Changes in Rhizosphere Soil Nutrients, Enzyme Activities, and Microbial Communities at Different Stages of Industrial Hemp Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Experiment
2.3. Soil Sample Determination
2.3.1. Physical and Chemical Properties of Soil
2.3.2. Soil Enzyme Activities
2.3.3. Count of Bacteria, Fungi, and Actinomycetes in Soil
2.3.4. DNA Extraction, PCR, and Sequencing of the 16S and ITS rRNA Gene
2.4. Statistical Analyses
3. Results
3.1. Soil Properties at Different Growth Stages of Industrial Hemp
3.2. Soil Enzyme Activities at Different Growth Stages of Industrial Hemp
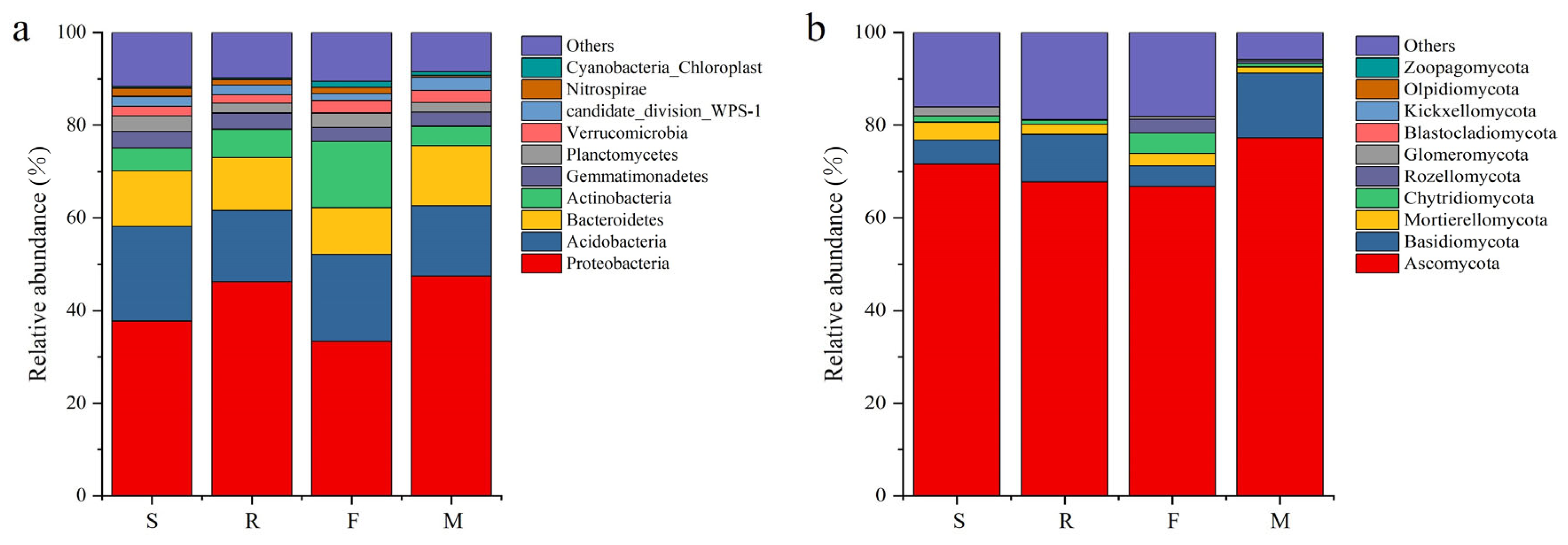
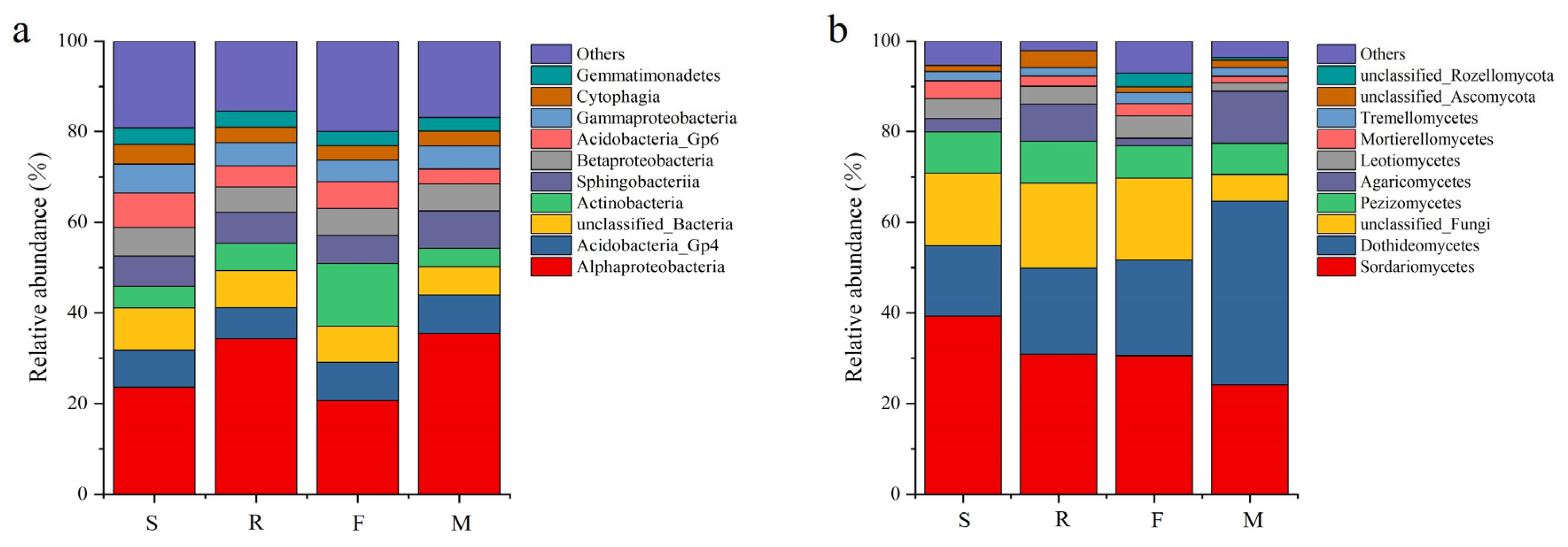
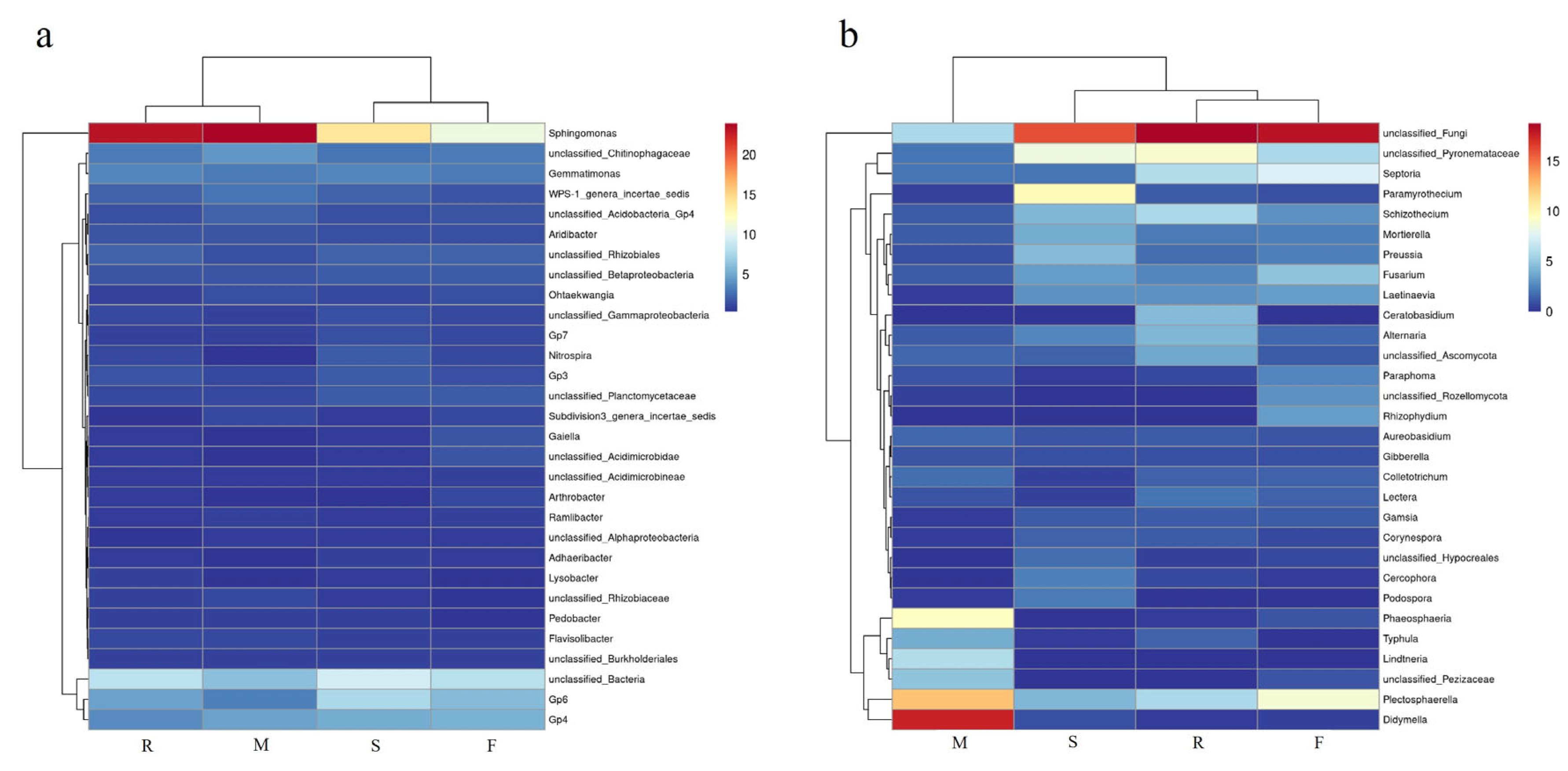
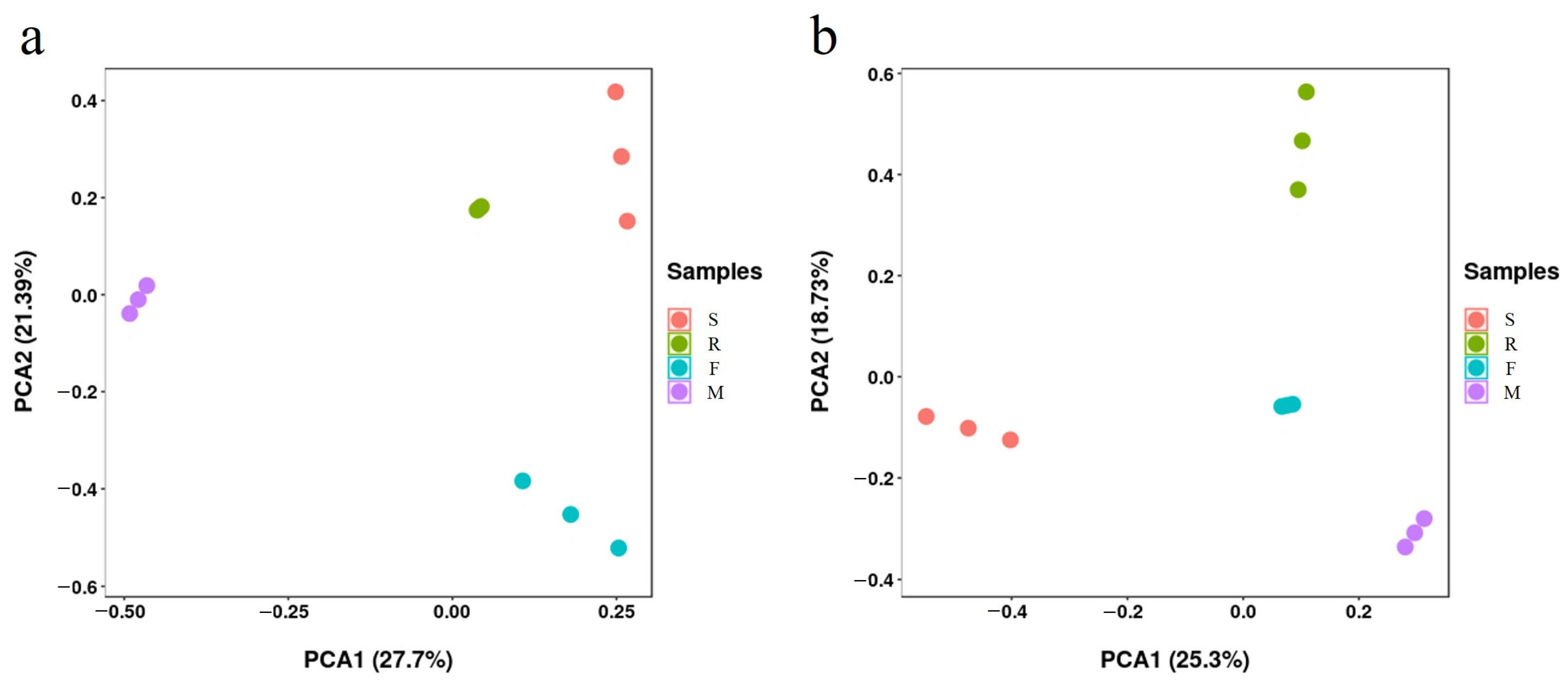
3.3. Soil Microbial Quantity and Community at Different Growth Stages of Industrial Hemp
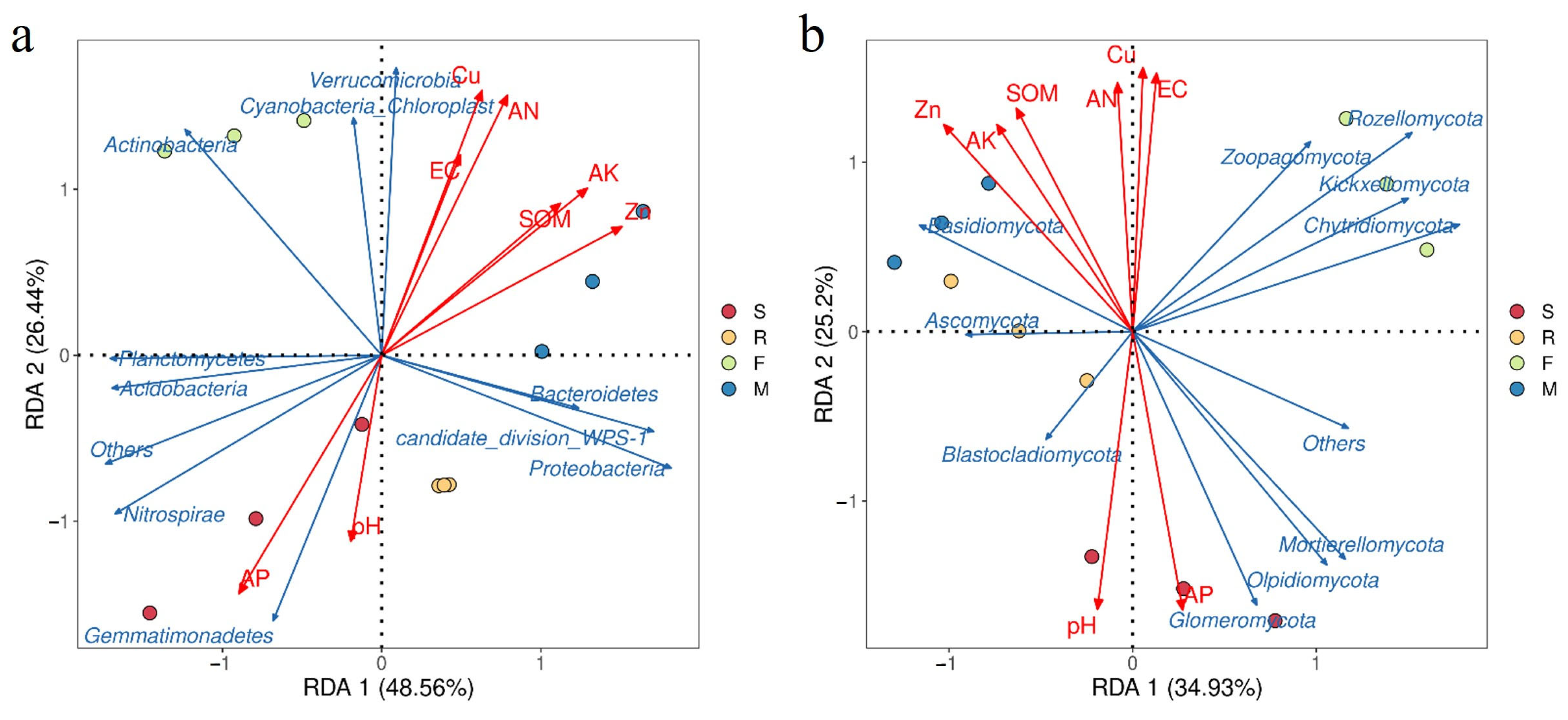
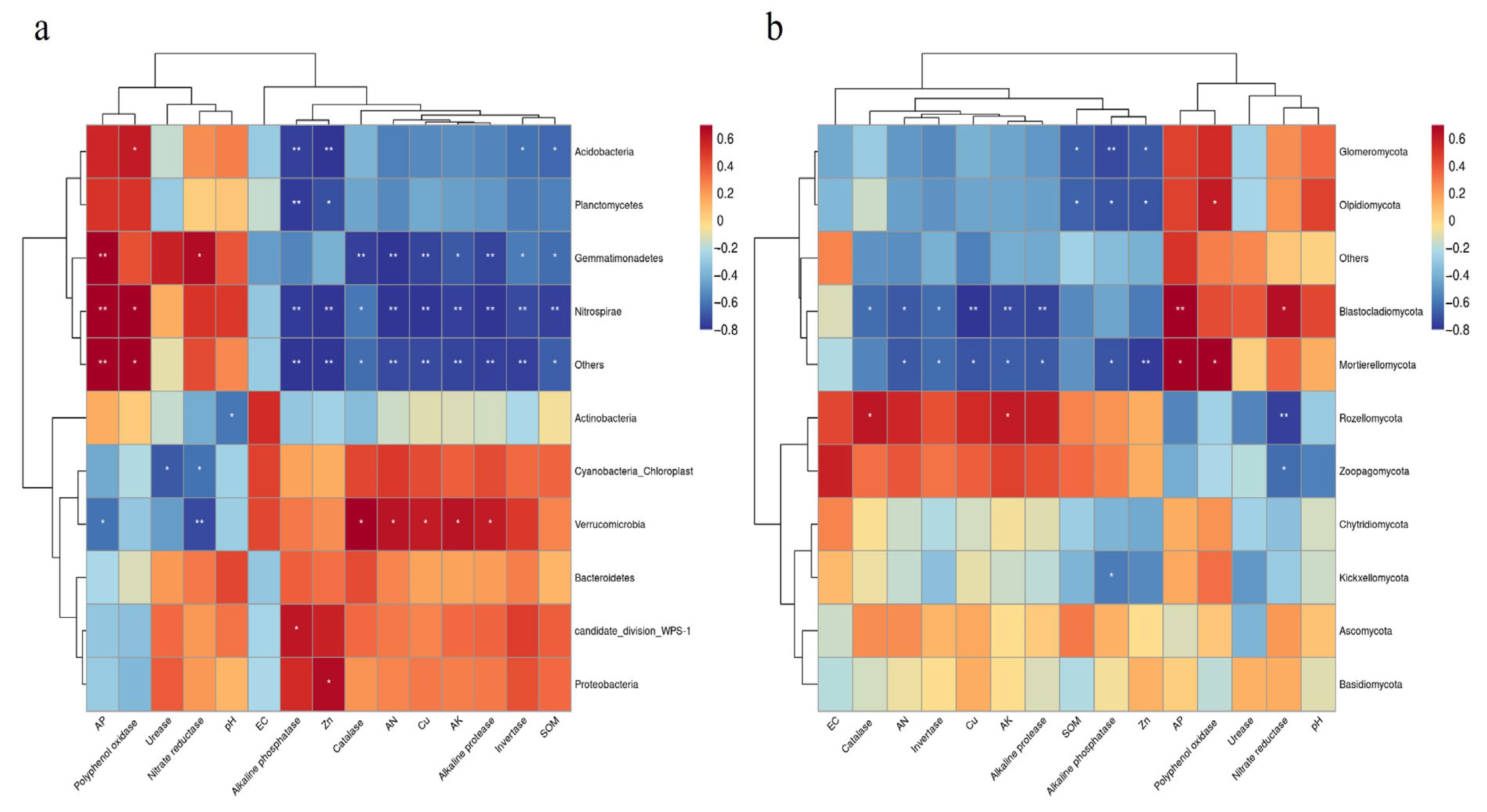
3.4. Impact of Soil Factors on Bacterial and Fungal Communities
4. Discussion
4.1. Responses of Soil Nutrients and Enzyme Activities to Different Growth Stages of Industrial Hemp
4.2. Different Growth Stages Altered the Rhizosphere Soil Microbial Community Composition of Industrial Hemp
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of Cannabinoids in Flowers of Industrial Hemp (Cannabis sativa L.): A Pilot Study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef] [PubMed]
- Cherney, J.H.; Small, E. Industrial Hemp in North America: Production, Politics and Potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.P.; et al. Medical Cannabis and Industrial Hemp Tissue Culture: Present Status and Future Potential. Front. Plant Sci. 2021, 12, 627240. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Maghirang, E.; Tan, J.W.; Yoon, J.Y.; Armstrong, P.; Kachroo, P.; Hildebrand, D. NIR Spectroscopy for Rapid Measurement of Moisture and Cannabinoid Contents of Industrial Hemp (Cannabis sativa). Ind. Crops Prod. 2022, 184, 115007. [Google Scholar] [CrossRef]
