Attenuated Isolate Gibellulopsis nigrescens Vn-1 Enhances Resistance against Verticillium dahliae in Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Pathogens
2.2. Isolate Virulence Screening
- 0 = no disease symptoms;
- 1 = several chlorotic leaves in less than 25%;
- 2 = partial necrosis and wilting, 25–50% of the diseased leaves showed symptoms;
- 3 = more than 50% of the diseased leaves showed symptoms, with a few leaves shed;
- 4 = severe wilting or death.
2.3. Vn-1-Induced Immune Response to Verticillium wilt in Potato
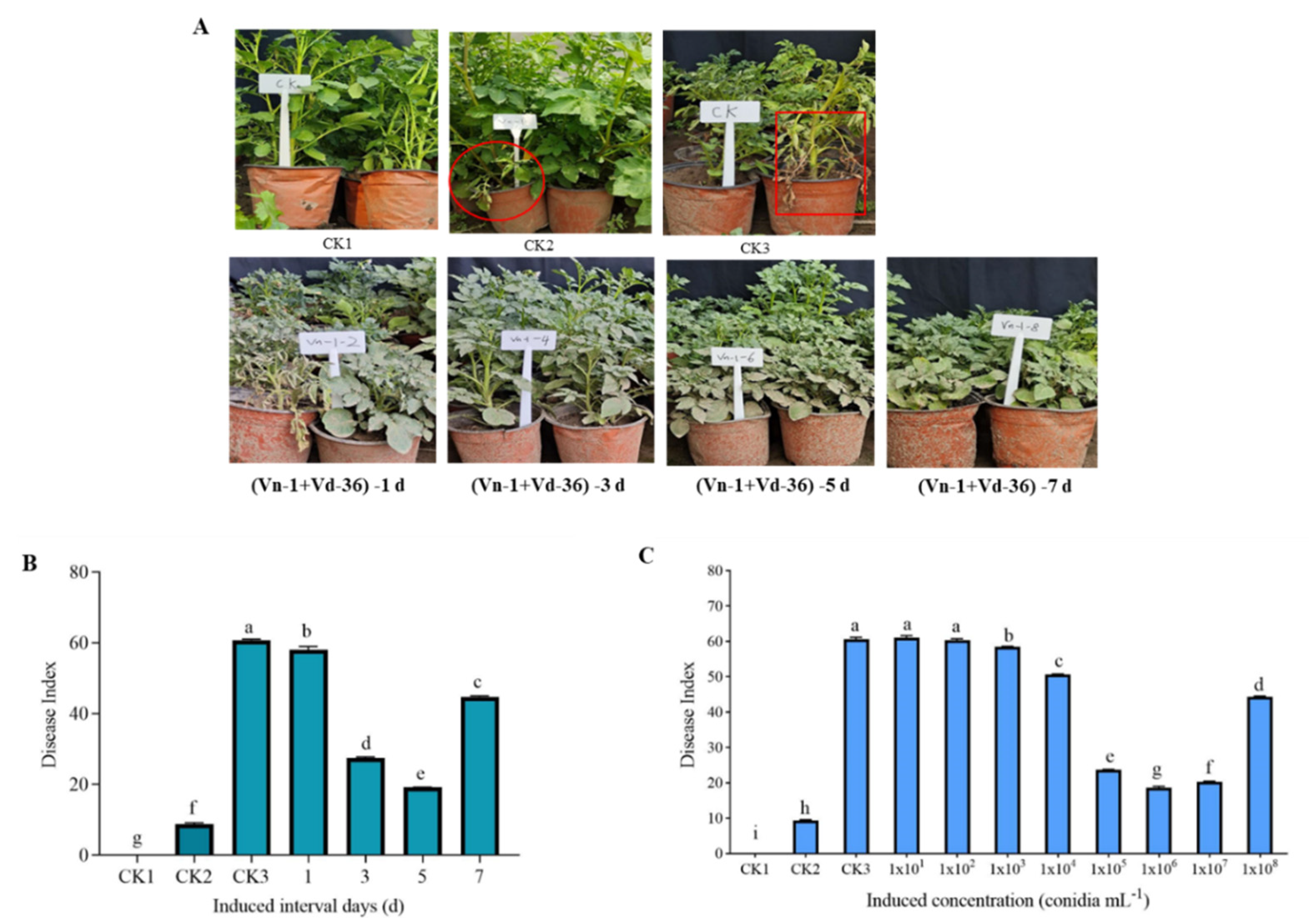
2.3.1. Optimal Interval
2.3.2. Optimum Concentration
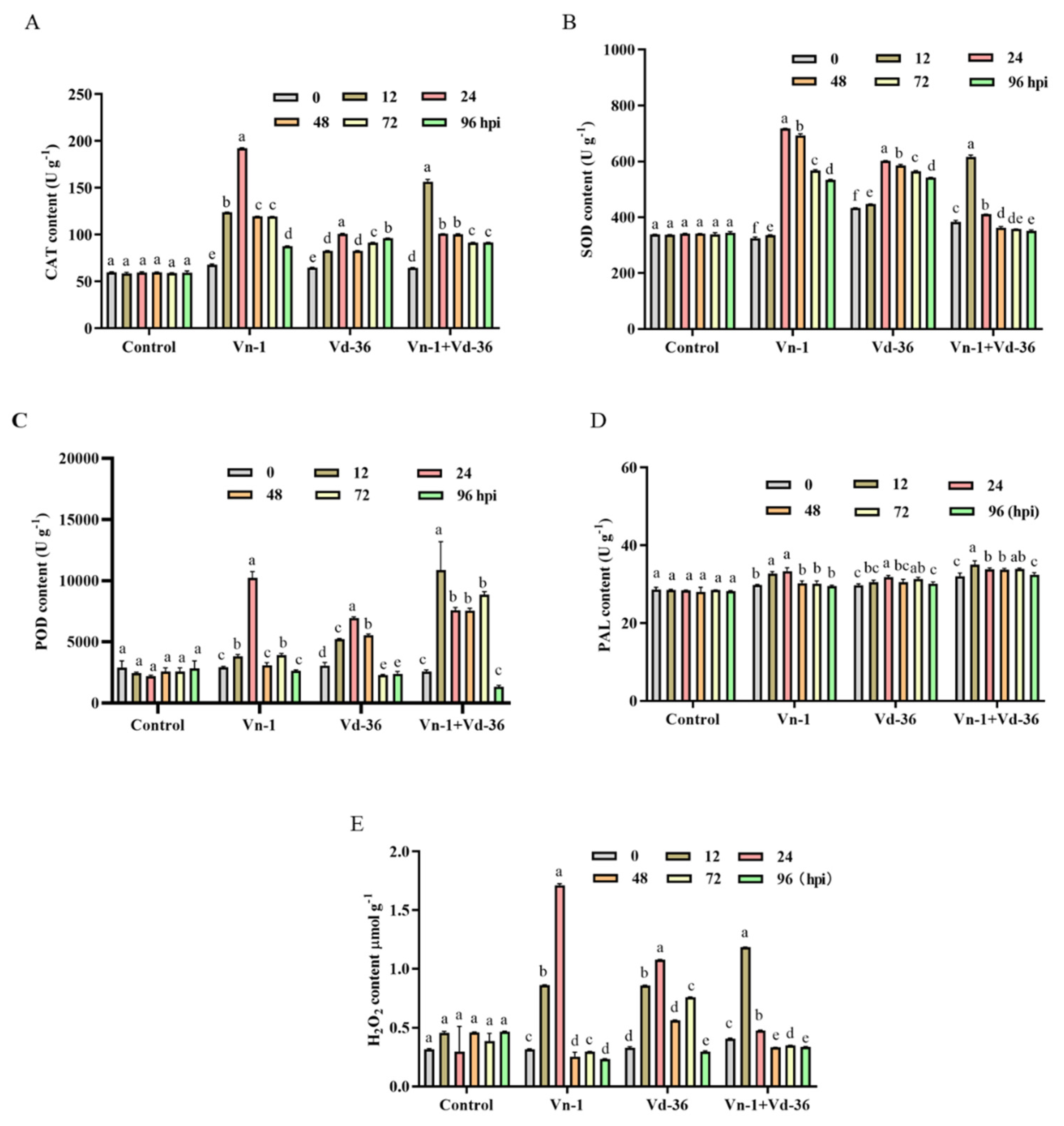
2.3.3. Reactive Oxygen Species (ROS), Hydrogen Peroxide (H2O2) Accumulation, and Respective Enzyme Activities
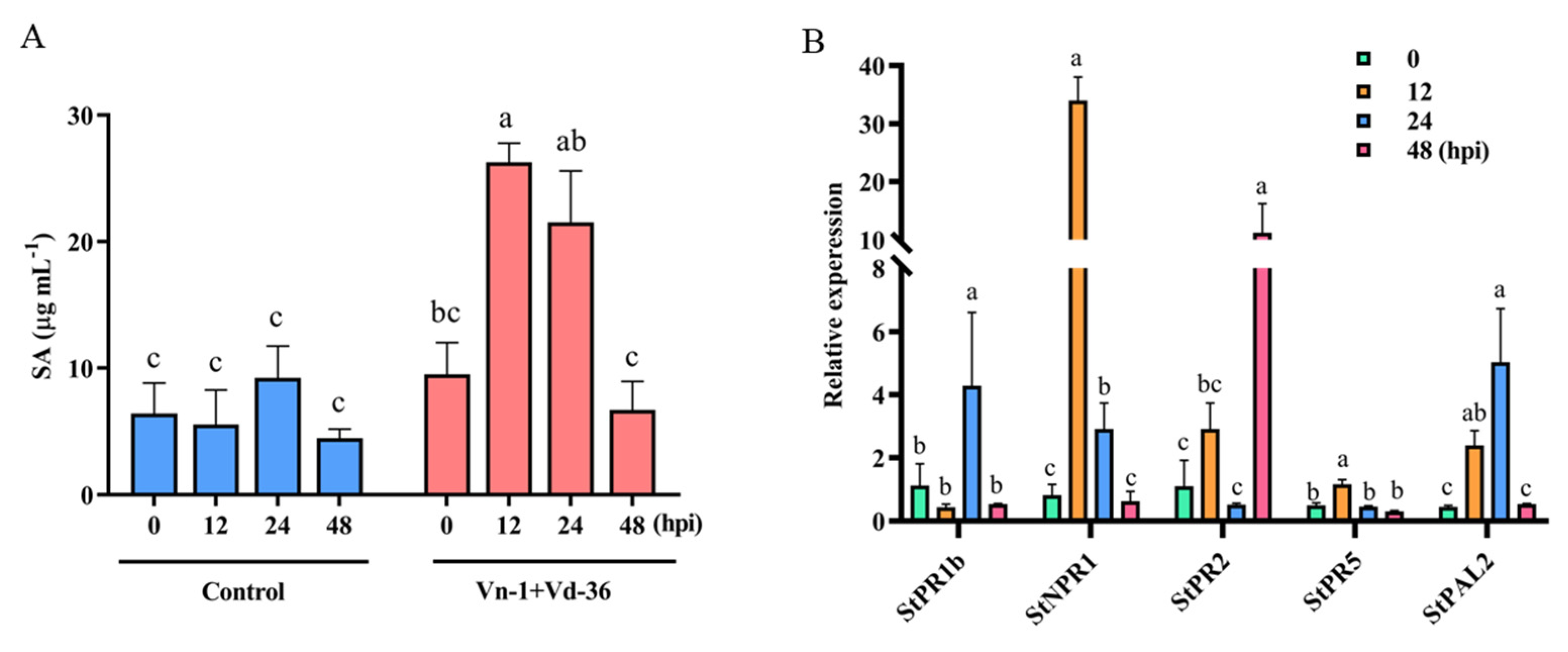
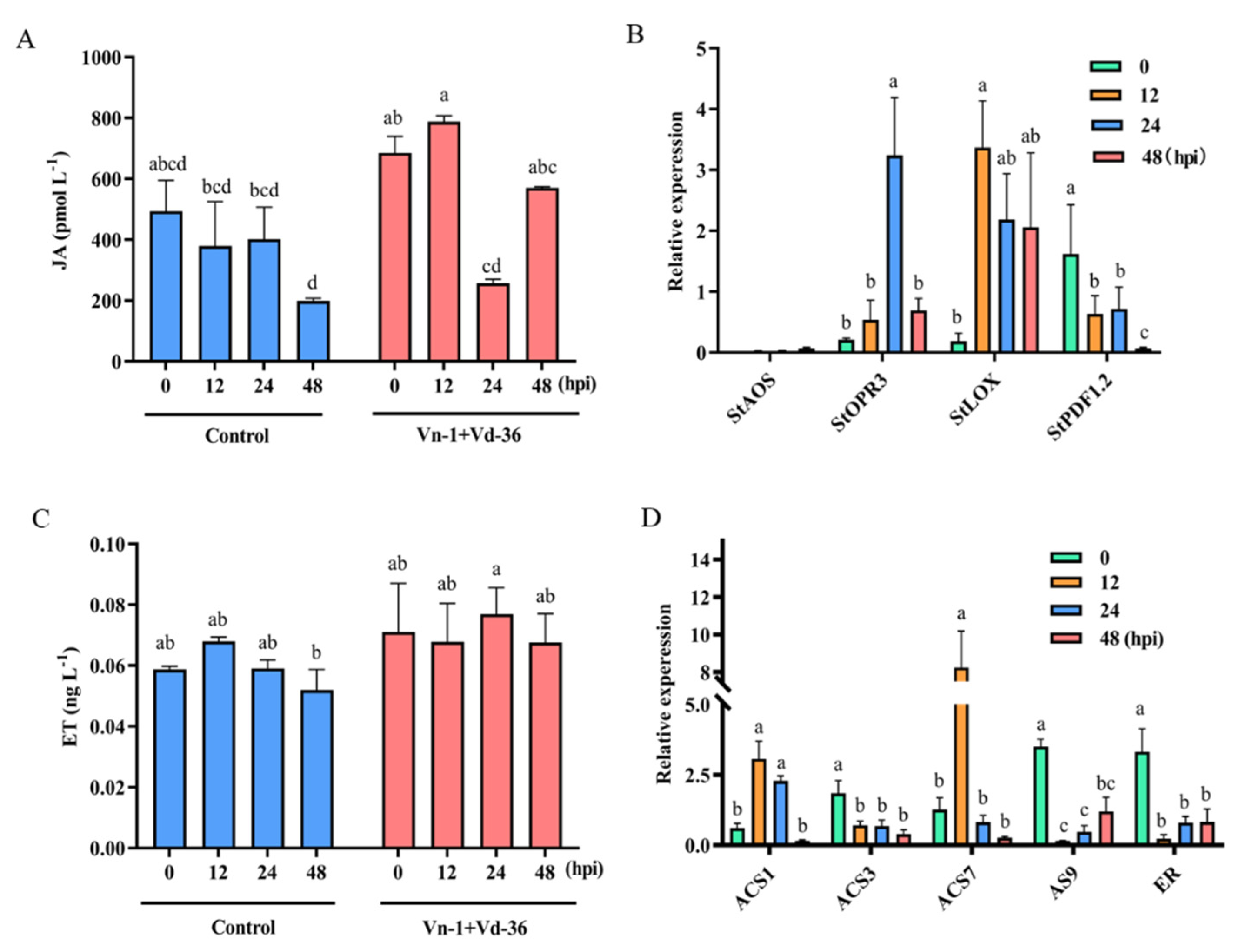
2.4. Analysis of SA, JA, and ET Signalling Pathways
2.5. Relative Expression Levels of Related Defense Genes in Signalling Pathways
2.6. Statistical Analyses
3. Results
3.1. Pathogenicity of V. dahliae
3.2. Inoculation Treatment of Attenuated Pathogen G. nigrescens Vn-1 Enhanced Potato Resistance against Verticillium Wilt
3.3. ROS Burst, H2O2 Accumulation, and Respective Enzyme Activities
3.4. H2O2 Accumulation and Related Enzyme Activities
3.5. Attenuated Vn-1 Mainly Protects Potatoes from Vd-36 Attack by Activating SA Signalling Pathway
3.6. JA/ET Signalling Pathways Are Activated Weakly by Attenuated Vn-1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez, W.; Alarcon, L.; Rojas, T.; Correa, Y.; Juarez, H.; Andrade-Piedra, J.L.; Anglin, N.L.; Elli, D. Screening South American Potato Landraces and Potato Wild Relatives for Novel Sources of Late Blight Resistance. Plant Dis. 2022, 106, 1845–1856. [Google Scholar] [CrossRef]
