1. Introduction
Sustainable agricultural growth requires that several significant and mounting environmental challenges be addressed, namely soil erosion, nutrient runoff, and pollinator decline [
1,
2,
3,
4]. Current production systems use intensive tillage practices and leave topsoil exposed throughout the winter months, facilitating increased rates of erosion and nutrient runoff [
5]. These problems have gained widespread attention in Minnesota, where it is estimated that up to 45% of cropland is experiencing unsustainable levels of soil erosion and a majority of water nutrient pollution is linked to agricultural sources [
6,
7]. These problems contribute to algal blooms in surface waters, and contamination of public drinking water supplies as nutrients leach into the groundwater aquifers [
3,
5,
8,
9,
10]. Pollinator populations have also declined significantly due to habitat loss and pesticide use associated with annual monoculture cropping systems, which are generally not capable of sustaining pollinator populations due to their limited flowering period [
2,
4,
11,
12,
13].
One proposed solution to these problems is increasing the amount of year-round cover on agricultural land through cover crops or perennial cropping systems [
14,
15]. This challenge has been the focus of the University of Minnesota Forever Green Initiative (FGI) for over a decade [
16]. The FGI brings together academic, industry, and legislative partners to advance the development of winter cover crops, as well as perennial or biennial species capable of overwintering in Minnesota [
16]. The core philosophy of the FGI is that all crops should provide economic benefits to the farmer, as well as ecosystem services that relieve the environmental impacts of agriculture. Perennial grain crops bred by FGI researchers, such as intermediate wheatgrass (
Thinopyrum intermedium (Host) Barkworth & D.R. Dewey subsp.
intermedium), have demonstrated increased rates of nutrient capture over a two-year period, proving that this is an effective method of reducing levels of agricultural pollutants to Minnesota waterways [
14,
17,
18,
19].
Domesticated annual flax (
L. usitatissimum L.), also known as common flax or linseed, is one of the oldest cultivated plants and was domesticated in the fertile crescent ~8000 B.C.E [
20,
21]. Throughout history, flax has been highly valued as a multi-use crop for fiber, feed, and industrial applications [
20]. Wild relatives of annual flax are being evaluated for domestication as a new perennial crop. Although wild species of
Linum L. have less total oil content, most share the unique oil profile of annual flax that is high in ω-3 fatty acids [
22,
23,
24]. The health benefits associated with consuming ω-3 fatty acids are expected to drive increased demand for flaxseed [
20,
23,
25]. Therefore, one of the primary objectives of FGI perennial flax breeding is to develop perennial OS flax with added ecosystem services, e.g., reduced soil erosion and water quality improvement. In a unique collaboration among agronomy and horticulture breeders, the FGI perennial flax breeding program is simultaneously pursuing both OS and ornamental breeding objectives [
26,
27]. Current breeding efforts are being facilitated using ideotype approaches [
27].
The genus
Linum contains ~180–200 species distributed throughout temperate and subtropical regions of the world [
21,
28,
29]. The genus can generally be divided into two major clades—a blue-flowered and a yellow-flowered clade [
21]. Most of the perennial flax species being evaluated by the FGI breeding program are from the section
Adenolinum (commonly called the
L. perenne group) within the blue-flowered clade. This section of the genus includes Eurasian species such as
L. perenne L. and
L. austriacum L., and North American
L. lewisii Pursh [
21,
30]. The blue-flowered clade also contains section
Linum, which encompasses notable species such as common flax (
L. usitatissimum) and its progenitor
L. bienne Mill. (=
L. angustifolium Huds.) [
21,
29].
The reproductive structures of flax are all quinquepartite: five sepals, five petals, five pistils and stamens, and a five chambered seed pod, or capsule. Each chamber of the seed pod can have up to two seeds, for a maximum of ten, although the average for cultivated flax is ~6 seeds per capsule [
31,
32]. Unlike most crops, shattering or capsular dehiscence is not completely fixed in domesticated flax. More northern (Canadian) types typically dehisce slightly at the apex, although not enough for seed shatter, which helps the seeds to resist weathering and disease by allowing excess moisture to escape the capsule [
33]. There is variation within the genus for all reproductive traits, including seed size and color, and degree of capsule opening [
22].
Perennial crop development will require significant decades-long investments in breeding to improve agronomically relevant traits [
34]. The FGI perennial flax program is evaluating natural variation in a diverse panel of
Linum spp.; meanwhile, a parallel effort maintained by the United States Department of Agriculture (USDA) is pursuing domestication of the North American
L. lewisii [
24]. These efforts are enhanced by the recent genome assembly of
L. lewisii developed in a joint effort between the USDA, University of Colorado—Boulder, and North Dakota State University, which will enable future developments in molecular breeding tools [
35]. These efforts to improve germplasm will be critical for realizing the goal of large-scale perennial flax production systems, as early agronomic trials have demonstrated significant yield losses due to shattering and weed competition [
36]. Thus, collaborations between agronomists, geneticists, breeders must be founded on quality perennial flax germplasm in order to achieve a viable perennial flax crop.
