Seed Meals from Allelopathic Crops as a Potential Bio-Based Herbicide on Herbicide-Susceptible and -Resistant Biotypes of Wild Oat (Avena fatua L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Seed Meals and Their Preparation
2.3. Herbicide Characteristics
2.4. Soil Characteristics
2.5. Set Up and Management of Pot Experiments
2.6. Measurement Range
2.7. Statistical Analysis
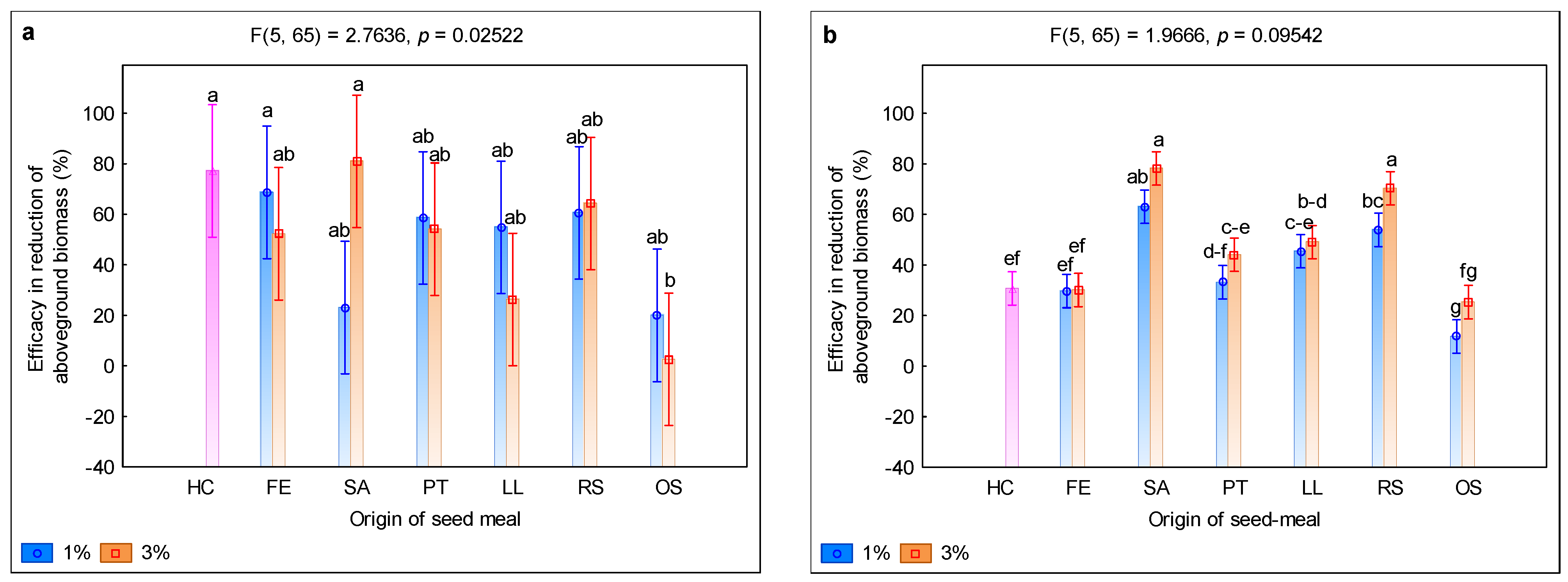
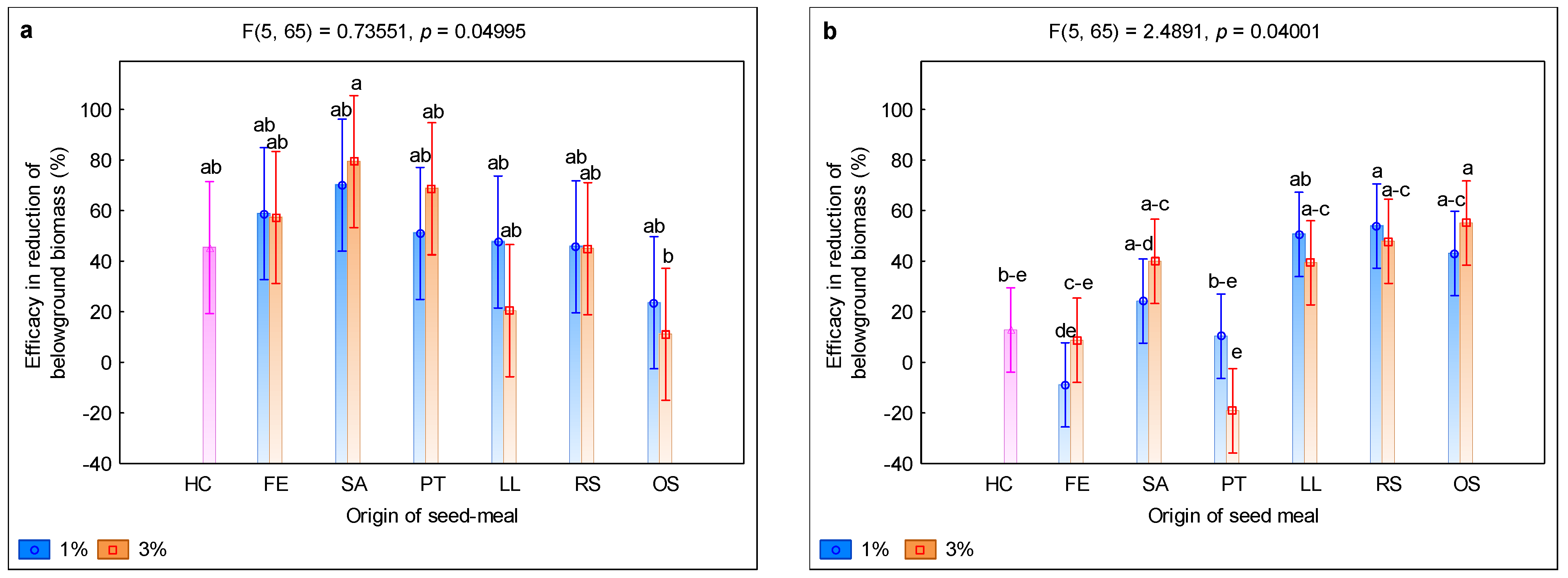
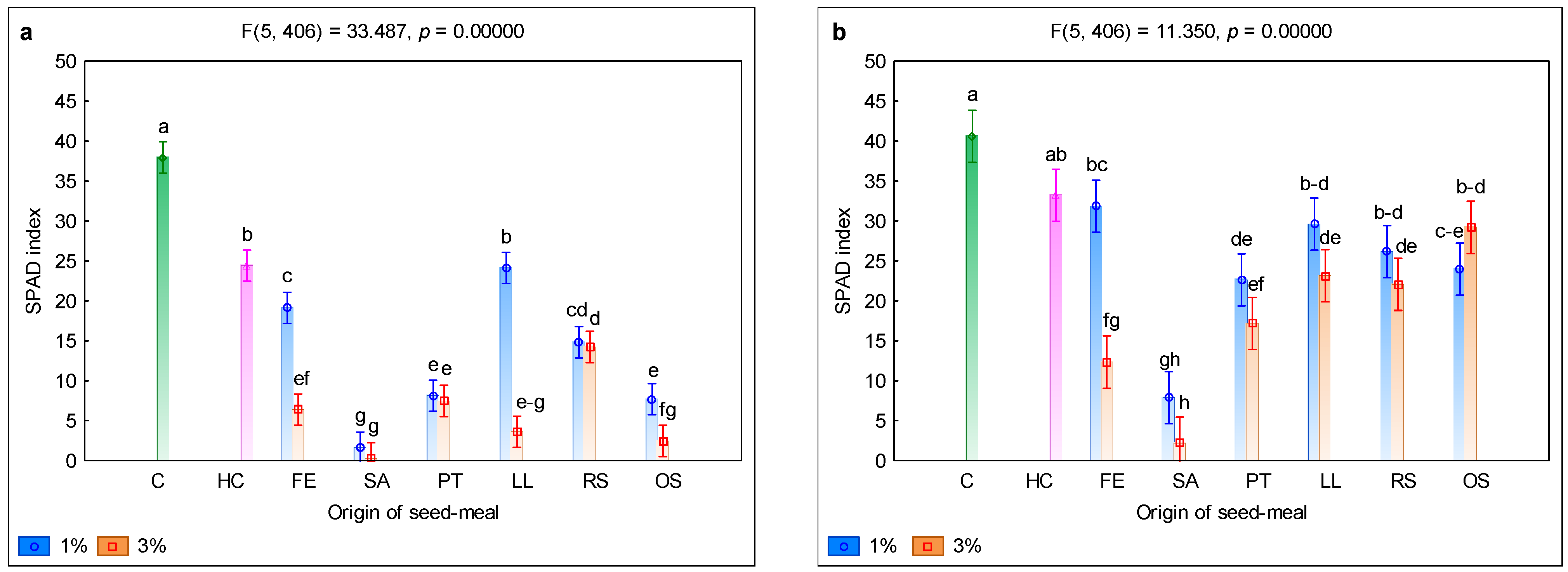
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heap, I. The International Herbicide-Resistant Weed Database. 2022. Available online: https://www.weedscience.org (accessed on 25 August 2022).
- Benedetti, L.; Rangani, G.; Ebeling Viana, V.; Carvalho-Moore, P.; Merotto, A., Jr.; Rabaioli Camargo, E.; Antonio de Avila, L.; Roma-Burgos, N. Rapid Reduction of Herbicide Susceptibility in Junglerice by Recurrent Selection with Sublethal Dose of Herbicides and Heat Stress. Agronomy 2020, 10, 1761. [Google Scholar] [CrossRef]
- Stankiewicz-Kosyl, M.; Synowiec, A.; Haliniarz, M.; Wenda-Piesik, A.; Domaradzki, K.; Parylak, D.; Wrochna, M.; Pytlarz, E.; Gala-Czekaj, D.; Marczewska-Kolasa, K.; et al. Herbicide Resistance and Management Options of Papaver rhoeas L. and Centaurea cyanus L. in Europe: A review. Agronomy 2020, 10, 874. [Google Scholar] [CrossRef]
- Vieira, B.C.; Luck, J.D.; Amundsen, K.L.; Werle, R.; Gaines, T.A.; Kruger, G.R. Herbicide drift exposure leads to reduced herbicide sensitivity in Amaranthus spp. Sci. Rep. 2020, 10, 2146. [Google Scholar] [CrossRef] [Green Version]
- Squires, C.C.; Coleman, G.R.; Broster, J.C.; Preston, C.; Boutsalis, P.; Owen, M.J.; Jalaludin, A.; Walsh, M.J. Increasing the value and efficiency of herbicide resistance surveys. Pest Manag. Sci. 2021, 77, 3881–3889. [Google Scholar] [CrossRef] [PubMed]