- Johnson, D.A.; Dung, J. Verticillium wilt of potato–the pathogen, disease and management. Can. J. Plant Pathol. 2010, 32, 58–67. [Google Scholar] [CrossRef]
- Rowe, R.C.; Davis, J.R.; Powelson, M.L.; Rouse, D.I. Potato early dying: Causal agents and management strategies. Plant Dis. 1987, 71, 482–489. [Google Scholar] [CrossRef]
- Powelson, M.L.; Rowe, R.C. Biology and management of early dying of potatoes. Annu. Rev. Phytopathol. 1993, 31, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Powelson, M.L. Potato early dying: Management challenges in a changing production environment. Plant Dis. 2002, 86, 1184–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, I. A comparative study of pathogenic isolates of Verticillium. Trans. Br. Mycol. Soc. 1949, 32, 137–157. [Google Scholar] [CrossRef]
- Skotland, C.B. Pathogenic and non-pathogenic Verticillium spp. from South Central Washington. Phytopathology 1971, 61, 435–436. [Google Scholar] [CrossRef]
- Slattery, R.J.; Eide, C.J. Prevalence of Verticillium wilt in potatoes in the Red River Valley area of Minnesota. Am. Potato J. 1980, 57, 293–299. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W.; Starink-Willemse, M.; Summerbell, R.C. Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae. Nova Hedwig. 2007, 85, 463–490. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Soliman, A.; Islam, M.R.; Adam, L.R.; Daayf, F. Verticillium dahliae’s is ochorismatase hydrolase is a virulence factor that contributes to interference with potato’s salicylate and jasmonate defense signaling. Front. Plant Sci. 2017, 8, 399. [Google Scholar] [CrossRef]
- Yun, L. Identification of Gibellulopsis Nigrescen from Sunflower and Study on its Biological Characteristics. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2014. [Google Scholar]
