Evaluation of Indian Mustard Genotypes for White Rust Resistance Using BjuWRR1Gene and Their Phenotypic Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Disease Reaction
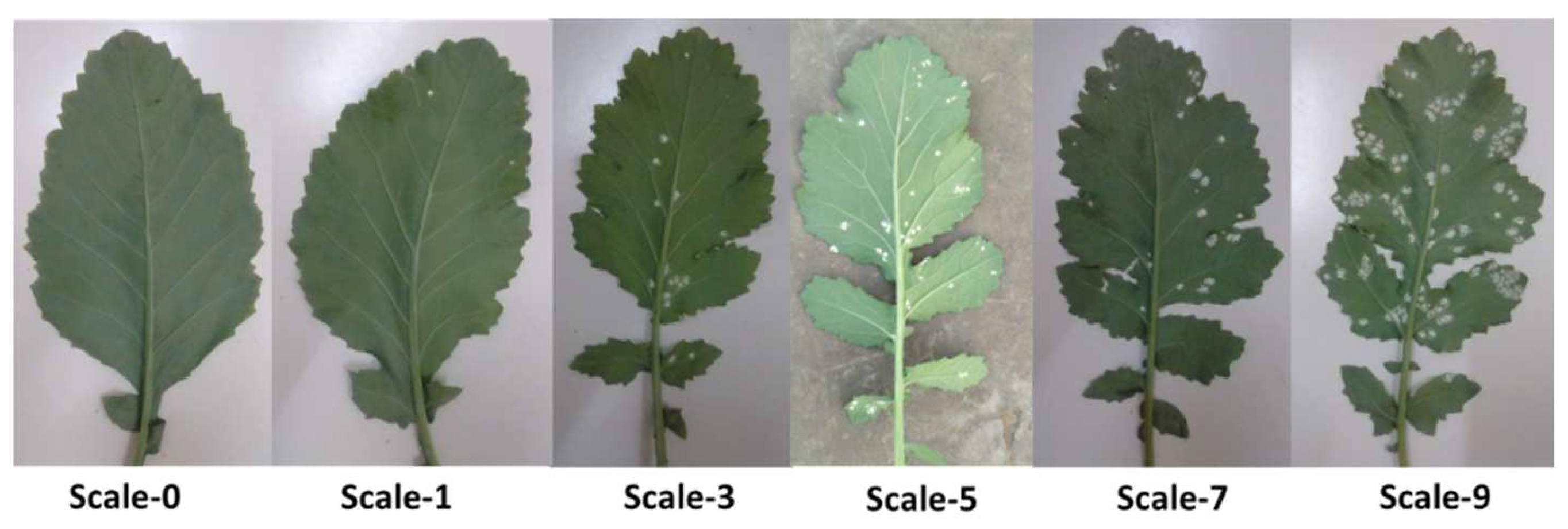
- Zero (immune for WR): no lesions on either cotyledon surface.
- One (HR): non-sporulating pin-point size necrotic spot/a >5% leaf area covered by lesions.
- Three (R): slightly sporulating minute 1–2 mm diameter necrotic spot/5–10% leaf area covered by lesions.
- Five (MR): moderately sporulating small pustules of about a 2–4 mm diameter for larger spots/a 11–25% leaf area covered by the spots.
- Seven (S): moderately sporulating, many large pustules of about a 4–5 mm diameter/a 26–50% leaf area covered by the lesions.
- Nine (HS): profusely sporulating, large coalescing pustules of a > 6 mm diameter/a > 50% leaf area covered by the lesions without margins.
2.3. Phenotypic Evaluation
- Days to 50% flowering: The number of days counted from the date of sowing to the date of attainment of a 50% flowering was recorded on plot basis.
- Days to 80% maturity: The number of days taken from the date of sowing to 80% siliqua turning yellow was recorded on plot basis.
- 1000 seed weight (g): The mean weight of a 1000 seed weight was recorded from the selected plants.
- Seed yield/Plant (g): The total yield produced by individual plants were recorded from the selected plants.
- Oil content (%): The total oil content of the samples was recorded using a pre-calibrated seed grader machine (FOSS InfratecTM1241 Grain Analyzer) of selected plants for each genotype.
