Advances in the Agro-Environment Migration of Organic Chemical Pollutants and Their Biotransformation in Crops
Abstract
:1. Introduction
2. The Main TOPs Existing in Agroecosystems
2.1. Types of TOPs
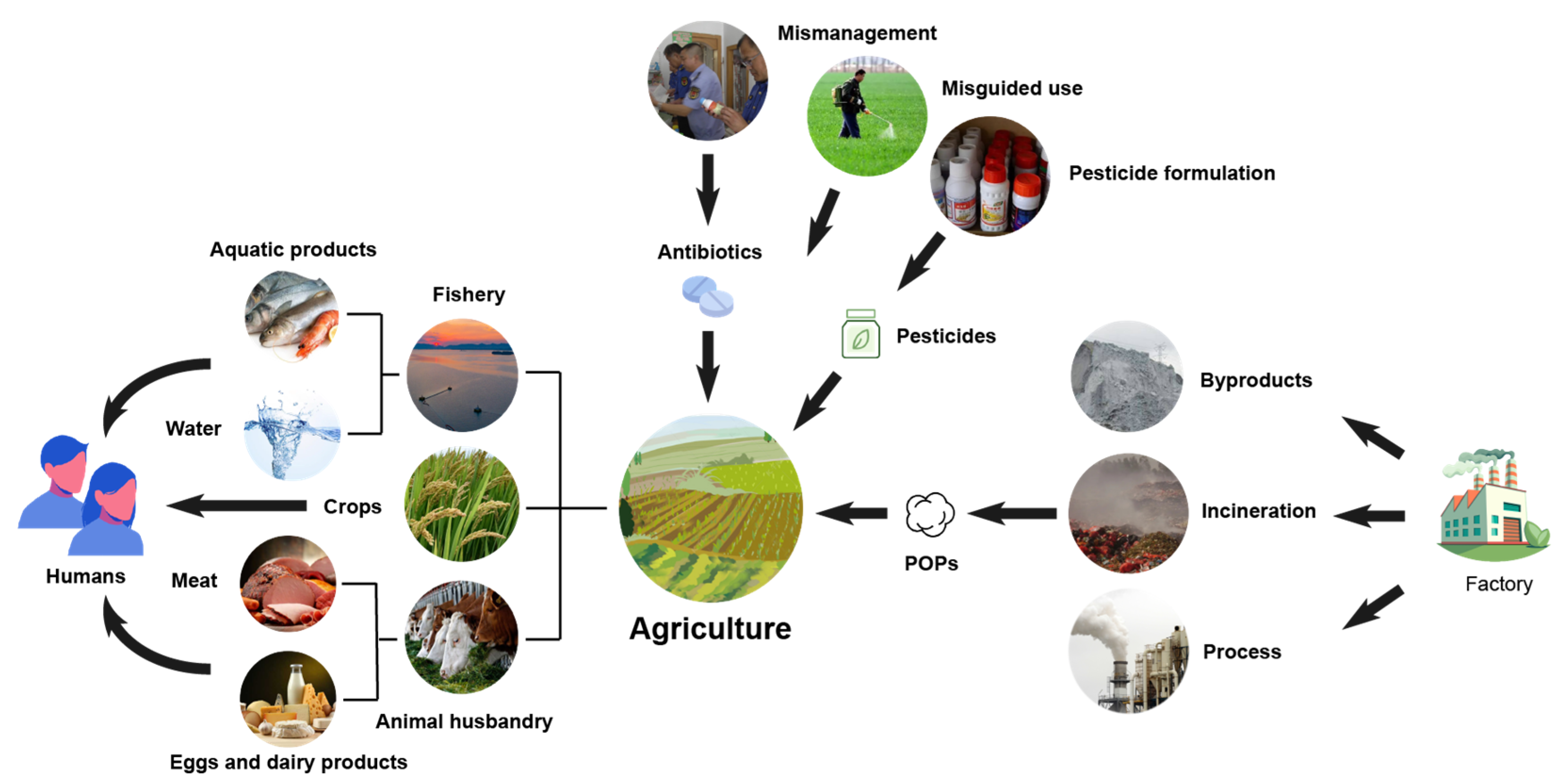
2.2. Environmental Sources
2.3. Hazards of Organic Chemical Pollutants on the Agricultural Environment
| TOPs | Sources | Environmental Hazards | Reference |
|---|---|---|---|
| Pesticide |
|
| [57,70,71,72,73,74,75,76,77,78] |
| Antibiotic |
|
| [79,80,81,82,83,84] |
| POPs |
|
| [85,86,87,88,89,90,91,92,93] |
3. Transport Behavior of Organic Chemical Pollutants in the Agricultural Environment
4. Transport and Transformation of Organic Chemical Pollutants in Crops
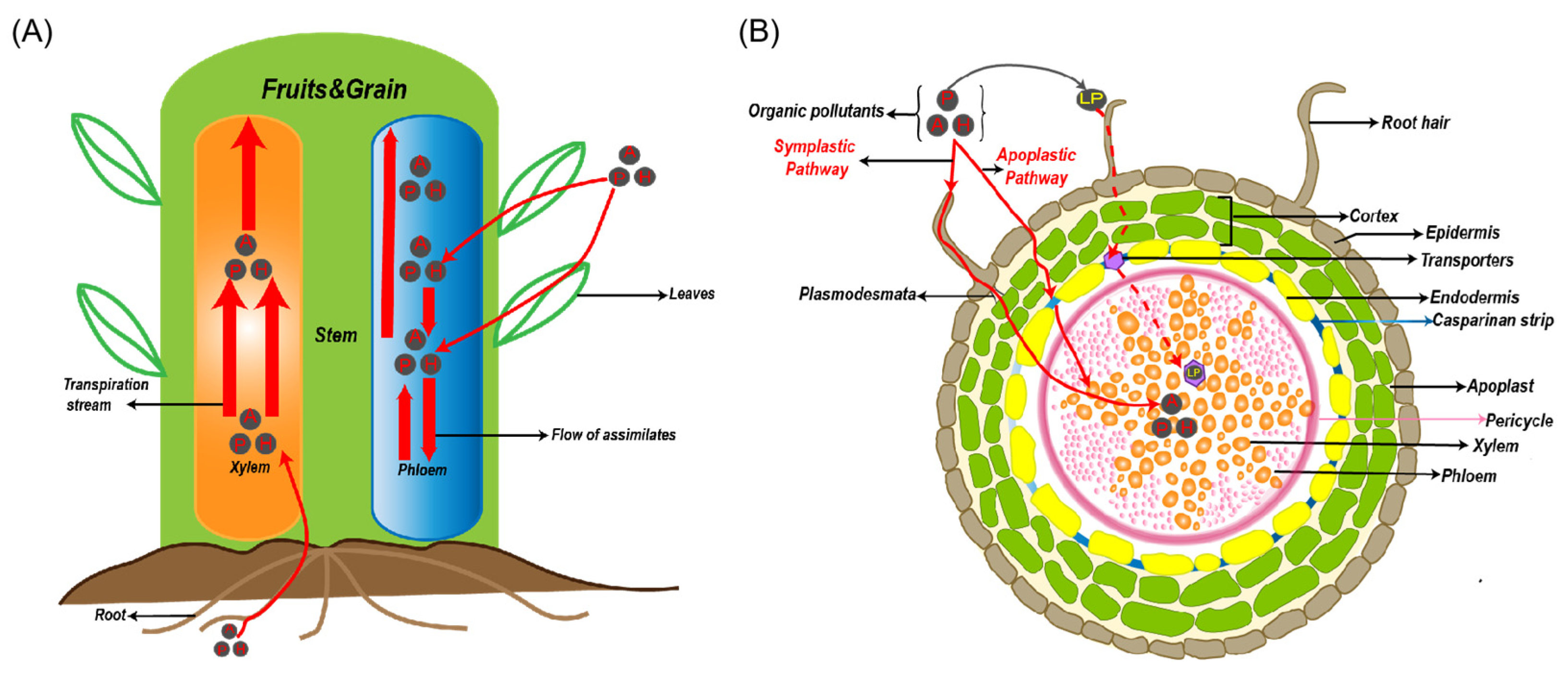
4.1. Biotransportation Processes in Soil–Plant
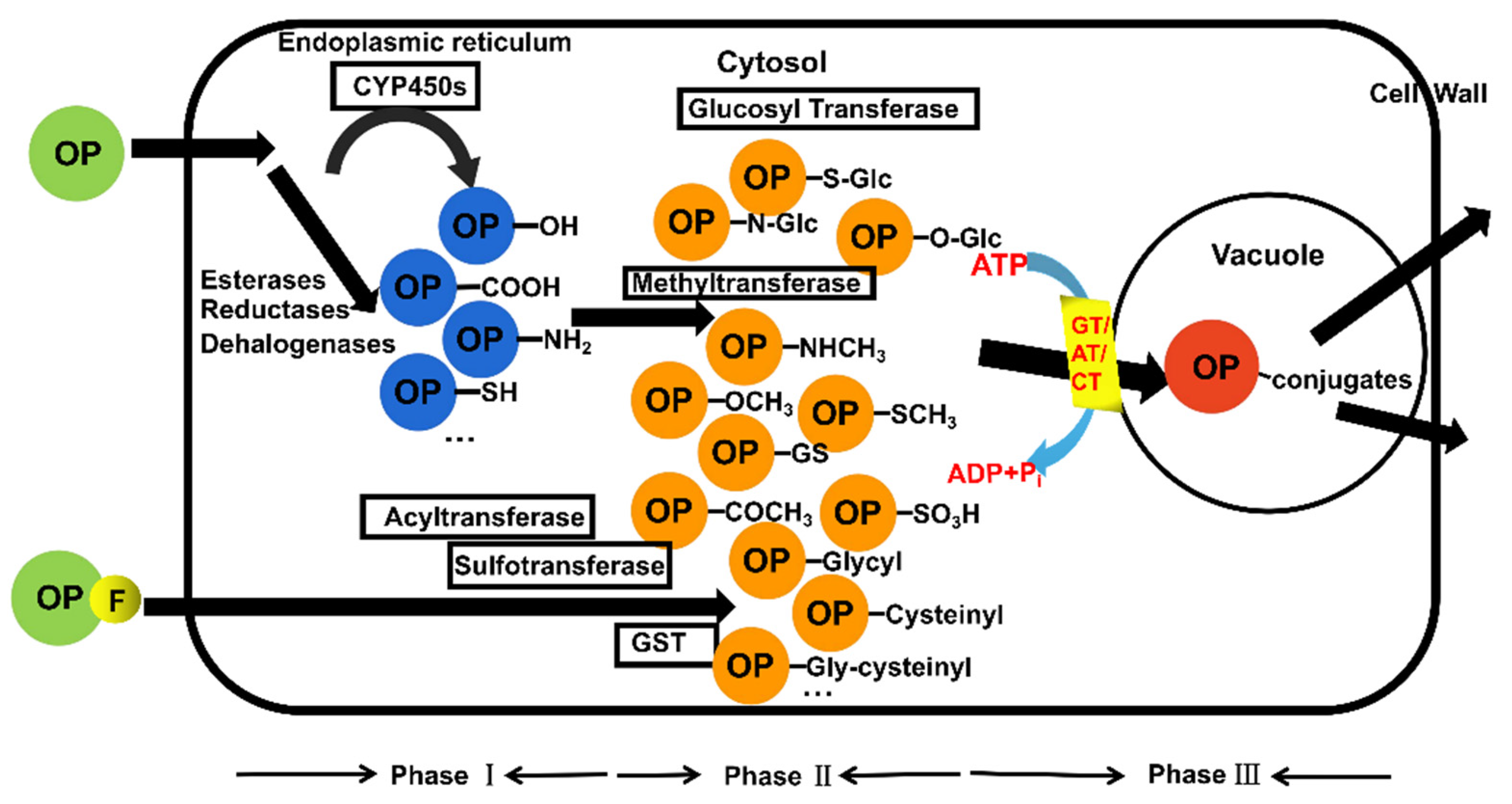
4.2. Biotransformation Process in Plant
5. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Griffiths, M.R.; Strobel, B.W.; Hama, J.R.; Cedergreen, N. Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environ. Sci. Eur. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Yang, H. Metabolism and Detoxification of Pesticides in Plants. Sci. Total Environ. 2021, 790, 148034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chu, G.; Wei, C.; Ye, J.; Li, Z.; Liang, Y. The Growth and Antioxidant Defense Responses of Wheat Seedlings to Omethoate Stress. Pestic. Biochem. Physiol. 2011, 100, 273–279. [Google Scholar] [CrossRef]
- Liang, C.-P.; Sack, C.; McGrath, S.; Cao, Y.; Thompson, C.J.; Robin, L.P. US Food and Drug Administration Regulatory Pesticide Residue Monitoring of Human Foods: 2009–2017. Food Addit. Contam. Part A 2021, 38, 1520–1538. [Google Scholar] [CrossRef]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An Overview on the Accumulation, Distribution, Transformations, Toxicity and Analytical Methods for the Monitoring of Persistent Organic Pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xia, Z.; Zhang, Q.; Yin, J.; Zhou, Y.; Yang, H. Distribution and Health Risk Assessment on Dietary Exposure of Polycyclic Aromatic Hydrocarbons in Vegetables in Nanjing, China. J. Chem. 2016, 2016, 1581253. [Google Scholar] [CrossRef] [Green Version]
- Fakinle, B.S.; Odekanle, E.L.; Ike-Ojukwu, C.; Sonibare, O.O.; Falowo, O.A.; Olubiyo, F.W.; Oke, D.O.; Aremu, C.O. Quantification and Health Impact Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) Emissions from Crop Residue Combustion. Heliyon 2022, 8, e09113. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Ashaolu, T.J. A Study on Polycyclic Aromatic Hydrocarbon and Heavy Metal Concentrations of Commercial Grilled Meat (Suya) and Smoked Catfish (Clarias Gariepinus Burchell, 1822) Fish from South-West, Nigeria. Polycycl. Aromat. Compd. 2022, 42, 3281–3290. [Google Scholar] [CrossRef]
- Yan, L.; Hua, G.; Guanhua, H.; Quanzhong, H.; Honglu, L. Contamination and Health Risk Assessment of PAHs in Irrigation District in Southeastern Suburb of Beijing. Trans. Chin. Soc. Agric. Mach. 2017, 48, 237–249. [Google Scholar]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of Polycyclic Aromatic Hydrocarbons in Vegetables Grown in an Industrial Area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, L.; Li, G.; Fang, L.; Yu, X.; Tang, Y.-T.; Li, M.; Chen, L. Veterinary Antibiotics Can Reduce Crop Yields by Modifying Soil Bacterial Community and Earthworm Population in Agro-Ecosystems. Sci. Total Environ. 2022, 808, 152056. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Feng, Y.; Wu, G.; Zhang, R.; Li, B.; Yin, Q.; Luo, L. Detection and Degradation Characterization of 16 Quinolones in Soybean Sprouts by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. Foods 2022, 11, 2500. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Liu, C.; Wang, Z.; Dong, J.; Zhu, G.; Huang, X. Potential Effect and Accumulation of Veterinary Antibiotics in Phragmites Australis under Hydroponic Conditions. Ecol. Eng. 2013, 53, 138–143. [Google Scholar] [CrossRef]
- Christou, A.; Michael, C.; Fatta-Kassinos, D.; Fotopoulos, V. Can the Pharmaceutically Active Compounds Released in Agroecosystems Be Considered as Emerging Plant Stressors? Environ. Int. 2018, 114, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M.; Yoon, Y.-E.; Lee, Y.B. Veterinary Antibiotics (VAs) Contamination as a Global Agro-Ecological Issue: A Critical View. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Saxena, G.; Bharagava, R.N. (Eds.) Bioremediation of Industrial Waste for Environmental Safety: Volume I: Industrial Waste and Its Management; Springer: Singapore, 2020; pp. 53–76. ISBN 9789811318900. [Google Scholar]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Deng, Y.; Li, W.; Du, W.; Gu, Y.; Li, J.; Xu, X. Phytoremediation of Antibiotic-Contaminated Wastewater: Insight into the Comparison of Ciprofloxacin Absorption, Migration, and Transformation Process at Different Growth Stages of E. Crassipes. Chemosphere 2021, 283, 131192. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, A.; Zhang, Y.; Si, C.; Chen, Z.; Qian, H.; Zhao, Z. Research on the Adsorption and Migration of Sulfa Antibiotics in Underground Environment. Environ. Earth Sci. 2016, 75, 1252. [Google Scholar] [CrossRef]
