Evaluation of Allelopathic Activity Interactions of Some Medicinal Plants Using Fractional Inhibitory Concentration and Isobologram
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Assessment of Allelopathic Effect on Lettuce Seed and Germination Traits
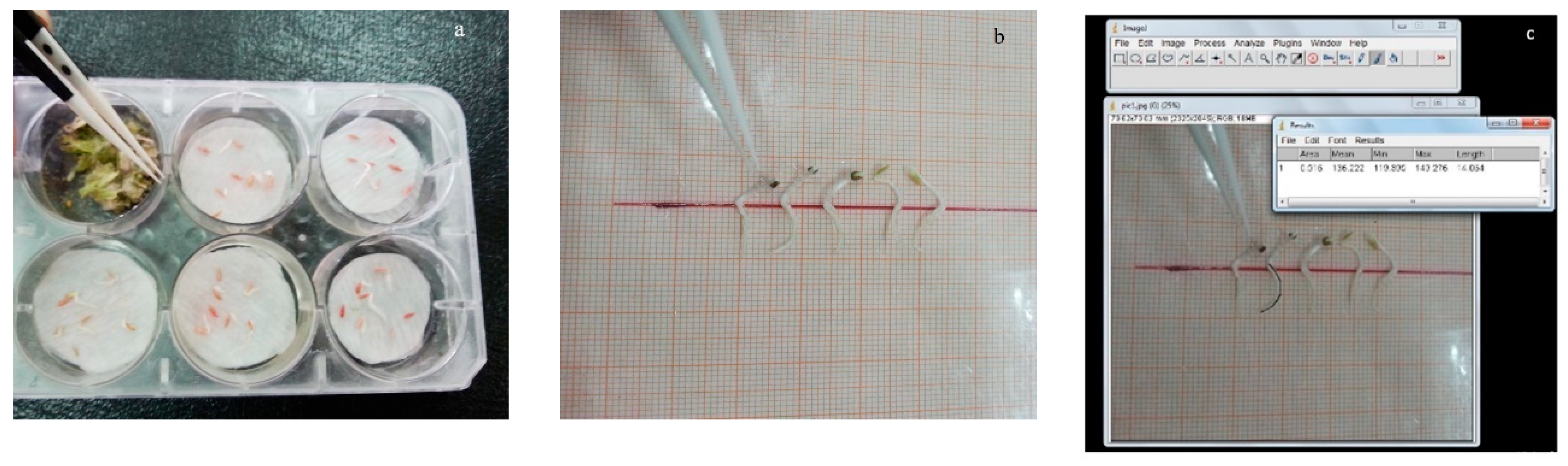
2.2.1. Dish Pack Method
2.2.2. Germination and Seed Traits
2.3. Assessment of Medicinal Plants’ Allelopathic Effects on Lettuce Seedling Growth
2.4. Statistical Analysis
2.5. Headspace Gas Chromatography-Mass Spectrometry (HS-GC-MS)
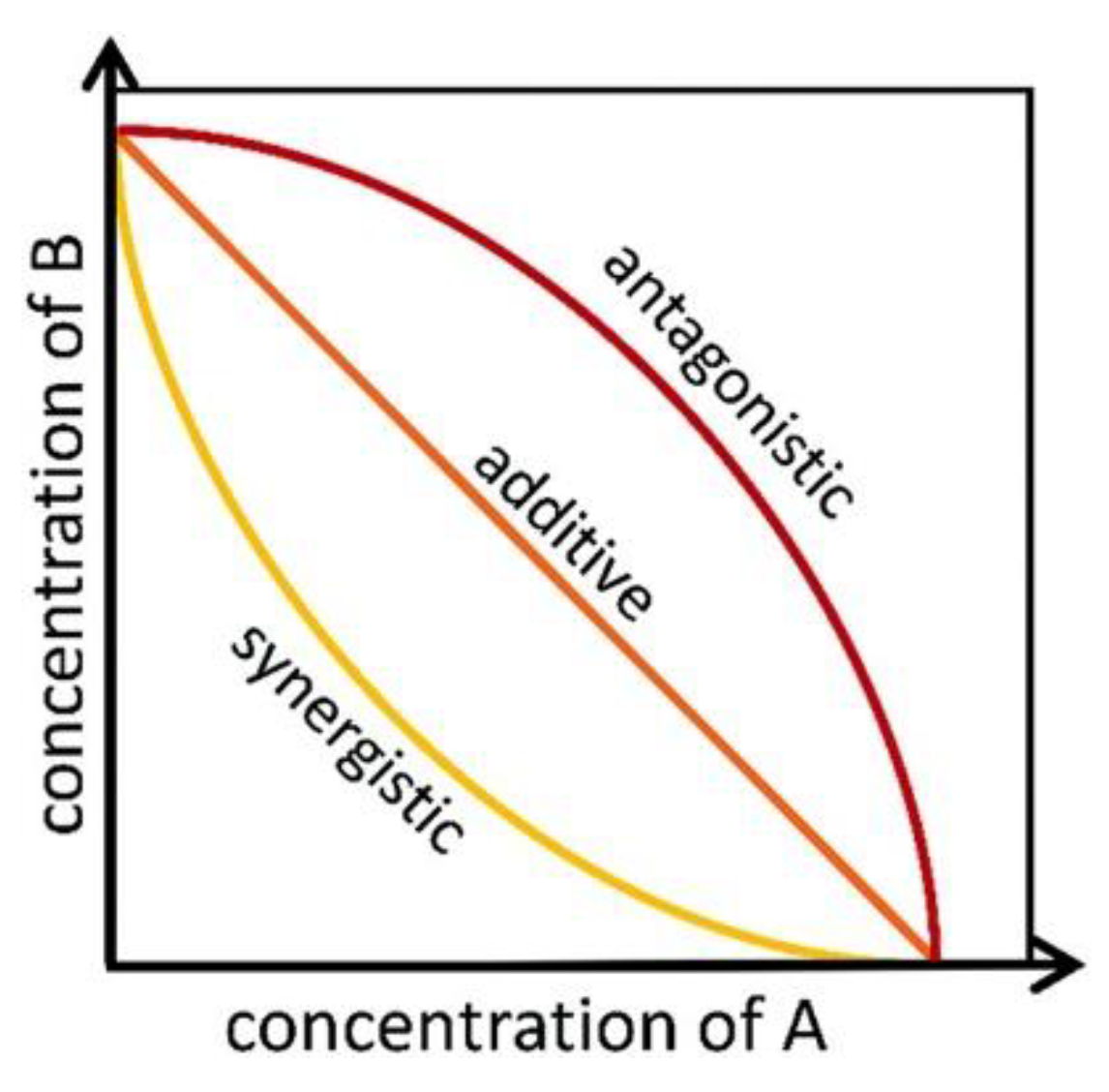
2.6. Allelopathic Interaction Effects
2.6.1. Determination of EC50 and EC25
2.6.2. Plants Combinations
- Fractional inhibitory concentration (FIC) was calculated using the following equation:
- Isobologram curves
3. Results
3.1. Allelopathic Effects of Medicinal Plants on Lettuce Seed Growth and Germination Specifications
3.1.1. Radicle Growth (R %)
3.1.2. Hypocotyl Growth (H %)
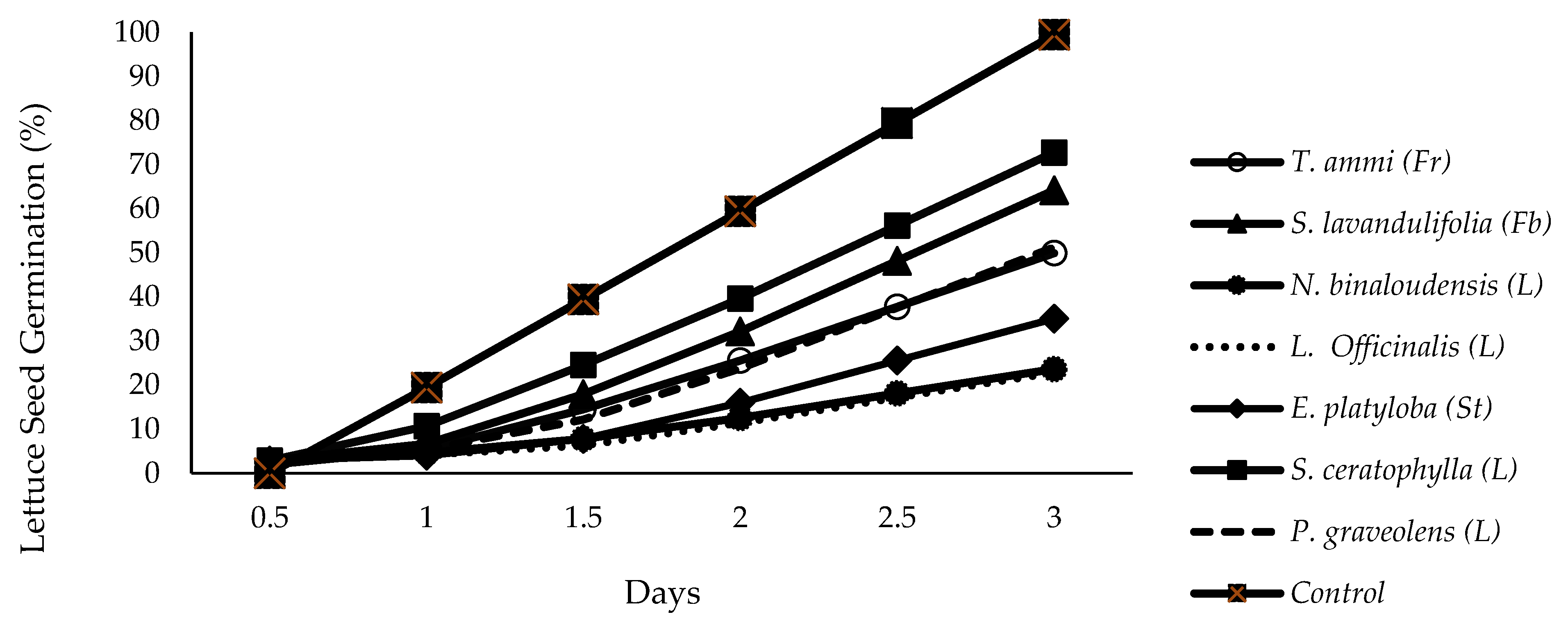
3.1.3. Germination Percentage (G %)
3.1.4. Germination Rate
3.2. Headspace Gas Chromatography-Mass Spectrometry (HS-GC-MS)
3.3. The Specification of Effective Concentrations of the Most Inhibitory Plants
3.4. Allelopathic Interaction of the Most Inhibitory Plants
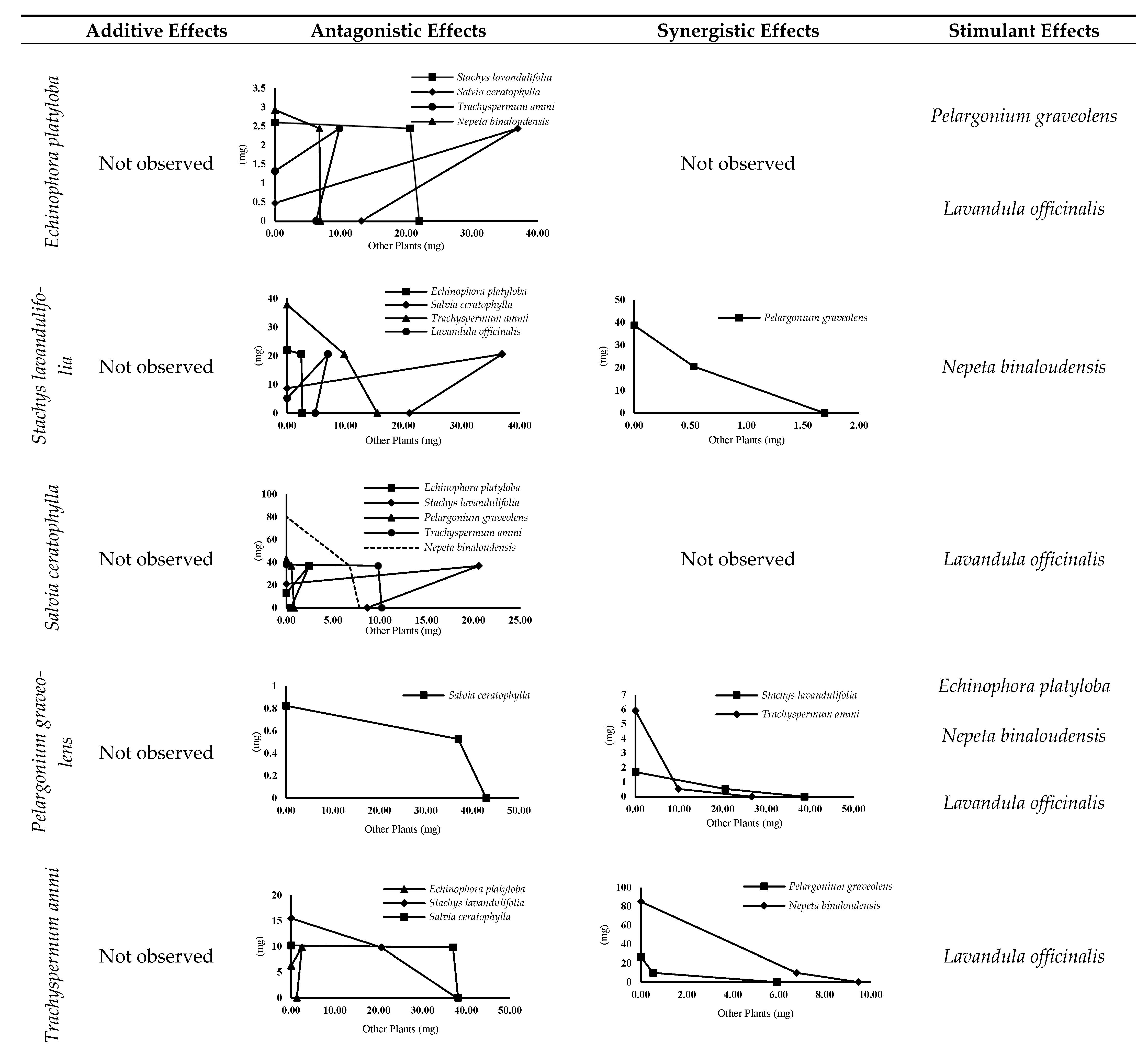
3.4.1. Interaction Result
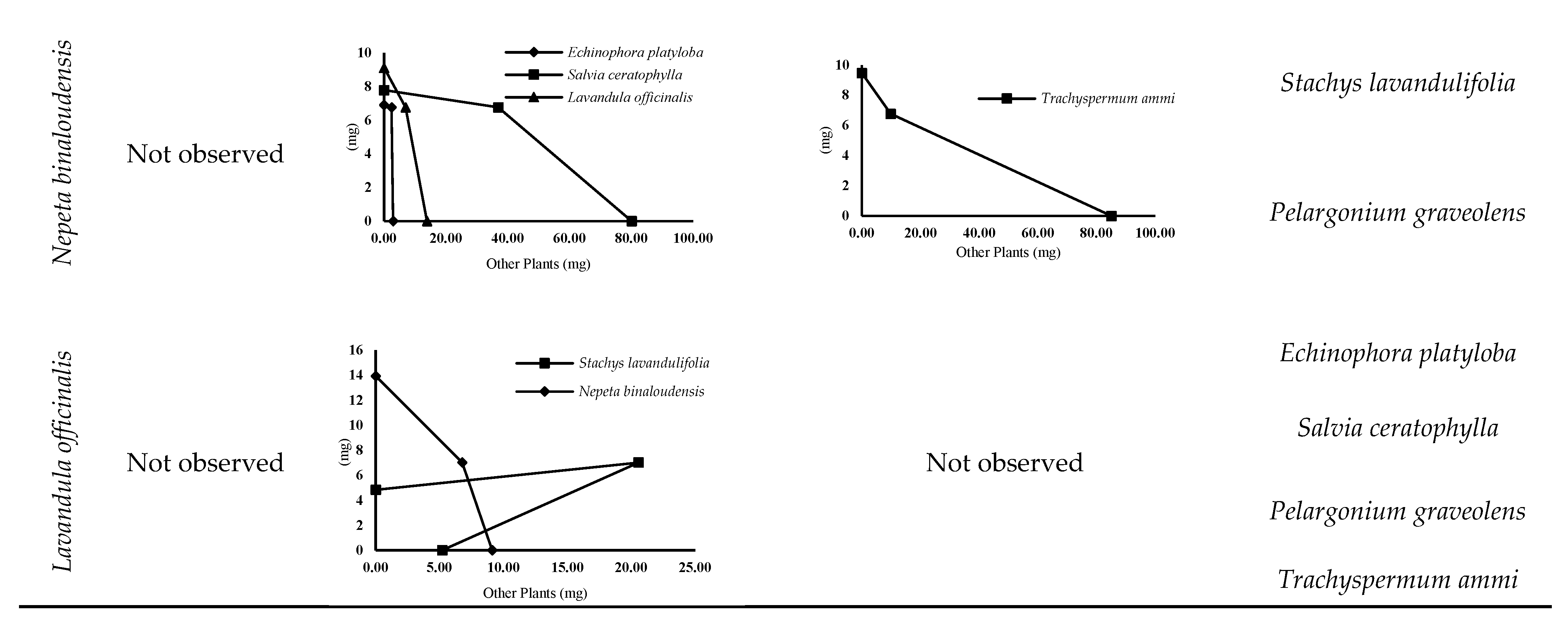
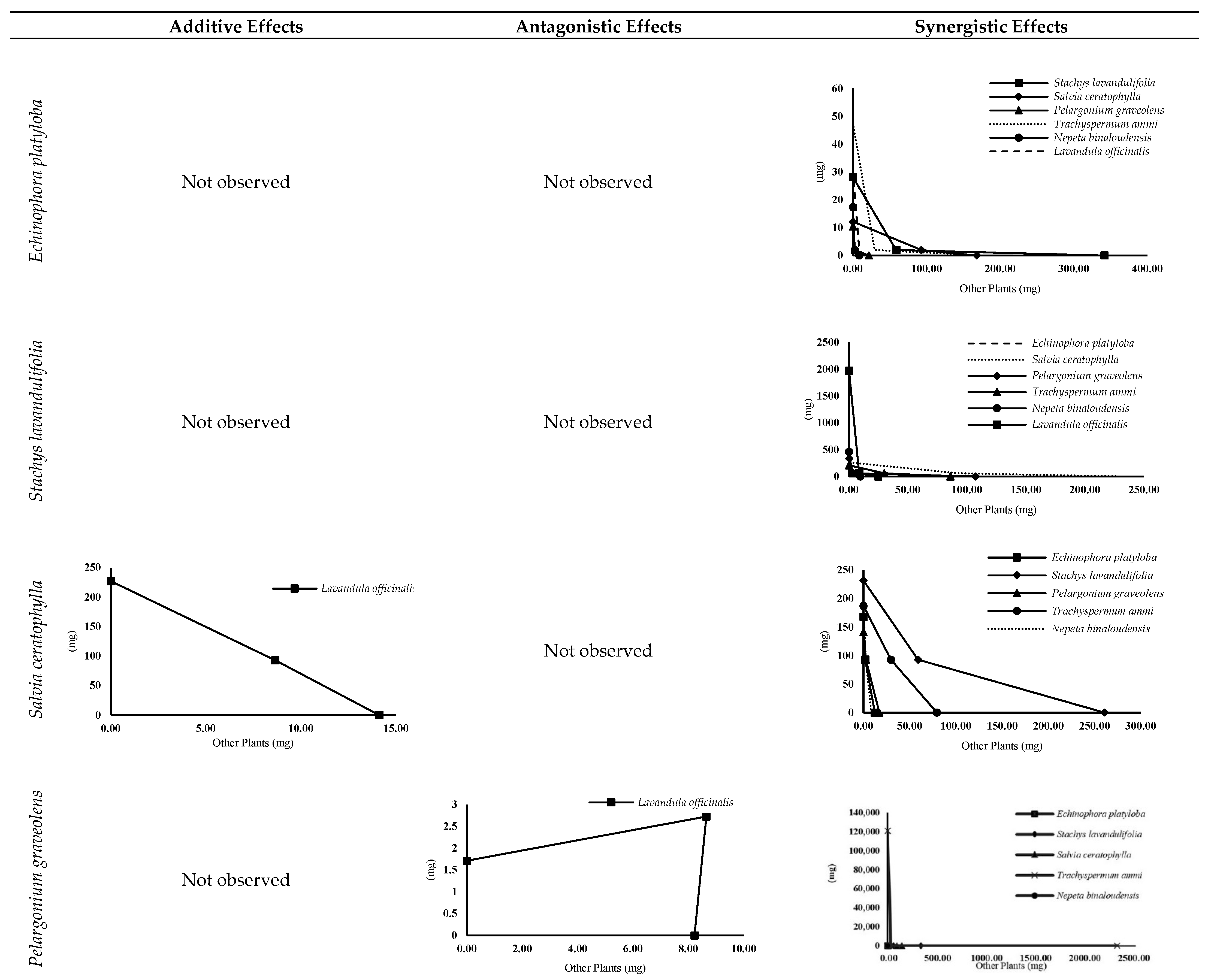
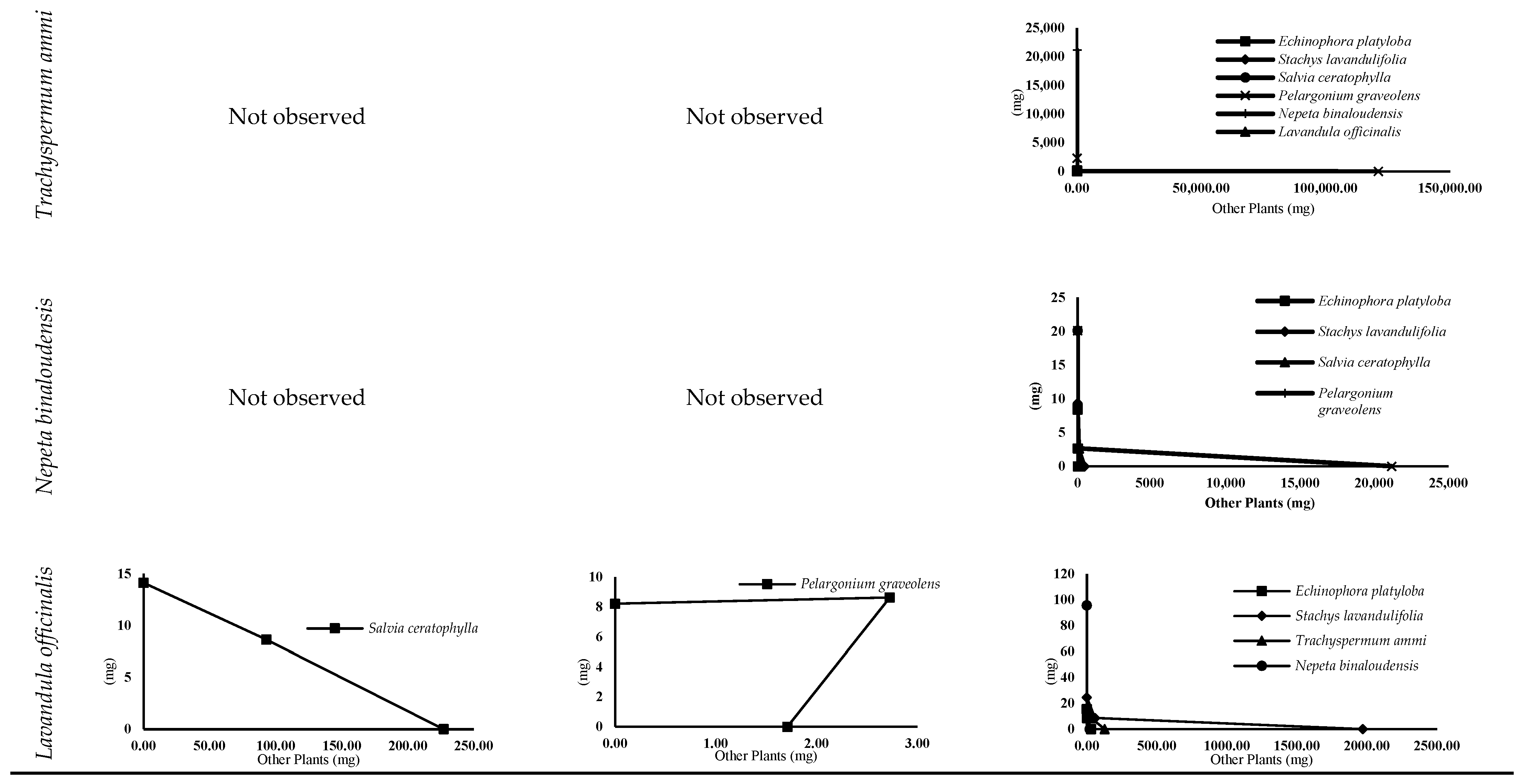
3.4.2. Isobologram Curves
3.4.3. Comparison of Evaluation Methods of Allelopathic Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batish, D.R.; Arora, K.; Singh, H.P.; Kohli, R. Potential utilization of dried powder of Tagetes minuta as a natural herbicide for managing rice weeds. Crop Prot. 2007, 26, 566–571. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Fundamentals of Weed Science, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Hesammi, E. The Allopathic effects of Sorghum halepense and Amaranthus retroflexsus extract on the Germination of Corn Grain. Aust. J. Basic Appl. Sci. 2011, 5, 2249–2253. [Google Scholar]
- Mohamadi, N.; Rajaie, P.; Fahimi, H. The allelopathic assay of Eucalyptus camaldulensis Labill on morphological and physiological parameters on monocot and dicot plants. ACECR Sci. Inf. Database 2012, 25, 456–464. [Google Scholar]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Sekine, T.; Appiah, K.S.; Azizi, M.; Fujii, Y. Plant Growth Inhibitory Activities and Volatile Active Compounds of 53 Spices and Herbs. Plants 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, M.; Tavana, M.; Farsi, M.; Oroojalian, F. Yield performance of Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst.(higher Basidiomycetes), using different waste materials as substrates. Int. J. Med. Mushrooms 2012, 14, 521–527. [Google Scholar] [CrossRef]
- Heidari, S.; Azizi, M.; Soltani, F.; Hadian, J. Foliar application of Ca (NO3)2 and KNO3 affects growth, essential oil content, and oil composition of French tarragon. Ind. Crops Prod. 2014, 62, 526–532. [Google Scholar] [CrossRef]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Ghorbanpour, M. Multi-walled carbon nanotubes stimulate growth, redox reactions and biosynthesis of antioxidant metabolites in Thymus daenensis celak. in vitro. Chemosphere 2020, 249, 126069. [Google Scholar] [CrossRef]