The primary objectives of this research were (1) to compare OS ideotype trait values among wild accessions and breeding populations which had undergone 1–5 yr. of selection in a common garden; (2) identify top candidate species for future breeding as well as significant phenotypes which might be introgressed into existing breeding populations; (3) evaluate changes in postharvest trait values during years 1–3 (Y1–3) of growth. Traits recorded, based on perennial flax OS ideotypes, included: yield per plant (Y1–3, 2019–2021), 1000 seed weight (Y1–2, 2019–2020), capsule diameter (Y1, 2019), number of seeds per capsule (Y1–2, 2019–2020), and percent germination (Y1, 2019). Trait data differed by year due to the COVID-19 pandemic, which severely limited the number of people allowed in the greenhouse and field trial sites at any given time. We hypothesize that the OS, as well as CF selection populations will possess mean trait values exceeding the species accessions for the OS traits under selection. It is expected that yield will be greater in Y2, but that no significant differences will be observed for 1000 seed weight and/or capsule traits.
2. Materials and Methods
2.1. Germplasm
We have published the species populations used to create the initial breeding lines and subsequent perennial flax selections during 2005–2008 by the breeding program [
37]. In fall 2018, selections for OS and CF traits were made from a 2018 elite restart nursery and a nursery of open-pollinated
L. austriacum,
L. lewisii, and
L. perenne. Additional accessions of wild perennial flax for this experiment were obtained from the Germplasm Resources Information Network (GRIN) of the USDA-ARS, Plant Gene Resources of Canada (GRIN-CA), the Kew Millennium Seed Bank, and additional commercial sources. In total, 137 accessions were studied of
L. alatum Ledeb. ex Juz.,
L. altaicum Ledeb. ex Juz.,
L. aristatum Engelm.,
L. austriacum,
L. baicalense Juz.,
L. bienne Mill.,
L. decumbens Desf.,
L. flavum L.,
L. grandiflorum Desf.,
L. hirsutum L.,
L. hudsonoides Planch.,
L. leonii F.W.Schultz,
L. lewisii, L. narbonense L.,
L. pallescens Bunge,
L. perenne,
L. stelleroides Planch,
L. strictum L.,
L. sulcatum Riddell,
L. virgultorum Boiss. & Heldr. ex Planch, and
L. viscosum L. (
Supplemental Table S1). Eight accessions of domesticated annual flax,
L. usitatissimum, were included as check lines in Y1 (
Supplemental Table S1).
The ‘Selections—OS’ population consists of 25 genotypes selected in 2018 for OS traits, primarily yield and seed size (1000 seed wt.) (
Supplemental Table S1). The population ‘Selections—CF’ contains of 17 selections made in 2018 for the CF ideotype [
27], based on growth habit, stem length, and flower diameter. Within both populations, the top 9–10 parent genotypes were propagated as vegetative cuttings from field plants in fall 2018. Ten stem tip cuttings per genotype (>5 cm length) were harvested from the crown, labeled, sealed in bags [1.2 mL Get Reddi
® Sandwich Bags, United States Plastic Corporation], and put into a cooler on ice for transport to MN Ag. Exp. Station Plant Growth Facility, University of Minnesota (44°59′17.8″ N, −93°10′51.6″ W) before rooting. Cuttings were trimmed to 5–7 cm length using a sterile razor [GEM Carbon Steel Extra Sharp Single Edge Blade, The Razor Blade Co., Van Nuys, CA, USA], the lower leaves removed, and the cut stem base dipped into 1000 ppm Indole-3-butyric Acid (IBA), after which cuttings were inserted into pre-moistened foam propagation strips [ROOTCUBES
® PLUS WEDGE
®, Oasis Grower Solutions, Kent, OH, USA]. Cuttings were rooted for 5 weeks in a glass mist house (21/21 °C, day/night, 16 h; 600–2200 h lighting with high pressure sodium high intensity discharge lamps or HIDs at a minimum set point of 150 μmol m
−2 s
−1 at plant level). An intermittent mist system, at a mist frequency of 10 min intervals (mist nozzles, reverse osmosis water) during 600–2200 h with a 7 s duration was used. After rooting, cuttings were transplanted into 10.12 cm square deep pots [SVD-355-DEEP-BK-40, T.O. Plastics, Clearwater, MN, USA] filled with a soilless medium [Promix Mycorrhizae, Premier Horticulture Inc., Quakertown, PA, USA] and grown in a glass greenhouse at 16.7/15.5 °C day/night daily integral and a 16 h photoperiod (600–2200 h; long days). Supplemental lighting was supplied during winter months and cloudy days by 400 w high pressure sodium high intensity discharge (HPS-HID) lamps, at a minimum of 150 μmol m
−2 s
−1 at plant level, with an 16 h photoperiod. These were grown as stock plants and then for cuttings in spring 2019. The open-pollinated (OP) seed from these clonal genotypes was also planted for evaluation in 2019 if ≥59 seeds were available, in addition to the other selections.