- Feng, J. The Molecular Regulatory Mechanism of SalicylicAcid-Primed Strawberry Resistance against Podosphaera aphanis. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar]
- Chang, M.; Chen, H.; Liu, F.; Fu, Z.Q. PTI and ETI: Convergent pathways with diverse elicitors. Trends Plant Sci. 2021, 27, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shao, S.; Zheng, C.; Sun, Z.; Shi, J.; Yu, J.; Shi, K. Induction of systemic resistance in tomato against Botrytis cinerea by N-decanoyl-homoserine lactone via jasmonic acid signaling. Planta 2018, 247, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, M.; Guo, L.; Yang, X.; Qiu, D. A novel protein elicitor (PeBA1) from Bacillus amyloliquefaciens NC6 induces systemic resistance in tobacco. Int. J. Biol. Sci. 2016, 12, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Feng, H.; Yuan, Y.; Jin, Y.; Shi, Y.; Zhang, C.; Li, F. Induced Immunity Effect and Mechanism of the Weak Pathogenicity Isolate of Verticillium dahliae Vd171 Against Verticillium Wilt in Cotton. Sci. Agric. Sin. 2018, 51, 1067–1078. [Google Scholar]
- Xue, X.; Geng, T.; Liu, H.; Yang, W.; Zhong, W.; Zhang, Z.; Zhu, C.; Chu, Z. Foliar application of silicon enhances resistance against Phytophthora infestans through the ET/JA-and NPR1-dependent signaling pathways in potato. Front. Plant Sci. 2021, 12, 609870. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic acquired resistance (SAR) and induced systemic resistance (ISR): Role and mechanism of action against phytopathogens. In Fungal Biotechnology and Bioengineering; Hesham, A.E., Upadhyay, R.S., Sharma, G.D., Manoharachary., C., Gupta, V.K., Eds.; Springer: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar]