- Tarakemeh, A.; Azizi, M.; Rowshan, V.; Salehi, H.; Spina, R.; Dupire, F.; Arouie, H.; Laurain-Mattar, D. Screening of Amaryllidaceae alkaloids in bulbs and tissue cultures of Narcissus papyraceus and four varieties of N. tazetta. J. Pharm. Biomed. Anal. 2019, 172, 230–237. [Google Scholar] [CrossRef]
- Koiou, K.; Vasilakoglou, I.; Dhima, K. Herbicidal potential of lavender (Lavandula angustifolia Mill.) essential oil components on bristly foxtail (Setaria verticillata (L.) P. Beauv.): Comparison with carvacrol, carvone, thymol and eugenol. Arch. Biol. Sci. 2020, 72, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Belmain, S.; Stevenson, P. Ethnobotanicals in Ghana: Reviving and modernising age-old farmer practice. Pestic. Outlook 2001, 12, 233–238. [Google Scholar] [CrossRef]
- Putnam, A. Allelopathic chemicals: Can natural plant herbicides help control weeds? Weeds Today 1984, 15, 6–8. [Google Scholar]
- Mardani, H.; Sekine, T.; Azizi, M.; Mishyna, M.; Fujii, Y. Identification of Safranal as the Main Allelochemical from Saffron (Crocus sativus). Nat. Prod. Commun. 2015, 10, 775–777. [Google Scholar] [CrossRef] [Green Version]
- Amini, S.; Azizi, M.; Joharchi, M.R.; Shafei, M.N.; Moradinezhad, F.; Fujii, Y. Determination of allelopathic potential in some medicinal and wild plant species of Iran by dish pack method. Theor. Exp. Plant Physiol. 2014, 26, 189–199. [Google Scholar] [CrossRef]
- Albuquerque, M.; Dos Santos, R.C.; Lima, L.M.; Filho, P.D.A.M.; Nogueira, R.J.M.C.; Da Câmara, C.A.G.; Ramos, A.D.R. Allelopathy, an alternative tool to improve cropping systems. A review. Agron. Sustain. Dev. 2010, 31, 379–395. [Google Scholar] [CrossRef] [Green Version]
- D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Simonet, A.M.; Zarrelli, A. Potential allelochemicals from Sambucus nigra. Phytochemistry 2001, 58, 1073–1081. [Google Scholar] [CrossRef]
- Fujii, Y.; Matsuyama, M.; Hiradate, S.; Nakatani, K. Development of new bioassay for volatile allelochemicals: Dish-pack method. J. Weed Sci. Technol. 2000, 45, 80–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.-C.; Lin, J.-H.; Zhang, S.; Lou, C.-W.; Li, T.-T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450. [Google Scholar] [CrossRef]
- Bankier, C.; Matharu, R.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antibacterial Activity of Green Synthesized Silver Nanomaterials with Colistin Antibiotic against Multidrug-Resistant Bacterial Pathogens. Crystals 2022, 12, 1057. [Google Scholar] [CrossRef]
- Oroojalian, F.; Kasra-Kermanshahi, R.; Azizi, M.; Bassami, M. Phytochemical composition of the essential oils from three Apiaceae species and their antibacterial effects on food-borne pathogens. Food Chem. 2009, 120, 765–770. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Vu, K.D.; Harich, M.; Salmieri, S.; Lacroix, M. Evidence for synergistic activity of plant-derived essential oils against fungal pathogens of food. Food Microbiol. 2016, 53, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M. Two approaches to the study of germination data. Proc. Int. Seed Test. Ass. 1968, 33, 531–540. [Google Scholar]
