Ultraviolet-B Irradiation Induces Resistance against Powdery Mildew in Cucumber (Cucumis sativus L.) through a Different Mechanism Than That of Heat Shock-Induced Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Conditions of the Plant and Pathogen
2.2. UV-B Irradiation and Heat Shock Treatment
2.3. Pathogen Inoculation
2.4. RNA Extraction and Gene Expression Analysis
2.5. Statistical Analysis
3. Results and Discussion
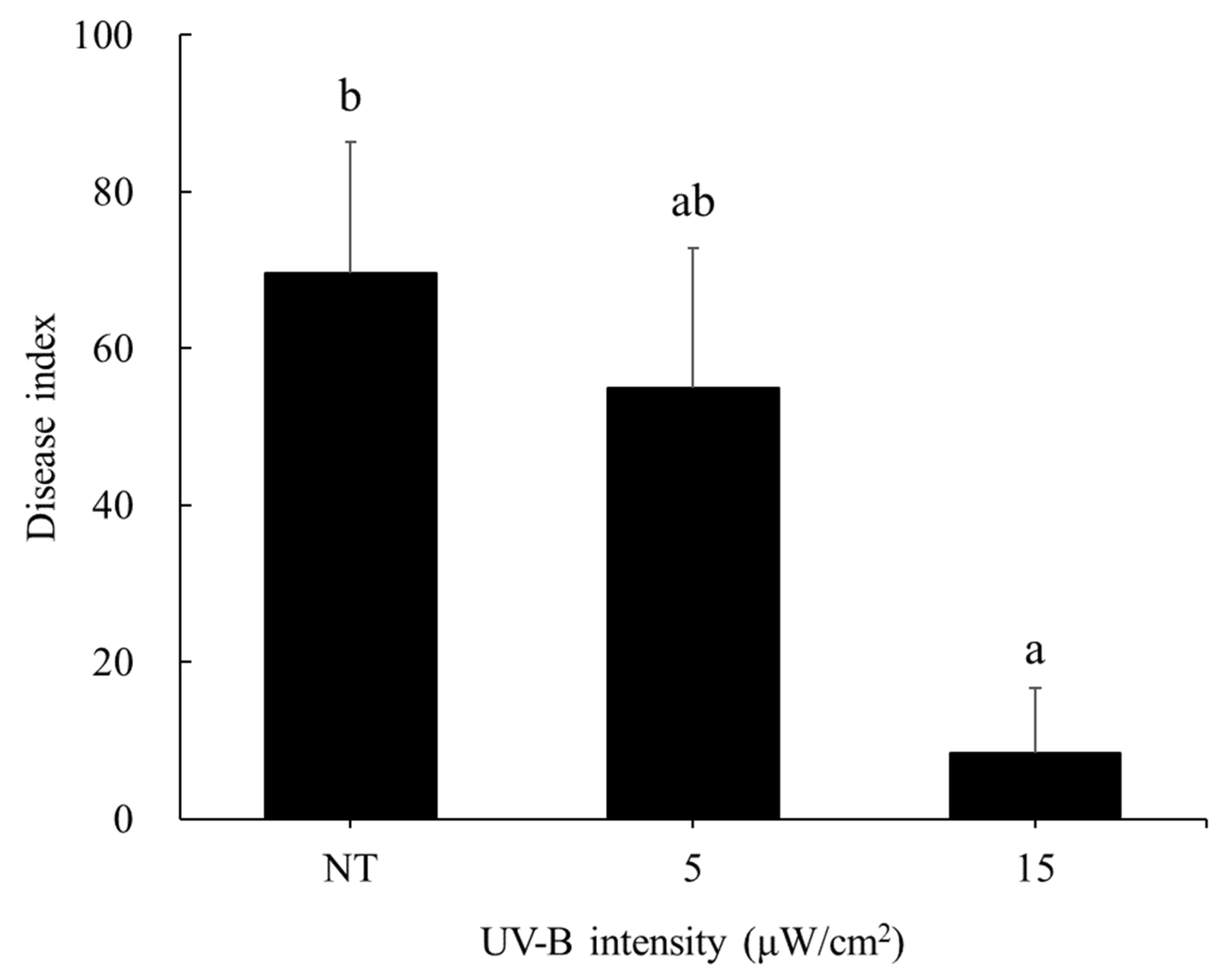
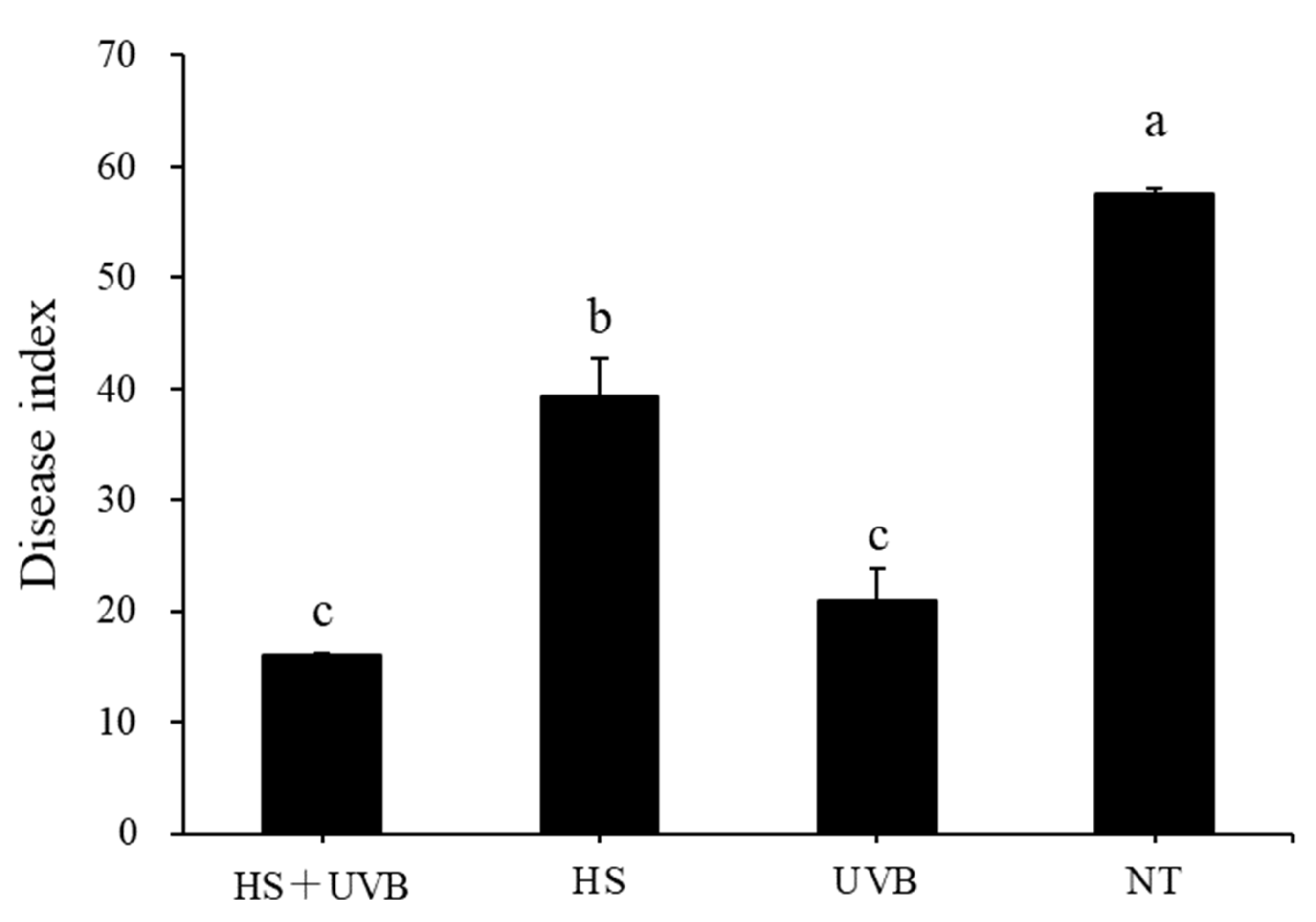
3.1. UV-B-Induced Resistance to Powdery Mildew in Cucumber
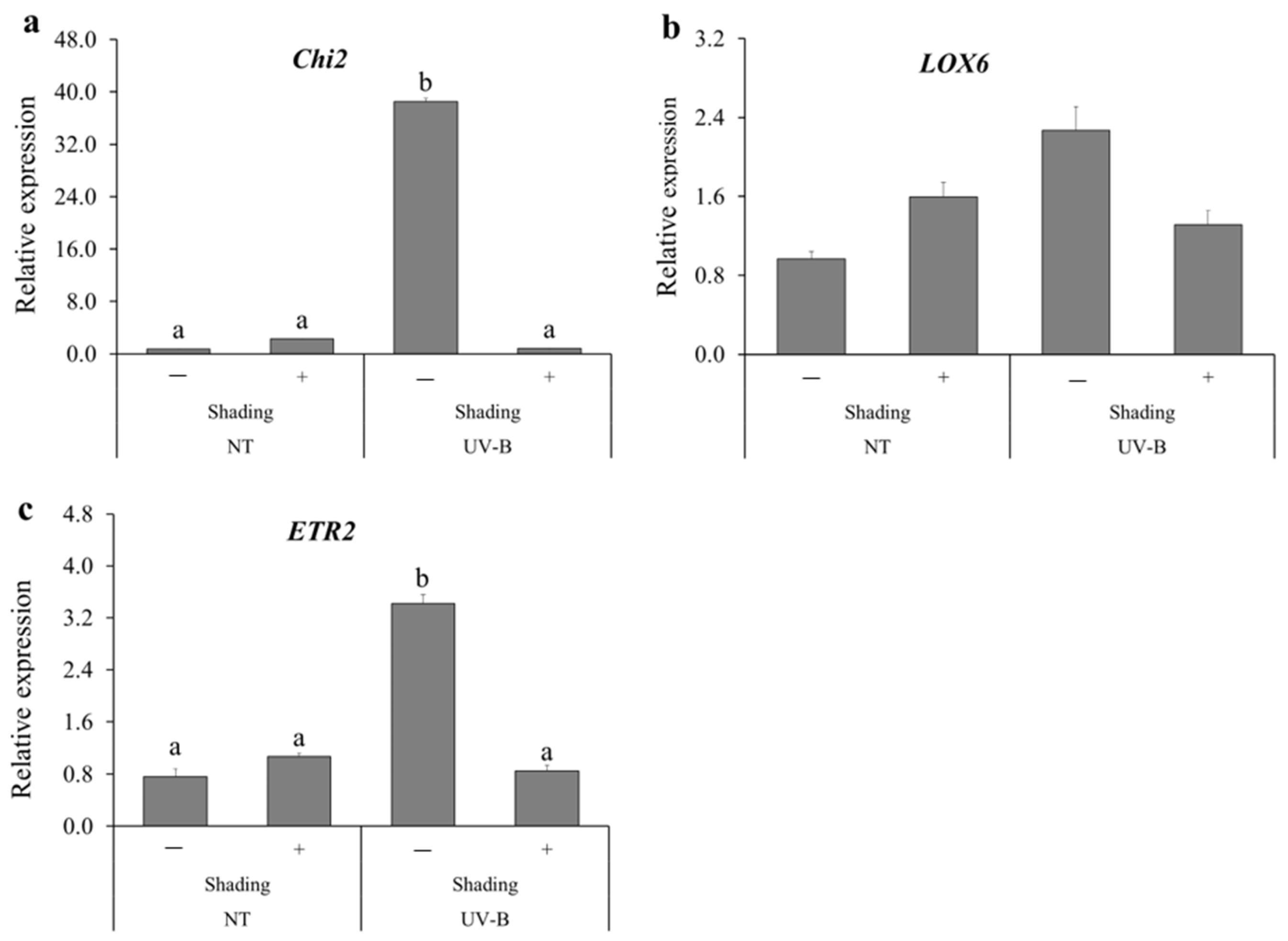
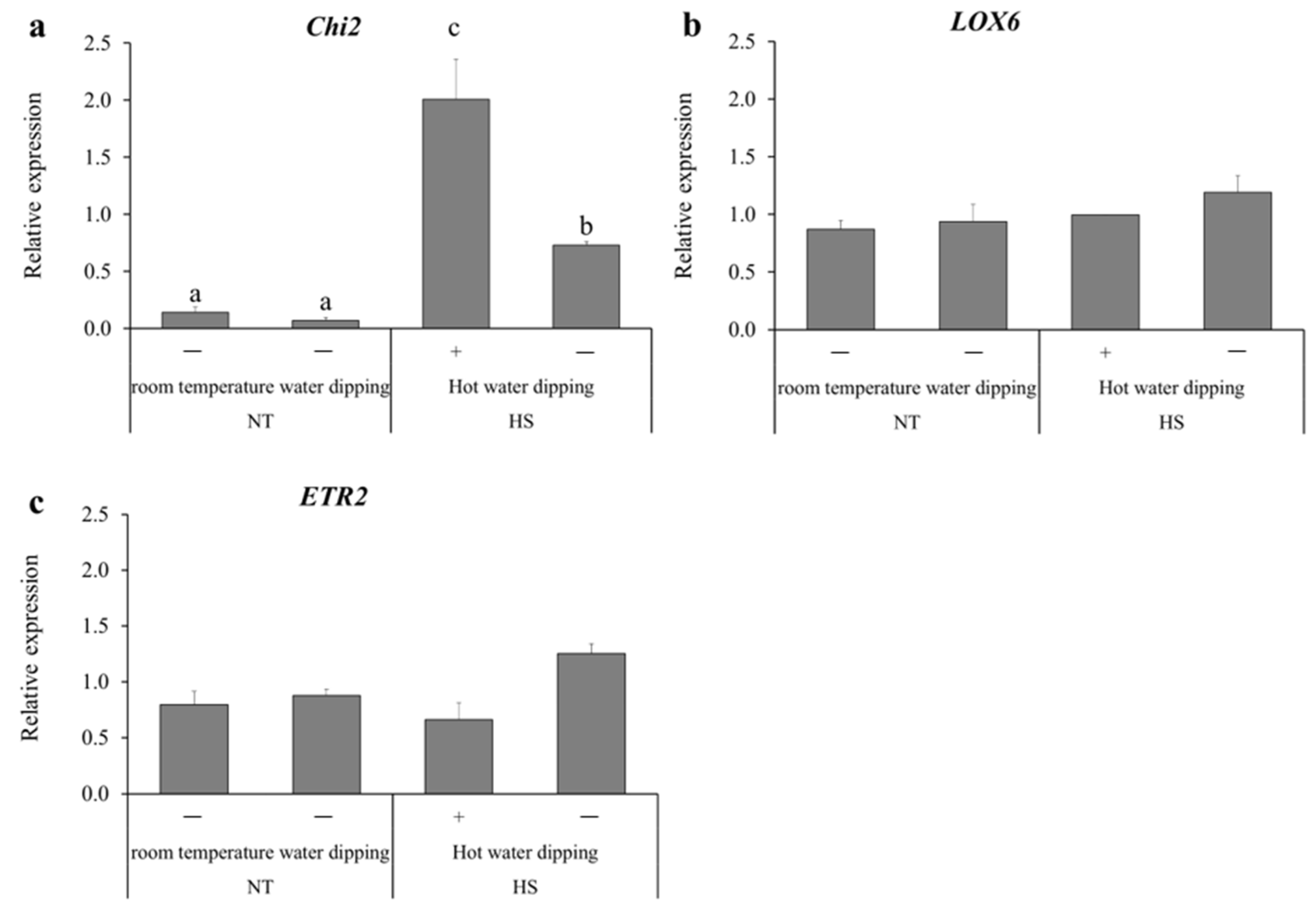
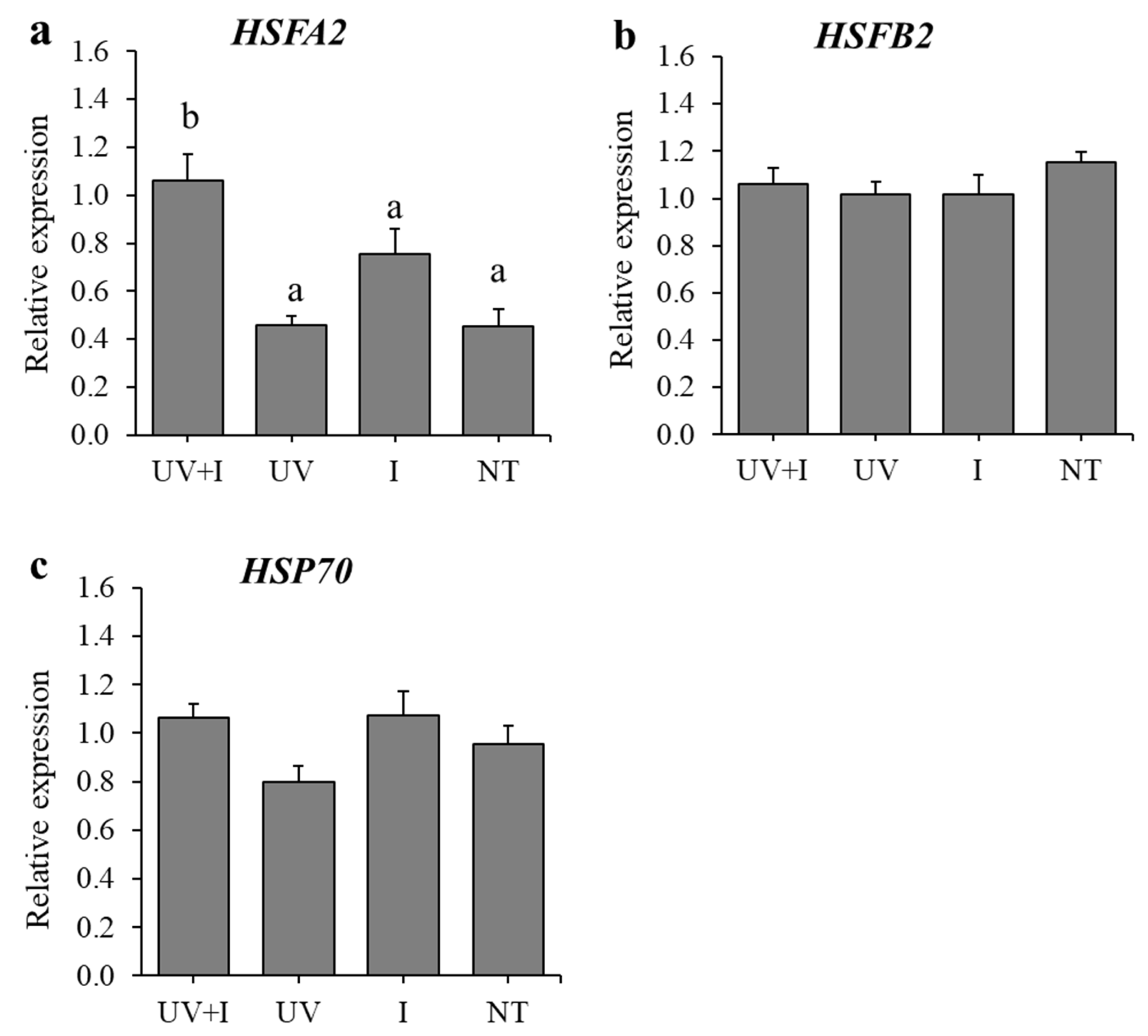
3.2. Expression Levels of Defense-Related Genes after UV-B Irradiation and HST
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirooka, T.; Ishii, H. Chemical control of plant diseases. Jpn. J. Phytopathol. 2014, 80, S172–S178. [Google Scholar] [CrossRef]
- Alewu, B.; Nosiri, C. Pesticides and human health. In Pesticides in the Modern World—Effects of Pesticides Exposure; Stoytcheva, M., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Konishi, K.; Ogawara, T.; Shimamoto, K.; Tomita, Y. Hot water spraying for the control of anthracnose and gray mold on strawberry. Boll. Hortic. Inst. Ibaraki Agric. Cent. 2010, 17, 43–46. [Google Scholar]
- Widiastuti, A.; Yoshino, M.; Hasegawa, M.; Nitta, Y.; Sato, T. Heat shock-induced resistance increases chitinase-1 gene expression and stimulates salicylic acid production in melon (Cucumis melo L.). Physiol Mol. Plant Pathol. 2013, 82, 51–55. [Google Scholar] [CrossRef]
