Honey Bee Exposure to the Fungicide Propiconazole in Lowbush Blueberry Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Propiconazole Exposure Experiments (2009–2013)
2.1.1. Propiconazole Residues on Lowbush Blueberry Flowers in Fields in Bloom (2009–2011)
2.1.2. Degradation of Propiconazole in the Field from the Time of Application until the End of Bloom (2010–2011)
2.1.3. Bioassay for Estimating Acute Contact Mortality in Worker Honey Bees (2013)
2.2. Experiment on the Detrimental Effects of Exposure to Propiconazole (2011–2013)
- (1)
- higher spring queen supersedure rates during bloom;
- (2)
- lower colony brood and worker population sizes during and after bloom;
- (3)
- lower queen oviposition rates during bloom;
- (4)
- lower egg proportion hatch during bloom;
- (5)
- lower brood proportion survival, reared after bloom;
- (6)
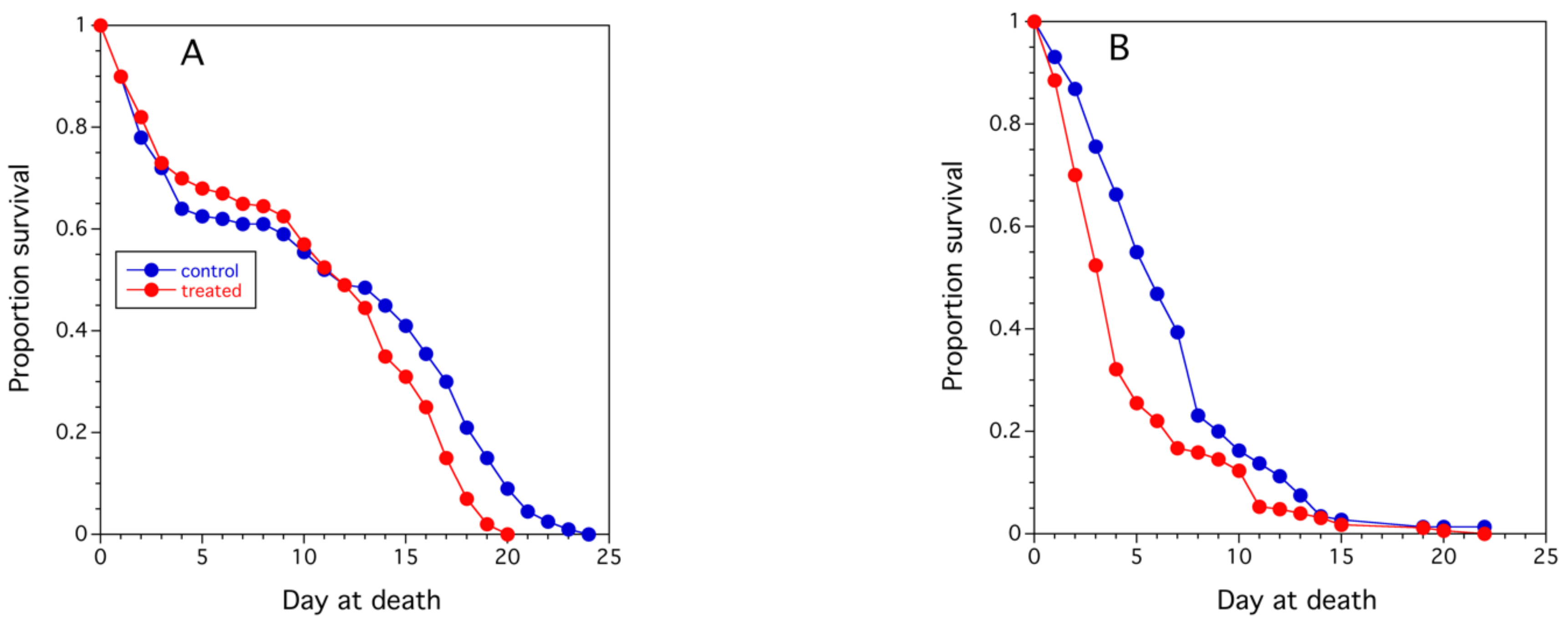
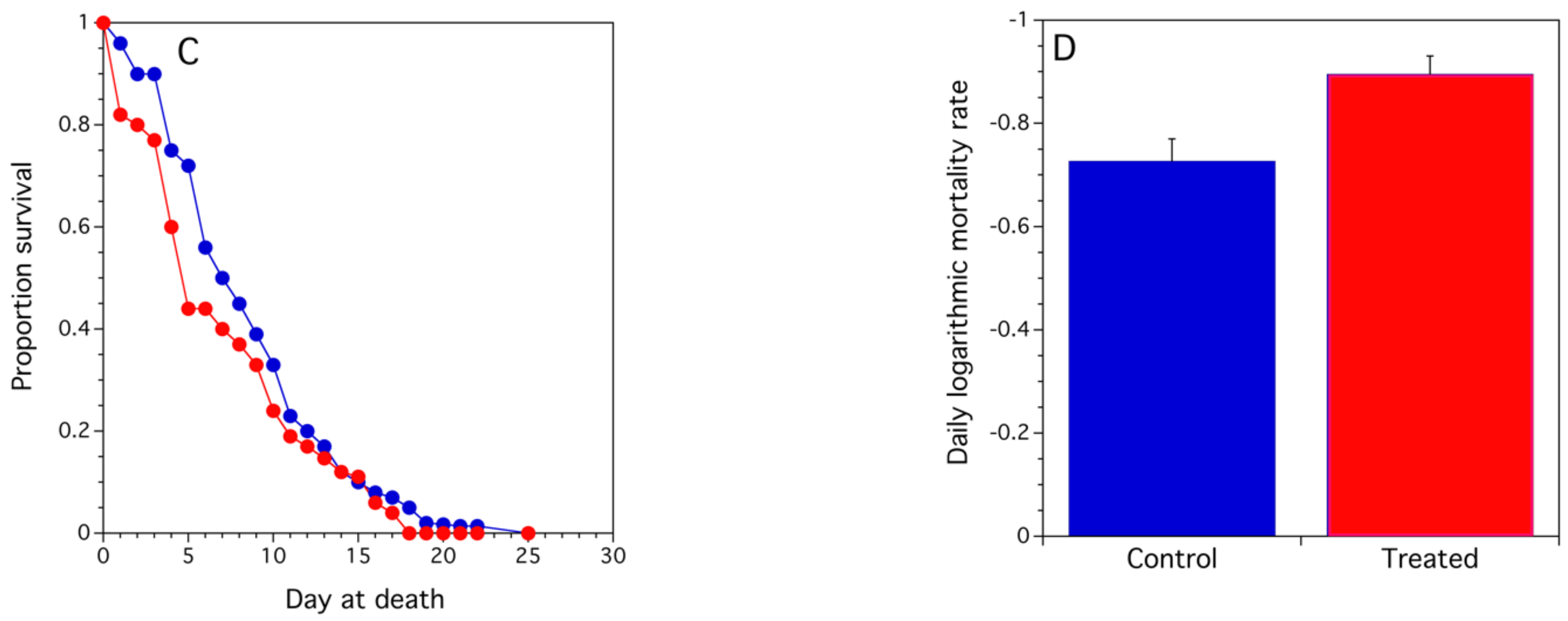
- shorter worker longevity after bloom;
- (7)
- lower royal jelly deposition in hives during bloom;