- Pytlarz, E.; Andrzejak, O. Threat of potential herbicide resistance biotypes of rye brome (Bromus secalinus L.) in Lower Silesia. Prog. Plant Prot. 2022, 62, 5–10. (In Polish) [Google Scholar] [CrossRef]
- Oerke, E. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Moss, S.; Ulber, L.; den Hoed, I. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Torra, J.; Montull, J.M.; Calha, I.M.; Osuna, M.D.; Portugal, J.; de Prado, R. Current Status of Herbicide Resistance in the Iberian Peninsula: Future Trends and Challenges. Agronomy 2022, 12, 929. [Google Scholar] [CrossRef]
- Moss, S. Integrated weed management (IWM): Why are farmers reluctant to adopt non-chemical alternatives to herbicides? Pest Manag. Sci. 2019, 75, 1205–1211. [Google Scholar] [CrossRef]
- El-Metwally, I.M.; El-Rokiek, K.G. Eucalyptus citriodora leaf extract as a source of allelochemicals for weed control in pea fields compared with some chemical herbicides. J. Plant Prot. Res. 2019, 59, 392–399. [Google Scholar] [CrossRef]
- Głąb, L.; Sowiński, J.; Bough, R.; Dayan, F.E. Allelopathic potential of sorghum (Sorghum bicolor (L.) Moench) in weed control: A comprehensive review. Adv. Agron. 2017, 145, 43–95. [Google Scholar] [CrossRef]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Gogaľová, Z.; Poráčová, J.; Camele, I.; De Feo, V. Thymol Chemotype Origanum vulgare L. Essential Oil as a Potential Selective Bio-Based Herbicide on Monocot Plant Species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef] [Green Version]
- Alsharekh, A.; El-Sheikh, M.A.; Alatar, A.A.; Abdel-Salam, E.M. Natural Control of Weed Invasions in Hyper-Arid Arable Farms: Allelopathic Potential Effect of Conocarpus erectus against Common Weeds and Vegetables. Agronomy 2022, 12, 703. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hananac, M.; Jamoussi, B. Reviews on phytotoxic effects of essential oils and their individual components: News approach for weeds management. Int. J. Appl. Biol. Pharm. 