- Widiastuti, A.; Yoshino, M.; Saito, H.; Maejima, K.; Zhou, S.; Odani, H.; Narisawa, K.; Hasegawa, M.; Nitta, Y.; Sato, T. Heat shock-induced resistance in strawberry against crown rot fungus Colletotrichum gloeosporioides. Physiol. Mol. Plant Pathol. 2013, 84, 86–91. [Google Scholar] [CrossRef]
- Arofatullah, N.A.; Hasegawa, M.; Tanabata, S.; Ogiwara, I.; Sato, T. Heat shock-induced resistance against Pseudomonas syringae pv. tomato (Okabe) Young. et al via heat shock transcription factors in tomato. Agronomy 2019, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Kharisma, A.D.; Arofatullah, N.A.; Yamane, K.; Tanabata, S.; Sato, T. Regulation of defense responses via heat shock transcription factors in Cucumis sativus L. against Botrytis cinerea. J. Gen. Plant Pathol. 2022, 88, 17–28. [Google Scholar] [CrossRef]
- Sato, T.; Saito, H.; Maejima, K.; Kuba, K.; Widiastuti, A.; Yoshino, M. Preventive effect and mode of action of repetitive hot water spraying against powdery mildew in strawberry. Hortic. J. 2018, 87, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Widiastuti, A.; Yoshino, M.; Saito, H.; Maejima, K.; Zhou, S.; Odani, H.; Hasegawa, M.; Nitta, Y.; Sato, T. Induction of disease resistance against Botrytis cinerea by heat shock treatment in melon (Cucumis melo L.). Physiol. Mol. Plant Pathol. 2011, 75, 157–162. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kanto, T.; Fujikawa, T.; Yamada, M.; Ishiwata, M.; Satou, M.; Hisamatsu, T. Supplemental UV radiation controls rose powdery mildew disease under the greenhouse conditions. Environ. Control. Biol. 2014, 51, 157–163. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ito, K.; Yokoda, Y.; Sato, T.; Yamada, M. Control of powdery mildew disease in cucumber and tomato seedlings by supplemental UV-B irradiation. Hort. Res. 2019, 18, 65–71. [Google Scholar] [CrossRef]