- (8)
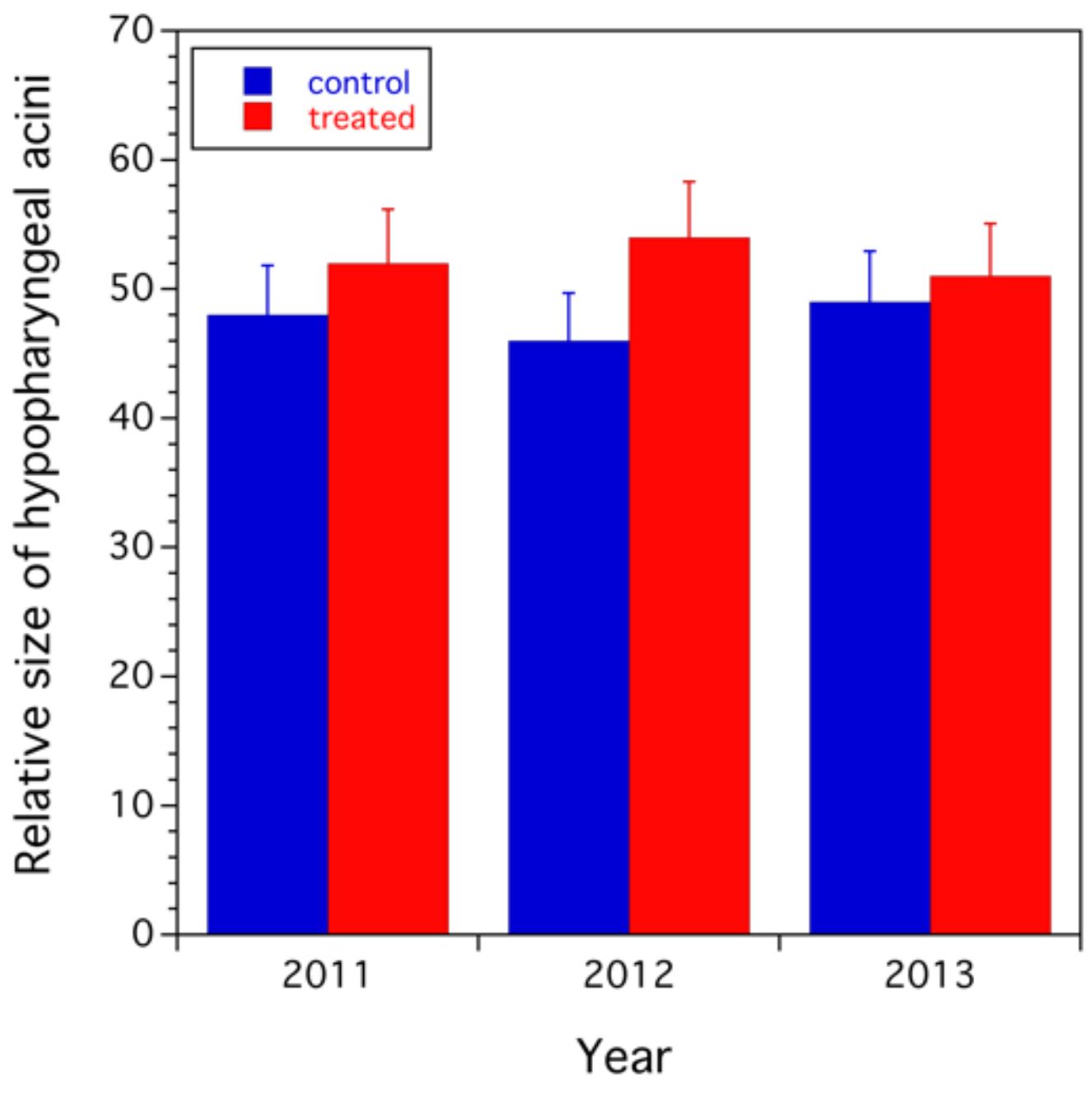
- different hypopharyngeal gland biomass of recently emerged workers;
- (9)
- lower numbers of worker foragers returning back to the hive during bloom;
- (10)
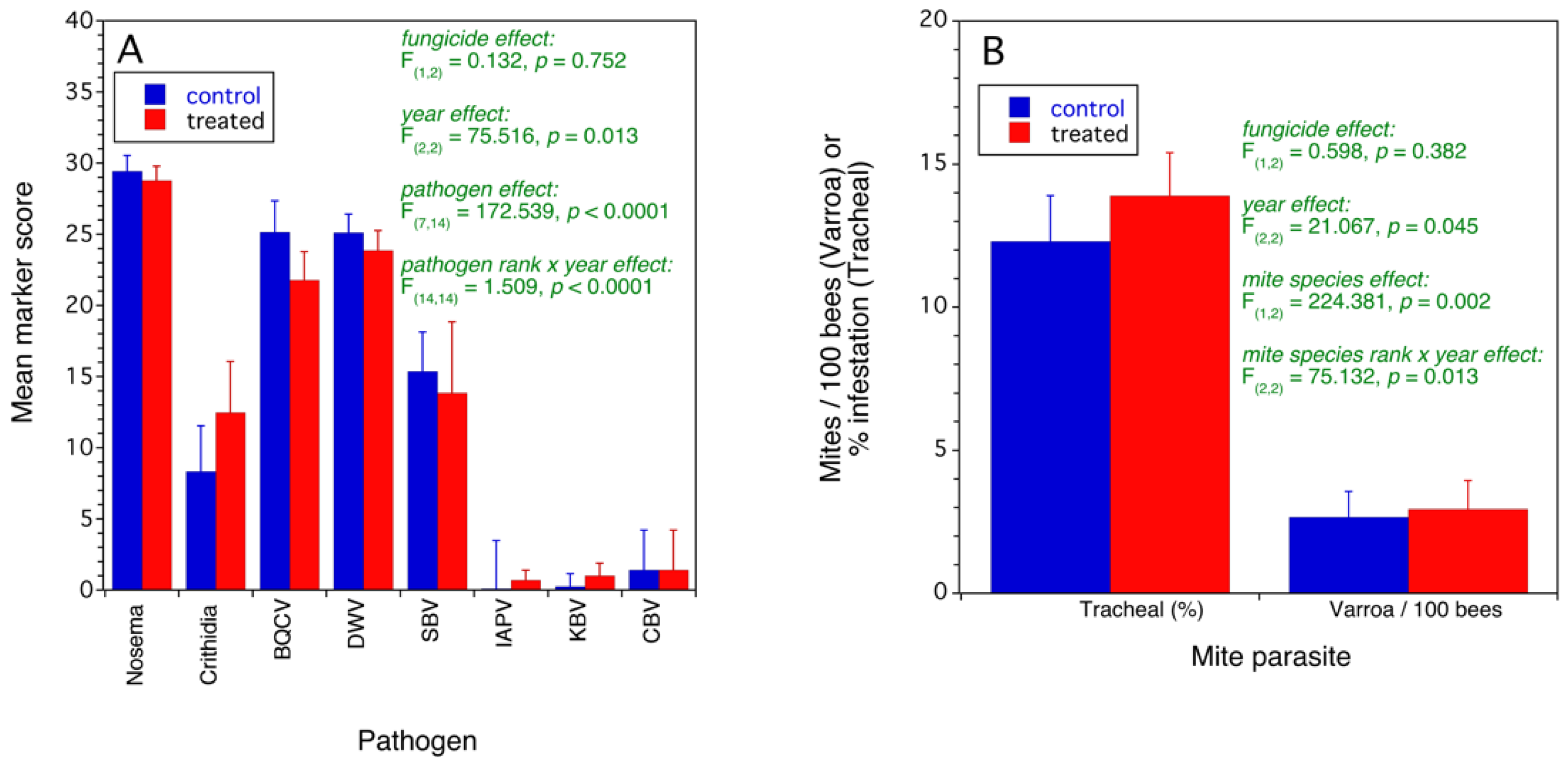
- higher Varroa and tracheal mite abundances;
- (11)
- higher virus, protozoan, and fungal disease incidence based upon molecular markers and;
- (12)
- higher colony losses over the winter and during the subsequent early spring.