Statistical Analysis of the Data
2.4. Physiological Evaluation (Stomatal Density Analysis)
2.5. Screening of BjuWRR1 Gene
2.6. Multiple Sequence Alignment ofBjuWRR1 Gene
3. Results
3.1. Disease Reaction and Phenotypic Evaluation
3.2. Stomatal Density with Disease Infection
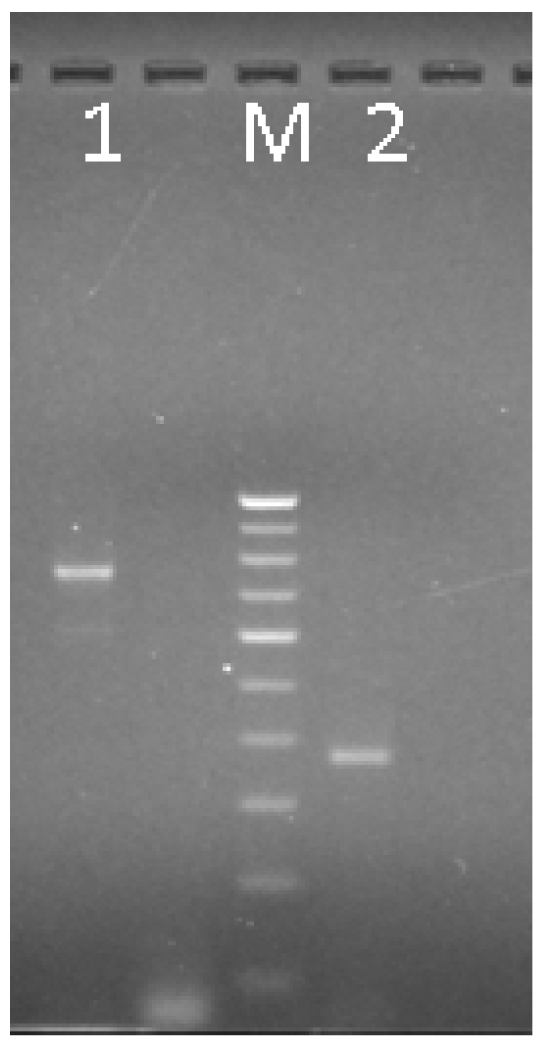
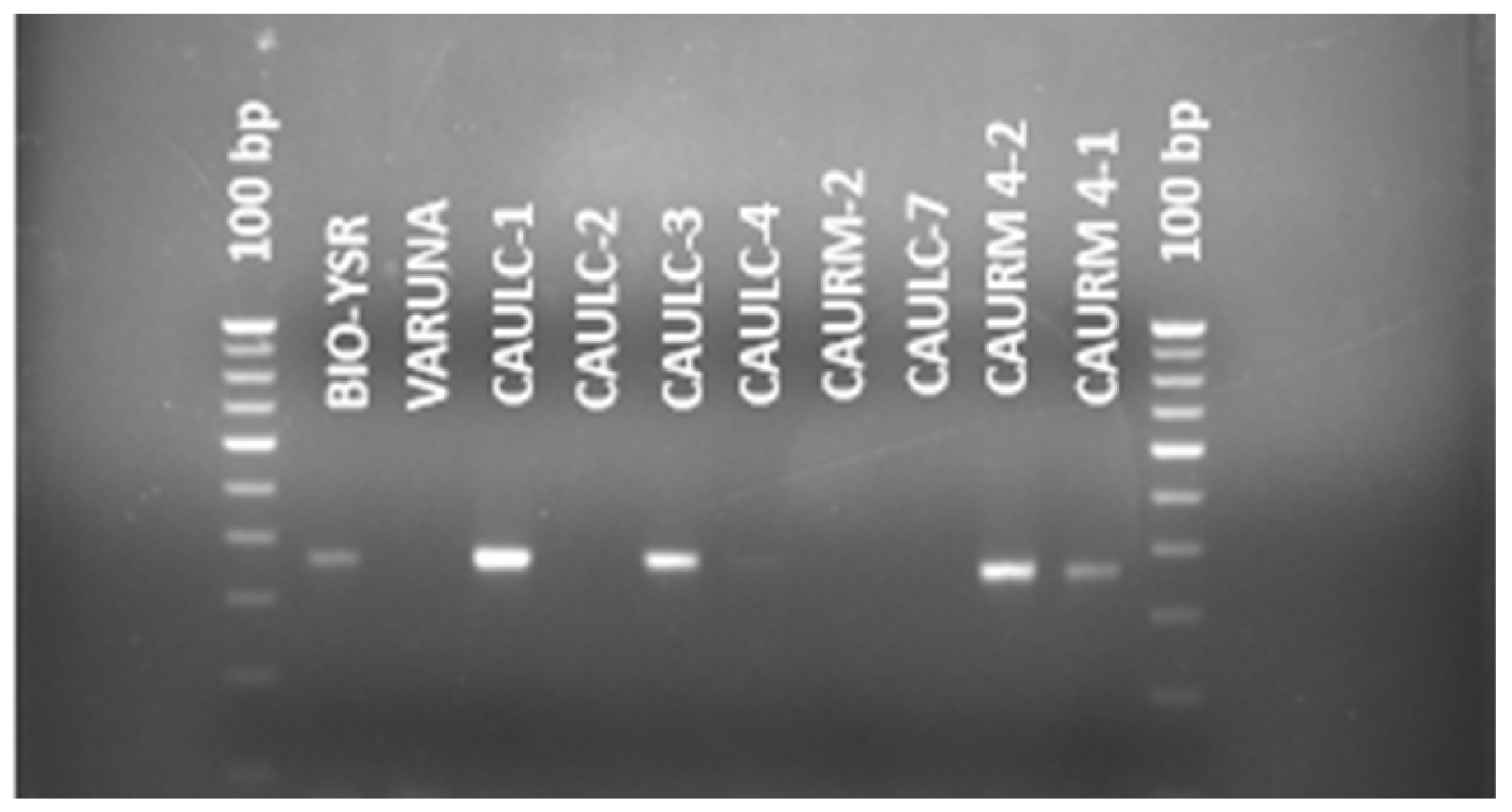
3.3. Validation and Screening of BjuWRR1 Gene
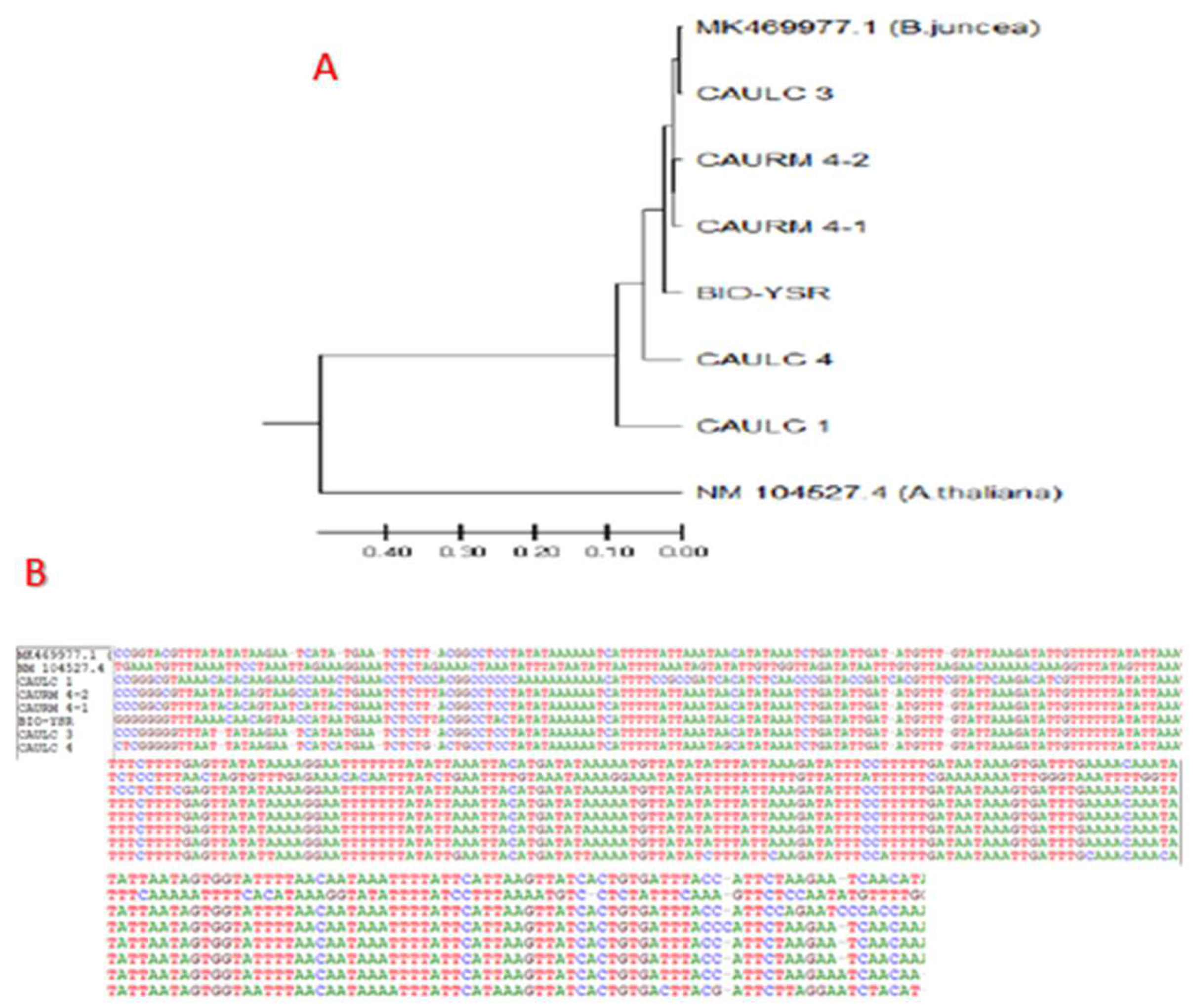
3.4. Multiple Sequence Alignment of BjuWRR1 Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Government of India. Agricultural Statistics at a Glance; Ministry of Agriculture & Farmers Welfare, Department of Agriculture & Farmers Welfare Directorate of Economics & Statistics: New Delhi, India, 2021.
- Sharma, D.; Nanjundan, J.; Singh, L.; Parmar, N.; Singh, K.H.; Verma, K.S.; Thakur, A.K. Genetic diversity and population structure analysis in Indian Mustard germplasm using phenotypic traits and SSR markers. Plant Mol. Biol. Rep. 2022, 40, 579–594. [Google Scholar] [CrossRef]
- Kamoun, S.; Furzer, O.; Jones, J.D.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Panjabi-Massand, P.; Yadava, S.K.; Sharma, P.; Kaur, A.; Kumar, A.; Arumugam, N.; Sodhi, Y.S.; Mukhopadhyay, A.; Gupta, V.; Pradhan, A.K.; et al. Molecular mapping reveals two independent loci conferring resistance to Albugo candida in the east European germplasm of oilseed mustard Brassica juncea. Theor. Appl. Genet. 