2013, 4, 96–114. [Google Scholar]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Pytlarz, E.; Gala-Czekaj, D. Possibilities of Using Seed Meals in Control of Herbicide-Susceptible and -Resistant Biotypes of Rye Brome (Bromus secalinus L.) in Winter Wheat. Plants 2022, 11, 331. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Rimando, A.M.; Schrader, K.K.; Aliotta, G.; Oliva, A. Chemicals from nature for weed management. Weed Sci. 2002, 50, 138–151. [Google Scholar] [CrossRef]
- Requesón, E.; Osuna, D.; Santiago, A.d.R.; Sosa, T. Evaluation of the Activity of Estragole and 2-Isopropylphenol, Phenolic Compounds Present in Cistus ladanifer. Agronomy 2022, 12, 1139. [Google Scholar] [CrossRef]
- Korsmo, E. Unkräuter im Ackerbau der Neuzeit; Springer: Berlin/Heidelberg, Germany, 1930; p. 582. (In German) [Google Scholar] [CrossRef]
- Gawęda, D.; Haliniarz, M. Yielding and weed infestation of oats (Avena sativa L.) grown in monoculture depending on weed control method and stubble catch crop. Prog. Plant Prot. 2013, 53, 297–302. (In Polish) [Google Scholar]
- Wrzesińska, B.; Kierzek, R.; Obrępalska-Stęplowska, A. Evaluation of six commonly used reference genes for gene expression studies in herbicide-resistant Avena fatua biotypes. Weed Res. 2016, 56, 284–292. [Google Scholar] [CrossRef]
- Jäck, O.; Menegat, A.; Gerhards, R. Winter wheat yield loss in response to Avena fatua competition and effect of reduced herbicide dose rates on seed production of this species. J. Plant Dis. Prot. 2017, 124, 371–382. [Google Scholar] [CrossRef]
- Ņečajeva, J.; Bleidere, M.; Jansone, Z.; Gailīte, A.; Ruņģis, D. Variability of Seed Germination and Dormancy Characteristics and Genetic Analysis of Latvian Avena fatua Populations. Plants 2021, 10, 235. [Google Scholar] [CrossRef]