- Meyer, P.; Van de Poel, B.; De Coninck, B. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021, 8, 194. [Google Scholar] [CrossRef]
- Kanto, T.; Matsuura, K.; Yamada, M.; Usami, T.; Amemiya, Y. UV-B radiation for control of strawberry powdery mildew. Acta Hortic. 2009, 842, 359–362. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Xie, C.; Zu, Y.; Zhan, F.; Mei, X.; Xia, Y.; Li, Y. Effects of UV-B radiation on the infectivity of Magnaporthe oryzae and rice disease-resistant physiology in Yuanyang terraces. Photochem. Photobiol. Sci. 2018, 17, 8–17. [Google Scholar] [CrossRef]
- Demkura, P.V.; Ballaré, C.L. UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol. Plant 2012, 5, 642–652. [Google Scholar] [CrossRef]
- Stermer, B.A.; Hammerschmidt, R. Heat shock induces resistance to Cladosporium cucumerinum and enhances peroxidase activity in cucumbers. Physiol. Plant Pathol. 1984, 25, 239–249. [Google Scholar] [CrossRef]
- Sato, T.; Kubo, M. Reducing the need for chemical spraying of summer greenhouse cucumber: Heat-shock controls disease and insect damage. Acta Hortic. 2002, 588, 165–170. [Google Scholar] [CrossRef]
- Sato, T.; Kubo, M.; Watanabe, S. Heat shock induces a systemic acquired resistance (SAR)-related gene via salicylic acid accumulation in cucumber (Cucumis sativus L.). Trop. Agric. 2003, 47, 77–82. [Google Scholar]
- Okayama, K.; Sugimura, T.; Matsutani, S. Effects of heat and high humidity treatment on the occurrence of strawberry powdery mildew. Boll. Nara Agric. Exp. Stn. 1997, 28, 29–34. [Google Scholar]