2.2. Establishment of Common Garden Nursery
In Y1 (spring 2019), all OS selections (25), CF selections (17), and species accessions (137) were grown in a common garden nursery in Rosemount, MN to compare phenotypic traits of interest within the same common garden environment (
Figure 1A). Accessions were sown in 288 plug trays [Landmark Plastic, Akron, OH, USA] with soilless germination media [Berger BM2 Germination Mix, Berger, Saint-Modeste, QC, Canada] and covered with fine vermiculite [Palmetto Vermiculite Medium A-2, Palmetto Vermiculite, Woodruff, SC, USA] in weeks 14 and 15 (5 and 12 April 2019). Due to the limited quantity of seed, four accessions (59 seeds each) were planted by hand in each 288 plug tray, leaving an empty row between accessions to prevent contamination. For all breeding populations, n ≤ 288 seeds/genotype were sown using a vacuum seeder [E-Z Seeder, E-Z Seeder, Inc., WI, USA]. All plug trays were placed in a mist house for 4 h to moisten the soilless medium using an intermittent mist system (St. Paul MN Plant Growth Facility, University of Minnesota; 44°59′17.8″ N, −93°10′51.6″ W) at a mist frequency of 10 min intervals (mist nozzles, reverse osmosis water) during 600–2200 h with a 7 s duration (21/21 °C, day/night, 16 h; 600–2200 h) with lighting supplied by high pressure sodium high intensity discharge (HID) lamps at a minimum set point of 150 μmol m
−2 s
−1. Once watered in, the trays were covered with plastic dome lids [Super Sprouter Standard Vented Humidity Dome 7″, Hawthorne Gardening Company, Vancouver, WA, USA] and transferred to a walk-in cooler for 2 weeks at 4/4 °C day/night in darkness to break seed dormancy (cold stratification), which is recommended for most wild
Linum species (K. Betts, personal communication, 2018; Barbara Atkins, STA laboratories, Longmont, CO, USA). Trays were uncovered and misted by hand, as needed, over this 2 week period to maintain adequate moisture levels in the soilless medium. After the 2 week stratification, the dome lids were removed, and the trays were returned to the mist house for an additional 3 weeks. During this 5 week (total) germination period, the number of seeds germinated per week was recorded using different colored toothpicks inserted into the media [
38,
39]. Plug trays were then moved onto capillary mats in a greenhouse at 16.7/15.5 °C day/night daily integral and a 16 h photoperiod (600–2200 h; long days) on weeks 19 and 20 (10 and 17 May 2019). Supplemental lighting was supplied during cloudy days by 400 w high pressure sodium high intensity discharge (HPS-HID) lamps, at a minimum of 150 μmol m
−2 s
−1 at plant level. Fertigation (Mondays-Fridays) provided nutrients at a constant liquid feed (CLF) rate of 125 ppm N from water soluble 20-10-20 fertilizer. Accessions remained in the greenhouse until transplanting in week 24 (Y1).
During week 15 and 16 (Y1), 100 vegetative cuttings from the top CF and OS selections (indicated by ‘clone’;
Supplemental Table S1) were harvested to bulk up these genotypes for field trials. The propagation protocol was identical to the one outlined above, except that cuttings were sourced from greenhouse stock plants. In week 21 the rooted cuttings were moved to the identical greenhouse as the seedlings with the same fertigation regime; these also remained in the greenhouse until transplanting in week 24 (Y1).
The common garden nursery was located at the Rosemount Research and Outreach Center, Rosemount, MN (44°42′58.2″ N, −93°5′54.9″ W). Accessions and seed propagated selections were randomized. In total, 20 seedlings of each accession and/or ten rooted cuttings per genotype were transplanted, with selection for early germination among genotypes within accessions. Planting spacing was 45.7 cm on center (OC) within rows with 1.83 m row widths (
Figure 1B). The field was irrigated post-planting with 2.54 cm water. Irrigation continued throughout the summer to maintain a minimum of 2.54 cm water per week when there was insufficient rainfall. Weed control consisted of weekly mechanical tillage between rows, pre-emergent herbicide applications [Fortress
®, OHP Inc., Bluffton, SC, USA] at the recommended rates, and bi-weekly hand weeding within rows.
2.3. Postharvest Traits
Perennial flax requires an extended growing season to mature and set seed following establishment, so the Y1 plants with mature seed pods were harvested once on a per plant basis in weeks 43–45. In Y2–Y3, however, flax plants flower and set seed much earlier, and in many cases yield a second harvest which matures very late in the fall. Harvest 1 occurred in midsummer (~weeks 28–29), followed by harvest 2 in late fall (~weeks 44–46). Plants were clipped at the base using pruners, then placed into labeled harvest bags for transport to St. Paul, MN facilities, where they were dried for 5 d at 32.2 °C and moved into dry, room temperature (~21 °C, day/night) storage. During Y2–Y3 due to the COVID-19 pandemic, each accession was bulk harvested into a single bag, rather than on a per-plant basis. The number of plants in each bulk was recorded for yield estimates (g) on an averaged per plant basis, which also accounted for changes to the total number of plants per genotype due to winter or summer mortality.