- de Luna, L.Z.; Kennedy, A.C.; Hansen, J.C.; Paulitz, T.C.; Gallagher, R.S.; Fuerst, E.P. Mycobiota on wild oat (Avena fatua L.) seed and their caryopsis decay potential. Plant Health Prog. 2011, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Beckie, H.; Warwick, S.; Sauder, C. Basis for Herbicide Resistance in Canadian Populations of Wild Oat (Avena fatua). Weed Sci. 2012, 60, 10–18. [Google Scholar] [CrossRef]
- Beckie, H.J.; Francis, A.; Hall, L.M. The Biology of Canadian Weeds. 27. Avena fatua L. (updated). Can. J. Plant Sci. 2012, 92, 1329–1357. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, G.; Chauhan, B. Seed longevity and seedling emergence behavior of wild oat (Avena fatua) and sterile oat (Avena sterilis ssp. ludoviciana) in response to burial depth in eastern Australia. Weed Sci. 2021, 69, 362–371. [Google Scholar] [CrossRef]
- Adamczewski, K.; Matysiak, K.; Kierzek, R.; Kaczmarek, S. Significant increase of weed resistance to herbicides in Poland. J. Plant Prot. Res. 2019, 59, 139–150. [Google Scholar] [CrossRef]
- Cavan, G.; Biss, P.; Moss, S.R. Herbicide resistance and gene flow in wild-oats (Avena fatua and Avena sterilis ssp. ludoviciana). Ann. appl. Biol. 1998, 133, 207–217. [Google Scholar] [CrossRef]
- Friesen, L.F.; Jones, T.L.; Van Acker, R.C.; Morrison, I.N. Identification of Avena fatua populations resistant to imazamethabenz, flamprop, and fenoxaprop-P. Weed Sci. 2000, 48, 532–540. [Google Scholar] [CrossRef]
- Beckie, H.J.; Hall, L.M.; Meers, S.; Laslo, J.J.; Stevenson, F.C. Management Practices Influencing Herbicide Resistance in Wild Oat. Weed Technol. 2004, 18, 853–859. [Google Scholar] [CrossRef]
- Yu, Q.; Ahmad-Hamdani, M.S.; Han, H.; Christoffers, M.J.; Powles, S.B. Herbicide resistance-endowing ACCase gene mutations in hexaploid wild oat (Avena fatua): Insights into resistance evolution in a hexaploid species. Heredity 2012, 110, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, A.O. Control of wild oat (Avena fatua) using some phenolic compounds I—Germination and some growth parameters. Saudi J. Biol. Sci. 2012, 19, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Walsh, K.D.; Sanderson, D.; Hall, L.M.; Mugo, S.; Hills, M.J. Allelopathic effects of camelina (Camelina sativa) and canol (Brassica napus) on wild oat, flax and radish. Allelopathy J. 2014, 33, 83–96. [Google Scholar]