- Wylie, S.E.; Ristvey, A.G.; Fiorellino, N.M. Fertility Management for Industrial Hemp Production: Current Knowledge and Future Research Needs. GCB Bioenergy 2021, 13, 517–524. [Google Scholar] [CrossRef]
- Emmett, B.D.; Buckley, D.H.; Drinkwater, L.E. Plant Growth Rate and Nitrogen Uptake Shape Rhizosphere Bacterial Community Composition and Activity in an Agricultural Field. New Phytol. 2020, 225, 960–973. [Google Scholar] [CrossRef]
- Gan, D.; Feng, J.; Han, M.; Zeng, H.; Zhu, B. Rhizosphere Effects of Woody Plants on Soil Biogeochemical Processes: A Meta-Analysis. Soil Biol. Biochem. 2021, 160, 108310. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Y.; Shen, M.; Dong, S.; Zhang, F.; Gao, Q.; He, P.; Shen, G.; Yang, J.; Wang, Z.; et al. Soil Conditioner Affects Tobacco Rhizosphere Soil Microecology. Microb. Ecol. 2022, 1–14. [Google Scholar] [CrossRef]
- Herre, M.; Heitkötter, J.; Heinze, S.; Rethemeyer, J.; Preusser, S.; Kandeler, E.; Marschner, B. Differences in Organic Matter Properties and Microbial Activity between Bulk and Rhizosphere Soil from the Top- and Subsoils of Three Forest Stands. Geoderma 2022, 409, 115589. [Google Scholar] [CrossRef]
- Feng, H.; Fu, R.; Hou, X.; Lv, Y.; Zhang, N.; Liu, Y.; Xu, Z.; Miao, Y.; Krell, T.; Shen, Q.; et al. Chemotaxis of Beneficial Rhizobacteria to Root Exudates: The First Step towards Root–Microbe Rhizosphere Interactions. Int. J. Mol. Sci. 2021, 22, 6655. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Zhai, L.; Zhang, B.; Peng, X.; Liu, X.; Zhang, J. Effects of Mineral-Solubilizing Microorganisms on Root Growth, Soil Nutrient Content, and Enzyme Activities in the Rhizosphere Soil of Robinia Pseudoacacia. Forests 2021, 12, 60. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in Soil Bacterial and Fungal Community Composition and Functional Groups during the Succession of Boreal Forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Tian, P.; Razavi, B.S.; Zhang, X.; Wang, Q.; Blagodatskaya, E. Microbial Growth and Enzyme Kinetics in Rhizosphere Hotspots Are Modulated by Soil Organics and Nutrient Availability. Soil Biol. Biochem. 