- Shan, Q.; Liu, M.; Li, R.; Shi, Q.; Li, Y.; Gong, B. γ-Aminobutyric Acid (GABA) Improves Pesticide Detoxification in Plants. Sci. Total Environ. 2022, 835, 155404. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. IJERPH 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.A.; El Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of Pesticides on Environmental and Human Health. In Toxicology Studies-Cells, Drugs and Environment; Andreazza, A.C., Scola, G., Eds.; InTech: London, UK, 2015; pp. 196–223. ISBN 978-953-51-2140-4. [Google Scholar]
- Zhao, L.; Teng, Y.; Luo, Y.M. Present Pollution Status and Control Strategy of Pesticides in Agricultural Soils in China: A Review. Soils 2017, 49, 417–427. [Google Scholar]
- Ajiboye, T.O.; Oladoye, P.O.; Olanrewaju, C.A.; Akinsola, G.O. Organophosphorus Pesticides: Impacts, Detection and Removal Strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Y.; Hu, R.; Huang, J.; Huang, X.; Li, Y.; Yin, Y.; Chen, Z. A Comparison of the Effects of Agricultural Pesticide Uses on Peripheral Nerve Conduction in China. Sci. Rep. 2018, 8, 9621. [Google Scholar] [CrossRef] [Green Version]
- Gerbig, S.; Brunn, H.E.; Spengler, B.; Schulz, S. Spatially Resolved Investigation of Systemic and Contact Pesticides in Plant Material by Desorption Electrospray Ionization Mass Spectrometry Imaging (DESI-MSI). Anal. Bioanal. Chem. 2015, 407, 7379–7389. [Google Scholar] [CrossRef]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, History, and Classification. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–42. ISBN 978-0-12-819304-4. [Google Scholar]
- Nayak, P.; Solanki, H. Pesticides and Indian agriculture—A review. Int. J. Res. Granthaalayah 2021, 9, 250–263. [Google Scholar] [CrossRef]
- Ran, L.; Yang, Y.; Zhou, X.; Jiang, X.; Hu, D.; Lu, P. The Enantioselective Toxicity and Oxidative Stress of Dinotefuran on Zebrafish (Danio Rerio). Ecotoxicol. Environ. Saf. 2021, 226, 112809. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L.; Cang, T.; Tang, T.; Guo, M.; Zhou, B.; Zhu, G.; Zhao, M. Toxicological Effect and Molecular Mechanism of the Chiral Neonicotinoid Dinotefuran in Honeybees. Environ. Sci. Technol. 2022, 56, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Gagneten, A.M.; Regaldo, L.; Carriquiriborde, P.; Reno, U.; Kergaravat, S.V.; Butinof, M.; Agostini, H.; Alvarez, M.; Harte, A. Atrazine Characterization: An Update on Uses, Monitoring, Effects, and Environmental Impact, for the Development of Regulatory Policies in Argentina. Integr. Environ. Assess. Manag. 2022; online ahead of print. [Google Scholar]
- Smith, L.L. Mechanism of Paraquat Toxicity in Lung and Its Relevance to Treatment. Hum. Toxicol. 1987, 6, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Q.E.; Zhou, X.; Wang, F.H.; Muurinen, J.; Virta, M.P.; Brandt, K.K.; Zhu, Y.G. Antibiotic Resistome in the Livestock and Aquaculture Industries: Status and Solutions. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2159–2196. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Li, Z.; Guo, S.; Li, K.; Xu, P.; Ok, Y.S.; Jones, D.L.; Zou, J. Antibiotics and Antibiotic Resistance Genes in Agricultural Soils: A Systematic Analysis. Crit. Rev. Environ. Sci. Technol. 2022, 1–18. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Shi, M.; Qiu, T.; Gao, M.; Tian, S.; Wang, X. Effect of Antibiotic Type and Vegetable Species on Antibiotic Accumulation in Soil-Vegetable System, Soil Microbiota, and Resistance Genes. Chemosphere 2021, 263, 128099. [Google Scholar] [CrossRef]
- Upmanyu, N.; Malviya, V.N. Antibiotics: Mechanisms of Action and Modern Challenges. In Microorganisms for Sustainable Environment and Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 367–382. ISBN 978-0-12-819001-2. [Google Scholar]
- Bull, K. Protocol to the 1979 Convention on Long-Range Transboundary Air Pollution on Persistent Organic Pollutants: The 1998 Agreement for the UNECE Region. In Persistent Organic Pollutants; Fiedler, H., Ed.; The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2003; Volume 3, pp. 1–11. ISBN 978-3-540-43728-4. [Google Scholar]
- Jeong, Y.; Lee, Y.; Park, K.J.; An, Y.-R.; Moon, H.-B. Accumulation and Time Trends (2003–2015) of Persistent Organic Pollutants (POPs) in Blubber of Finless Porpoises (Neophocaena Asiaeorientalis) from Korean Coastal Waters. J. Hazard. Mater. 2020, 385, 121598. [Google Scholar] [CrossRef]
- Chen, Y.; Zhi, D.; Zhou, Y.; Huang, A.; Wu, S.; Yao, B.; Tang, Y.; Sun, C. Electrokinetic Techniques, Their Enhancement Techniques and Composite Techniques with Other Processes for Persistent Organic Pollutants Remediation in Soil: A Review. J. Ind. Eng. Chem. 2021, 97, 163–172. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Farmers’ Behaviour in Pesticide Use: A Key Concept for Improving Environmental Safety. Curr. Opin. Environ. Sci. Health 2018, 4, 27–30. [Google Scholar] [CrossRef]