- Pużyńska, K.; Jop, B.; Gala-Czekaj, D.; Synowiec, A.; Bocianowski, J. Effect of allelopathic seed meals on the weed infestation and yielding of maize. Acta Physiol. Plant 2019, 41, 193. [Google Scholar] [CrossRef]
- Rice, A.R.; Johnson-Maynard, J.L.; Thill, D.C.; Morra, M.J. Vegetable crop emergence and weed control following amendment with different Brassicaceae seed meals. Renew. Agric. Food Syst. 2007, 22, 204–212. [Google Scholar] [CrossRef]
- Webber, C.L., III; White, P.M., Jr.; Boydston, R.; Shrefer, J. Impact of mustard seed meal applications on direct-seeded cucurbits and weed control. J. Agric. Sci. 2017, 9, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Boydston, R.A.; Anderson, T. Mustard (Sinapis alba) seed meal suppresses weeds in container-grown ornamentals. HortScience 2008, 43, 800–803. [Google Scholar] [CrossRef] [Green Version]
- Messiha, N.K.; El-Dabaa, M.A.T.; El-Masry, R.R.; Ahmed, S.A.A. The allelopathic influence of Sinapis alba seed powder (white mustard) on the growth and yield of Vicia faba (faba bean) with Orobanche crenata (broomrape). Middle East J. Appl. Sci. 2018, 8, 418–425. [Google Scholar]
- El-Rokiek, K.G.; Ahmed, S.A.A.; Messiha, N.K.; Mohamed, S.A.; El-Masry, R.R. Controlling the Grassy weed Avena fatua associating wheat plants with the seed powder of two brassicaceae plants Brassica rapa and Sinapis alba. Middle East J. Agric. Res. 2017, 6, 1014–1020. [Google Scholar]
- Ahmed, S.A.; Messiha, N.K.; El-Rokiek, K.G.; Mohamed, S.A.; El-Masry, R.R. The allelopathic Efficiency of two Brassicaceae plant seeds in controlling weeds associating sunflower plants. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 158–165. [Google Scholar]
