q., Chen, L., Lu, Y., & Gao, S. (2022). Imidacloprid Disturbs the Nitrogen Metabolism and Triggers an Overall Stress Response in Maize Seedlings. Agronomy, 12(12), 3161. https://doi.org/10.3390/agronomy12123161