- Azizi, M.; Fujii, Y. Allelopathic Effect of Some Medicinal Plant Substances on Seed Germination of Amaranthus retroflexus and Portulaca oleraceae. Acta Hortic. 2006, 699, 61–68. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G.D. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Doern, C.D. When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [Green Version]
- Callaway, R.M. Experimental designs for the study of allelopathy. Plant Soil 2003, 256, 1–11. [Google Scholar] [CrossRef]
- Fujii, Y.; Hiradate, S. Allelopathy: New Concepts & Methodology; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hashemi, M.; Ehsani, A.; Jazani, N.H.; Aliakbarlu, J.; Mahmoudi, R. Chemical composition and in vitro antibacterial activity of essential oil and methanol extract of Echinophora platyloba D.C against some of food-borne pathogenic bacteria. Veter- Res. Forum Int. Q. J. 2013, 4, 123–127. [Google Scholar]
- Bingol, M.N.; Bursal, E. LC-MS/MS analysis of phenolic compounds and in vitro antioxidant potential of stachys lavandulifolia vahl. var. brachydon boiss. Int. Lett. Nat. Sci. 2018, 72, 28–36. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Adiguzel, A.; Ozer, H.; Sokmen, M.; Gulluce, M.; Sokmen, A.; Kilic, H.; Sahin, F.; Baris, O. Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria. Pol. J. Microbiol. 2009, 58, 69–76. [Google Scholar] [PubMed]
- Birkett, M.A.; Hassanali, A.; Hoglund, S.; Pettersson, J.; Pickett, J.A. Repellent activity of catmint, Nepeta cataria, and iridoid nepetalactone isomers against Afro-tropical mosquitoes, ixodid ticks and red poultry mites. Phytochemistry 2011, 72, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavela, R. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia 2005, 76, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P.S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Zunino, M.P.; Zygadlo, J.A. Effect of monoterpenes on lipid oxidation in maize. Planta 2004, 219, 303–309. [Google Scholar]
- Sfara, V.; Zerba, E.N.; Alzogaray, R.A. Fumigant Insecticidal Activity and Repellent Effect of Five Essential Oils and Seven Monoterpenes on First-Instar Nymphs of Rhodnius prolixus. J. Med. Èntomol. 2009, 46, 511–515. [Google Scholar] [CrossRef]
- Klocke, J.A.; Darlington, M.V.; Balandrin, M.F. 1,8-Cineole (Eucalyptol), a mosquito feeding and ovipositional repellent from volatile oil of Hemizonia fitchii (Asteraceae). J. Chem. Ecol. 1987, 13, 2131–2141. [Google Scholar] [CrossRef]
- Bag, A.; Chattopadhyay, R.R. Evaluation of Synergistic Antibacterial and Antioxidant Efficacy of Essential Oils of Spices and Herbs in Combination. PLoS ONE 2015, 10, e0131321. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]


| No | Family | Plant Scientific Name | Part Used | Inhibition Activity (%) | Criteria | |||
|---|---|---|---|---|---|---|---|---|
| Average (41 mm) | Average (Whole Wells) | |||||||
| Radicle | Hypocotyl | Radicle | Hypocotyl | |||||