- Rother, H.-A. Pesticide Labels: Protecting Liability or Health?–Unpacking “Misuse” of Pesticides. Curr. Opin. Environ. Sci. Health 2018, 4, 10–15. [Google Scholar] [CrossRef]
- Devi, P.I.; Manjula, M.; Bhavani, R.V. Agrochemicals, Environment, and Human Health. Annu. Rev. Environ. Resour. 2022, 47, 399–421. [Google Scholar] [CrossRef]
- Sriram, A.; Kalanxhi, E.; Kapoor, G.; Craig, J.; Balasubramanian, R.; Brar, S.; Criscuolo, N.; Hamilton, A.; Klein, E.; Tseng, K.; et al. The State of the World’s Antibiotics 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers; Center for disease Dynamics, Economics &Policy: Washington, DC, USA, 2021. [Google Scholar]
- Chen, Y.; Jiang, C.; Wang, Y.; Song, R.; Tan, Y.; Yang, Y.; Zhang, Z. Sources, Environmental Fate, and Ecological Risks of Antibiotics in Sediments of Asia’s Longest River: A Whole-Basin Investigation. Environ. Sci. Technol. 2022, 56, 14439–14451. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A Global Perspective on the Use, Sales, Exposure Pathways, Occurrence, Fate and Effects of Veterinary Antibiotics (VAs) in the Environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Aravind kumar, J.; Krithiga, T.; Sathish, S.; Renita, A.A.; Prabu, D.; Lokesh, S.; Geetha, R.; Namasivayam, S.K.R.; Sillanpaa, M. Persistent Organic Pollutants in Water Resources: Fate, Occurrence, Characterization and Risk Analysis. Sci. Total Environ. 2022, 831, 154808. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Agriculture, Pesticides, Food Security and Food Safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Virág, D.; Naár, Z.; Kiss, A. Microbial Toxicity of Pesticide Derivatives Produced with UV-Photodegradation. Bull. Environ. Contam. Toxicol. 2007, 79, 356–359. [Google Scholar] [CrossRef]
- Vallotton, N.; Eggen, R.I.L.; Chèvre, N. Effect of Sequential Isoproturon Pulse Exposure on Scenedesmus Vacuolatus. Arch. Environ. Contam. Toxicol. 2009, 56, 442–449. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Zhang, J.J.; Luo, F.; Huang, M.T.; Yang, H. RNA-Sequencing Oryza Sativa Transcriptome in Response to Herbicide Isoprotruon and Characterization of Genes Involved in IPU Detoxification. RSC Adv. 2016, 6, 18852–18867. [Google Scholar] [CrossRef]
- Pascal-Lorber, S.; Alsayeda, H.; Jouanin, I.; Debrauwer, L.; Canlet, C.; Laurent, F. Metabolic Fate of [14 C]Diuron and [14 C]Linuron in Wheat (Triticum Aestivum) and Radish (Raphanus Sativus). J. Agric. Food Chem. 2010, 58, 10935–10944. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yuan, S.; Zhou, Y.; Li, X.; Duan, L.; Huang, L.; Zhou, X.; Ma, Y.; Pang, S. Microplastics Reduce the Bioaccumulation and Oxidative Stress Damage of Triazole Fungicides in Fish. Sci. Total Environ. 2022, 806, 151475. [Google Scholar] [CrossRef]
- Vela, N.; Fenoll, J.; Navarro, G.; Garrido, I.; Navarro, S. Trial of Solar Heating Methods (Solarization and Biosolarization) to Reduce Persistence of Neonicotinoid and Diamide Insecticides in a Semiarid Mediterranean Soil. Sci. Total Environ. 2017, 590–591, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in Agriculture and the Risk to Human Health: How Worried Should We Be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Litskas, V.D.; Karamanlis, X.N.; Prousali, S.P.; Koveos, D.S. The Xenobiotic Doxycycline Affects Nitrogen Transformations in Soil and Impacts Earthworms and Cultivated Plants. J. Environ. Sci. Health Part A 2019, 54, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, I.; Moreno Terrazas, E.G.; Vilca, F.Z. Application of Duckweed (Lemna sp.) and Water Fern (Azolla sp.) in the Removal of Pharmaceutical Residues in Water: State of Art Focus on Antibiotics. Sci. Total Environ. 2022, 838, 156565. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, R.; Uddin, M.; Qiao, X.; Chen, J.; Gu, G. Uptake and Metabolism of Clarithromycin and Sulfadiazine in Lettuce. Environ. Pollut. 2019, 247, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, X.; Ma, Y.; Cade-Menun, B.J. Pollutant and Soil Types Influence Effectiveness of Soil-Applied Absorbents in Reducing Rice Plant Uptake of Persistent Organic Pollutants. Pedosphere 2017, 27, 537–547. [Google Scholar] [CrossRef]
- Li, Y.; Sallach, J.B.; Zhang, W.; Boyd, S.A.; Li, H. Characterization of Plant Accumulation of Pharmaceuticals from Soils with Their Concentration in Soil Pore Water. Environ. Sci. Technol. 2022, 56, 9346–9355. [Google Scholar] [CrossRef]
- Lei, B.; Zhang, K.; An, J.; Zhang, X.; Yu, Y. Human Health Risk Assessment of Multiple Contaminants Due to Consumption of Animal-Based Foods Available in the Markets of Shanghai, China. Environ. Sci. Pollut. Res. 2015, 22, 4434–4446. [Google Scholar] [CrossRef]