- Bangarwa, S.K.; Nosworthy, J.K.; Mattice, J.D.; Gbur, E.E. Glucosinolate and Isothiocyanate Production from Brassicaceae Cover Crops in a Plasticulture Production System. Weed Sci. 2011, 59, 247–254. [Google Scholar] [CrossRef]
- Sharara, F.A.; El-Rokiek, K.G.; Gaweesh, S.S. Effect of soil fumigation on growth, development, yield of wheat (Triticum aestivum L.) and associated weeds. Int. J. Aca. Res. 2011, 3, 780–785. [Google Scholar]
- Latif, S.; Gurusinghe, S.; Weston, P.A.; Brown, W.B.; Quinn, J.C.; Piltz, J.W.; Weston, L.A. Performance and weed-suppressive potential of selected pasture legumes against annual weeds in south-eastern Australia. Crop Pasture Sci. 2019, 70, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Latif, S.; Gurusinghe, S.; Weston, P.A.; Quinn, J.C.; Piltz, J.W.; Weston, L.A. Metabolomic approaches for the identification of flavonoids associated with weed suppression in selected Hardseeded annual pasture legumes. Plant Soil 2020, 447, 199–218. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Masi, M.; Cimmino, A.; Vilariño, S.; Evidente, A. Allelopathic Effect of Quercetin, a Flavonoid from Fagopyrum esculentum Roots in the Radicle Growth of Phelipanche ramosa: Quercetin Natural and Semisynthetic Analogues Were Used for a Structure-Activity Relationship Investigation. Plants 2021, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Szwed, M.; Wiczkowski, W.; Szawara-Nowak, D.; Obendorf, R.L.; Horbowicz, M. Allelopathic influence of common buckwheat root residues on selected weed species. Acta Physiol. Plant 2019, 41, 92. [Google Scholar] [CrossRef]
- Kalinova, J.; Vrchotova, N.; Triska, J. Exudation of Allelopathic Substances in Buckwheat (Fagopyrum esculentum Moench). J. Agric. Food Chem. 2007, 55, 6453–6459. [Google Scholar] [CrossRef]