2010, 121, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.P.; Nashaat, N.I.; Kolte, S.J.; Tewari, A.K.; Meena, P.D.; Bhatt, R. Screening of putative resistant sources against Indian and exotic isolates of Albugo candida inciting white rust in rapeseed-mustard. J. Oilseed Brassica 2012, 1, 27–37. [Google Scholar]
- Lakra, B.S.; Saharan, G.S. Correlation of leaf and stag head infection intensities of white rust with yield and yield components of mustard. Indian J. Mycol. Plant Pathol. 1989, 19, 279–281. [Google Scholar]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Vignesh, M.; Yadava, D.K.; Sujata, V.; Mohapatra, T.; Jain, N.; Yadav, A.K.; Malik, D.; Yadav, M.S.; Prabhu, K.V. Genetics of white rust resistance in [Brassica juncea (L.) Czern. & Coss.] and allelic relationship between interspecific sources of resistance. Indian J. Genet. Plant Breed. 2009, 69, 205–208. [Google Scholar]
- Vignesh, M.; Yadava, D.K.; Sujata, V.; Yadav, A.K.; Mohapatra, T.; Prabhu, K.V. Characterization of an Indian mustard (Brassica juncea) indigenous germplasm line Bio-YSR for white rust resistance. Indian J. Plant Genet. Resour. 2011, 24, 40–42. [Google Scholar]
- Prabhu, K.V.; Somers, D.J.; Rakow, G.; Gugel, R.K. Molecular markers linked to white rust resistance in mustard Brassica juncea. Theor. Appl. Genet. 1998, 97, 865–870. [Google Scholar] [CrossRef]
- Varshney, A.; Mohapatra, T.; Sharma, R.P. Development and validation of CAPS and AFLP markers for white rust resistance gene in Brassica juncea. Theor. Appl. Genet. 2004, 109, 153–159. [Google Scholar] [CrossRef]