- Vogt, R.; Bennett, D.; Cassady, D.; Frost, J.; Ritz, B.; Hertz-Picciotto, I. Cancer and Non-Cancer Health Effects from Food Contaminant Exposures for Children and Adults in California: A Risk Assessment. Environ. Health 2012, 11, 83. [Google Scholar] [CrossRef] [Green Version]
- Polder, A.; Savinova, T.N.; Tkachev, A.; Løken, K.B.; Odland, J.O.; Skaare, J.U. Levels and Patterns of Persistent Organic Pollutants (POPS) in Selected Food Items from Northwest Russia (1998–2002) and Implications for Dietary Exposure. Sci. Total Environ. 2010, 408, 5352–5361. [Google Scholar] [CrossRef]
- Fernandes, A.; Mortimer, D.; Rose, M.; Gem, M. Dioxins (PCDD/Fs) and PCBs in Offal: Occurrence and Dietary Exposure. Chemosphere 2010, 81, 536–540. [Google Scholar] [CrossRef]
- Schwarz, M.A.; Lindtner, O.; Blume, K.; Heinemeyer, G.; Schneider, K. Dioxin and Dl-PCB Exposure from Food: The German LExUKon Project. Food Addit. Contam. Part A 2014, 31, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.H.; Rose, G.; Odell, E.; Zhang, P.; Bui, A.; Pettigrove, V. Household Herbicide Use as a Source of Simazine Contamination in Urban Surface Waters. Environ. Pollut. 2022, 299, 118868. [Google Scholar] [CrossRef] [PubMed]
- Rosas, J.M.; Vicente, F.; Saguillo, E.G.; Santos, A.; Romero, A. Remediation of Soil Polluted with Herbicides by Fenton-like Reaction: Kinetic Model of Diuron Degradation. Appl. Catal. B Environ. 2014, 144, 252–260. [Google Scholar] [CrossRef]
- Khan, M.A.; Costa, F.B.; Fenton, O.; Jordan, P.; Fennell, C.; Mellander, P.-E. Using a Multi-Dimensional Approach for Catchment Scale Herbicide Pollution Assessments. Sci. Total Environ. 2020, 747, 141232. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, S.; Zhang, L.; Liu, R.; Deng, Y.; Nie, Y.; Zhou, Z.; Diao, J. Effects of Simazine Herbicide on a Plant-Arthropod-Lizard Tritrophic Community in Territorial Indoor Microcosms: Beyond the Toxicity. Sci. Total Environ. 2021, 781, 146723. [Google Scholar] [CrossRef]
- Yan, X.; Wang, J.; Zhu, L.; Wang, J.; Li, S.; Kim, Y.M. Oxidative Stress, Growth Inhibition, and DNA Damage in Earthworms Induced by the Combined Pollution of Typical Neonicotinoid Insecticides and Heavy Metals. Sci. Total Environ. 2021, 754, 141873. [Google Scholar] [CrossRef] [PubMed]
- Khataei, M.M.; Epi, S.B.H.; Lood, R.; Spégel, P.; Yamini, Y.; Turner, C. A Review of Green Solvent Extraction Techniques and Their Use in Antibiotic Residue Analysis. J. Pharm. Biomed. Anal. 2022, 209, 114487. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What Can We Learn from Commercial Insecticides? Efficacy, Toxicity, Environmental Impacts, and Future Developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Hu, H.; Wu, R.; Ling, J.; Yue, S.; Yang, D.; Yu, W.; Du, W.; Shen, G.; et al. Neonicotinoid Pollution in Marine Sediments of the East China Sea. Sci. Total Environ. 2022, 842, 156658. [Google Scholar] [CrossRef]
- Satapute, P.; Jogaiah, S. A Biogenic Microbial Biosurfactin That Degrades Difenoconazole Fungicide with Potential Antimicrobial and Oil Displacement Properties. Chemosphere 2022, 286, 131694. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tong, D.; Zhang, Y.; Wang, J.; Sun, H. A Simple Judgment Method for Joint Action of Antibacterial Agents on Bacterial Resistance. MethodsX 2022, 9, 101700. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, L.; Fang, L.; Yang, Y.; Chen, E.; Yuan, K.; Zou, S.; Wang, X.; Luan, T. Complex Pollution of Antibiotic Resistance Genes Due to Beta-Lactam and Aminoglycoside Use in Aquaculture Farming. Water Res. 2018, 134, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, S.; Zhang, Q.; Long, Z.; Yu, Y.; Fang, H. Fungicides Enhanced the Abundance of Antibiotic Resistance Genes in Greenhouse Soil. Environ. Pollut. 2020, 259, 113877. [Google Scholar] [CrossRef] [PubMed]
- Delis, G.A.; Siarkou, V.I.; Vingopoulou, E.I.; Koutsoviti-Papadopoulou, M.; Batzias, G.C. Pharmacodynamic Interactions of Amikacin with Selected β -Lactams and Fluoroquinolones against Canine Escherichia Coli Isolates. Res. Vet. Sci. 2018, 117, 187–195. [Google Scholar] [CrossRef]
- Buelow, E.; Ploy, M.-C.; Dagot, C. Role of Pollution on the Selection of Antibiotic Resistance and Bacterial Pathogens in the Environment. Curr. Opin. Microbiol. 2021, 64, 117–124. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, W.; Liang, B.; Han, J.; Cheng, H.; Haider, M.R.; Wang, H.; Liu, W.; Liu, S.; Wang, A. UV Photolysis as an Efficient Pretreatment Method for Antibiotics Decomposition and Their Antibacterial Activity Elimination. J. Hazard. Mater. 2020, 392, 122321. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, B.; Xu, X.; Liu, A.; Zheng, L. Distribution Characteristics, Source Analysis and Risk Assessment of Organochlorine Pesticides in Ny-Ålesund, Arctic. Mar. Pollut. Bull. 2022, 181, 113862. [Google Scholar] [CrossRef]
- Adithya, S.; Jayaraman, R.S.; Krishnan, A.; Malolan, R.; Gopinath, K.P.; Arun, J.; Kim, W.; Govarthanan, M. A Critical Review on the Formation, Fate and Degradation of the Persistent Organic Pollutant Hexachlorocyclohexane in Water Systems and Waste Streams. Chemosphere 2021, 271, 129866. [Google Scholar] [CrossRef]