- Füzy, A.; Kovács, R.; Cseresnyés, I.; Parádi, I.; Szili-Kovács, T.; Kelemen, B.; Rajkai, K.; Takács, T. Selection of plant physiological parameters to detect stress effects in pot experiments using principal component analysis. Acta Physiol. Plant. 2019, 41, 56. [Google Scholar] [CrossRef] [Green Version]
- Ku, Y.S.; Au-Yeung, W.K.; Yung, Y.L.; Li, M.W.; Wen, C.Q.; Liu, X.; Lam, H.M. Drought stress and tolerance in soybean. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; Board, J.E., Ed.; IntechOpen: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Cannella, D.; Möllers, K.B.; Frigaard, N.U.; Jensen, P.E.; Bjerrum, M.J.; Johansen, K.S.; Felby, C. Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme. Nat. Commun. 2016, 7, 11134. [Google Scholar] [CrossRef] [Green Version]
- Houborg, R.; McCabe, M.; Cescatti, A.; Gao, F.; Schull, M.; Gitelson, A. Joint leaf chlorophyll content and leaf area index retrieval from Landsat data using a regularized model inversion system (REGFLEC). Regist. Superann. Entity RSE 2015, 159, 203–221. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of Chlorophyll, Carotenoid and SPAD-502 Measurement to Salinity and Nutrient Stress in Wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Sanglard, L.M.; Martins, S.C.; Detmann, K.C.; Silva, P.E.; Lavinsky, A.O.; Silva, M.M.; Detmann, E.; Araujo, W.L.; DaMatta, F.M. Silicon nutrition alleviates the negative impacts of arsenic on the photosynthetic apparatus of rice leaves: An analysis of the key limitations of photosynthesis. Physiol. Plant. 2014, 152, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]



| Biotype | ED50 (g ha–1) | Site (Coordinate) |
|---|---|---|
| S | 14.75 | Wrocław (51.1360 N 17.1150 E) |
| R | 57.96 | Środa Śląska (51.1767 N 16.6687 E) |
| Name | Cultivar | Abbreviation | |
|---|---|---|---|
| English | Latin | ||
| Common buckwheat | Fagopyrum esculentum Moench. | Panda | FE |
| White mustard | Sinapis alba L. | Bardena | SA |
| Lacy phacelia | Phacelia tanacetifolia Benth. | Anabela | PT |
| Yellow lupin | Lupinus luteus L. | Mister | LL |