| 1 | Amaranthaceae | Amaranthus blitoides | Flower | 30.12 | 36.08 | 30.70 | 40.00 | ** |
| 2 | Amaranthus hypochondriacus | Leaf | 79.03 | 85.62 | 62.52 | 63.52 | *** | |
| 3 | Atriplex halimus | Leaf | 36.98 | 16.34 | 29.50 | 20.42 | ** | |
| 4 | Kochia prostrata | Leaf | 24.60 | 15.02 | 28.27 | 15.92 | * | |
| 5 | Amaryllidaceae | Allium sativum | Leaf | 30.03 | 18.04 | 25.27 | 18.25 | * |
| 6 | Ungernia trisphaera | Leaf | 9.72 | −6.32 | −21.64 | −39.28 | + | |
| 7 | Apiaceae | Conium maculatum | Leaf | 82.57 | 85.93 | 66.70 | 73.57 | *** |
| 8 | Echinophora platyloba | Stem | 100.00 | 100.00 | 84.62 | 82.85 | **** | |
| 9 | Ferula szowitsiana | Leaf | 26.94 | 29.22 | 5.57 | −6.90 | * | |
| 10 | Ferula xylorhachis | Leaf | 11.85 | 17.78 | −2.08 | 0.77 | + | |
| 11 | Foeniculum vulgare | Fruit | 87.03 | 85.23 | 79.91 | 76.73 | *** | |
| 12 | Seseli transcaucasicum | Flower | 12.47 | 35.98 | −8.70 | −6.82 | + | |
| 13 | Seseli transcaucasicum | Leaf | 37.94 | 38.03 | 1.61 | −22.83 | * | |
| 14 | Seseli transcaucasicum | Stem | 33.63 | 29.21 | −8.06 | −55.43 | + | |
| 15 | Seseli transcaucasicum | Root | 28.04 | 31.69 | 13.91 | 1.52 | * | |
| 16 | Trachyspermum ammi | Fruit | 99.41 | 100.00 | 80.20 | 82.73 | **** | |
| 17 | Apocynaceae | Vinca minor | Leaf | 77.62 | 80.55 | 52.23 | 51.49 | ** |
| 18 | Asparagaceae | Asparagus officinalis | Leaf | 78.25 | 86.54 | 66.35 | 72.66 | *** |
| 19 | Asteraceae | Achillea nobilis | Flower | 94.56 | 97.10 | 82.39 | 79.58 | *** |
| 20 | Achillea filipendula | Leaf | 55.23 | 66.35 | 50.82 | 60.69 | ** | |
| 21 | Achillea biebersteinii | Leaf | 90.05 | 88.37 | 85.72 | 83.40 | *** | |
| 22 | Achillea wilhelmsii | Flower | 87.98 | 91.68 | 62.13 | 62.93 | *** | |
| 23 | Achillea millefolium | Flower | 27.90 | 18.02 | 25.82 | 16.74 | * | |
| 24 | Achillea pachycephala | Leaf | 70.17 | 65.36 | 60.49 | 53.56 | *** | |
| 25 | Artemisia absinthium | Leaf | 39.75 | 37.50 | 40.47 | 50.54 | ** | |
| 26 | Artemisia scoparia | Leaf | 40.81 | 35.56 | 35.16 | 27.38 | ** | |
| 27 | Artemisia tournefortiana | Leaf | 11.36 | 16.12 | −3.64 | −13.16 | + | |
| 28 | Calandula officinalis | Leaf | 50.54 | 46.87 | 58.12 | 59.35 | ** | |
| 29 | Centaurea behen | Leaf | 82.11 | 83.86 | 71.31 | 70.17 | *** | |
| 30 | Cousinia raddeana | Flower | 26.61 | 27.43 | 34.69 | 37.11 | ** | |
| 31 | Codonocephalum peacockianum | Leaf | 36.40 | 20.91 | 30.27 | 30.48 | ** | |
| 32 | Grindelia robusta | Leaf | 31.90 | 31.46 | 48.49 | 44.94 | ** | |
| 33 | Helichrysum italicum | Leaf | 51.91 | 59.15 | 44.62 | 45.42 | ** | |
| 34 | Lactuca persica | Leaf | 20.45 | 03.04 | −12.73 | −9.21 | + | |
| 35 | Matricaria chamomilla | Flower | 64.70 | 68.19 | 46.51 | 52.23 | ** | |
| 36 | Pseudohandelia umbellifera | Leaf | 55.14 | 59.79 | 44.50 | 40.47 | ** | |
| 37 | Pulicaria gnaphalodes | Leaf | 28.55 | 30.69 | 16.11 | 13.50 | * | |
| 38 | Pulicaria gnaphalodes | Seed | 91.07 | 89.72 | 84.68 | 86.79 | *** | |
| 39 | Santolina chamaecyparissus | Leaf | 85.02 | 92.05 | 66.25 | 69.57 | *** | |
| 40 | Tanacetum balsamita | Leaf | 45.24 | 43.94 | 23.17 | 28.87 | * | |