- Koitabashi, M.; Nakashima, M.; Kashio, T.; Nishimura, N. Pest control test of powdery mildew and spider mite by hot water treatment on strawberry. Jpn. J. Phytopathol. 2002, 68, 197. [Google Scholar]
- Arofatullah, N.A.; Widiastuti, A.; Chinta, Y.D.; Kobayashi, T.; Tanabata, S.; Sato, T. Prevention of powdery mildew disease in tomato nursery by improved hot water spraying device. Jpn. J. Farm. Work Res. 2019, 54, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Suthaparan, A.; Stensvand, A.; Solhaug, K.A.; Torre, S.; Telfer, K.H.; Ruud, A.K.; Mortensen, L.M.; Gadoury, D.M.; Seem, R.C.; Gislerød, H.R. Suppression of cucumber powdery mildew by supplemental UV-B radiation in greenhouses can be augmented or reduced by background radiation quality. Plant Dis. 2014, 98, 1349–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, C.N.; Wilcox, W.F. Effects of sunlight exposure on grapevine powdery mildew development. Phytopathology 2012, 102, 857–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wargent, J.J.; Taylor, A.; Paul, N.D. UV supplementation for growth regulation and disease control. Acta Hortic. 2006, 711, 333–338. [Google Scholar] [CrossRef]
- Satou, M.; Kobayashi, M.; Kanto, T.; Sugawara, K.; Yamada, M.; Ishiwata, M. Suppression of anthracnose of Russel prairie gentian by UV-B irradiation. Annu. Rep. Kanto-Tosan Plant Prot. Soc. 2013, 60, 79–81. [Google Scholar] [CrossRef]
- Kanto, T.; Matsuura, K.; Ogawa, T.; Yamada, M.; Ishiwata, M.; Usami, T.; Amemiya, Y. A new UV-B lighting system controls powdery mildew of strawberry. Acta Hortic. 2014, 1049, 655–660. [Google Scholar] [CrossRef]