- Bae, H.; Kim, M.S.; Sicher, R.C.; Bae, H.J.; Bailey, B.A. Necrosis-and ethylene-inducing peptide from Fusarium oxysporum induces a complex cascade of transcripts associated with signal transduction and cell death in Arabidopsis. Plant Physiol. 2006, 141, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Denoux, C.; Galletti, R.; Mammarella, N.; Gopalan, S.; Werck, D.; De Lorenzo, G.; Dewdney, J. Activation of defense response pathways by OGs and Flg22 elicitors in Arabidopsis seedlings. Mol. Plant 2008, 1, 423–445. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, M.T.; Akita, M.; Frank, W.; Reski, R.; Valkonen, J.P. Involvement of a class III peroxidase and the mitochondrial protein TSPO in oxidative burst upon treatment of moss plants with a fungal elicitor. Mol. Plant-Microbe Interact. 2012, 25, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, J.; Feng, H.; Wen, C. Study on growth promotion and signaling pathway of induced systemic resistance elicitation mediated by endophytic bacteria strain EBS05 in tomato plant. J. Henan Agric. Univ. 2018, 45, 1212–1219. [Google Scholar]
- Zhao, X.; Zhang, J.; Zhang, G.; Zhang, Y.; Zhou, H.; Zhao, J. Identification of the vegetative compatibility groups (VCGs), races, mating types, and pathogenicity differentiation of pathogenic bacteria of potato Verticillium wilt. J. Plant Prot. 2018, 45, 1212–1219. [Google Scholar]
- Davis, J.R.; Huisman, O.C.; Everson, D.O.; Nolte, P.; Sorensen, L.H.; Schneider, A.T. Ecological relationships of Verticillium wilt suppression of potato by green manures. Am. J. Potato Res. 2010, 87, 315–326. [Google Scholar] [CrossRef]
- Ochiai, N.; Powelson, M.L.; Dick, R.P.; Crowe, F.J. Effects of green manure type and amendment rate on Verticillium wilt severity and yield of Russet Burbank potato. Plant Dis. 2007, 91, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y. The Mechanism of Resistance to Verticillium Wilt on Sunflower Induced by Gibellulopsis nigrescens Vn-1. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2014. [Google Scholar]