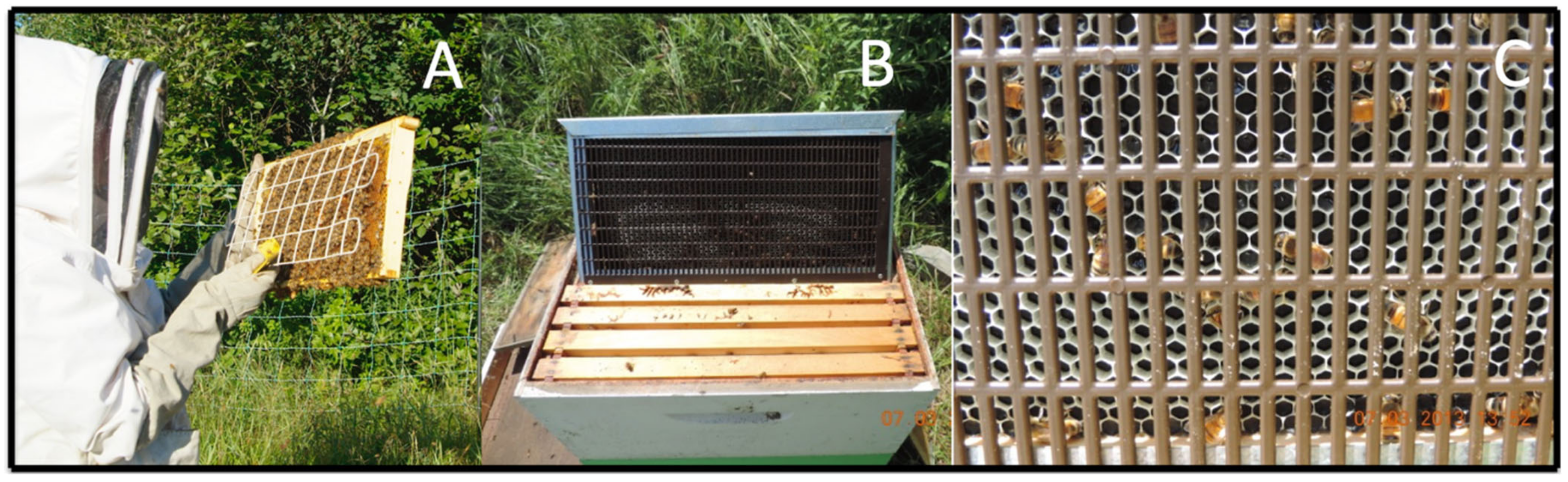
2.2.1. Propiconazole Residues on Flowers, Trapped Honey Bee Pollen, and Foraging Honey Bees (2011–2013)
2.2.2. Assessment of Colony Health (2011–2013)
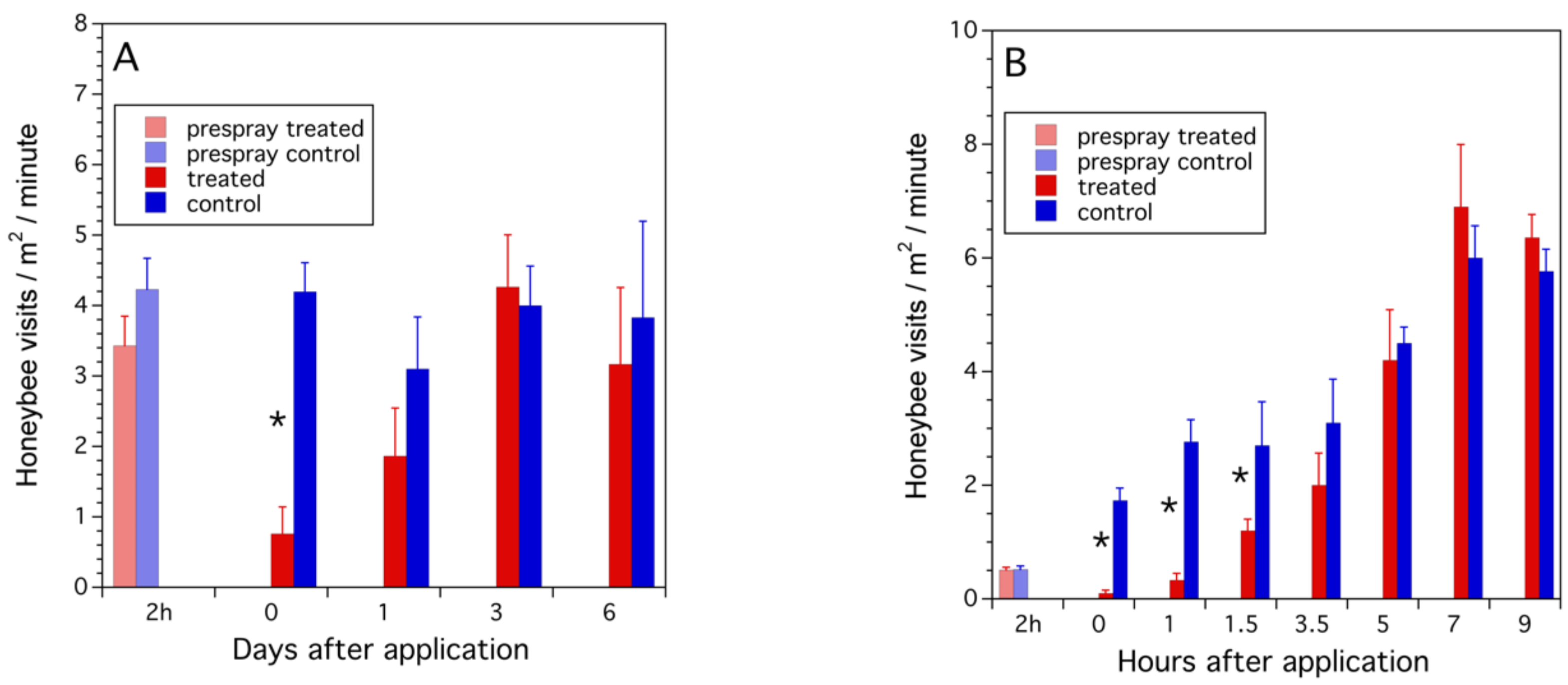
2.2.3. Repellency of Bloom Sprayed with Propiconazole (2013–2014)
3. Results
3.1. Propiconazole Exposure Experiments
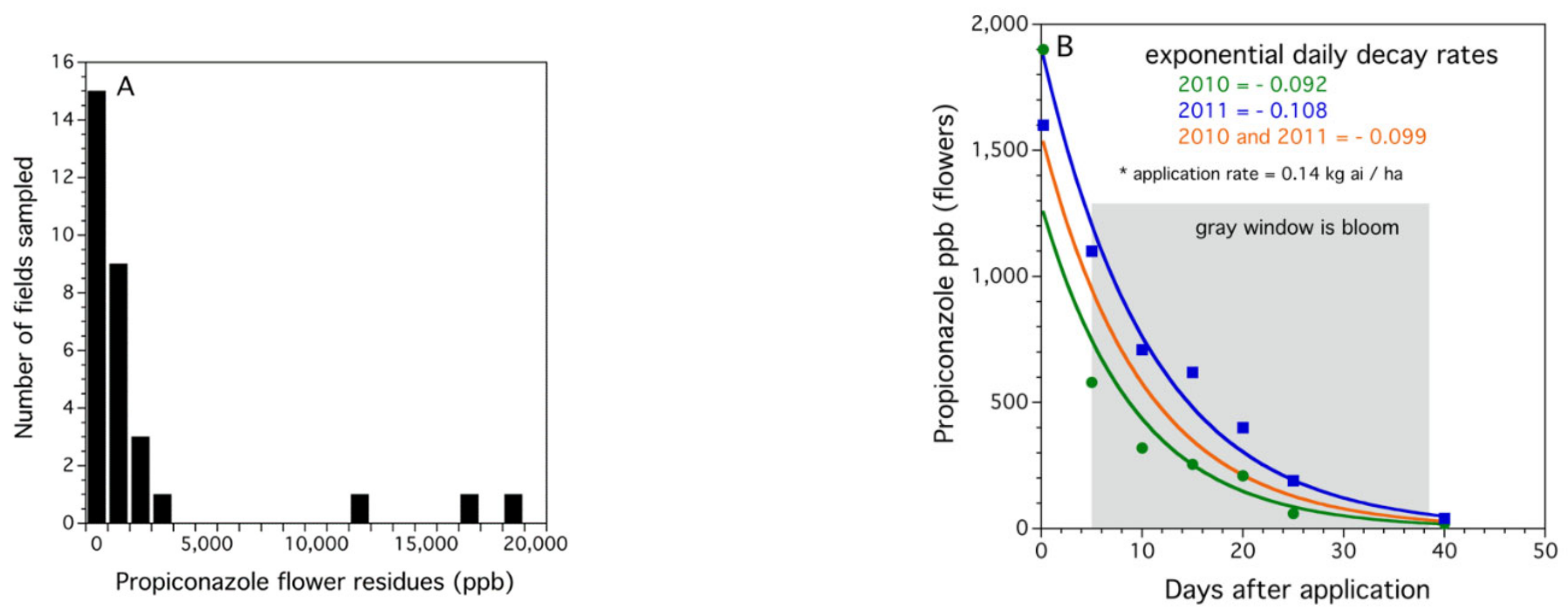
3.1.1. Propiconazole Residues on Lowbush Blueberry Flowers in Fields in Bloom
3.1.2. Degradation of Propiconazole in the Field from the Time of Application until the End of Bloom
3.1.3. Bioassay for Estimating Acute Contact Mortality in Worker Honey Bees
3.2. Experiment on the Detrimental Effects of Exposure to Propiconazole
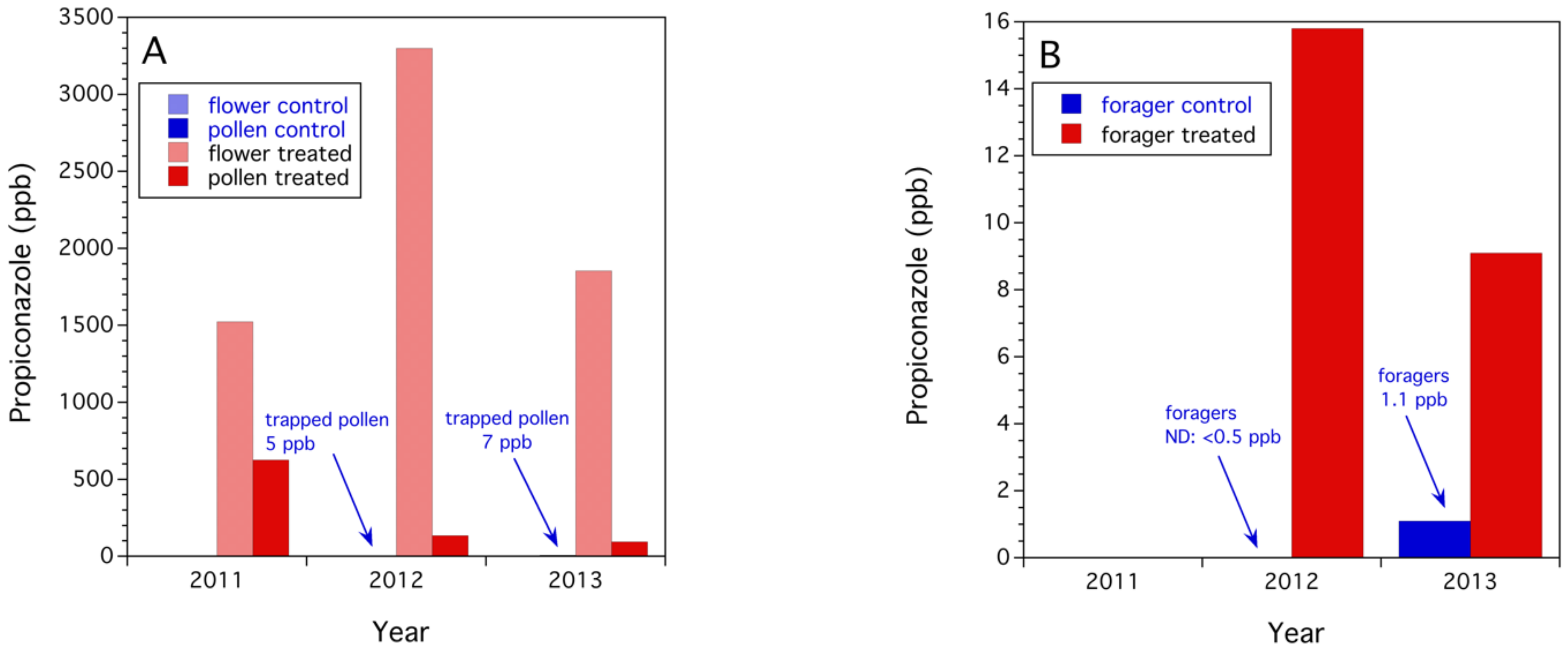
3.2.1. Propiconazole Residues on Flowers, Trapped Honey Bee Pollen, and Foraging Honey Bees
3.2.2. Assessment of Colony Health
3.2.3. Repellency of Bloom Sprayed with Propiconazole
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Penman, L.N.; Annis, S.L. Leaf and flower blight caused by Monilinia vaccinii-corymbosi on lowbush blueberry: Effects on yield and relationship to bud phenology. Phytopathology 2005, 95, 1174–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batra, L.R.; Batra, S.W. Floral mimicry induced by mummy-berry fungus exploits host’s pollinators as vectors. Science 1985, 228, 1011–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngugi, H.K.; Scherm, H. Pollen mimicry during infection of blueberry flowers by conidia of Monilinia vaccinii-corymbosi. Physiol. Mol. Plant Pathol. 2004, 64, 113–123. [Google Scholar] [CrossRef]