| Fodder radish | Raphanus sativus L. var. oleiformis Pers. | Adagio | RS |
| Common birdsfoot | Ornithopus sativus Brot. | Bydgoska 1 | OS |
| Origin of Seed Meals | Biotype | |
|---|---|---|
| S | R | |
| C | 87.0 ± 4.7 | 83.3 ± 4.1 |
| FE1 | 94.4 ± 2.6 | 81.5 ± 3.7 |
| FE3 | 31.5 ± 4.2 | 51.9 ± 3.6 |
| SA1 | 7.4 ± 1.5 | 35.2 ± 1.7 |
| SA3 | 1.9 ± 0.6 | 9.3 ± 1.3 |
| PT1 | 37.0 ± 2.5 | 50.0 ± 2.3 |
| PT3 | 33.3 ± 4.0 | 48.1 ± 3.9 |
| LL1 | 44.4 ± 2.9 | 66.7 ± 4.7 |
| LL3 | 9.3 ± 2.5 | 55.6 ± 3.8 |
| RS1 | 79.6 ± 6.7 | 77.8 ± 5.9 |
| RS3 | 70.4 ± 3.2 | 79.6 ± 6.2 |
| OS1 | 22.2 ± 3.6 | 74.1 ± 5.3 |
| OS3 | 11.1 ± 0.6 | 66.7 ± 4.6 |
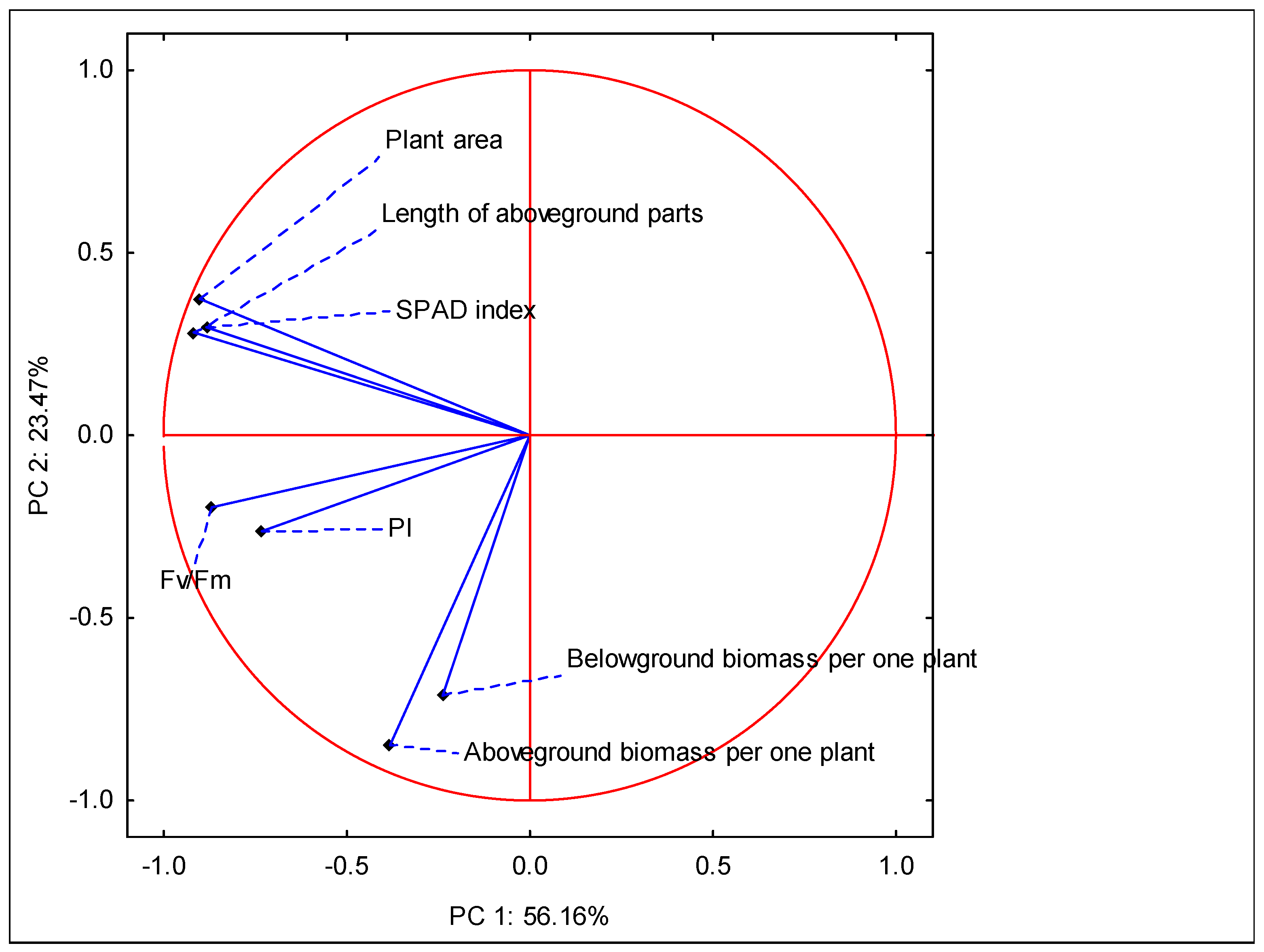
| PARAMETER | PC 1 | PC 2 |
|---|---|---|
| Eigenvalues | 3.93 | 1.64 |
| % of total variance | 56.16 | 23.47 |
| Variables | Factor loadings of variables | |
| Aboveground biomass per one plant | −0.382 | −0.849 |
| Belowground biomass per one plant | −0.239 | −0.713 |
| Plant area | −0.901 | 0.374 |
| SPAD index | −0.884 | 0.296 |
| Fv/Fm | −0.869 | −0.197 |
| PI | −0.732 | −0.262 |
| Length of aboveground parts | −0.919 | 0.282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytlarz, E.; Gala-Czekaj, D. Seed Meals from Allelopathic Crops as a Potential Bio-Based Herbicide on Herbicide-Susceptible and -Resistant Biotypes of Wild Oat (Avena fatua L.). Agronomy 2022, 12, 3083. https://doi.org/10.3390/agronomy12123083
Pytlarz E, Gala-Czekaj D. Seed Meals from Allelopathic Crops as a Potential Bio-Based Herbicide on Herbicide-Susceptible and -Resistant Biotypes of Wild Oat (Avena fatua L.). Agronomy. 2022; 12(12):3083. https://doi.org/10.3390/agronomy12123083
Chicago/Turabian StylePytlarz, Elżbieta, and Dorota Gala-Czekaj. 2022. "Seed Meals from Allelopathic Crops as a Potential Bio-Based Herbicide on Herbicide-Susceptible and -Resistant Biotypes of Wild Oat (Avena fatua L.)" Agronomy 12, no. 12: 3083. https://doi.org/10.3390/agronomy12123083