- When, C.; Zhao, Y.; Dong, B.; Meng, H.; Zhao, J.; Zhou, H. The resistant identification to Vertichillium wilt for main cultivars of potato in Inner Monglia. J. Plant Prot. 2018, 45, 1220–1226. [Google Scholar]
- Hao, J.; Wang, D.; Hussain, A.; Wang, Y.; Zhou, H. Techniques for Evaluating Early Pathogenicity Detection of Potato Verticillium Wilt. PAKJAS, 2022; submitted. [Google Scholar]
- de Oliveira, M.V.; Xu, G.; Li, B.; de Souza Vespoli, L.; Meng, X.; Chen, X.; He, P. Specific control of Arabidopsis BAK1/SERK4-regulated cell death by protein glycosylation. Nat. Plants 2016, 2, 15218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. Oxidative stress and antioxidative systems: Recipes for successful data collection and interpretation. Plant Cell Environ. 2016, 39, 1140–1160. [Google Scholar] [CrossRef] [Green Version]
- Elbasuney, S.; El-Sayyad, G.S.; Attia, M.S.; Abdelaziz, A.M. Ferric oxide colloid: Towards green nano-fertilizer for tomato plant with enhanced vegetative growth and immune response against fusarium wilt disease. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4270–4283. [Google Scholar] [CrossRef] [PubMed]
- Miles, T.D.; Schilder, A.C. Host defenses associated with fruit infection by Colletotrichum species with an emphasis on anthracnose of blueberries. Plant Health Prog. 2013, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, S.; Wang, M.; Zhang, J.; Yang, M.; Dong, W. Expression of rice immunity genes involved in interaction between attenuated Rhizoctonia solani and rice. China Plant Prot. 2019, 39, 5–14. [Google Scholar]