- Bouzid, I.; Maire, J.; Laurent, F.; Broquaire, M.; Fatin-Rouge, N. Controlled Treatment of a High Velocity Anisotropic Aquifer Model Contaminated by Hexachlorocyclohexanes. Environ. Pollut. 2021, 268, 115678. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wang, J.; Lu, S.; Cao, Y.; Tang, C.; Yan, Z.; Zheng, L. History Traces of HCHs and DDTs by Groundwater Dating and Their Behaviours and Ecological Risk in Northeast China. Chemosphere 2020, 257, 127212. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Albanese, S.; Lima, A.; Hope, D.; Pond, P.; Fortelli, A.; Romano, N.; Cerino, P.; Pizzolante, A.; De Vivo, B. The Occurrence of OCPs, PCBs, and PAHs in the Soil, Air, and Bulk Deposition of the Naples Metropolitan Area, Southern Italy: Implications for Sources and Environmental Processes. Environ. Int. 2019, 124, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.G. Variation in Natural Attenuation Rates of Polychlorinated Biphenyls (PCBs) in Fish from Streams and Reservoirs in East Tennessee Observed over a 35-Year Period. J. Hazard. Mater. 2022, 438, 29427. [Google Scholar] [CrossRef] [PubMed]
- Hasan, G.M.M.A.; Shaikh, M.A.A.; Satter, M.A.; Hossain, M.S. Detection of Indicator Polychlorinated Biphenyls (I-PCBs) and Polycyclic Aromatic Hydrocarbons (PAHs) in Cow Milk from Selected Areas of Dhaka, Bangladesh and Potential Human Health Risks Assessment. Toxicol. Rep. 2022, 9, 1514–1522. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, G.; Yang, Y.; Chen, M.; Yang, W.; Cheng, C.; Huang, B.; Fu, Z.; Bi, X.; Zhou, Z.; et al. Measurement of the Mixing State of PAHs in Individual Particles and Its Effect on PAH Transport in Urban and Remote Areas and from Major Sources. Environ. Res. 2022, 214, 114075. [Google Scholar] [CrossRef]
- Chakravarty, P.; Chowdhury, D.; Deka, H. Ecological Risk Assessment of Priority PAHs Pollutants in Crude Oil Contaminated Soil and Its Impacts on Soil Biological Properties. J. Hazard. Mater. 2022, 437, 129325. [Google Scholar] [CrossRef]
- Lewis, S.E.; Silburn, D.M.; Kookana, R.S.; Shaw, M. Pesticide Behavior, Fate, and Effects in the Tropics: An Overview of the Current State of Knowledge. J. Agric. Food Chem. 2016, 64, 3917–3924. [Google Scholar] [CrossRef]
- Zhang, P.; Ren, C.; Sun, H.; Min, L. Sorption, Desorption and Degradation of Neonicotinoids in Four Agricultural Soils and Their Effects on Soil Microorganisms. Sci. Total Environ. 2018, 615, 59–69. [Google Scholar] [CrossRef]
- Bento, C.P.M.; Yang, X.; Gort, G.; Xue, S.; van Dam, R.; Zomer, P.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Persistence of Glyphosate and Aminomethylphosphonic Acid in Loess Soil under Different Combinations of Temperature, Soil Moisture and Light/Darkness. Sci. Total Environ. 2016, 572, 301–311. [Google Scholar] [CrossRef]
- Dores, E.F.G.C.; Spadotto, C.A.; Weber, O.L.S.; Dalla Villa, R.; Vecchiato, A.B.; Pinto, A.A. Environmental Behavior of Chlorpyrifos and Endosulfan in a Tropical Soil in Central Brazil. J. Agric. Food Chem. 2016, 64, 3942–3948. [Google Scholar] [CrossRef]
- Dolliver, H.; Gupta, S.; Noll, S. Antibiotic Degradation during Manure Composting. J. Environ. Qual. 2008, 37, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, P.A.; Kay, P.; Ashauer, R.; Boxall, A.B.A. Effects of Agricultural Conditions on the Leaching Behaviour of Veterinary Antibiotics in Soils. Chemosphere 2009, 75, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review: Antibiotic Pollution in the Environment. Clean. Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Hong, B.; Yu, S.; Zhou, M.; Li, J.; Li, Q.; Ding, J.; Lin, Q.; Lin, X.; Liu, X.; Chen, P.; et al. Sedimentary Spectrum and Potential Ecological Risks of Residual Pharmaceuticals in Relation to Sediment-Water Partitioning and Land Uses in a Watershed. Sci. Total Environ. 2022, 817, 152979. [Google Scholar] [CrossRef] [PubMed]
- Le, T.X.; Munekage, Y. Residues of Selected Antibiotics in Water and Mud from Shrimp Ponds in Mangrove Areas in Viet Nam. Mar. Pollut. Bull. 2004, 49, 922–929. [Google Scholar] [CrossRef]
- Ternes, T.A.; Herrmann, N.; Bonerz, M.; Knacker, T.; Siegrist, H.; Joss, A. A Rapid Method to Measure the Solid–Water Distribution Coefficient (Kd) for Pharmaceuticals and Musk Fragrances in Sewage Sludge. Water Res. 2004, 38, 4075–4084. [Google Scholar] [CrossRef]
- De Voogt, P.; Jansson, B. Vertical and Long-Range Transport of Persistent Organics in the Atmosphere. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Springer: New York, NY, USA, 1993; Volume 132, pp. 1–27. ISBN 978-1-4684-7067-3. [Google Scholar]
- Ashraf, M.A. Persistent Organic Pollutants (POPs): A Global Issue, a Global Challenge. Environ. Sci. Pollut. Res. 2017, 24, 4223–4227. [Google Scholar] [CrossRef]
