Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. × intermedia
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Station’s Location
2.2. Details of Field Experiment
2.3. Essential Oil Isolation and Analyses
2.4. The Antimicrobial Activity of Essential Oils
2.5. Data Analysis and Visualisations
3. Results
3.1. Composition of Essential Oils
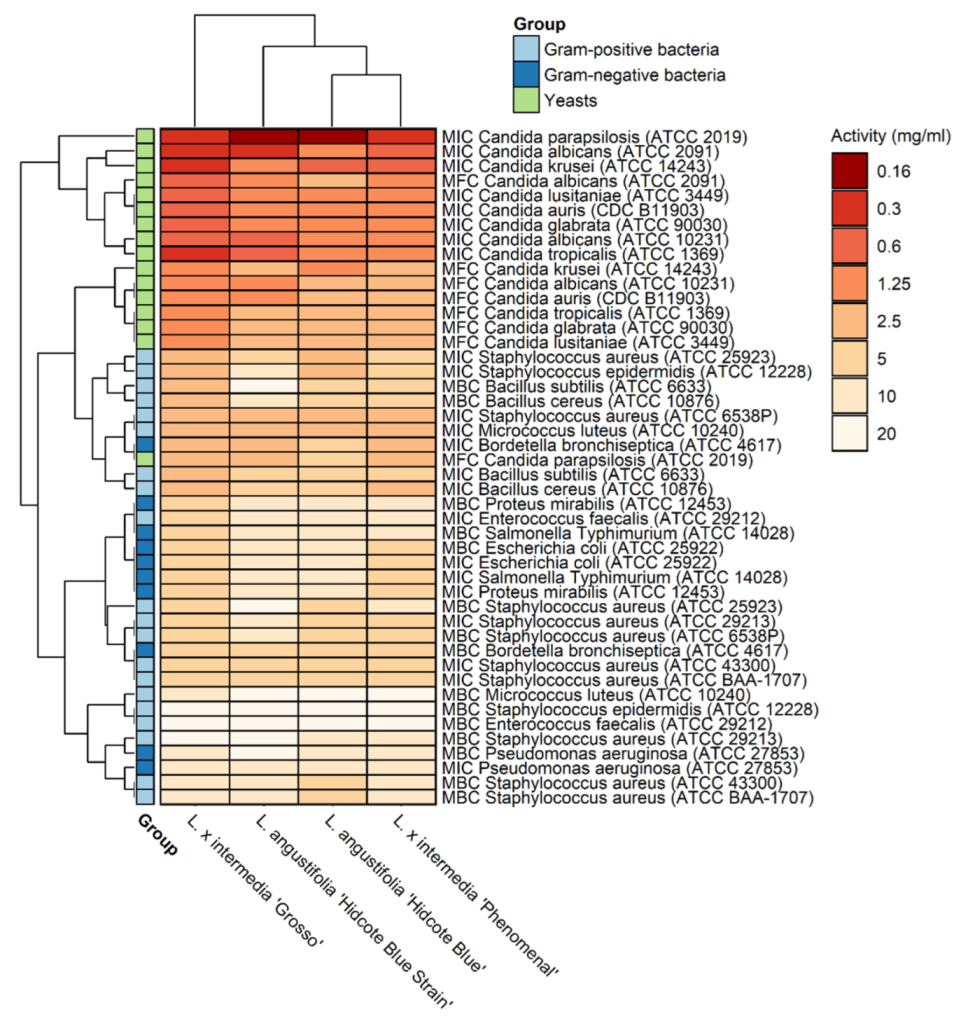
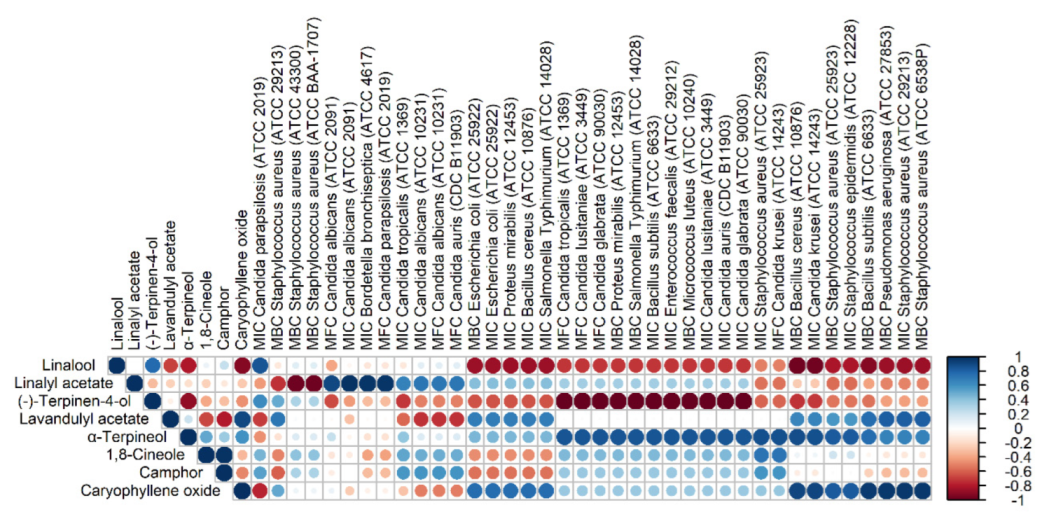
3.2. Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erbaş, S.; Baydar, H. Effects of harvest time and drying temperature on essential oil content and composition in lavandin (Lavandula × intermedia Emerice × Loisel.). Turk. J. Field Crops. 2008, 13, 24–31. [Google Scholar]
- Royal Botanic Gardens, Kew. Plants of the World Online. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:20960-1 (accessed on 13 November 2022).