- Zeier, J. Metabolic regulation of systemic acquired resistance. Curr. Opin. Plant Biol. 2021, 62, 102050. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, C.; Yong, D.; Li, G.; Dong, X.; Li, B. Induction of resistance mediated by an attenuated strain of Valsa mali var. mali using pathogen-apple callus interaction system. Sci. World J. 2014, 2014, 201382. [Google Scholar] [CrossRef] [Green Version]
- Ju, L.; Liu, Y.; Yun, X. Ctontent Variations of Several Physiological and Biochemical Substances in Cucumber Leaves Inoculated with the Attenuated Strains for Fusarium oxysporum f. sp. Cucumerinum. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2016, 37, 10–16. [Google Scholar]
- Liu, J.; Wang, Y.; Yun, X. PIAS Induced Resistance to Tomato Late Blight and Enzyme Activity in Leaves. J. Inn. Mong. Agric. Univ. (Nat. Sci. Ed.) 2021, 42, 12–19. [Google Scholar]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Frotiers Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef]
- Duan, Y.; Jiang, Y.; Ye, S.; Karim, A.; Ling, Z.; He, Y.; Luo, K. PtrWRKY73, a salicylic acid-inducible poplar WRKY transcription factor, is involved in disease resistance in Arabidopsis thaliana. Plant Cell Rep. 2015, 34, 831–841. [Google Scholar] [CrossRef]
- Abdullah-Zawawi, M.R.; Ahmad-Nizammuddin, N.F.; Govender, N.; Harun, S.; Mohd-Assaad, N.; Mohamed-Hussein, Z.A. Comparative genome-wide analysis of WRKY, MADS-box and MYB transcription factor families in Arabidopsis and rice. Sci. Rep. 2021, 11, 19678. [Google Scholar] [CrossRef]
- Arena, G.D.; Ramos-Gonzalez, P.L.; Nunes, M.A.; Ribeiro-Alves, M.; Camargo, L.E.; Kitajima, E.W.; Freitas-Astua, J. Citrus leprosis virus C infection results in hypersensitive-like response, suppression of the JA/ET plant defense pathway and promotion of the colonization of its mite vector. Front. Plant Sci. 2016, 7, 1757. [Google Scholar] [CrossRef] [Green Version]