- Tian, X.; Liu, J.; Zhou, G.; Peng, P.; Wang, X.; Wang, C. Estimation of the Annual Scavenged Amount of Polycyclic Aromatic Hydrocarbons by Forests in the Pearl River Delta of Southern China. Environ. Pollut. 2008, 156, 306–315. [Google Scholar] [CrossRef]
- Yuan, X.; Lee, J.; Han, H.; Ju, B.; Park, E.; Shin, Y.; Lee, J.; Kim, J.-H. Translocation of Residual Ethoprophos and Tricyclazole from Soil to Spinach. Appl. Biol. Chem. 2021, 64, 47. [Google Scholar] [CrossRef]
- Fujita, K.; Inui, H. How Does the Cucurbitaceae Family Take up Organic Pollutants (POPs, PAHs, and PPCPs)? Rev. Environ. Sci. Biotechnol. 2021, 20, 751–779. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant Uptake and Translocation of Contaminants of Emerging Concern in Soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Inui, H.; Wakai, T.; Gion, K.; Kim, Y.-S.; Eun, H. Differential Uptake for Dioxin-like Compounds by Zucchini Subspecies. Chemosphere 2008, 73, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Fryer, M.; Grosso, A. Plant Uptake of Non-Ionic Organic Chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.; Chang, H.; Li, Z.; Xue, J. Uptake and Translocation of Organic Pollutants in Plants: A Review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.D.; Zhu, Y.G.; Liang, Y.C.; Zhang, J.; Smith, F.A.; Yang, M. Uptake of Oxytetracycline and Its Phytotoxicity to Alfalfa (Medicago Sativa L.). Environ. Pollut. 2007, 147, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.D. A Semi-Quantitative Approach to Deriving a Model Structure for the Uptake of Organic Chemicals by Vegetation. Int. J. Phytoremediation 2008, 10, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Eggen, T.; Asp, T.N.; Grave, K.; Hormazabal, V. Uptake and Translocation of Metformin, Ciprofloxacin and Narasin in Forage- and Crop Plants. Chemosphere 2011, 85, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Desalme, D.; Binet, P.; Chiapusio, G. Challenges in Tracing the Fate and Effects of Atmospheric Polycyclic Aromatic Hydrocarbon Deposition in Vascular Plants. Environ. Sci. Technol. 2013, 47, 3967–3981. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Dong, F.; Sallach, J.B.; Wu, X.; Liu, X.; Xu, J.; Zheng, Y.; Li, Y. Uptake Kinetics and Accumulation of Pesticides in Wheat (Triticum Aestivum L.): Impact of Chemical and Plant Properties. Environ. Pollut. 2021, 275, 116637. [Google Scholar] [CrossRef]
- St-Amand, A.D.; Mayer, P.M.; Blais, J.M. Modeling PAH Uptake by Vegetation from the Air Using Field Measurements. Atmos. Environ. 2009, 43, 4283–4288. [Google Scholar] [CrossRef]
- Su, Y.; Wania, F.; Harner, T.; Lei, Y.D. Deposition of Polybrominated Diphenyl Ethers, Polychlorinated Biphenyls, and Polycyclic Aromatic Hydrocarbons to a Boreal Deciduous Forest. Environ. Sci. Technol. 2007, 41, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Yang, P.; Tian, F.; Qiao, X.; Bian, H.; Ge, L. Distribution of PAHs in Pine (Pinus Thunbergii) Needles and Soils Correlates with Their Gas-Particle Partitioning. Environ. Sci. Technol. 2009, 43, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Loftin, K.A.; Adams, C.D.; Meyer, M.T.; Surampalli, R. Effects of Ionic Strength, Temperature, and PH on Degradation of Selected Antibiotics. J. Environ. Qual. 2008, 37, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, B.; Li, H.; Ma, L.Q. Effects of PH and Ionic Strength on Sulfamethoxazole and Ciprofloxacin Transport in Saturated Porous Media. J. Contam. Hydrol. 2011, 126, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Ashraf, M.; Aksoy, A.; Ahmad, M.S.A.; Hakeem, K.R. (Eds.) Plants, Pollutants and Remediation; Springer: Dordrecht, The Netherlands, 2015; pp. 251–268. ISBN 978-94-017-7193-1. [Google Scholar]
- Chedik, L.; Bruyere, A.; Bacle, A.; Potin, S.; Le Vée, M.; Fardel, O. Interactions of Pesticides with Membrane Drug Transporters: Implications for Toxicokinetics and Toxicity. Expert Opin. Drug Metab. Toxicol. 2018, 14, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Chandler, A.M.; Basler, E.; Santelmann, P.W. Uptake and Translocation of Alachlor in Soybean and Wheat. Weed Sci. 1974, 22, 253–258. [Google Scholar] [CrossRef]
- Su, Y.-H.; Zhu, Y.-G. Transport Mechanisms for the Uptake of Organic Compounds by Rice (Oryza Sativa) Roots. Environ. Pollut. 2007, 148, 94–100. [Google Scholar] [CrossRef]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root Uptake of Pharmaceuticals and Personal Care Product Ingredients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef]
- Xi, J.; Xu, P.; Xiang, C.-B. Loss of AtPDR11, a Plasma Membrane-Localized ABC Transporter, Confers Paraquat Tolerance in Arabidopsis Thaliana: A Paraquat Transporter in Arabidopsis. Plant J. 2012, 69, 782–791. [Google Scholar] [CrossRef]
- Wang, J.; Lin, H.; Sun, W.; Xia, Y.; Ma, J.; Fu, J.; Zhang, Z.; Wu, H.; Qian, M. Variations in the Fate and Biological Effects of Sulfamethoxazole, Norfloxacin and Doxycycline in Different Vegetable–Soil Systems Following Manure Application. J. Hazard. Mater. 2016, 304, 49–57. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, J.; Zhou, C.; Ma, L.; Chen, X.; Li, Y.; Sun, X.; Yan, X.; Geng, R.; Wan, Q.; et al. Uptake Kinetics and Subcellular Distribution of Three Classes of Typical Pesticides in Rice Plants. Sci. Total Environ. 2022, 858, 159826. [Google Scholar] [CrossRef]