2020, 141, 107662. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Shen, Z.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Pre-Colonization of PGPR Triggers Rhizosphere Microbiota Succession Associated with Crop Yield Enhancement. Plant Soil 2019, 439, 553–567. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A Review of Root Exudates and Rhizosphere Microbiome for Crop Production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Banerjee, S.; Dini-Andreote, F.; Xu, Y.; Shen, Q.; Jousset, A.; Wei, Z. Small Changes in Rhizosphere Microbiome Composition Predict Disease Outcomes Earlier than Pathogen Density Variations. ISME J. 2022, 16, 2448–2456. [Google Scholar] [CrossRef]
- Gu, S.; Wei, Z.; Shao, Z.; Friman, V.-P.; Cao, K.; Yang, T.; Kramer, J.; Wang, X.; Li, M.; Mei, X.; et al. Competition for Iron Drives Phytopathogen Control by Natural Rhizosphere Microbiomes. Nat. Microbiol. 2020, 5, 1002–1010. [Google Scholar] [CrossRef]
- Tang, L.; Fan, C.; Yuan, H.; Wu, G.; Sun, J.; Zhang, S. The Effect of Rotational Cropping of Industrial Hemp (Cannabis sativa L.) on Rhizosphere Soil Microbial Communities. Agronomy 2022, 12, 2293. [Google Scholar] [CrossRef]
- Guo, L.; Chen, X.; Li, Z.; Wang, M.; Che, Y.; Zhang, L.; Jiang, Z.; Jie, S. Effects of Continuous Cropping on Bacterial Community and Diversity in Rhizosphere Soil of Industrial Hemp: A Five-Year Experiment. Diversity 2022, 14, 250. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; An, Z.; Du, J.; Zhang, X.; Chang, S.X. Tree Species Identity and Mixing Ratio Affected the Release of Several Metallic Elements from Mixed Litter in Coniferous-Broadleaf Plantations in Subtropical China. Sci. Total Environ. 2022, 838, 156143. [Google Scholar] [CrossRef] [PubMed]