- Rasoolizadeh, A.; Labbé, C.; Sonah, H.; Deshmukh, R.K.; Belzile, F.; Menzies, J.G. Silicon protects soybean plants against Phytophthora sojae by interfering with effector-receptor expression. BMC Plant Biol 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hong, Y.; Yi, T.; Li, D. Advances in biological control of tomato bacterial wilt. Plant Prot. 2007, 2, 15–20. [Google Scholar]
- Qiu, D. Progress and Prospect of Plant Immunity Inducer. J. Agric. Sci. Technol. 2014, 16, 39–45. [Google Scholar]
- Zheng, X.; Zhu, Y.; Liu, B.; Yu, Q.; Lin, N. Rapid differentiation of Ralstonia solanacearum avirulent and virulent strains by cell fractioning of an isolate using high performance liquid chromatography. Microb. Pathog. 2016, 90, 84–92. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Y.; Liu, B.; Ge, C. Preparation of colloidal suspension agent used as plant vaccine against tomato bacterial wiltdisease and its control efficacy. Plant Prot. 2017, 43, 208–211. [Google Scholar]


| Isolate | Disease Index | Isolate | Disease Index | Isolate | Disease Index |
|---|---|---|---|---|---|
| Vd-1 | 40.36 | Vd-25 | 37.31 | Vd-49 | 43.28 |
| Vd-2 | 44.83 | Vd-26 | 40.22 | Vd-50 | 62.26 |
| Vd-3 | 38.4 | Vd-27 | 31.93 | Vd-51 | 33.02 |
| Vd-4 | 39.46 | Vd-28 | 32.22 | Vd-52 | 38.34 |
| Vd-5 | 43.99 | Vd-29 | 25.07 | Vd-53 | 34.95 |
| Vd-6 | 30.32 | Vd-30 | 28.77 | Vd-54 | 34.76 |
| Vd-7 | 29.11 | Vd-31 | 33.33 | Vd-55 | 40.7 |
| Vd-8 | 31.27 | Vd-32 | 36.16 | Vd-56 | 39.04 |
| Vd-9 | 18.21 | Vd-33 | 36.57 | Vd-57 | 41.1 |
| Vd-10 | 17.28 | Vd-34 | 34 | Vd-58 | 39.93 |
| Vd-11 | 31.08 | Vd-35 | 44.09 | Vd-59 | 39.82 |
| Vd-12 | 30.95 | Vd-36 * | 65.62 * | Vd-60 | 46.77 |
| Vd-13 | 28.87 | Vd-37 | 50.62 | Vd-61 | 41.76 |
| Vd-14 | 31.45 | Vd-38 | 38.18 | Vd-62 | 40.73 |
| Vd-15 | 54.24 | Vd-39 | 42.02 | Vd-63 | 48.13 |
| Vd-16 | 40.37 | Vd-40 | 44.57 | Vd-64 | 43.34 |