Before each sample was cleaned, the capsule diameter (mm) and number of seeds per capsule were recorded for five randomly selected mature capsules/genotype. Samples were cleaned using a belt thresher [hand fabricated at the UMN; D. Vellekson, 2019, pers. comm.], followed by a fractionating aspirator [CFZ1 and CFZ2 Fractionating Aspirator Test Models, Carter Day International Inc., Minneapolis, MN]. If additional cleaning was required, sieves of various sizes were used before picking out the remaining chaff by hand. Once cleaned, the total weight of the sample was recorded to measure total seed yield (g) on a per-plant basis.
A seed counter [DATA Count S-JR, DATA Detection Technologies Ltd., Jerusalem, Israel] was used to subsample 1000 seeds and the 1000 seed wt. (g) was recorded. If a sample had <1000 seeds total, the total number was recorded and used to calculate an estimated 1000 seed weight with the equation:
The validity and rigor of this statistical estimate (methodology) was tested for 1000 seed wt. for populations and genotypes and their respective interactions.
Due to constraints of the COVID-19 pandemic, the recording of capsule diameter (mm) was dropped Y2–Y3 of the study. Additionally, in Y3, 1000 seed wt. (g) and average number of seeds per capsule (SPC) were dropped from the study.
2.4. Ornamental Traits
Each individual plant in the nursery was monitored weekly for flowering (≥1 open flower) [
40]. Flowering data collection began in weeks 27 through 43, or 17 weeks total. The total number of weeks in flower was used to compare the flowering periods. Transplant survival was also recorded on a per-plant basis in week 43. Winter survival was recorded the following spring in week 19 (2020). Other plant measurements recorded during the growing seasons [
27,
40] included plant height (cm) measured from the soil surface to the highest point of the plant; plant width 1 (cm; measure at the widest point of the plant), plant width 2 (cm; measured at a 90° angle from width 1); height:width ratio was calculated as:
the semi-ellipsoid volume (cm
3) represents the most accurate plant shape metric and was determined using the formula:
the eccentricity of an ellipse (
e) quantifies plant shape, being calculated as:
circumference (cm) was determined using:
the base area of the ellipse (cm
2) is based on the formula:
stem length (cm) was the distance from the plant crown to stem apex; stem diameter (mm) was measured 30 cm basipetally from the stem apex; flower diameter (mm).
2.4.1. Statistical Analysis (Y1 Data)
Initially, separate two-way analysis of variances (ANOVAs) were used to compare 1000 seed weight methodologies (actual vs. estimated) and whether there were population × methodology or genotype × methodology interactions. Once the validity of the 1000 seed weight estimate was confirmed, the effect of population and genotype factors on yield per plant, 1000 seed weight (estimate), number of seeds per capsule, and capsule diameter were analyzed using independent, one-way ANOVAs and mean separations (5% Tukey’s honestly significant difference, HSD) using the Statistical Package for Social Sciences (SPSS, v. 25 for Windows, SPSS, Inc., Chicago, IL, USA). For population comparison analysis, data were pooled by population. There was large variability in the sample size of each population due to constraints in seed availability, seed germination, stand establishment and/or rooting of OS and CF clonal selections. To maintain statistical power, any species accession or selection with