- Taye, Z.M.; Helgason, B.L.; Bell, J.K.; Norris, C.E.; Vail, S.; Robinson, S.J.; Parkin, I.A.P.; Arcand, M.; Mamet, S.; Links, M.G.; et al. Core and Differentially Abundant Bacterial Taxa in the Rhizosphere of Field Grown Brassica Napus Genotypes: Implications for Canola Breeding. Front. Microbiol. 2020, 10, 3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Natalia Umaña, M.; Li, W.; Fang, M.; Chen, Y.; Lu, H.; Yu, S. Linking Soil Nutrients and Traits to Seedling Growth: A Test of the Plant Economics Spectrum. For. Ecol. Manag. 2022, 505, 119941. [Google Scholar] [CrossRef]
- Huo, C.; Lu, J.; Yin, L.; Wang, P.; Cheng, W. Coupled of Carbon and Nitrogen Mineralization in Rhizosphere Soils along a Temperate Forest Altitudinal Gradient. Plant Soil 2022, 1–15. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000; pp. 355–356. [Google Scholar]
- Jiang, J.; Yang, Y.; Wang, L.; Cao, S.; Long, T.; Liu, R. Effects of Chlorothalonil Application on the Physio-Biochemical Properties and Microbial Community of a Yellow–Brown Loam Soil. Agriculture 2022, 12, 608. [Google Scholar] [CrossRef]
- Hou, W.; Wang, J.; Nan, Z.; Christensen, M.J.; Xia, C.; Chen, T.; Zhang, Z.; Niu, X. Epichloë Gansuensis Endophyte-Infection Alters Soil Enzymes Activity and Soil Nutrients at Different Growth Stages of Achnatherum Inebrians. Plant Soil 2020, 455, 227–240. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root Exudates Drive Soil-Microbe-Nutrient Feedbacks in Response to Plant Growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Han, L.; Li, C.; Xiao, B.; Wang, H.; Yang, C. Extensive Secretion of Phenolic Acids and Fatty Acids Facilitates Rhizosphere PH Regulation in Halophyte Puccinellia Tenuiflora under Alkali Stress. Physiol. Plant. 2022, 174, e13678. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z. Afforestation Drives Soil Carbon and Nitrogen Changes in China. Land Degrad. Dev. 2017, 28, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Ren, L.; Zhang, X.-C. Effect of Biochar on the Soil Nutrients about Different Grasslands in the Loess Plateau. Catena 2016, 137, 554–562. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, X.; Zhou, B.; Lei, L.; Xiao, W. Variations in Rhizosphere Soil Total Phosphorus and Bioavailable Phosphorus with Respect to the Stand Age in Pinus Massoniana Lamb. Front. Plant Sci. 2022, 13, 939683. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jin, Z.; Wang, X.; George, T.S.; Feng, G.; Zhang, L. Simulated Root Exudates Stimulate the Abundance of Saccharimonadales to Improve the Alkaline Phosphatase Activity in Maize Rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]
- Gomola, C.E.; McKay, J.K.; Wallenstein, M.D.; Wagg, C.; O’Brien, M.J. Within-Species Trade-Offs in Plant-Stimulated Soil Enzyme Activity and Growth, Flowering, and Seed Size. Ecol. Evol. 2018, 8, 11717–11724. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, G.; Xue, S.; Song, Z. Rhizosphere Soil Microbial Activity under Different Vegetation Types on the Loess Plateau, China. Geoderma 2011, 161, 115–125. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; He, H. Effects of Rehabilitation through Afforestation on Soil Aggregate Stability and Aggregate-Associated Carbon after Forest Fires in Subtropical China. Geoderma 2020, 376, 114548. [Google Scholar] [CrossRef]
- Hanaka, A.; Plak, A.; Zagórski, P.; Ozimek, E.; Rysiak, A.; Majewska, M.; Jaroszuk-Ściseł, J. Relationships between the Properties of Spitsbergen Soil, Number and Biodiversity of Rhizosphere Microorganisms, and Heavy Metal Concentration in Selected Plant Species. Plant Soil 2019, 436, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zuo, J.; Zhang, H.; Zu, M.; Liu, S. The Chinese Medicinal Plants Rhizosphere: Metabolites, Microorganisms, and Interaction. Rhizosphere 2022, 22, 100540. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, M.; Zhao, Q.; Wang, F.; Gao, J.; Sheng, H.; An, L. Complete Genome Sequence of Sphingomonas Sp. Cra20, a Drought Resistant and Plant Growth Promoting Rhizobacteria. Genomics 2020, 112, 3648–3657. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Lee, S.-H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and Composition of Soil Acidobacteria and Proteobacteria Communities as a Bacterial Indicator of Past Land-Use Change from Forest to Farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Cunha, A.; Figueiredo, S.; Faustino, P.; Patinha, C.; Silva, H.; Sierra-Garcia, I.N. The Root Microbiome of Salicornia Ramosissima as a Seedbank for Plant-Growth Promoting Halotolerant Bacteria. Appl. Sci. 2021, 11, 2233. [Google Scholar] [CrossRef]
- Górska, E.B.; Stępien, W.; Cunha, A.; Sierra-Garcia, I.N.; Szyszkowska, K.; Gozdowski, D.; Gworek, B.; Sas-Paszt, L.; Lisek, A.; Hewelke, E.; et al. Microbial Diversity as an Indicator of a Diversified Cropping System for Luvisoils in a Moderate Climate. Case Study—Long Term Experiments from Poland. Ecol. Indic. 2022, 141, 109133. [Google Scholar] [CrossRef]
- You, Y.; Aho, K.; Lohse, K.A.; Schwabedissen, S.G.; Ledbetter, R.N.; Magnuson, T.S. Biological Soil Crust Bacterial Communities Vary Along Climatic and Shrub Cover Gradients Within a Sagebrush Steppe Ecosystem. Front. Microbiol. 2021, 12, 569791. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Wang, L.; Wang, T.; Jiao, Z.J.; Pang, F.H.; Tao, A.L.; Niu, X.R.; Lu, W.H. First Report of Didymella Leaf Blight on Cornus Officinalis Caused by Didymella Glomerata in China. Plant Dis. 2017, 102, 1031. [Google Scholar] [CrossRef]
- Carson, M.L.; Stuber, C.W.; Senior, M.L. Quantitative Trait Loci Conditioning Resistance to Phaeosphaeria Leaf Spot of Maize Caused by Phaeosphaeria Maydis. Plant Dis. 2005, 89, 571–574. [Google Scholar] [CrossRef]
| Growing Stage | Sampling Time | Growth Status |
|---|---|---|
| SES | 7 June | All seeds sprouted with an average height of 10 cm. |
| RGS | 12 July | The seedling production speed is fast, and the average plant height is 100 cm. |
| FLS | 8 August | More than 50% of male plants in the plots are in full bloom. |