- McGovern, K.B.; Annis, S.L.; Yarborough, D.E. Efficacy of organically acceptable materials for control of mummy berry disease on lowbush blueberries in Maine. Int. J. Fruit Sci. 2012, 12, 188–204. [Google Scholar] [CrossRef]
- Fisher II, A.; DeGrandi-Hoffman, G.; Smith, B.H.; Ozturk, C.; Kaftanoglu, O.; Fewell, J.H.; Harrison, J.F. Field cross-fostering and in vitro rearing demonstrate negative effects of both larval and adult exposure to a widely used fungicide in honey bees (Apis mellifera). Ecotoxicol. Environ. Saf. 2021, 217, 112251. [Google Scholar] [CrossRef]
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Shepherd, W.S. Pesticide exposure to honey bees in a four-year nationwide study. Insects 2019, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Mussen, E.C.; Lopez, J.E.; Peng, C.Y. Effects of selected fungicides on growth and development of larval honey bees, Apis mellifera L. (Hymenoptera: Apidae). Environ. Entomol. 2004, 33, 1151–1154. [Google Scholar] [CrossRef] [Green Version]
- Atkins, E.L.; Kellum, D. Comparative morphogenic and toxicity studies on the effect of pesticides on honeybee brood. J. Apic. Res. 1986, 25, 242–255. [Google Scholar] [CrossRef]
- Ladurner, E.; Bosch, J.; Kemp, W.P.; Maini, S. Assessing delayed and acute toxicity of five formulated fungicides to Osmia lignaria Say and Apis mellifera. Apidologie 2005, 36, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, 77547. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Disruption of quercetin metabolism by fungicide affects energy production in honey bees (Apis mellifera). Proc. Natl. Acad. Sci. USA 2017, 114, 2538–2543. [Google Scholar] [CrossRef] [Green Version]