n < 10 observations was dropped from the analysis. For analysis of genotypic differences, the chosen cutoff was n = 3 observations per genotype, which captured the majority of genotypes tested. Pearson correlations and descriptive statistics were calculated using SPSS to compare all traits analyzed by ANOVA. Correlations with the ornamental traits ([
40]; see
Section 2.4 above]) were also calculated using SPSS.
2.4.2. Statistical Analysis (Y1–Y3 Data)
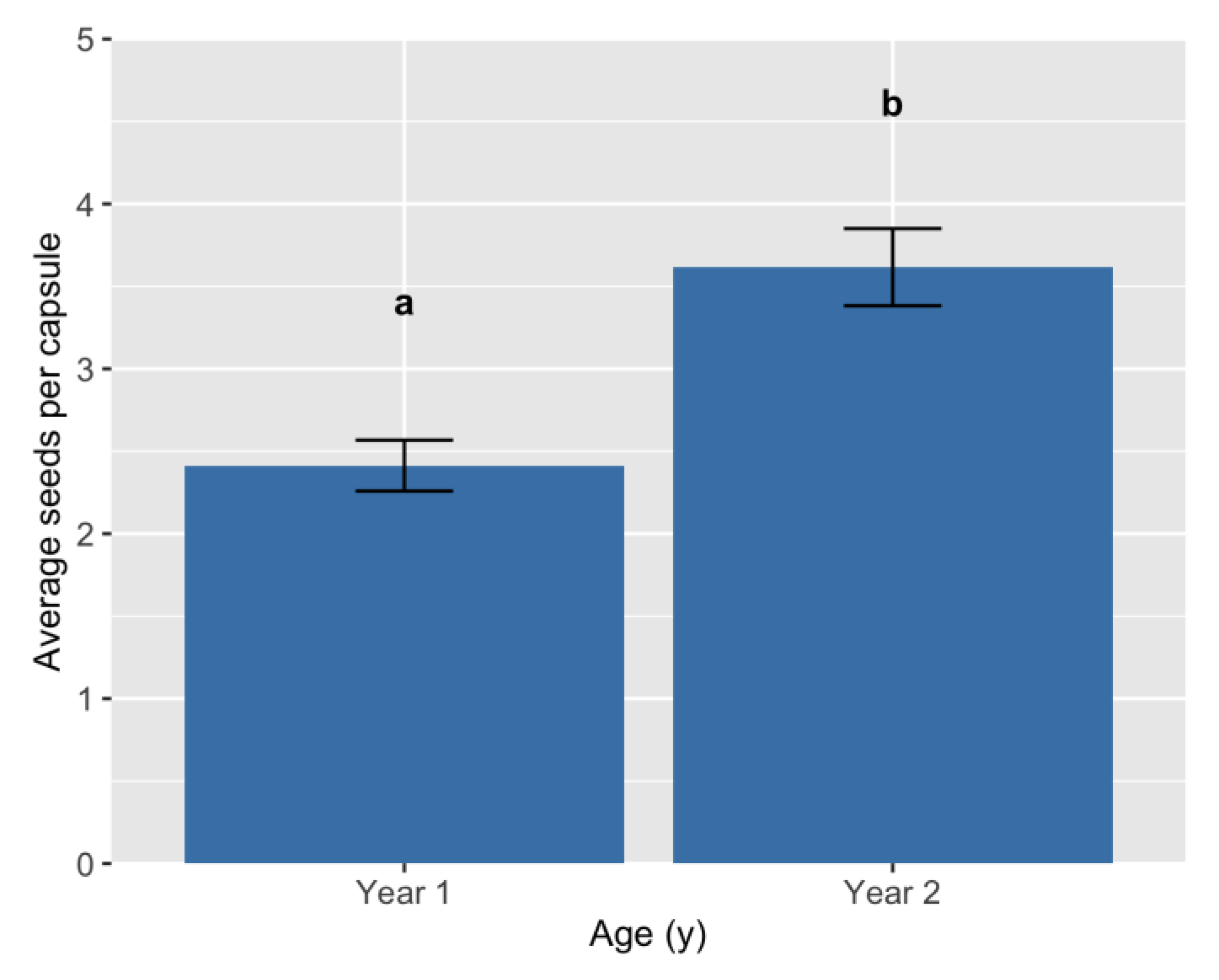
Analysis of multi-year comparisons required a different approach due to the modifications to the harvest method in Y2–Y3, namely bulk harvesting of the accessions (all plants bagged together) as opposed to the per-plant harvest conducted in Y1. This eliminated the possibility of conducting genotypic comparisons for Y2–Y3 data, as variance could not be calculated within genotypes. Thus, multi-year comparisons are a separate set of analyses from those conducted in Y1. First, one-way ANOVA and mean separations (5% Tukey’s HSD) were used to evaluate trait differences based on the factors of Age (A; Y1–Y3), population (P), and their interaction. For all analyses, genotypes were dropped if the number of plants harvested was n < 3 individuals and populations were dropped if they contained n < 5 total genotypes. The average yield per plant (g) was evaluated for all years (Y1–Y3), and the first and second harvest entries were combined, when applicable, so that the average yield per plant (g) represented the total for that year. This was achieved using the formula [(Harvest 1 total yield (g) + Harvest 2 total yield (g)/(Harvest 1 number of plants)], such that plants which yielded a second harvest were not double counted, which would reductionally bias the final value. The traits 1000 seed wt. (g) and average number of seeds per capsule (SPC) were evaluated for Y1–Y2 only. ANOVAs and trait correlations were calculated using the Statistical Package for Social Sciences (SPSS, v. 25 for Windows, SPSS, Inc., Chicago, IL, USA). Additional correlations were calculated separately for each population using SPSS.
T-tests were calculated using Microsoft® Excel (Version 16.76, © 2023 Microsoft, Redmond, WA, USA) to compare the average per-plant yield (g) for harvests 1 and 2 in Y2–Y3. First, Y1 data was removed, as was any genotype with <3 plants harvested. Then, any genotype that did not have two harvests within a single growth year was removed. The resulting dataset contained 45 total ‘genotype × year’ entries. The Excel add-in ‘Analysis ToolPak’ was used to first conduct a two-sample F-test to check for equal variances. The result was highly significant; thus, a two-sample t-test assuming unequal variances was conducted. Following this, the data was divided by population and the analysis repeated. All F-tests were highly significant. Thus, two-sample t-tests assuming unequal variances were used for each comparison. The mean and standard error were also calculated for each ‘population × harvest number’ group using Excel. Lastly, separate correlations for harvests 1, 2, and pooled harvest data were calculated using the Statistical Package for Social Sciences (SPSS, v. 25 for Windows, SPSS, Inc., Chicago, IL, USA). For pooled harvest data, if multiple harvest entries were present for 1000 seed weight. and SPC in Y2, an average was taken to arrive at a single value for each genotype before running correlations.