- Oka, K.; Yamada, M.; Ishiwata, M.; Okada, K. Expression of UV-B-induced resistant response in eggplant and control of leaf mold on greenhouse-grown eggplant. Jpn. J. Phytopathol. 2011, 77, 23–27. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Li, X.; Zhan, F.; Xie, C.; Zu, Y.; Li, Y.; Yue, M. Resistance-related physiological response of rice leaves to the compound stress of enhanced UV-B radiation and Magnaporthe oryzae. J. Plant Interact. 2018, 13, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Mackerness, S.A.-H.; Surplus, S.L.; Blake, P.; John, C.F.; Buchanan-Wollaston, V.; Jordan, B.R.; Thomas, B. Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: Role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ. 1999, 22, 1413–1423. [Google Scholar] [CrossRef]
- Fujibe, T.; Watanabe, K.; Nakajima, N.; Ohashi, Y.; Mitsuhara, I.; Yamamoto, K.T.; Takeuchi, Y. Accumulation of pathogenesis-related proteins in tobacco leaves irradiated with UV-B. J. Plant Res. 2000, 113, 387–394. [Google Scholar] [CrossRef]
- Green, R.; Fluhr, R. UV-B-induced PR-1 accumulation is mediated by active oxygen species. Plant Cell 1995, 7, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, M.; Widiastuti, A.; Hasegawa, M.; Sato, T. Induction of disease resistance against gray mold by heat shock using hot water dipping in cucumber and its underlying mechanism. Hort. Res. 2011, 10, 429–433. [Google Scholar] [CrossRef] [Green Version]
- Casati, P.; Walbot, V. Rapid transcriptome responses of maize (Zea mays) to UV-B in irradiated and shielded tissues. Genome Biol. 2004, 5, R16. [Google Scholar] [CrossRef] [Green Version]