| 41 | Berberidaceae | Berberis integerrima | Root | 59.00 | 33.91 | 46.26 | 34.12 | ** |
| 42 | Berberis vulgaris | Leaf | 59.07 | 48.68 | 40.30 | 31.62 | ** | |
| 43 | Boraginaceae | Trichodesma incanum | Leaf | 58.96 | 59.97 | 57.33 | 61.59 | ** |
| 44 | Cannabaceae | Cannabis sativa | Leaf | 32.14 | −4.59 | −18.57 | −33.67 | + |
| 45 | Capparaceae | Capparis spinosa | Leaf | 87.86 | 92.02 | 57.69 | 68.82 | ** |
| 46 | Cleomaceae | Cleome chorassanica | Leaf | 8.63 | 27.69 | 4.59 | 18.94 | * |
| 47 | Ephedraceae | Ephedra major | Leaf | −0.86 | −29.55 | −14.81 | −54.96 | + |
| 48 | Euphorbiaceae | Chrozophora tinctoria | Leaf | 60.62 | 61.59 | 49.55 | 55.67 | ** |
| 49 | Ricinus communis | Fruit | 56.67 | 49.65 | 20.26 | 27.47 | * | |
| 50 | Euphorbia petiolata | Leaf | 7.95 | 4.61 | −10.30 | −18.42 | + | |
| 51 | Euphorbia serpens | Leaf | 82.74 | 88.23 | 75.23 | 75.29 | *** | |
| 52 | Euphorbia aellenii | Leaf | 26.96 | 10.15 | 20.26 | 12.25 | * | |
| 53 | Euphorbia granulata | Leaf | 82.47 | 88.39 | 60.35 | 63.68 | *** | |
| 54 | Fabaceae | Genista tinctoria | Leaf | 41.69 | 37.77 | 34.12 | 22.73 | ** |
| 55 | Frankeniaceae | Frankenia spp | Leaf | 33.52 | 4.76 | 27.63 | 31.92 | * |
| 56 | Geraniaceae | Pelargonium graveolens | Leaf | 100.00 | 100.00 | 83.35 | 88.23 | **** |
| 57 | Hypericaceae | Hypericum helianthemoides | Root | 51.68 | 59.62 | 37.52 | 48.88 | ** |
| 58 | Hypericum perforatum | Leaf | 71.81 | 63.71 | 49.21 | 52.58 | ** | |
| 59 | Hypericum scabrum | Leaf | 34.91 | 35.45 | −11.50 | −36.67 | + | |
| 60 | Lamiaceae | Acinos graveolens | Leaf | 63.97 | 79.21 | 37.26 | 52.02 | ** |
| 61 | Ballota nigra | Leaf | 44.93 | 61.84 | 26.13 | 25.00 | * | |
| 62 | Hyssopus angustifolius | Leaf | 18.48 | 3.63 | 9.82 | −7.34 | * | |
| 63 | Hyssopus angustifolius | Flower | 39.83 | 28.13 | 30.44 | 28.13 | ** | |
| 64 | Lavandula officinalis | Flower | 100.00 | 100.00 | 80.29 | 82.49 | **** | |
| 65 | Lavandula officinalis | Leaf | 84.56 | 92.14 | 82.24 | 88.39 | *** | |
| 66 | Melissa officinalis | Leaf | −12.36 | −1.22 | 23.64 | 22.37 | * | |
| 67 | Melissa officinalis | Root | −12.37 | −1.23 | −13.39 | −1.50 | + | |
| 68 | Mentha piperita | Leaf | −10.03 | 10.44 | 0.62 | 18.36 | * | |
| 69 | Mentha spicata | Leaf | 18.06 | 29.40 | 9.12 | 7.31 | * | |
| 70 | Mentha longifolia | Leaf | 90.03 | 88.55 | 67.20 | 77.57 | *** | |
| 71 | Nepeta binaloudensis | Leaf | 100.00 | 100.00 | 96.37 | 98.28 | **** | |
| 72 | Nepeta sintenisii | Leaf | 29.52 | 18.82 | 14.06 | 16.37 | * | |
| 73 | Origanum major | Leaf | 57.07 | 75.54 | 16.16 | 17.39 | * | |
| 74 | Origanum vulgare | Leaf | 64.12 | 92.96 | −26.70 | −26.53 | + | |
| 75 | Perovskia abrotanoides | Leaf | 95.32 | 89.65 | 81.81 | 77.13 | *** | |
| 76 | Perovskia abrotanoides | Seed | 31.21 | 20.94 | 20.81 | 16.78 | * | |
| 77 | Rosmarinus officinalis | Leaf | 90.67 | 91.63 | 80.86 | 85.41 | *** | |
| 78 | Salvia aethiopis | Leaf | 94.62 | 92.56 | 55.38 | 36.90 | ** | |
| 79 | Salvia tebesana | Leaf | 12.50 | −22.64 | −1.00 | −31.13 | + | |
| 80 | Salvia nemorosa | Leaf | 71.68 | 71.45 | 63.16 | 65.95 | *** | |