- Borhan, H.M.; Brose, E.; Beynon, J.L.; Holub, E.B. White rust (Albugo candida) resistance loci on three Arabidopsis chromosomes are closely linked to downy mildew (Peronospora parasitica) resistance loci. Mol. Plant Pathol. 2001, 2, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Borhan, M.H.; Gunn, N.; Cooper, A.; Gulden, S.; Tor, M.; Rimmer, S.R.; Holub, E.B. WRR4 encodes a TIR-NB-LRR protein that confers broad-spectrum white rust resistance in Arabidopsis thaliana to four physiological races of Albugo candida. Mol. Plant Microbe Interact. 2008, 21, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Borhan, M.H.; Holub, E.B.; Kindrachuk, C.; Omidi, M.; Bozorgmanesh-Frad, G.; Rimmer, S.R. WRR4, a broad-spectrum TIR-NB-LRR gene from Arabidopsis thaliana that confers white rust resistance in transgenic oilseed brassica crops. Mol. Plant Pathol. 2010, 11, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Padmaja, K.L.; Paritosh, K.; Mukhi, N.; Tewari, A.K.; Mukhopadhyay, A.; Gupta, V.; Pradhan, A.K. BjuWRR1, a CC-NB-LRR gene identified in Brassica juncea, confers resistance to white rust caused by Albugo candida. Theor. Appl. Genet. 