- Belsky, J.; Joshi, N.K. Effects of fungicide and herbicide chemical exposure on Apis and non-Apis bees in agricultural landscape. Front. Environ. Sci. 2020, 8, 81. [Google Scholar] [CrossRef]
- Christen, V.; Krebs, J.; Fent, K. Fungicides chlorothanolin, azoxystrobin and folpet induce transcriptional alterations in genes encoding enzymes involved in oxidative phosphorylation and metabolism in honey bees (Apis mellifera) at sublethal concentrations. J. Hazard. Mater. 2019, 377, 215–226. [Google Scholar] [CrossRef]
- Traynor, K.S.; Van Englesdorp, D.; Lamas, Z.S. Social disruption: Sublethal pesticides in pollen lead to Apis mellifera queen events and brood loss. Ecotoxicol. Environ. Saf. 2021, 214, 112105. [Google Scholar] [CrossRef]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar] [CrossRef]
- DesJardins, N.S.; Fisher II, A.; Ozturk, C.; Fewell, J.H.; DeGrandi-Hoffman, G.; Harrison, J.F.; Smith, B.H. A common fungicide, Pristine®, impairs olfactory associative learning performance in honey bees (Apis mellifera). Environ. Pollut. 2021, 288, 117720. [Google Scholar] [CrossRef]
- Annis, S.; Schwab, J.; Tooley, B.; Calderwood, L. 2022 Pest Management Guide: Disease. 2022. Available online: https://extension.umaine.edu/blueberries/wp-content/uploads/sites/41/2022/02/2022-fungicide-chart.pdf. (accessed on 31 October 2022).