| 81 | Salvia leriifolia | Root | 86.21 | 78.93 | 55.88 | 54.49 | ** | |
| 82 | Salvia leriifolia | Leaf | 67.54 | 65.89 | 85.06 | 83.94 | *** | |
| 83 | Salvia chloroleuca | Leaf | 67.16 | 59.85 | 29.36 | 26.37 | ** | |
| 84 | Salvia ceratophylla | Leaf | 100.00 | 100.00 | 83.87 | 81.54 | **** | |
| 85 | Salvia macrosiphon | Leaf | 71.56 | 70.51 | 57.79 | 51.84 | ** | |
| 86 | Salvia officinalis | Leaf | 17.86 | 6.49 | 11.43 | −8.16 | * | |
| 87 | Salvia virgata | Leaf | 32.99 | 35.00 | 40.17 | 41.90 | ** | |
| 88 | Salvia sahendica | Leaf | 17.85 | 26.07 | 24.52 | 18.59 | * | |
| 89 | Salvia sclarea | Leaf | 62.24 | 73.54 | 60.19 | 68.31 | *** | |
| 90 | Stachys byzantina | Leaf | 43.43 | 47.05 | 40.05 | 45.20 | ** | |
| 91 | Stachys lavandulifolia | Flowering branch | 100.00 | 100.00 | 89.52 | 88.65 | **** | |
| 92 | Thuspeinanta brahuica | Leaf | 18.18 | −0.32 | −10.61 | −12.72 | + | |
| 93 | Teucrium chamaedrys | Leaf | 21.36 | 7.84 | 17.57 | 13.74 | * | |
| 94 | Malvaceae | Althaea officinalis | Leaf | 21.94 | 51.12 | 21.61 | 40.32 | * |
| 95 | Malva sylvestris | Flower | 76.13 | 82.20 | 76.75 | 78.11 | *** | |
| 96 | Malva sylvestris | Leaf | 49.41 | 54.58 | 16.28 | 10.13 | * | |
| 97 | Myrtaceae | Peganum harmala | Leaf | 46.20 | 49.02 | 36.22 | 44.65 | ** |
| 98 | Eucalyptus globulus | Leaf | 100.00 | 100.00 | 80.29 | 82.49 | *** | |
| 99 | Nitrariaceae | Eucalyptus globulus | Seed | −12.99 | −59.00 | 2.01 | −14.22 | * |
| 100 | Onagraceae | Epilobium hirsutum | Leaf | 30.02 | 38.02 | 27.22 | 32.99 | * |
| 101 | Oenothera biennis | Leaf | 63.00 | 61.01 | 47.50 | 52.41 | ** | |
| 102 | Papaveraceae | Corydalis aitchisonii | Leaf | 34.43 | 34.83 | 19.27 | 18.55 | * |
| 103 | Glaucium flavum | Leaf | 14.69 | 10.18 | 14.85 | −8.59 | * | |
| 104 | Plantaginaceae | Plantago major | Leaf | 29.96 | 28.87 | 14.94 | 17.75 | * |
| 105 | Polygonaceae | Polygonum aviculare | Leaf | 68.71 | 59.09 | 45.12 | 47.84 | ** |
| 106 | Polygonum patulum | Leaf | 53.91 | 60.01 | 48.00 | 54.02 | ** | |
| 107 | Rosaceae | Filipendula ulmaria | Leaf | 16.07 | 2.08 | 18.18 | 20.58 | * |
| 108 | Rosa foetida | Leaf | 58.44 | 61.15 | 60.81 | 57.81 | *** | |
| 109 | Rutaceae | Haplophyllum furfuraceum | Leaf | 76.91 | 76.12 | 37.13 | 27.99 | ** |
| 110 | Ruta graveolens | Leaf | 33.52 | 41.44 | 24.40 | 30.36 | * | |
| 111 | Solanaceae | Datura innoxia | Leaf | 37.72 | 40.01 | 39.16 | 33.78 | ** |
| 112 | Datura stramonium | Leaf | 12.33 | 12.88 | −3.40 | 2.00 | + | |
| 113 | Lycium depressum | Leaf | 52.60 | 47.90 | 45.99 | 48.99 | ** | |
| 114 | Lycium ruthenicum | Leaf | 55.37 | 55.35 | 59.24 | 61.67 | *** | |
| 115 | Solanum nigrum | Leaf | 38.83 | 36.60 | 32.42 | 28.80 | ** | |
| 116 | Urticaceae | Urtica dioica | Leaf | 77.65 | 70.97 | 68.39 | 71.68 | *** |
| 117 | Urtica dioica | Root | −45.30 | 21.10 | −30.00 | 20.01 | + | |
| 118 | Verbenaceae | Lippia citriodora | Leaf | 91.35 | 87.32 | 33.02 | 22.83 | ** |
| 119 | Vitex pseudo-negundo | Leaf | 53.90 | 45.75 | 23.99 | 20.33 | * | |
| 120 | Vitex pseudo-negundo | Seed | 20.22 | 38.54 | 15.22 | 12.02 | * | |
| 121 | Lantana montevidensis | Leaf | 19.67 | 15.33 | 29.50 | 36.49 | ** | |