2019, 132, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Bhayana, L.; Paritosh, K.; Arora, H.; Yadava, S.K.; Singh, P.; Nandan, D.; Mukhopadhyay, A.; Gupta, V.; Pradhan, A.K.; Pental, D. A Mapped locus on LG A6 of Brassica juncealine Tumida conferring resistance to white rust contains a CNL Type R Gene. Front. Plant Sci. 2020, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.T.; Williams, P.H. Correlation of spore production by Albugo candida in Brassica campestris and a visual rating scale. Can. J. Plant Pathol. 1984, 6, 175–176. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kumar, S.; Tamura, K.; Nei, M. MEGA: Molecular evolutionary genetics analysis software for microcomputers. Bioinformatics 1994, 10, 189–191. [Google Scholar] [CrossRef]
- Yadava, S.K.; Arumugam, N.; Mukhopadhyay, A.; Sodhi, Y.S.; Gupta, V.; Pental, D.; Pradhan, A.K. QTL mapping of yield-associated traits in Brassica juncea: Meta-analysis and epistatic interactions using two different crosses between east European and Indian gene pool lines. Theor. Appl. Genet. 2012, 125, 1553–1564. [Google Scholar] [CrossRef]
- Singh, B.K.; Nandan, D.; Ambawat, S.; Ram, B.; Kumar, A.; Singh, T.; Meena, H.S.; Kumar, V.; Singh, V.V.; Rai, P.K.; et al. Validation of molecular markers for marker assisted pyramiding of white rust resistance loci in Indian mustard (Brassica junceaL.). Can. J. Plant Sci. 2015, 95, 939–945. [Google Scholar] [CrossRef]
- Yadava, J.S.; Singh, N.B. Strategies to Enhance Yield Potential of Rapeseed-Mustard in India. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999; Available online: www.regional.org.au/au/gcirc/6/634.htm-34k (accessed on 12 January 2019).