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Dioscorides, P. De Materia Medica—Being an Herbal with Many Other Medicinal Materials, Written in Greek in the First Century of the Common Era: A New Indexed Version in Modern English; Osbaldeston, T.A., Wood, R.P.A., Eds.; Ibidis Press: Johannesburg, South Africa, 2000. [Google Scholar]
- Bingen, H. Physica–Uzdrawiające Dzieło Stworzenia. Naturalna Siła Oddziaływania Rzeczy; Polskie Centrum Świętej Hildegardy: Legnica, Poland, 2014. [Google Scholar]
- Gattefosse, R.-M. Gattefosse’s Aromatherapy; Random House: New York, NY, USA, 2012. [Google Scholar]
- Cavanagh, H.M.; Wilkinson, J.M. Lavender essential oil: A review. Aust. Infect. Control 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Adaszyńska, M.; Swarcewicz, M.; Dzięcioł, M.; Dobrowolska, A. Comparison of chemical composition and antibacterial activity of lavender varieties from Poland. Nat. Prod. Res. 2013, 27, 1497–1501. [Google Scholar] [CrossRef]
- Prusinowska, R.; Smigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L.). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Barreca, S.; La Bella, S.; Maggio, A.; Licata, M.; Buscemi, S.; Leto, C.; Pace, A.; Tuttolomondo, T. Flavouring extra-virgin olive oil with aromatic and medicinal plants essential oils stabilizes oleic acid composition during photo-oxidative stress. Agriculture 2021, 11, 266. [Google Scholar] [CrossRef]
- Herraiz-Peñalver, D.; Cases, M.Á.; Varela, F.; Navarrete, P.; Sánchez-Vioque, R.; Usano-Alemany, J. Chemical characterization of Lavandula latifolia Medik. essential oil from Spanish wild populations. Biochem. Syst. Ecol. 2013, 46, 59–68. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Hristov, A.N. Lavender and hyssop productivity, oil content, and bioactivity as a function of harvest time and drying. Ind. Crops Prod. 2012, 36, 222–228. [Google Scholar] [CrossRef]
- Sienkiewicz, M.; Głowacka, A.; Kowalczyk, E.; Wiktorowska-Owczarek, A.; Jóźwiak-Bębenista, M.; Łysakowska, M. The biological activities of cinnamon, geranium and lavender essential oils. Molecules 2014, 19, 20929–20940. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between the bioactivity and chemical composition of commercial plant essential oils. Flavour Fragr. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Lis-Balchin, M. (Ed.) Lavender: The Genus Lavandula; CRC press: Boca Raton, FL, USA, 2002; Available online: www.books.google.pl (accessed on 20 October 2022).