4. Implications for Breeding: Oilseed Potential of Wild Species
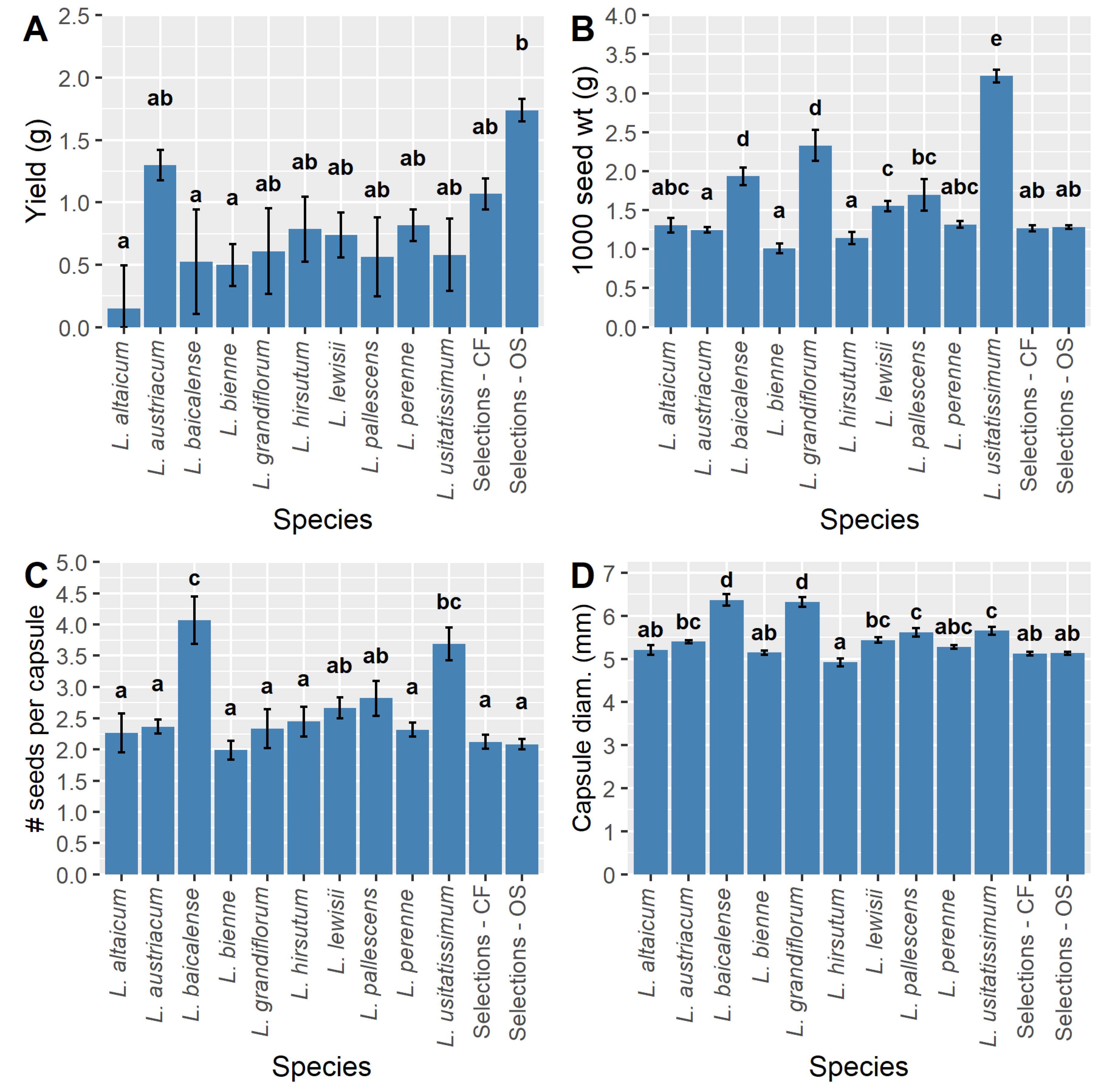
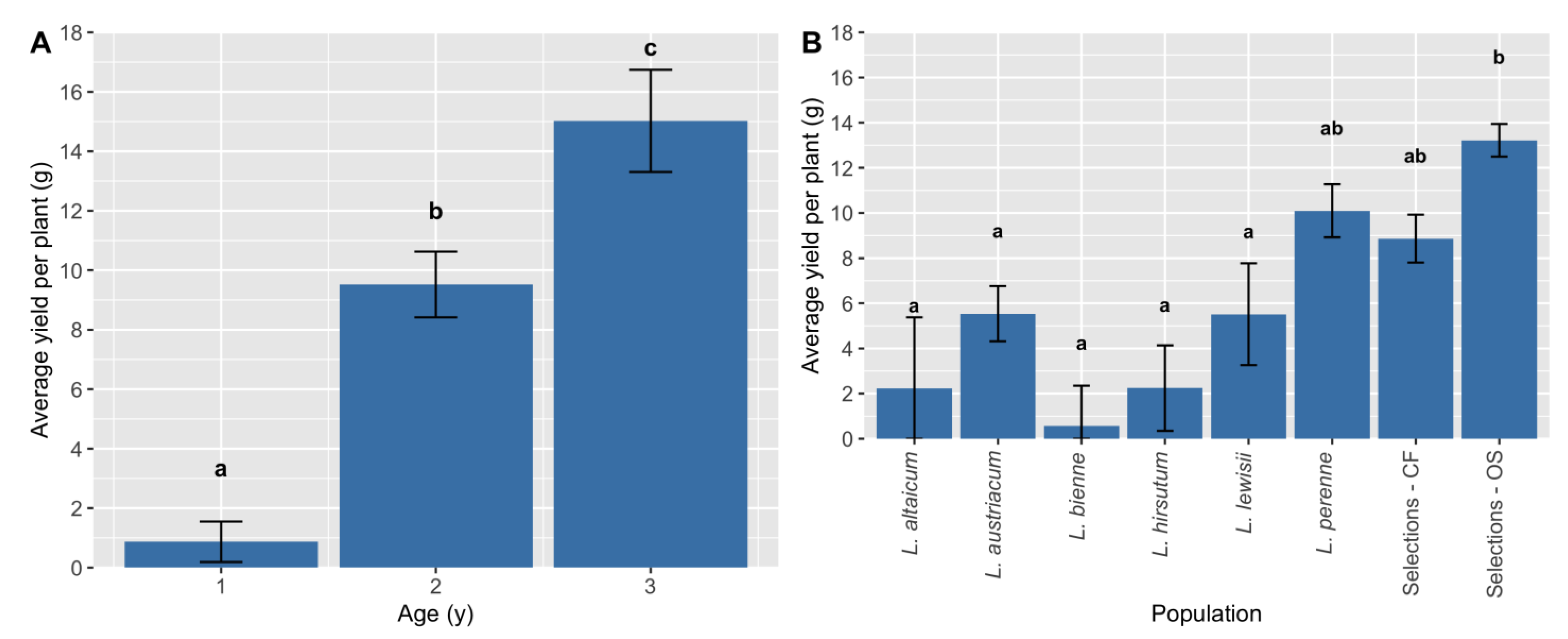
One of the primary objectives of this study was to determine the most promising wild species for future breeding efforts. Among the wild species tested,
L. austriacum first appeared to be an ideal candidate for OS selection overall, as it has high mean Y1 yield per plant (g;
Figure 2A) and is reported to have high oil content [
23]. However, Y1–3 data demonstrated that
L. perenne has higher yield potential in Y2–3 compared to
L. austriacum (
Figure 3B), and is reported to have a comparable oil content [
22].
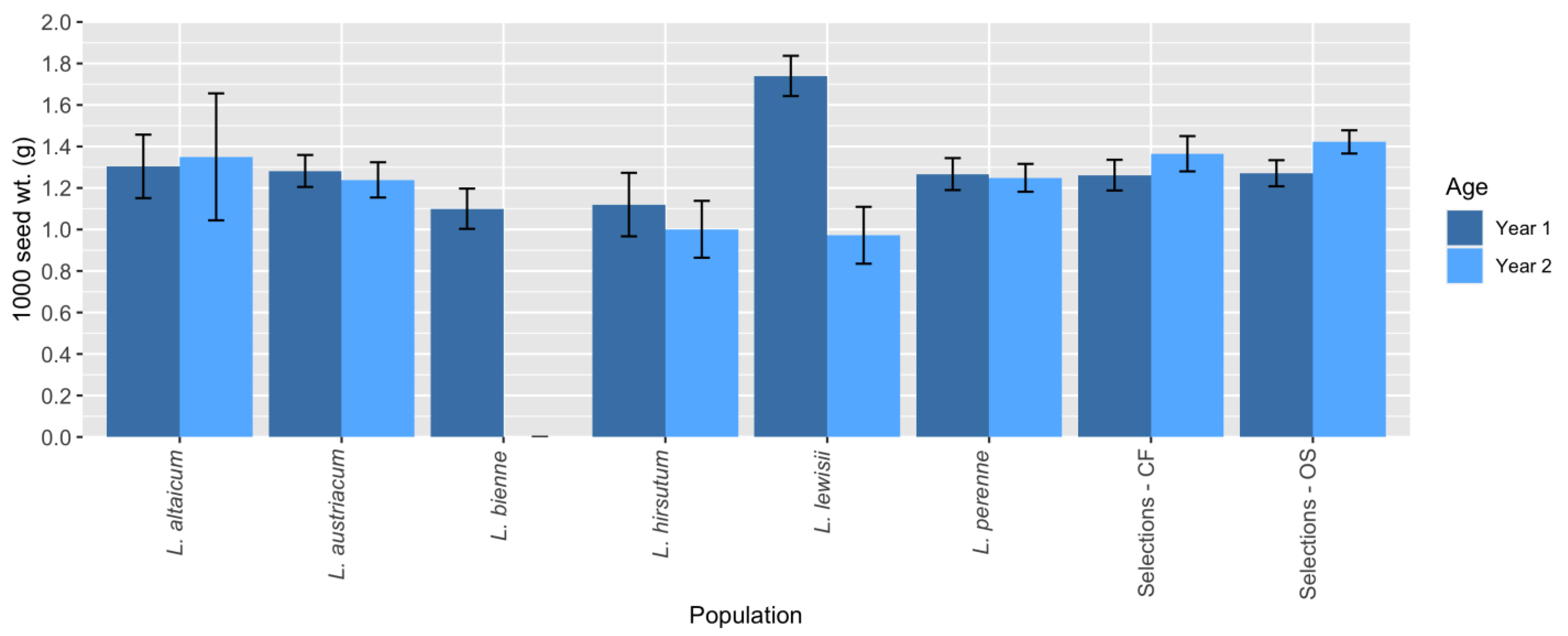
Linum lewisii, however, had the highest 1000 seed weights overall, despite the fact that it expressed low stability for this trait (
Figure 4). For the purposes of trait introgression,
L. baicalense appeared very promising in Y1, as it had high seed weight and low shattering compared to the other species (
Figure 2B,C). However, the small size and short flowering period of
L. baicalense [
40] suggests that the germplasm would have little value otherwise once traits are introgressed into progeny, if that proves to be possible. Similarly, but to a lesser extent, the same is true for
L. pallescens, which has relatively large seeds and low shattering (
Figure 2B,C). Unlike
L. baicalense,
L. pallescens grew to moderate size and had the longest stems of any population tested [
40]. Arguably, there is more value to be gained by attempting wide crosses between OS breeding lines and
L. pallescens. Finally, although
L. baicalense had a higher seed germination rate compared to
L. pallescens, the latter species had higher summer and winter survival [
40], suggesting that it is better suited to growing conditions in Minnesota. Altogether, future evaluations of wild flax species for OS potential should focus efforts on
L. austriacum, L. perenne, and
L. lewisii while attempting wide interspecific crosses with
L. pallescens and/or
L. baicalense to introgress shattering resistance.
5. Conclusions