- Widiastuti, A.; Arofatullah, N.A.; Kharisma, A.D.; Sato, T. Upregulation of heat shock transcription factors, Hsp70, and defense-related genes in heat shock-induced resistance against powdery mildew in cucumber. Physiol. Mol. Plant Pathol. 2021, 116, 101730. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow TransPlant 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Suthaparan, A.; Torre, S.; Mortensen, L.M.; Gislerød, H.R.; Stensvand, A.; Solhaug, K.A.; Gadoury, D.M. Interruption of the night period by UV-B suppresses powdery mildew of rose and cucumber. Acta Hortic. 2012, 956, 617–620. [Google Scholar] [CrossRef]
- Suthaparan, A.; Solhaug, K.A.; Bjugstad, N.; Gislerød, H.R.; Gadoury, D.M.; Stensvand, A. Suppression of powdery mildews by UV-B: Application frequency and timing, dose, reflectance, and automation. Plant Dis. 2016, 100, 1643–1650. [Google Scholar] [CrossRef] [Green Version]
- Shoresh, M.; Yedidia, I.; Chet, I. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 2005, 95, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Pieterse, C.M.; Mauch-Mani, B.; Mauch-Mani, B. Priming in plant-pathogen interactions. Trends Plant Sci. 2002, 7, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Oyoshi, K.; Katano, K.; Yunose, M.; Suzuki, N. Memory of 5-min heat stress in Arabidopsis thaliana. Plant Signal. Behav. 2020, 8, 1778919. [Google Scholar] [CrossRef]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-Mediated Accumulation of Flavonoid Phytoalexins in Cucumber. Biochem. Cell Biol. 1998, 8, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Sävenstrand, H.; Brosché, M.; Strid, A. Ultraviolet-B signalling: Arabidopsis brassinosteroid mutants are defective in UV-B regulated defence gene expression. Plant Physiol. Biochem. 