- Jørgensen, L.N.; Heick, T.M. Azole use in agriculture, horticulture, and wood preservation–is it indispensable? Front. Cell Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef]
- Price, C.L.; Parker, J.E.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. Azole fungicides-understanding resistance mechanisms in agricultural fungal pathogens. Pest Manag. Sci. 2015, 71, 1054–1058. [Google Scholar] [CrossRef]
- Siegel, M.R. Sterol-inhibiting fungicides: Effects on sterol biosynthesis and sites of action. Plant Dis. 1981, 65, 986–989. [Google Scholar] [CrossRef]
- Buchenhauer, H. Modern Selective Fungicides; Lyr, H., Ed.; VEB Gustav Fischer Verlag: Jena, Germany; Longman Group UK Ltd.: London, UK, 1987; pp. 205–232. [Google Scholar]
- Rutkowski, D.; Litsey, E.; Maalouf, I.; Vannette, R.L. Bee-associated fungi mediate effects of fungicides on bumble bees. Ecol. Entomol. 2022, 47, 411–422. [Google Scholar] [CrossRef]
- Chalvet-Monfray, K.; Sabatier, P.; Belzunces, L.P.; Colin, M.E.; Fléché, C. Synergy between deltamethrin and prochloraz in bees: A modeling approach. Environ. Toxicol. Chem. Int. J. 1996, 15, 525–534. [Google Scholar] [CrossRef]
- Vandame, R.; Belzunces, L.P. Joint actions of deltamethrin and azole fungicides on honey bee thermoregulation. Neurosci. Lett. 1998, 251, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Papaefthimiou, C.; Theophilidis, G. The cardiotoxic action of the pyrethroid insecticide deltamethrin, the azole fungicide prochloraz, and their synergy on the semi-isolated heart of the bee Apis mellifera macedonica. Pestic. Biochem. Physiol. 2001, 69, 77–91. [Google Scholar] [CrossRef]
- Haas, J.; Nauen, R. Pesticide risk assessment at the molecular level using honey bee cytochrome P450 enzymes: A complementary approach. Environ. Int. 2021, 147, 106372. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Motoyama, N.; Ambrose, J.T.; Roe, R.M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot. 2004, 23, 371–378. [Google Scholar] [CrossRef]
- Bart, S.; Short, S.; Jager, T.; Eagles, E.J.; Robinson, A.; Badder, C.; Lahive, E.; Spurgeon, D.J.; Ashauer, R. How to analyse and account for interactions in mixture toxicity with toxicokinetic-toxicodynamic models. Sci. Total Environ. 2022, 843, 157048. [Google Scholar] [CrossRef]
- Drummond, F. Reproductive biology of wild blueberry (Vaccinium angustifolium Aiton). Agric. Spec. Issue N. Am. Nativ. Food Crops 2019, 9, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- SAS Institute. JMP(R), Version 16; SAS Institute Inc.: Cary, NC, USA, 2022. [Google Scholar]
- Drummond, F.A.; Ballman, E.S.; Eitzer, B.D.; Du Clos, B.; Dill, J. Exposure of honeybee colonies to pesticides in pollen, a statewide survey in Maine. Environ. Entomolology 2018, 47, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Delaplane, K.S.; Van der Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Souza Cunha, A.E.; Rose, J.; Prior, J.; Aumann, H.; Emanetoglu, N.; Drummond, F.A. A novel non-invasive radar to monitor honey bee colony health. Comput. Electron. Agric. 2020, 170, 105241. [Google Scholar] [CrossRef]
- Miranda, M.A.; Bicout, D.; Bøtner, A.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Gortazar Schmidt, C.; et al. EFSA Panel on Animal Health and Welfare (AHAW). Assessing the health status of managed honeybee colonies (HEALTHY-B): A toolbox to facilitate harmonised data collection. J. Eur. Food Saf. 2016, 14, e04578. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2016.4578 (accessed on 27 October 2022).