In the generations of domestication of OS and CF perennial flax to date, simultaneous selection for multiple crop ideotypes has been achieved [
40]. The most important outcome of the early stage domestication is increased vigor through adaptation to the local environment. Tork et al. [
40] conducted an ornamental phenotypic trait analysis in the same common garden using the herbaceous perennial flax crop ideotype for the identical germplasm (OS and CF selections, wild
Linum species) tested herein. Selection for OS and CF traits within the same population produced differential outcomes, depending on the trait. Of the phenotypic traits studied for the CF ideotype, i.e., flower diameter, flowering period, stem length and diameter, plant width and height, summer and winter survival, several illustrate the need for directive selection of germplasm depending on the desired crop ideotype. For example, both OS and CF selections had longer flowering periods, larger plant size, and more uniform growth than wild species [
40]. While longer flowering periods are highly desirable in the herbaceous perennial and cut flower flax crop ideotypes [
27,
40] it would be undesirable for the OS ideotype since this would delay seed set and maturation, as well as harvest 1 which could eliminate the possibility of harvest 2 in northern latitudes due to the onset of winter. Larger flower diameters would be irrelevant for the OS ideotype creating an unnecessary carbohydrate sink, provided smaller flower diameters continue to attract pollinators. Additionally, plant vigor in OS types would be limited to achieving upright, strong-stemmed selections without side branching at the crown to facilitate ease of mechanical harvesting [
27].
Selection for higher yield in OS types has already had an initial effect. The mean yield per plant increase observed for the OS selections, relative to the CF selections and wild species [
40], meant that only the OS selections outperformed all wild species for Y1 yield. Thus, while the efficiency of breeding for multiple traits in perennial flax simultaneously has been successful [