- Yadav, V.K.; Srivastava, K.K.; Mishra, V.K.; Negi, S. Studies on Genetic Variability, Character Association and Genetic Divergence in Indian Mustard (Brassica juncea L. Czern and Coss.). Int. J. Curr. Microbiol. App. Sci. 2020, 10, 132–143. [Google Scholar]
- Tateda, C.; Obara, K.; Abe, Y.; Sekine, R.; Nekoduka, S.; Hikage, T.; Nishihara, M.; Sekine, K.T.; Fujisaki, K. The host stomatal density determines resistance to Septoria gentianae in Japanese Gentian. Mol. Plant Microbe Interact. 2019, 32, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Dutton, C.; Hõrak, H.; Hepworth, C.; Mitchell, A.; Ton, J.; Hunt, L.; Gray, J.E. Bacterial infection systemically suppresses stomatal density. Plant Cell Environ. 2019, 42, 2411–2421. [Google Scholar] [CrossRef]
- Negi, M.S.; Devic, M.; Delseny, M.; Lakshmikumaran, M. Identification of AFLP fragments linked to seed coat colour in Brassica juncea and conversion to a SCAR marker for rapid selection. Theor. Appl. Genet. 2000, 101, 146–152. [Google Scholar] [CrossRef]

| Sl. No. | Genotypes | Source | Sl. No. | Genotypes | Source |
|---|---|---|---|---|---|
| 1. | CAULC-1 | Awang Potshangbam, Manipur | 16. | Pusa Mustard-28 | DRMR, Bharatpur, Rajasthan |
| 2. | CAULC-2 | Kakching Wairi, Manipur | 17. | Laxmi | DRMR, Bharatpur, Rajasthan |
| 3. | CAULC-3 | Kakching Lamjao, Manipur | 18. | Bio-YSR | DRMR, Bharatpur, Rajasthan |
| 4. | CAULC-4 | Sekmai, Manipur | 19. | JD-6 | DRMR, Bharatpur, Rajasthan |
| 5. | CAURM-1 | AICRP (RM) CAU, Imphal Centre | 20. | Urvashi | DRMR, Bharatpur, Rajasthan |
| 6. | CAURM-2 | AICRP (RM) CAU, Imphal Centre | 21. | GM-2 | DRMR, Bharatpur, Rajasthan |
| 7. | CAURM-5 | AICRP (RM) CAU, Imphal Centre | 22. | Rajendra Suflam | DRMR, Bharatpur, Rajasthan |
| 8. | CAURM 4-3 | AICRP (RM) CAU, Imphal Centre | 23. | Pusa Bold | DRMR, Bharatpur, Rajasthan |
| 9. | CAURM 4-2 | AICRP (RM) CAU, Imphal Centre | 24. | RH-30 | DRMR, Bharatpur, Rajasthan |
| 10. | CAULR-7 | AICRP (RM) CAU, Imphal Centre | 25. | JM-1 | DRMR, Bharatpur, Rajasthan |
| 11. | BPR-547 | AICRP (RM) CAU, Imphal Centre | 26. | Basanti | DRMR, Bharatpur, Rajasthan |
| 12. | CAUMC-28 | AICRP (RM) CAU, Imphal Centre | 27. | NRCHB-101 | DRMR, Bharatpur, Rajasthan |
| 13. | CAURM-4 | AICRP (RM) CAU, Imphal Centre | 28. | RH-749 | DRMR, Bharatpur, Rajasthan |
| 14. | CAURM-4-1 | AICRP (RM) CAU, Imphal Centre | 29. | Varuna | DRMR, Bharatpur, Rajasthan |
| 15. | JM-2 | DRMR, Bharatpur, Rajasthan | 30. | NRCDR-2 | DRMR, Bharatpur, Rajasthan |
| Gene | Primer | Sequence (5′-3′) | Tm (°C) | Expected Amplicon | |
|---|---|---|---|---|---|
| Varuna (Susceptible) | Donskaja-IV (Resistant) | ||||
| BjuWRR1 | F-DV | GGCATAGTATTCCCTAGAAGAGAGATAAC | 58 | 761 bp | 366 bp |
| R-D | TGTTGATTCTTAGAATGGTAAATCACAG | ||||