- Danh, L.T.; Han, L.N.; Triet, N.D.A.; Zhao, J.; Mammucari, R.; Foster, N. Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food Bioprocess Tech. 2013, 6, 3481–3489. [Google Scholar] [CrossRef]
- Andrys, D.; Kulpa, D.; Grzeszczuk, M.; Bihun, M.; Dobrowolska, A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated in vitro. Folia Hortic. 2017, 29, 161–180. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula × intermedia) grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Lammerink, J.; Wallace, A.R.; Porter, N.G. Effects of harvest time and postharvest drying on oil from lavandin (Lavandula × intermedia). N. Z. J. Crop Hortic. Sci. 1989, 17, 315–326. [Google Scholar] [CrossRef]
- Sadowska, U. The influence of the lavender and lavendine drying method on the plant material quality. J. Res. Appl. Agric. Eng. 2012, 57, 83–85. [Google Scholar]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Jeliazkova, E. Distillation time effect on lavender essential oil yield and composition. J. Oleo Sci. 2013, 62, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.H.; Salehi, P.; Vala, M.M.; Ghorbanpour, M. The effect of drying methods on yield and chemical constituents of the essential oil in Lavandula angustifolia Mill. (Lamiaceae). Plant Physiol. Rep. 2019, 24, 96–103. [Google Scholar] [CrossRef]
- Kuş, Ç.; Duru, M.E. Effects of post-harvest drying times of Lavandula angustifolia and L. intermedia species on chemical components of their essential oils. Avrupa Bilim Teknol. Derg. 2021, 21, 501–505. [Google Scholar] [CrossRef]
- Adaszyńska, M.; Swarcewicz, M.; Markowska-Szczupak, A. Porównanie składu chemicznego i aktywności przeciwdrobnoustrojowej olejku eterycznego otrzymanego z różnych krajowych odmian lawendy wąskolistnej (Lavandula angustifolia L.). Post Fitoter 2013, 2, 90–96. Available online: http://polona.pl/item/46238610 (accessed on 20 October 2022).
- Bombarda, I.; Dupuy, N.; Le Van Da, J.P.; Gaydou, E.M. Comparative chemometric analyses of geographic origins and compositions of lavandin var. Grosso essential oils by mid infrared spectroscopy and gas chromatography. Anal. Chim. Acta 2008, 613, 31–39. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. Dis. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Malm, A.; Grzegorczyk, A.; Biernasiuk, A.; Baj, T.; Rój, E.; Tyśkiewicz, K.; Dębczak, A.; Stolarski, M.J.; Krzyżaniak, M.; Olba-Zięty, E. Could supercritical extracts from the aerial parts of Helianthus salicifolius A. Dietr. and Helianthus tuberosus L. be regarded as potential raw materials for biocidal purposes? Agriculture 2021, 11, 10. [Google Scholar] [CrossRef]
- Wells, R.; Lis-Balchin, M. Perfumery uses of lavender and lavandin oils. In Lavender: The Genus Lavandula; Lis-Balchin, M., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 208–213. [Google Scholar]
- Charles, D.J.; Renaud, E.N.; Simon, J.E. Comparative study of essential oil quantity and composition from ten cultivars of organically grown lavender and lavandin. In Lavender: The Genus Lavandula; Lis-Balchin, M., Ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 246–256. [Google Scholar]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Kokina, M.; Salević, A.; Kalušević, A.; Lević, S.; Pantić, M.; Pljevljakušić, D.; Nedović, V. Characterization, antioxidant and antibacterial activity of essential oils and their encapsulation into biodegradable material followed by freeze drying. Food Sci. Biotechnol. 2019, 57, 282. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.A.; Holden, M.T. Staphylococcus aureus: Superbug, super genome? Trends Microbiol. 2004, 12, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antimicrobial properties and potential application in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- De Rapper, S.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial effects of Lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Malm, A. Olejki eteryczne w profilaktyce i leczeniu chorób infekcyjnych (Essential oils in the prevention and treatment of infectious diseases). PZWL Wydaw. Lek. 2021, 25–28, 42–48. [Google Scholar]
- Marín, I.; Sayas-Barberá, E.; Viuda-Martos, M.; Navarro, C.; Sendra, E. Chemical composition, antioxidant and antimicrobial activity of essential oils from organic fennel, parsley, and lavender from Spain. Foods 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C.A. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J. Appl. Microbiol. 2006, 101, 1232–1240. [Google Scholar] [CrossRef] [PubMed]


| Variety | Oil Content |
|---|---|
| L. angustifolia ‘Hidcote Blue Strain’ | 3.6 |
| L. angustifolia ‘Hidcote Blue’ | 3.1 |
| L. × intermedia ‘Phenomenal’ | 8.1 |
| L. × intermedia ‘Grosso’ | 4.4 |
| Name | Retention Time | Retention Index | L. angustifolia ‘Hidcote Blue Strain’ | L. angustifolia ‘Hidcote Blue’ | L. × intermedia ‘Phenomenal’ | L. × intermedia ‘Grosso’ | |
|---|---|---|---|---|---|---|---|
| 1. | β-Pinene | 8.103 | 941 | 0.51 | - | 0.54 | - |
| 2. | 1-Octen-3-ol | 8.170 | 944 | 0.06 | - | 0.32 | 0.57 |
| 3. | 3-Oktanone | 8.243 | 946 | 0.56 | 1.04 | - | 0.83 |
| 4. | β-Myrcene | 8.337 | 949 | 0.84 | 0.52 | 0.61 | 0.51 |
| 5. | n-Hexyl acetate | 8.827 | 965 | 0.90 | 0.15 | 0.29 | 0.33 |
| 6. | o-Cymene | 9.143 | 971 | 0.17 | - | - | 0.68 |
| 7. | p-Cymene | 9.153 | 975 | 0.57 | 0.33 | 0.25 | - |
| 8. | Limonene | 9.253 | 978 | 0.64 | 0.53 | 0.80 | 0.32 |
| 9. | 1.8-Cineole | 9.320 | 981 | 2.77 | 1.19 | 9.10 | 0.98 |
| 10. | (E)-β-Ocimene | 9.363 | 982 | 0.48 | 0.84 | - | 0.53 |
| 11. | trans-Linalool oxide | 10.147 | 1007 | 4.19 | 0.77 | 0.62 | 1.00 |
| 12. | 6-Methyl-2-(2-oxiranyl)-5-hepten-2-ol | 10.477 | 1018 | 3.22 | 0.51 | 0.24 | 0.32 |
| 13. | Linalool | 10.757 | 1027 | 15.10 | 21.77 | 25.53 | 29.56 |
| 14. | 1-Octene-3-ol acetate | 10.833 | 1029 | 1.99 | 1.51 | 0.97 | 2.22 |
| 15. | Camphor | 11.757 | 1058 | 0.56 | 0.55 | 9.57 | 0.14 |
| 16. | Cyclohexanone | 11.770 | 1059 | 1.25 | - | - | - |
| 17. | Lavandulol | 12.033 | 1067 | 1.08 | 1.19 | 0.52 | 2.46 |
| 18. | Borneol | 12.290 | 1075 | 2.45 | 1.92 | 4.58 | 0.71 |
| 19. | Terpinen-4-ol | 12.423 | 1079 | 1.79 | 2.91 | 3.52 | 18.08 |
| 20. | n- Hexyl butyrate | 12.503 | 1058 | - | 0.71 | 0.32 | 0.53 |
| 21. | Cryptone | 12.530 | 1083 | 2.78 | 1.01 | 0.26 | - |
| 22. | α-Terpineol | 12.713 | 1088 | 4.30 | 3.50 | 3.97 | 2.82 |
| 23. | 2-Pinene-4-on | 12.920 | 1095 | - | 0.07 | - | 0.60 |
| 24. | Cis-Geraniol | 13.213 | 1104 | 0.76 | 0.46 | 0.46 | 0.45 |
| 25. | Linalyl acetate | 13.607 | 1117 | 16.87 | 34.22 | 21.05 | 18.56 |
| 26. | Geraniol | 13.670 | 1119 | 1.71 | 1.47 | 1.36 | 1.24 |
| 27. | Lavandulyl acetate | 14.203 | 1137 | 8.26 | 5.80 | 3.52 | 5.90 |
| 28. | 3-Nonanol | 15.290 | 1173 | 0.70 | 0.38 | 0.10 | - |
| 29. | Neryl acetate | 15.317 | 1180 | 1.07 | 1.14 | 0.79 | 0.73 |
| 30. | Geranyl acetate | 15.850 | 1191 | 2.17 | 1.90 | 1.51 | 1.36 |
| 31. | Santalene | 16.637 | 1218 | 0.24 | 0.87 | 0.16 | 0.24 |
| 32. | Caryopyllene | 16.713 | 1221 | 0.46 | 1.67 | 0.74 | 1.47 |
| 33. | Cis- β-Farnesene | 17.107 | 1234 | 0.09 | 0.63 | 0.43 | 1.46 |