| Vd-17 | 32.82 | Vd-41 | 38.04 | Vd-65 | 22.06 |
| Vd-18 | 40.2 | Vd-42 | 31.09 | Vd-66 | 17.18 |
| Vd-19 | 45.23 | Vd-43 | 36.13 | Vd-67 | 53 |
| Vd-20 | 31.18 | Vd-44 | 31.8 | Vd-68 | 45.93 |
| Vd-21 | 28.89 | Vd-45 | 36.31 | Vd-69 | 52.5 |
| Vd-22 | 40.9 | Vd-46 | 40.1 | Vd-70 | 37.02 |
| Vd-23 | 28.03 | Vd-47 | 46.98 | Vd-71 | 51.43 |
| Vd-24 | 45.35 | Vd-48 | 35 | Vd-72 | 30.42 |
| Treatments | Inoculation Mode | Inoculation Concentration | Inoculation Dosage |
|---|---|---|---|
| Vn-1 + Vd-36 | Potatoes inoculated with Vn-1 for 5 d and then challenged with Vd-36 | Vn-1: 1 × 106 conidia mL−1 Vd-36: 1 × 107 conidia mL−1 | 20 mL 20 mL |
| Vn-1 | Potatoes inoculated with Vn-1 alone | 1 × 107 conidia mL−1 | 20 mL |
| Vd-36 | Potatoes inoculated with Vd-36 alone | 1 × 107 conidia mL−1 | 20 mL |
| CK | Potatoes inoculated with sterile water | 20 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Wang, D.; Wang, Y.; Zhou, H. Attenuated Isolate Gibellulopsis nigrescens Vn-1 Enhances Resistance against Verticillium dahliae in Potato. Agronomy 2022, 12, 3082. https://doi.org/10.3390/agronomy12123082
Hao J, Wang D, Wang Y, Zhou H. Attenuated Isolate Gibellulopsis nigrescens Vn-1 Enhances Resistance against Verticillium dahliae in Potato. Agronomy. 2022; 12(12):3082. https://doi.org/10.3390/agronomy12123082
Chicago/Turabian StyleHao, Jianxiu, Dong Wang, Yu Wang, and Hongyou Zhou. 2022. "Attenuated Isolate Gibellulopsis nigrescens Vn-1 Enhances Resistance against Verticillium dahliae in Potato" Agronomy 12, no. 12: 3082. https://doi.org/10.3390/agronomy12123082
APA StyleHao, J., Wang, D., Wang, Y., & Zhou, H. (2022). Attenuated Isolate Gibellulopsis nigrescens Vn-1 Enhances Resistance against Verticillium dahliae in Potato. Agronomy, 12(12), 3082. https://doi.org/10.3390/agronomy12123082