- Lin, H.; Tao, S.; Zuo, Q.; Coveney, R.M. Uptake of Polycyclic Aromatic Hydrocarbons by Maize Plants. Environ. Pollut. 2007, 148, 614–619. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Yu, S.; He, S.; Cao, D.; Yao, S.; Fang, H.; Yu, Y. Chemical Factors Affecting Uptake and Translocation of Six Pesticides in Soil by Maize (Zea Mays L.). J. Hazard. Mater. 2021, 405, 124269. [Google Scholar] [CrossRef]
- Fan, T.; Chen, X.; Xu, Z.; Liu, L.; Shen, D.; Dong, S.; Zhang, Q. Uptake and Translocation of Triflumezopyrim in Rice Plants. J. Agric. Food Chem. 2020, 68, 7086–7092. [Google Scholar] [CrossRef]
- Benvidi, A.; Dadras, A.; Abbasi, S.; Tezerjani, M.D.; Rezaeinasab, M.; Tabaraki, R.; Namazian, M. Experimental and Computational Study of the p K a of Coumaric Acid Derivatives. J. Chin. Chem. Soc. 2019, 66, 589–593. [Google Scholar] [CrossRef]
- Christou, A.; Papadavid, G.; Dalias, P.; Fotopoulos, V.; Michael, C.; Bayona, J.M.; Piña, B.; Fatta-Kassinos, D. Ranking of Crop Plants According to Their Potential to Uptake and Accumulate Contaminants of Emerging Concern. Environ. Res. 2019, 170, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cui, K.; Yan, H.; Li, Y.; Chai, Y.; Liu, X.; Cheng, J.; Yu, X. Uptake and Translocation of Imidacloprid, Thiamethoxam and Difenoconazole in Rice Plants. Environ. Pollut. 2017, 226, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.; Collins, C.D. (Eds.) Organic Xenobiotics and Plants: From Mode of Action to Ecophysiology; Plant Ecophysiology; Springer: Dordrecht, The Netherlands, 2011; Volume 8, pp. 125–148. ISBN 978-90-481-9851-1. [Google Scholar]
- Coleman, J.; Blake-Kalff, M.; Davies, E. Detoxification of Xenobiotics by Plants: Chemical Modification and Vacuolar Compartmentation. Trends Plant Sci. 1997, 2, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Kreuz, K.; Martinoia, E. Herbicide Metabolism in Plants: Integrated Pathways of Detoxification. In Pesticide Chemistry and Bioscience; Elsevier: Amsterdam, The Netherlands, 1999; pp. 279–287. ISBN 978-1-85573-810-2. [Google Scholar]
- Morant, M.; Bak, S.; Møller, B.L.; Werck-Reichhart, D. Plant Cytochromes P450: Tools for Pharmacology, Plant Protection and Phytoremediation. Curr. Opin. Biotechnol. 2003, 14, 151–162. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, X.; Ren, T. Expression of a Wheat Cytochrome P450 Monooxygenase CDNA in Yeast Catalyzes the Metabolism of Sulfonylurea Herbicides. Pestic. Biochem. Physiol. 2006, 85, 1–6. [Google Scholar] [CrossRef]
- Chu, W.K.; Wong, M.H.; Zhang, J. Accumulation, Distribution and Transformation of DDT and PCBs by Phragmites Australis and Oryza Sativa L.: II. Enzyme Study. Environ. Geochem. Health 2006, 28, 169–181. [Google Scholar] [CrossRef]
- Li, R.N.; Wang, H.L. Herbicide-induced passivation mechanism by plants N-dealkylation reaction. World Pestic. 1982, 5, 50–52. [Google Scholar]
- Woo, H.; Lee, J.; Park, D.; Jung, E. Protective Effect of Mulberry (Morus Alba L.) Extract against Benzo[a]Pyrene Induced Skin Damage through Inhibition of Aryl Hydrocarbon Receptor Signaling. J. Agric. Food Chem. 2017, 65, 10925–10932. [Google Scholar] [CrossRef]
- Lu, Y.C.; Luo, F.; Pu, Z.J.; Zhang, S.; Huang, M.T.; Yang, H. Enhanced Detoxification and Degradation of Herbicide Atrazine by a Group of O -Methyltransferases in Rice. Chemosphere 2016, 165, 487–496. [Google Scholar] [CrossRef]
- Liscombe, D.K.; Louie, G.V.; Noel, J.P. Architectures, Mechanisms and Molecular Evolution of Natural Product Methyltransferases. Nat. Prod. Rep. 2012, 29, 1238. [Google Scholar] [CrossRef]
- Rezek, J.; Macek, T.; Mackova, M.; Triska, J.; Ruzickova, K. Hydroxy-PCBs, Methoxy-PCBs and Hydroxy-Methoxy-PCBs: Metabolites of Polychlorinated Biphenyls Formed In Vitro by Tobacco Cells. Environ. Sci. Technol. 2008, 42, 5746–5751. [Google Scholar] [CrossRef]
- Bauer, A.; Luetjohann, J.; Rohn, S.; Jantzen, E.; Kuballa, J. Development of a Suspect Screening Strategy for Pesticide Metabolites in Fruit and Vegetables by UPLC-Q-Tof-MS. Food Anal. Methods 2018, 11, 1591–1607. [Google Scholar] [CrossRef]
- Dudley, S.; Sun, C.; Jiang, J.; Gan, J. Metabolism of Sulfamethoxazole in Arabidopsis Thaliana Cells and Cucumber Seedlings. Environ. Pollut. 2018, 242, 1748–1757. [Google Scholar] [CrossRef]
- Butler, J.M.; Groeger, A.W.; Fletcher, J.S. Characterization of Monochlorinated Biphenyl Products Formed by Paul’s Scarlet Rose Cells. Bull. Environ. Contam. Toxicol. 1992, 49, 821–826. [Google Scholar] [CrossRef]
- Levsen, K.; Schiebel, H.-M.; Behnke, B.; Dötzer, R.; Dreher, W.; Elend, M.; Thiele, H. Structure Elucidation of Phase II Metabolites by Tandem Mass Spectrometry: An Overview. J. Chromatogr. A 2005, 1067, 55–72. [Google Scholar] [CrossRef]