| MAS | 28 September | More than 50% of the seeds in the plots are mature. |
| Growing Stage | SOM (g/kg) | Cu (mg/kg) | Zn (mg/kg) | pH | EC (ms/cm) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| SES | 23.95 ± 0.35 b | 9.13 ± 0.29 a | 21.24 ± 0.39 c | 8.55 ± 0.01 a | 198.20 ± 0.7 b | 132.80 ± 1.28 d | 16.51 ± 0.67 a | 183.11 ± 2.80 d |
| RGS | 26.13 ± 0.3 a | 11.7 ± 0.53 b | 30.70 ± 0.49 b | 8.40 ± 0.03 b | 203.33 ± 0.73 a | 142.65 ± 2.52 c | 13.92 ± 0.29 b | 224.57 ± 4.74 c |
| FLS | 26.14 ± 0.25 a | 20.46 ± 0.21 c | 29.73 ± 0.78 b | 8.35 ± 0.03 b | 204.50 ± 0.29 a | 174.51 ± 1.59 b | 11.78 ± 0.41 c | 259.98 ± 14.88 b |
| MAS | 27.46 ± 0.75 a | 21.90 ± 0.14 d | 45.64 ± 0.74 a | 8.41 ± 0.02 b | 203.00 ± 0.58 a | 185.28 ± 3.83 a | 10.75 ± 0.30 c | 371.07 ± 7.22 a |
| Growing Stage | Urease (mg/g·d) | Protease (mg/g·d) | Phosphatase (mg/g·d) | Catalase (mg/g·d) | Sucrase (mg/g·d) | Polyphenol Oxidase (mg/g·d) | Nitrate Reductase (mg/g·d) |
|---|---|---|---|---|---|---|---|
| SES | 1.82 ± 0.03 ab | 3.19 ± 0.02 d | 1.72 ± 0.03 c | 182.14 ± 0.49 d | 24.03 ± 0.32 c | 45.58 ± 0.64 a | 0.34 ± 0.01 a |
| RGS | 1.85 ± 0.03 a | 3.32 ± 0.02 c | 2.18 ± 0.03 b | 182.85 ± 0.48 c | 25.59 ± 0.61 bc | 25.35 ± 10.81 b | 0.28 ± 0.01 b |
| FLS | 1.69 ± 0.04 b | 3.72 ± 0.03 b | 2.15 ± 0.05 b | 191.65 ± 0.35 b | 26.42 ± 0.73 b | 25.69 ± 1.25 b | 0.16 ± 0.01 d |
| MAS | 1.77 ± 0.05 ab | 3.94 ± 0.04 a | 2.60 ± 0.03 a | 206.54 ± 0.30 a | 30.52 ± 0.67 a | 20.41 ± 0.76 c | 0.19 ± 0.01 c |
| Growing Stage | Bacteria (106cfu/g·soil) | Fungus (103cfu/g·soil) | Actinomyces (106cfu/g·soil) |
|---|---|---|---|
| SES | 1.97 ± 0.01 a | 0.46 ± 0.01 a | 2.25 ± 0.02 a |
| RGS | 1.47 ± 0.02 c | 0.34 ± 0.01 c | 0.87 ± 0.02 c |
| FLS | 0.87 ± 0.08 d | 0.20 ± 0.02 d | 0.64 ± 0.01 d |
| MAS | 1.63 ± 0.02 b | 0.38 ± 0.01 b | 1.04 ± 0.02 b |
| Type | Growing Stage | Ace Index | Chao1 Index | Shannon Index | Simpson Index | Coverage Index |
|---|---|---|---|---|---|---|
| Bacteria | SES | 2594.77 ± 39.51 bc | 2602.79 ± 54.06 ab | 0.81 ± 0.01 b | 0.99 ± 0.01 a | 0.99 ± 0.01 a |