| 34. | γ-Cadinene | 18.813 | 1294 | 0.53 | 0.18 | - | - |
| 35. | Caryophyllene Oxide | 19.300 | 1311 | 6.42 | 2.20 | 0.44 | 1.02 |
| 36. | γ-Muurolene | 20.153 | 1343 | 2.10 | 0.65 | 0.98 | - |
| 37. | Androstan-17-on | 20.598 | 1359 | 0.85 | - | - | - |
| 38. | Bisabolol | 20.727 | 1364 | - | - | 1.87 | - |
| Sum | 88.44 | 91.55 | 95.42 | 95.62 |
| Microorganisms | L. angustifolia ‘Hidcote Blue Strain’ | L. angustifolia ‘Hidcote Blue’ | L. × intermedia ‘Phenomenal’ | L. × intermedia ‘Grosso’ | ||||
|---|---|---|---|---|---|---|---|---|
| Gram-positive bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Staphylococcus aureus ATCC 29213 | 10 | 20 | 5 | 10 | 5 | 10 | 5 | 20 |
| Staphylococcus aureus ATCC 6538P | 2.5 | 10 | 2.5 | 5 | 2.5 | 5 | 2.5 | 5 |
| Staphylococcus aureus ATCC 25923 | 5 | 20 | 2.5 | 5 | 5 | 10 | 2.5 | 5 |
| Staphylococcus aureus ATCC 43300 | 5 | 10 | 5 | 5 | 5 | 10 | 5 | 10 |
| Staphylococcus aureus ATCC BAA-1707 | 5 | 10 | 5 | 5 | 5 | 10 | 5 | 10 |
| Staphylococcus epidermidis ATCC 12228 | 10 | 20 | 2.5 | 20 | 5 | 20 | 2.5 | 20 |
| Enterococcus faecalis ATCC 29212 | 10 | 20 | 10 | 20 | 10 | 20 | 5 | 20 |
| Micrococcus luteus ATCC 10240 | 2.5 | 20 | 2.5 | 20 | 2.5 | 20 | 2.5 | 10 |
| Bacillus subtilis ATCC 6633 | 5 | 20 | 5 | 5 | 5 | 5 | 2.5 | 2.5 |
| Bacillus cereus ATCC 10876 | 5 | 10 | 5 | 5 | 2.5 | 5 | 2.5 | 2.5 |
| Gram-negative bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Salmonella Typhimurium ATCC 14028 | 10 | 10 | 10 | 10 | 5 | 10 | 5 | 5 |
| Proteus mirabilis ATCC 12453 | 10 | 10 | 10 | 10 | 5 | 10 | 5 | 5 |
| Bordetella bronchiseptica ATCC 4617 | 2.5 | 5 | 5 | 5 | 2.5 | 5 | 2.5 | 5 |
| Escherichia coli ATCC 25922 | 10 | 10 | 10 | 10 | 5 | 5 | 5 | 5 |
| Pseudomonas aeruginosa ATCC 27853 | 10 | 20 | 10 | 10 | 10 | 10 | 10 | 10 |
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| Candida albicans ATCC 2091 | 0.3 | 1.25 | 1.25 | 2.5 | 0.6 | 1.25 | 0.3 | 0.6 |
| Candida albicans ATCC 10231 | 0.6 | 1.25 | 1.25 | 2.5 | 1.25 | 2.5 | 0.6 | 1.25 |
| Candida auris CDC B11903 | 1.25 | 1.25 | 1.25 | 2.5 | 1.25 | 2.5 | 0.6 | 1.25 |
| Candida glabrata ATCC 90030 | 1.25 | 2.5 | 1.25 | 2.5 | 1.25 | 2.5 | 0.6 | 1.25 |
| Candida parapsilosis ATCC 2019 | 0.16 | 2.5 | 0.16 | 5 | 0.3 | 2.5 | 0.3 | 2.5 |
| Candida krusei ATCC 14243 | 1.25 | 2.5 | 0.6 | 1.25 | 0.6 | 2.5 | 0.3 | 1.25 |
| Candida lusitaniae ATCC 3449 | 1.25 | 2.5 | 1.25 | 2.5 | 1.25 | 2.5 | 0.6 | 1.25 |
| Candida tropicalis ATCC 1369 | 0.6 | 2.5 | 1.25 | 2.5 | 1.25 | 2.5 | 0.3 | 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walasek-Janusz, M.; Grzegorczyk, A.; Zalewski, D.; Malm, A.; Gajcy, S.; Gruszecki, R. Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. × intermedia. Agronomy 2022, 12, 2955. https://doi.org/10.3390/agronomy12122955
Walasek-Janusz M, Grzegorczyk A, Zalewski D, Malm A, Gajcy S, Gruszecki R. Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. × intermedia. Agronomy. 2022; 12(12):2955. https://doi.org/10.3390/agronomy12122955
Chicago/Turabian StyleWalasek-Janusz, Magdalena, Agnieszka Grzegorczyk, Daniel Zalewski, Anna Malm, Sylwia Gajcy, and Robert Gruszecki. 2022. "Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. × intermedia" Agronomy 12, no. 12: 2955. https://doi.org/10.3390/agronomy12122955
APA StyleWalasek-Janusz, M., Grzegorczyk, A., Zalewski, D., Malm, A., Gajcy, S., & Gruszecki, R. (2022). Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. × intermedia. Agronomy, 12(12), 2955. https://doi.org/10.3390/agronomy12122955