2004, 42, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Kunz, B.A.; Dando, P.K.; Grice, D.M.; Mohr, P.G.; Schenk, P.M.; Cahill, D.M. UV-induced DNA damage promotes resistance to the biotrophic pathogen Hyaloperonospora parasitica in Arabidopsis. Plant Physiol. 2008, 148, 1021–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Ghaouth, A.; Wilson, C.L.; Callahan, A.M. Induction of chitinase, β-1,3-glucanase, and phenylalanine ammonia lyase in peach fruit by UV-C treatment. Phytopathology 2003, 93, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, A.; Mercier, L.; Franchel, J.; Baillieul, F.; Benizri, E.; Mauro, M.C. Response of grapevine defenses to UV-C exposure. Am. J. Enol. Vitic. 2004, 55, 51–59. [Google Scholar] [CrossRef]
- Kalbin, G.; Hidema, J.; Brosché, M.; Kumagai, T.; Bornman, J.F.; Strid, Å. UV-B-induced DNA damage and expression of defence genes under UV-B stress: Tissue-specific molecular marker analysis in leaves. Plant Cell Environ. 2001, 24, 983–990. [Google Scholar] [CrossRef]


| Target Genes | Properties | Sequences (5′–3′) |
|---|---|---|
| Chi2 | acidic endochitinase | AGGTCCTCCTCTCTATCGGTG |
| NM_001308904.1 | GGCGCGGCAGATAAAATGAC | |
| ETR2 | ethylene receptor 2 | GAGTGTCAGCGTGTGAGTGA |
| NM_001308840.1 | GGAAACCAGGGCGGTAAGAA | |
| LOX6 | lipoxygenase 6 | CTGGGAGGTTGAAGGCTCTG |
| XM_004135257.3 | CATGGTCCCTCAGCCAAGAA | |
| PR1 | pathogenesys-related protein 1 | CGGGACAGACTCACCTCAAG |
| XM_011660558.2 | GGCTTCTCATCCACCCACAA | |
| HSFB2 | heat shock transcription factor B2 | CGGCAAGACAGGTGATGGAA |
| XM_031885273.1 | TCATCCAACGGTTCCGCTTT | |
| HSFA2 | heat shock transcription factor A2 | AGAAGCACGTTAATGGCGGA |
| XM_031880532.1 | CACTTGGGCTGGCAGTTAGT | |
| HSP70 | heat shock protein 70 | GGGACCAGTGAAGAAAGCCA |
| NM_001305774.1 | GAGGGGCAACGTCAAGAAGA | |