| 122 | Zygophyllaceae | Tribulus terrestris | Leaf | 56.88 | 67.94 | 46.14 | 41.68 | ** |
| 123 | Zygophyllum fabago | Leaf | 12.94 | 11.27 | 20.22 | 16.21 | * | |
| Sample | Part of Use | Main Identified Components | RT | %Area |
|---|---|---|---|---|
| Stachys lavandulifolia | Flowering branch | Thymol | 20.13 | 7.96 |
| Salvia ceratophylla | Leaf | Carvacrol | 17.9 | 1.42 |
| trans-Caryophyllene | 18.14 | 0.7 | ||
| Thymol acetate | 20.13 | 11.82 | ||
| Chavicol | 25.13 | 0.98 | ||
| Echinophora platyloba | Stem | Thymol acetate | 20.11 | 5.31 |
| Trachyspermum ammi | Fruit | P-Cymene | 8.82 | 0.82 |
| β-Pinene | 10.10 | 0.69 | ||
| Borneol | 14.46 | 0.53 | ||
| Carvacrol Methyl Ether | 17.9 | 0.77 | ||
| α-Ionone | 18.12 | 0.35 | ||
| Thymol | 20.12 | 40.02 | ||
| Carvacrol | 20.48 | 0.92 | ||
| trans-Caryophyllene | 25.13 | 0.62 | ||
| Propene | 33.98 | 0.56 | ||
| α-terpinolene | 35.29 | 1.18 | ||
| 4-Cumylphenol | 35.49 | 1.02 | ||
| s-Indacene | 37.42 | 0.95 | ||
| Lavandula officinalis | Flower | 1,8-Cineole | 9.1 | 14.3 |
| Camphor | 13.55 | 2.46 | ||
| 1-Borneol | 14.47 | 1.04 | ||
| Carvacrol Methyl Ether | 17.9 | 0.42 | ||
| Thymol | 20.11 | 35.71 | ||
| Linalool | 20.49 | 0.71 | ||
| Chromolaenin | 35.28 | 1.3 | ||
| α-Amorphene | 35.49 | 1.12 | ||
| Bornyl acetate | 37.4 | 0.7 | ||
| Nepeta binaloudensis | Leaf | p-Cymene | 8.84 | 0.57 |
| 1,8-Cineole | 9.11 | 8.47 | ||
| α-Pinene | 10.15 | 0.34 | ||
| Carvacrol Methyl Ether | 17.92 | 0.21 | ||
| Verbenone | 19.2 | 0.14 | ||
| Thymol | 20.11 | 28.44 | ||
| α-iso-methyl ionone | 20.5 | 0.71 | ||
| 4-Cumylphenol | 35.27 | 0.86 | ||
| β-Caryophyllene | 35.49 | 0.92 | ||
| α-Terpinene | 37.4 | 0.79 | ||
| Pelargonium graveolens | Leaf | β-pinene | 7.17 | 1.22 |
| 1,8-Cineole | 9.14 | 1.13 | ||
| α-Pinene | 10.13 | 0.7 | ||
| Thymol | 20.12 | 13.93 | ||
| Carvacrol | 20.49 | 0.85 | ||
| 4-Cumylphenol | 35.28 | 0.74 | ||
| Isomenthone | 35.49 | 0.56 |
| Plant Scientific Name | Part of Use | Radicle Inhibition | Hypocotyl Inhibition | ||
|---|---|---|---|---|---|
| EC25 | EC50 | EC25 | EC50 | ||
| mg/well | |||||
| Stachys lavandulifolia | Flowering branch | 20.57 | 72.80 | 58.97 | 107.8 |
| Salvia ceratophylla | Leaf | 36.96 | 83.70 | 92.87 | 130.5 |
| Echinophora platyloba | Stem | 2.44 | 8.99 | 1.97 | 7.915 |
| Trachyspermum ammi | Fruit | 9.81 | 25.48 | 29.72 | 52.19 |
| Lavandula officinalis | Flower | 7.01 | 9.86 | 8.65 | 11.21 |
| Nepeta binaloudensis | Leaf | 6.76 | 7.85 | 2.64 | 7.923 |
| Pelargonium graveolens | Leaf | 0.53 | 5.31 | 2.72 | 13.71 |
| Plant Interactions | FIC | ||
|---|---|---|---|
| Radicle | Hypocotyl | ||
| Echinophora platyloba × | Stachys lavandulifolia | 0.52 An | 1.46 S |
| Salvia ceratophylla | 0.15 An | 1.17 Ad | |
| Pelargonium graveolens | −0.09 St | 1.11 Ad | |
| Trachyspermum ammi | 0.33 An | 1.61 S | |
| Nepeta binaloudensis | 0.56 An | 1.30 S | |
| Lavandula officinalis | −0.12 St | 1.49 S | |
| Stachys lavandulifolia × | Salvia ceratophylla | 0.27 An | 1.37 S |
| Pelargonium graveolens | 0.73 Ad | 1.46 S | |
| Trachyspermum ammi | 0.72 Ad | 1.28 S | |