| RGS | 2539.19 ± 5.25 c | 2557.94 ± 7.61 b | 0.76 ± 0.01 c | 0.97 ± 0.01 c | 0.99 ± 0.01 a | |
| FLS | 2691.50 ± 22.68 a | 2697.49 ± 34.15 a | 0.82 ± 0.01 a | 0.99 ± 0.01 a | 0.99 ± 0.01 a | |
| MAS | 2641.11 ± 24.47 ab | 2674.97 ± 13.37 a | 0.75 ± 0.01 c | 0.98 ± 0.01 b | 0.99 ± 0.01 a | |
| fungus | SES | 471.51 ± 2.67 b | 474.54 ± 2.60 c | 0.64 ± 0.01 a | 0.94 ± 0.01 a | 0.99 ± 0.01 a |
| RGS | 523.65 ± 14.51 a | 519.45 ± 12.26 b | 0.61 ± 0.01 ab | 0.94 ± 0.01 a | 0.99 ± 0.01 a | |
| FLS | 528.67 ± 6.72 a | 525.06 ± 6.57 ab | 0.64 ± 0.01 a | 0.94 ± 0.01 a | 0.99 ± 0.01 a | |
| MAS | 534.77 ± 3.70 a | 549.02 ± 10.02 a | 0.55 ± 0.04 b | 0.90 ± 0.03 a | 0.99 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Ma, L.; Wang, G.; Chen, X.; Li, Z.; Wang, M.; Che, Y.; Zhang, L.; Jie, S.; Jiang, Z. Changes in Rhizosphere Soil Nutrients, Enzyme Activities, and Microbial Communities at Different Stages of Industrial Hemp Development. Agronomy 2022, 12, 3159. https://doi.org/10.3390/agronomy12123159
Guo L, Ma L, Wang G, Chen X, Li Z, Wang M, Che Y, Zhang L, Jie S, Jiang Z. Changes in Rhizosphere Soil Nutrients, Enzyme Activities, and Microbial Communities at Different Stages of Industrial Hemp Development. Agronomy. 2022; 12(12):3159. https://doi.org/10.3390/agronomy12123159
Chicago/Turabian StyleGuo, Li, Lan Ma, Guijiang Wang, Xiangwei Chen, Zeyu Li, Mingze Wang, Ye Che, Ling Zhang, Siyuan Jie, and Zeyu Jiang. 2022. "Changes in Rhizosphere Soil Nutrients, Enzyme Activities, and Microbial Communities at Different Stages of Industrial Hemp Development" Agronomy 12, no. 12: 3159. https://doi.org/10.3390/agronomy12123159
APA StyleGuo, L., Ma, L., Wang, G., Chen, X., Li, Z., Wang, M., Che, Y., Zhang, L., Jie, S., & Jiang, Z. (2022). Changes in Rhizosphere Soil Nutrients, Enzyme Activities, and Microbial Communities at Different Stages of Industrial Hemp Development. Agronomy, 12(12), 3159. https://doi.org/10.3390/agronomy12123159