| Actin | Actin | TCTGTCCCTCTACGCTAGTGGAC |
| AB698859.1 | TCCAAACGGAGAATGGCATGAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fardhani, D.M.; Kharisma, A.D.; Kobayashi, T.; Arofatullah, N.A.; Yamada, M.; Tanabata, S.; Yokoda, Y.; Widiastuti, A.; Sato, T. Ultraviolet-B Irradiation Induces Resistance against Powdery Mildew in Cucumber (Cucumis sativus L.) through a Different Mechanism Than That of Heat Shock-Induced Resistance. Agronomy 2022, 12, 3011. https://doi.org/10.3390/agronomy12123011
Fardhani DM, Kharisma AD, Kobayashi T, Arofatullah NA, Yamada M, Tanabata S, Yokoda Y, Widiastuti A, Sato T. Ultraviolet-B Irradiation Induces Resistance against Powdery Mildew in Cucumber (Cucumis sativus L.) through a Different Mechanism Than That of Heat Shock-Induced Resistance. Agronomy. 2022; 12(12):3011. https://doi.org/10.3390/agronomy12123011
Chicago/Turabian StyleFardhani, Dinar Mindrati, Agung Dian Kharisma, Tomoyuki Kobayashi, Nur Akbar Arofatullah, Makoto Yamada, Sayuri Tanabata, Yumi Yokoda, Ani Widiastuti, and Tatsuo Sato. 2022. "Ultraviolet-B Irradiation Induces Resistance against Powdery Mildew in Cucumber (Cucumis sativus L.) through a Different Mechanism Than That of Heat Shock-Induced Resistance" Agronomy 12, no. 12: 3011. https://doi.org/10.3390/agronomy12123011
APA StyleFardhani, D. M., Kharisma, A. D., Kobayashi, T., Arofatullah, N. A., Yamada, M., Tanabata, S., Yokoda, Y., Widiastuti, A., & Sato, T. (2022). Ultraviolet-B Irradiation Induces Resistance against Powdery Mildew in Cucumber (Cucumis sativus L.) through a Different Mechanism Than That of Heat Shock-Induced Resistance. Agronomy, 12(12), 3011. https://doi.org/10.3390/agronomy12123011