| R-V | TTGAAAATCACATGTATACATATGGCTT | ||||
| Genotype | 50% DF | 80% DM | Y/P (IN) | Y/P (NIP) | 1000 SW (g) | OC (%) | PDI (%) | Stomata Density/mm2 |
|---|---|---|---|---|---|---|---|---|
| G1 (CAULC-1) | 66.21 | 121.80 | 4.62 | 4.74 | 3.26 | 43.84 | 4.11 (2.19) | 146.67 |
| G2 (CAULC-2) | 65.21 | 122.13 | 2.20 | 2.26 | 2.38 | 38.13 | 3.73 (2.11) | 130.67 |
| G3 (CAULC-3) | 67.54 | 123.46 | 2.39 | 2.34 | 2.07 | 40.44 | 2.80 (1.90) | 69.33 |
| G4 (CAULC-4) | 77.37 | 141.63 | 3.06 | 4.04 | 1.65 | 43.96 | 2.44 (1.89) | 74.67 |
| G5 (CAURM-1) | 57.71 | 119.46 | 3.28 | 3.15 | 4.88 | 43.11 | 15.07 (3.78) | 136.00 |
| G6 (CAURM-2) | 59.71 | 120.46 | 3.31 | 3.57 | 4.34 | 45.10 | 7.46 (2.92) | 106.67 |
| G7 (CAURM-5) | 56.71 | 121.46 | 3.87 | 4.44 | 4.77 | 45.45 | 12.95 (3.74) | 69.33 |
| G8 (CAURM 4-3) | 63.37 | 119.12 | 2.82 | 3.85 | 3.28 | 41.11 | 13.15 (3.77) | 138.67 |
| G9 (CAURM 4-2) | 64.71 | 118.79 | 2.93 | 3.52 | 4.48 | 45.31 | 8.64 (3.12) | 85.33 |
| G10 (CAULR-7) | 54.05 | 114.46 | 2.35 | 2.18 | 3.76 | 42.77 | 6.42 (2.34) | 117.33 |
| G11 (BPR-547) | 56.38 | 118.79 | 2.83 | 2.84 | 4.53 | 44.30 | 13.31 (3.54) | 109.33 |
| G12 (CAUMC-28) | 59.37 | 120.30 | 2.97 | 5.39 | 4.84 | 46.16 | 13.95 (3.96) | 130.67 |
| G13 (CAURM-4) | 64.37 | 121.79 | 3.27 | 4.07 | 4.36 | 48.91 | 6.99 (2.84) | 88.00 |
| G14 (CAURM-4-1) | 57.04 | 124.12 | 3.69 | 4.25 | 2.99 | 43.79 | 9.22 (3.21) | 93.33 |
| G15 (JM-2) | 59.54 | 120.46 | 4.06 | 4.38 | 4.45 | 43.29 | 16.55 (4.04) | 109.33 |
| G16 (Pusa Mustard-28) | 46.71 | 113.79 | 3.78 | 6.06 | 3.16 | 43.05 | 11.46 (4.71) | 80.00 |
| G17 (Laxmi) | 60.05 | 128.46 | 4.06 | 6.61 | 3.50 | 43.24 | 13.51 (3.56) | 128.00 |
| G18 (Bio-YSR) | 61.00 | 120.50 | 4.05 | 4.75 | 3.48 | 41.79 | 9.87 (3.17) | 98.67 |
| G19 (JD-6) | 55.87 | 117.80 | 2.67 | 3.79 | 3.49 | 43.62 | 11.91 (3.47) | 120.00 |
| G20 (Urbashi) | 61.54 | 125.46 | 5.17 | 7.81 | 6.92 | 42.40 | 12.38 (3.20) | 85.33 |
| G 21 (GM-2) | 74.71 | 136.63 | 3.82 | 6.58 | 3.92 | 44.05 | 11.24 (3.58) | 82.67 |
| G 22 (Rajendra Suflam) | 64.37 | 123.63 | 3.01 | 6.16 | 4.23 | 42.99 | 12.71 (3.72) | 117.33 |
| G 23 (Pusa Bold) | 66.04 | 122.96 | 4.02 | 5.86 | 3.50 | 43.51 | 12.18 (3.71) | 210.67 |
| G 24 (RH-30) | 62.71 | 127.63 | 3.56 | 7.69 | 4.73 | 43.04 | 21.09 (4.33) | 173.33 |
| G 25 (JM-1) | 64.50 | 120.50 | 3.42 | 4.30 | 3.95 | 42.87 | 11.31 (3.43) | 98.67 |
| G 26 (Basanti) | 60.71 | 121.79 | 3.04 | 3.09 | 3.85 | 43.94 | 14.22 (2.40) | 165.33 |
| G 27 (NRCHB-101) | 53.75 | 124.25 | 3.23 | 5.39 | 5.04 | 43.35 | 14.41 (4.06) | 157.33 |
| G 28 (RH-749) | 63.50 | 135.25 | 4.99 | 8.80 | 3.65 | 44.13 | 12.18 (3.81) | 133.33 |
| G 29 (Varuna) | 57.50 | 121.75 | 3.31 | 5.27 | 2.73 | 43.44 | 22.31 (4.78) | 114.67 |
| G 30 (NRCDR-2) | 64.00 | 126.50 | 3.67 | 5.44 | 3.61 | 44.36 | 12.06 (3.54) | 168.00 |