- Ahmad, S.; Khan, S.A.; Khan, K.A.; Li, J. Novel insight into the development and function of hypopharyngeal glands in honey bees. Front. Physiol. 2021, 11, 615830. [Google Scholar] [CrossRef]
- Frazier, M.; Mullin, C.; Frazier, J.; Ashcraft, S.; Leslie, T.; Mussen, E.; Drummond, F. Assessing honey bee (Apis mellifera L.) foraging populations and the potential impact of pesticides on eight U.S. Crops. J. Econ. Entomol. 2015, 108, 2141–2152. [Google Scholar] [CrossRef] [Green Version]
- Hendrickson, R. Field alcohol wash provides a consistent sampling method for determining colony Varroa mite loads. Am. Bee J. 2009, 149, 55–56. [Google Scholar]
- Sammataro, D. An easy dissection technique for finding the tracheal mite, Acarapis woodi (Rennie)(Acari: Tarsonemidae), in honey bees, with video link. Int. J. Acarol. 2006, 32, 339–343. [Google Scholar] [CrossRef]
- Cullen, M.G.; Thompson, L.J.; Carolan, J.C.; Stout, J.C.; Stanley, D.A. Fungicides, herbicides and bees: A systematic review of existing research and methods. PLoS ONE 2019, 14, e0225743. [Google Scholar] [CrossRef] [Green Version]
- Steffan-Dewenter, I.; Kuhn, A. Honeybee foraging in differentially structured landscapes. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Butler, C.G.; Haigh, J.C. A note on the use of honey-bees as pollinating agents in cages. J. Hortic. Sci. 1956, 31, 295–297. [Google Scholar] [CrossRef]
- Kubik, M.; Nowacki, J.; Pidek, A.; Warakomska, Z.; Michalczuk, L.; Goszczyñski, W. Pesticide residues in bee products collected from cherry trees protected during blooming period with contact and systemic fungicides. Apidologie 1999, 30, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Piechowicz, B.; Woś, I.; Podbielska, M.; Grodzicki, P. The transfer of active ingredients of insecticides and fungicides from an orchard to beehives. J. Environ. Sci. Health Part B 2018, 53, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.P.; Yang, F.W.; Li, J.W.; Xing, H.Z.; Ren, F.Z.; Pang, G.F.; Li, Y.X. Toxicities of neonicotinoid-containing pesticide mixtures on nontarget organisms. Environ. Toxicol. Chem. 2020, 39, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.H.; Pearlstein, D.J.; Wu, W.Y.; Kelley, A.G.; Montag, W.M.; Hsieh, E.M.; Berenbaum, M.R. Increase in longevity and amelioration of pesticide toxicity by natural levels of dietary phytochemicals in the honey bee, Apis mellifera. PLoS ONE 2020, 15, e0243364. [Google Scholar] [CrossRef] [PubMed]
- Almasri, H.; Tavares, A.D.; Tchamitchian, S.; Pélissier, M.; Sené, D.; Cousin, M.; Brunet, J.-L.; Belzunces, L.P. Toxicological status changes the susceptibility of the honey bee Apis mellifera to a single fungicidal spray application. Environ. Sci. Pollut. Res. 2021, 28, 42807–42820. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.; Thompson, H.M.; Walters, K.F. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 2013, 44, 563–574. [Google Scholar] [CrossRef] [Green Version]
- Manning, P.; Ramanaidu, K.; Cutler, G.C. Honey bee survival is affected by interactions between field-relevant rates of fungicides and insecticides used in apple and blueberry production. Facets 2017, 2, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Renzi, M.T.; Tosi, S.; Bogo, G.; Teper, D.; Porrini, C.; Molowny-Horas, R.; Bosch, J. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 2017, 73, 1236–1243. [Google Scholar] [CrossRef]
- Chandler, A.J.; Drummond, F.A.; Collins, J.A.; Lund, J.; Alnajjar, G. Assessment of synergism between acetamiprid and propiconazole on the Common Eastern Bumble bee, Bombus impatiens (Cresson), in Wild Blueberry. J. Agric. Urban Entomol. 2020, 36, 1–23. [Google Scholar] [CrossRef]
- Ricke, D.F.; Lin, C.H.; Johnson, R.M. Pollen treated with a combination of agrochemicals commonly applied during almond bloom reduces the emergence rate and longevity of honey bee (Hymenoptera: Apidae) queens. J. Insect Sci. 2021, 21, 5. [Google Scholar] [CrossRef]
- Yoder, J.A.; Jajack, A.J.; Rosselot, A.E.; Smith, T.J.; Yerke, M.C.; Sammataro, D. Fungicide contamination reduces beneficial fungi in bee bread based on an area-wide field study in honey bee, Apis mellifera, colonies. J. Toxicol. Environ. Health Part A 2013, 76, 587–600. [Google Scholar] [CrossRef]
- Kalaba, M.H.; Sultan, M.H.; Elbahnasawy, M.A.; El-Didamony, S.E.; El Bakary, N.M.; Sharaf, M.H. First report on isolation of Mucor bainieri from honeybees, Apis mellifera: Characterization and biological activities. Biotechnol. Rep. 2022, 36, e00770. [Google Scholar] [CrossRef]
- Pearlstein, D.J. Effects of an Insecticide/Fungicide Mixture on Queen Rearing Behavior and Hypopharyngeal Gland Morphology in Honey Bees (Apis mellifera L.) (Hymenoptera: Apidae). Master’s Thesis, University of Illinois, Urbana, IL, USA, 2020. Available online: http://hdl.handle.net/2142/109329 (accessed on 31 October 2022).
- Gupta, P.R.; Chandel, R.S. Effects of diflubenzuron and penfluron on workers of Apis cerana indica F. and Apis mellifera L. Apidologie 1995, 26, 3–10. [Google Scholar] [CrossRef]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and insects: Effects and interactions in agroecosystems. Sci. Total Environ. 2022, 825, 153899. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drummond, F.A. Honey Bee Exposure to the Fungicide Propiconazole in Lowbush Blueberry Fields. Agronomy 2022, 12, 3081. https://doi.org/10.3390/agronomy12123081
Drummond FA. Honey Bee Exposure to the Fungicide Propiconazole in Lowbush Blueberry Fields. Agronomy. 2022; 12(12):3081. https://doi.org/10.3390/agronomy12123081
Chicago/Turabian StyleDrummond, Francis Andrew. 2022. "Honey Bee Exposure to the Fungicide Propiconazole in Lowbush Blueberry Fields" Agronomy 12, no. 12: 3081. https://doi.org/10.3390/agronomy12123081
APA StyleDrummond, F. A. (2022). Honey Bee Exposure to the Fungicide Propiconazole in Lowbush Blueberry Fields. Agronomy, 12(12), 3081. https://doi.org/10.3390/agronomy12123081