26,
40], directive selection within each crop ideotype needs increased focus in subsequent breeding and selection efforts.
In general, there is still considerable similarity among OS and CF selection populations, suggesting that simultaneous selection for differing crop ideotypes should continue for at least several more generations (
Figure 1B). In these early stages of selection, multiple ideotypes can be selected out of the same diverse pool of germplasm, which will conserve time, resources, and prevent genetic bottlenecks from occurring early in the domestication program [
26]. The majority of this study focused on the opportunity to improve Y1 phenotypes. This was partly due to the interruptions caused by COVID-19, which prevented the same detailed phenotyping of Y1 from occurring in Y2–Y3. However, it was also noted at the start of the project that plants generally performed well in Y2, and that Y1 vigor was the area most in need of improvement [
50]. Therefore, the greatest challenge is to achieve acceptable stand establishment and yield in Y1. Since these tested species and selections were transplanted in spring, possible direct seed sowing in the previous fall season may enhance earlier growth rates in Y1 and increase Y1 yield [
36]. This is especially true of the OS selections, which will need to be capable of establishing from direct sowing in the fall or the spring. The quantity of seed generated between calendar Y1–Y3 might prove sufficient to begin answering some of the associated agronomic questions, such as determining the optimal row spacing, planting time, and planting density. For future breeders of perennial flax, the greatest opportunities and questions involve examining trait changes across Y1–Y3 of growth, determining the genetic basis for traits of interest, and finding effective ways of working around the high level of genetic diversity inherent in obligate outcrossing taxa. The vast and varied opportunities for utilizing perennial flax make this a prime target for continued crop domestication efforts [
26].