- Lu, F.F.; Liu, J.T.; Zhang, N.; Chen, Z.J.; Yang, H. OsPAL as a Key Salicylic Acid Synthetic Component Is a Critical Factor Involved in Mediation of Isoproturon Degradation in a Paddy Crop. J. Clean. Prod. 2020, 262, 121476. [Google Scholar] [CrossRef]
- Chen, C.-H. Xenobiotic Metabolic Enzymes: Bioactivation and Antioxidant Defense; Springer International Publishing: Cham, Switzerland, 2020; pp. 87–106. ISBN 978-3-030-41678-2. [Google Scholar]
- Ahmad, M.Z.; Li, P.; Wang, J.; Rehman, N.U.; Zhao, J. Isoflavone Malonyltransferases GmIMaT1 and GmIMaT3 Differently Modify Isoflavone Glucosides in Soybean (Glycine Max) under Various Stresses. Front. Plant Sci. 2017, 8, 735. [Google Scholar] [CrossRef]
- Kóňa, J.; Fabian, W.M.F. Hybrid QM/MM Calculations on the First Redox Step of the Catalytic Cycle of Bovine Glutathione Peroxidase GPX1. J. Chem. Theory Comput. 2011, 7, 2610–2616. [Google Scholar] [CrossRef] [PubMed]
- Pivato, M.; Fabrega-Prats, M.; Masi, A. Low-Molecular-Weight Thiols in Plants: Functional and Analytical Implications. Arch. Biochem. Biophys. 2014, 560, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Su, X.N.; Zhang, J.J.; Liu, J.T.; Zhang, N.; Ma, L.Y.; Lu, F.F.; Chen, Z.J.; Shi, Z.; Si, W.J.; Liu, C.; et al. Biodegrading Two Pesticide Residues in Paddy Plants and the Environment by a Genetically Engineered Approach. J. Agric. Food Chem. 2019, 67, 4947–4957. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xu, J.Y.; Lu, F.F.; Jin, S.F.; Yang, H. Detoxification of Atrazine by Low Molecular Weight Thiols in Alfalfa (Medicago Sativa). Chem. Res. Toxicol. 2017, 30, 1835–1846. [Google Scholar] [CrossRef]
- Farkas, M.H.; Berry, J.O.; Aga, D.S. Chlortetracycline Detoxification in Maize via Induction of Glutathione S -Transferases after Antibiotic Exposure. Environ. Sci. Technol. 2007, 41, 1450–1456. [Google Scholar] [CrossRef]
- Knights, K.M.; Sykes, M.J.; Miners, J.O. Amino Acid Conjugation: Contribution to the Metabolism and Toxicity of Xenobiotic Carboxylic Acids. Expert Opin. Drug Metab. Toxicol. 2007, 3, 159–168. [Google Scholar] [CrossRef]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) Transporters: Roles in Xenobiotic Detoxification and Bt Insecticidal Activity. IJMS 2019, 20, 2829. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC Transporters. Arab. Book 2011, 9, e0153. [Google Scholar] [CrossRef] [Green Version]
- Lespine, A.; Martin, S.; Dupuy, J.; Roulet, A.; Pineau, T.; Orlowski, S.; Alvinerie, M. Interaction of Macrocyclic Lactones with P-Glycoprotein: Structure–Affinity Relationship. Eur. J. Pharm. Sci. 2007, 30, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Ye, X.; Osburn, L.D.; Stewart, C.N.; Cheng, Z.-M. Transgenic Hybrid Aspen Overexpressing the Atwbc19 Gene Encoding an ATP-Binding Cassette Transporter Confers Resistance to Four Aminoglycoside Antibiotics. Plant Cell Rep. 2010, 29, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Yamaji, N.; Mitani, N.; Yano, M.; Nagamura, Y.; Ma, J.F. A Bacterial-Type ABC Transporter Is Involved in Aluminum Tolerance in Rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Weber, H. Fatty Acid-Derived Signals in Plants. Trends Plant Sci. 2002, 7, 217–224. [Google Scholar] [CrossRef]
- Ohkawa, H.; Inui, H. Metabolism of Agrochemicals and Related Environmental Chemicals Based on Cytochrome P450s in Mammals and Plants. Pest Manag. Sci. 2015, 71, 824–828. [Google Scholar] [CrossRef]
- Kato, S.; Yokota, Y.; Suzuki, R.; Fujisawa, Y.; Sayama, T.; Kaga, A.; Anai, T.; Komatsu, K.; Oki, N.; Kikuchi, A.; et al. Identification of a Cytochrome P450 Hydroxylase, CYP81E22, as a Causative Gene for the High Sensitivity of Soybean to Herbicide Bentazon. Theor. Appl. Genet. 2020, 133, 2105–2115. [Google Scholar] [CrossRef]
- Jin, M.; Chen, L.; Deng, X.W.; Tang, X. Development of herbicide resistance genes and their application in rice. Crop J. 2022, 10, 26–35. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, Y.; Yuan, Y.; Qin, Y.; Zhang, C.; Wang, X.; Feng, S.; Lu, Y. Advances in the Agro-Environment Migration of Organic Chemical Pollutants and Their Biotransformation in Crops. Agronomy 2022, 12, 3009. https://doi.org/10.3390/agronomy12123009
Hua Y, Yuan Y, Qin Y, Zhang C, Wang X, Feng S, Lu Y. Advances in the Agro-Environment Migration of Organic Chemical Pollutants and Their Biotransformation in Crops. Agronomy. 2022; 12(12):3009. https://doi.org/10.3390/agronomy12123009
Chicago/Turabian StyleHua, Yifei, Yi Yuan, Yi Qin, Chenyi Zhang, Xiaodong Wang, Shengjun Feng, and Yichen Lu. 2022. "Advances in the Agro-Environment Migration of Organic Chemical Pollutants and Their Biotransformation in Crops" Agronomy 12, no. 12: 3009. https://doi.org/10.3390/agronomy12123009
APA StyleHua, Y., Yuan, Y., Qin, Y., Zhang, C., Wang, X., Feng, S., & Lu, Y. (2022). Advances in the Agro-Environment Migration of Organic Chemical Pollutants and Their Biotransformation in Crops. Agronomy, 12(12), 3009. https://doi.org/10.3390/agronomy12123009