| Nepeta binaloudensis | −0.53 St | 1.56 S | |
| Lavandula officinalis | 0.18 An | 1.85 S | |
| Salvia ceratophylla × | Pelargonium graveolens | 0.58 An | 1.05 Ad |
| Trachyspermum ammi | 0.52 An | 1.24 S | |
| Nepeta binaloudensis | 0.97 Ad | 1.22 S | |
| Lavandula officinalis | −0.50 St | 1.36 S | |
| Pelargonium graveolens × | Trachyspermum ammi | 1.03 Ad | 1.97 S |
| Nepeta binaloudensis | −0.38 St | 1.46 S | |
| Lavandula officinalis | −0.56 St | 0.54 An | |
| Trachyspermum ammi × | Nepeta binaloudensis | 1.60 S | 2.00 S |
| Lavandula officinalis | −0.20 St | 1.47 S | |
| Nepeta binaloudensis × | Lavandula officinalis | 1.50 S | 2.00 S |
| Plant Interactions | FIC | Isobologram | |||
|---|---|---|---|---|---|
| Radicle | Hypocotyl | Radicle | Hypocotyl | ||
| Echinophora platyloba × | Stachys lavandulifolia | An | S | An | S |
| Salvia ceratophylla | An | Ad | An | S | |
| Pelargonium graveolens | St | Ad | St | S | |
| Trachyspermum ammi | An | S | An | S | |
| Nepeta binaloudensis | An | S | An | S | |
| Lavandula officinalis | St | S | St | S | |
| Stachys lavandulifolia × | Salvia ceratophylla | An | S | An | S |
| Pelargonium graveolens | Ad | S | S | S | |
| Trachyspermum ammi | Ad | S | An | S | |
| Nepeta binaloudensis | St | S | St | S | |
| Lavandula officinalis | An | S | An | S | |
| Salvia ceratophylla × | Pelargonium graveolens | An | Ad | An | S |
| Trachyspermum ammi | An | S | An | S | |
| Nepeta binaloudensis | Ad | S | An | S | |
| Lavandula officinalis | St | S | St | Ad | |
| Pelargonium graveolens × | Trachyspermum ammi | Ad | S | S | S |
| Nepeta binaloudensis | St | S | St | S | |
| Lavandula officinalis | St | An | St | An | |
| Trachyspermum ammi × | Nepeta binaloudensis | S | S | S | S |
| Lavandula officinalis | St | S | St | S | |
| Nepeta binaloudensis × | Lavandula officinalis | S | S | An | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadeqifard, S.; Mirmostafaee, S.; Joharchi, M.R.; Zandavifard, J.; Azizi, M.; Fujii, Y. Evaluation of Allelopathic Activity Interactions of Some Medicinal Plants Using Fractional Inhibitory Concentration and Isobologram. Agronomy 2022, 12, 3001. https://doi.org/10.3390/agronomy12123001
Sadeqifard S, Mirmostafaee S, Joharchi MR, Zandavifard J, Azizi M, Fujii Y. Evaluation of Allelopathic Activity Interactions of Some Medicinal Plants Using Fractional Inhibitory Concentration and Isobologram. Agronomy. 2022; 12(12):3001. https://doi.org/10.3390/agronomy12123001
Chicago/Turabian StyleSadeqifard, Somayeh, Somayeh Mirmostafaee, Mohammad Reza Joharchi, Jaleh Zandavifard, Majid Azizi, and Yoshiharu Fujii. 2022. "Evaluation of Allelopathic Activity Interactions of Some Medicinal Plants Using Fractional Inhibitory Concentration and Isobologram" Agronomy 12, no. 12: 3001. https://doi.org/10.3390/agronomy12123001
APA StyleSadeqifard, S., Mirmostafaee, S., Joharchi, M. R., Zandavifard, J., Azizi, M., & Fujii, Y. (2022). Evaluation of Allelopathic Activity Interactions of Some Medicinal Plants Using Fractional Inhibitory Concentration and Isobologram. Agronomy, 12(12), 3001. https://doi.org/10.3390/agronomy12123001