| Mean | 61.54 | 123.30 | 3.51 | 4.75 | 3.86 | 43.54 | 7.70 (3.36) | 117.96 |
| Range | 46.71–77.37 | 113.79 141.63 | 2.2–5.17 | 2.26–8.80 | 1.65–6.92 | 38.1–48.91 | 2.44–22.31 | |
| GCV (%) | 9.99 | 4.71 | 17.44 | 26.29 | 28.12 | 4.15 | 20.38 | |
| PCV (%) | 10.19 | 4.79 | 25.26 | 32.00 | 28.32 | 4.65 | 22.04 | |
| h2 (%) | 95.99 | 96.78 | 47.66 | 67.47 | 98.57 | 79.56 | 85.49 | |
| GAM (%) | 20.18 | 9.56 | 24.84 | 44.54 | 57.59 | 7.63 | 38.87 | |
| SE | 1.917 | 1.616 | 0.981 | 1.325 | 0.200 | 1.398 | 0.431 | |
| C.D. (5%) | 4.086 | 3.445 | - | 2.824 | 0.425 | - | 0.919 |
| Sl. No | Genotype Name | Collection Place | Amplicon Size (bp) | Query Gene | Accession No. | Query Coverage (%) | Total Score (S) | E-Value | Max. Identity (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | CAULC-1 | Manipur | 366 | BjuWRR1 | OM243099 | 67 | 372 | 2 × 10−98 | 94.65 |
| 2. | CAULC-3 | Manipur | 366 | BjuWRR1 | OM243103 | 97 | 595 | 9 × 10−166 | 99.10 |
| 3. | CAULC-4 | Manipur | 366 | BjuWRR1 | OM243167 | 89 | 532 | 7 × 10−153 | 94.33 |
| 4. | CAURM 4-1 | AICRP(R-M), CAU, Imphal Centre | 366 | BjuWRR1 | OM243101 | 97 | 573 | 4 × 10−159 | 97.63 |
| 5. | CAURM 4-2 | AICRP(R-M), CAU, Imphal Centre | 366 | BjuWRR1 | OM243100 | 96 | 564 | 3 × 10−156 | 97.33 |
| 6. | BIO-YSR | DRMR, Bharatpur | 366 | BjuWRR1 | OM243102 | 92 | 549 | 7 × 10−152 | 97.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, Y.S.; Devi, T.R.; Thakur, A.K.; Ngangkham, U.; Devi, H.N.; Kh., P.; Sinha, B.; Senjam, P.; Singh, N.B.; Mishra, L.K. Evaluation of Indian Mustard Genotypes for White Rust Resistance Using BjuWRR1Gene and Their Phenotypic Performance. Agronomy 2022, 12, 3122. https://doi.org/10.3390/agronomy12123122
Devi YS, Devi TR, Thakur AK, Ngangkham U, Devi HN, Kh. P, Sinha B, Senjam P, Singh NB, Mishra LK. Evaluation of Indian Mustard Genotypes for White Rust Resistance Using BjuWRR1Gene and Their Phenotypic Performance. Agronomy. 2022; 12(12):3122. https://doi.org/10.3390/agronomy12123122
Chicago/Turabian StyleDevi, Yengkhom Sanatombi, Th. Renuka Devi, Ajay Kumar Thakur, Umakanta Ngangkham, H. Nanita Devi, Pramesh Kh., Bireswar Sinha, Pushparani Senjam, N. Brajendra Singh, and Lokesh Kumar Mishra. 2022. "Evaluation of Indian Mustard Genotypes for White Rust Resistance Using BjuWRR1Gene and Their Phenotypic Performance" Agronomy 12, no. 12: 3122. https://doi.org/10.3390/agronomy12123122
APA StyleDevi, Y. S., Devi, T. R., Thakur, A. K., Ngangkham, U., Devi, H. N., Kh., P., Sinha, B., Senjam, P., Singh, N. B., & Mishra, L. K. (2022). Evaluation of Indian Mustard Genotypes for White Rust Resistance Using BjuWRR1Gene and Their Phenotypic Performance. Agronomy, 12(12), 3122. https://doi.org/10.3390/agronomy12123122






