Genetic and Genomics Resources of Cross-Species Vigna Gene Pools for Improving Biotic Stress Resistance in Mungbean (Vigna radiata L. Wilczek)
Abstract
1. Introduction
2. Approaches to Combat Biotic Stresses
2.1. Pathogen Characterization and Screening
2.2. Understanding the Genetics of Pathogen-Specific Biotic Stress Resistance
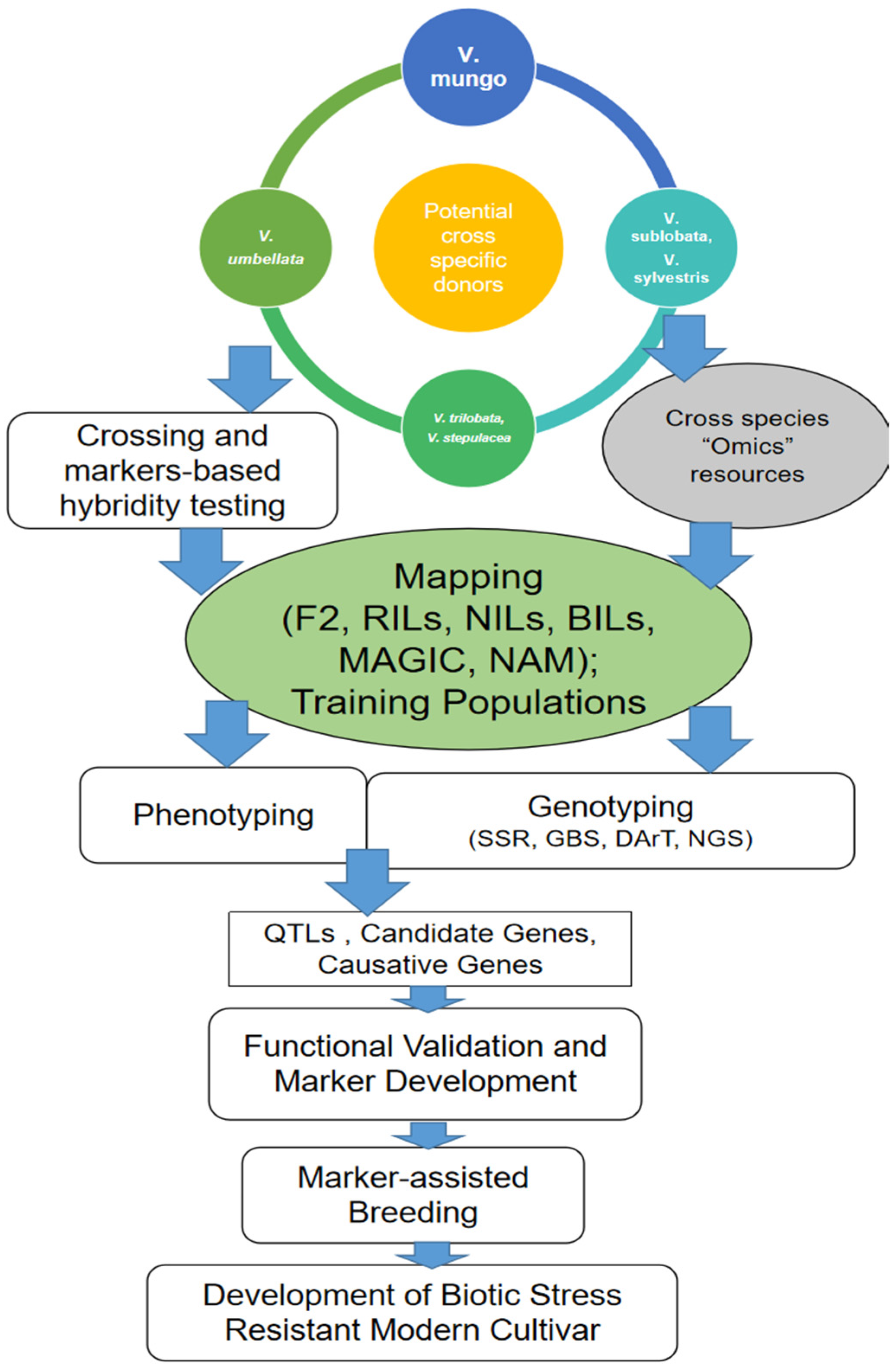
2.3. Exploring Cross-Specific Newer Gene Pools for Potential Donors
2.4. Characterizing Vigna Diversity: From Conventional to Omics Approaches
2.5. Highlights of Vigna Genomic Resources
2.6. Tagging, Mapping, and Exploiting QTL
2.7. Expanding Genomic Regions for Tagging New Candidate Genes
2.8. Comprehensive RNA-Seq Approach
2.9. Gene-Based Functional Markers
2.10. Developing Potential SCARs
2.11. Marker-Assisted Breeding
3. Conclusions and Way Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratap, A.; Gupta, S.; Rathore, M.; Basavaraja, T.; Singh, C.M.; Prajapati, U.; Singh, P.; Singh, Y.; Kumari, G. Mungbean. In The Beans and the Peas: From Orphan to Mainstream Crops; Pratap, A., Gupta, S., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–32. [Google Scholar]
- Sriphadet, S.; Lambrides, C.J.; Srinives, P. Inheritance of agronomic traits and their interrelationship in mungbean (Vigna radiata (L.) Wilczek). J. Crop Sci. Biotechnol. 2007, 10, 249–256. [Google Scholar]
- Keatinge, J.D.H.; Easdown, W.J.; Yang, R.Y.; Chadha, M.L.; Shanmugasundaram, S. Overcoming chronic malnutrition in a future warming world: The key importance of mungbean and vegetable soybean. Euphytica 2011, 180, 129–141. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Karuppudurai, T.; Sinha, P.B.; Kamarul, H.A.; Manoharan, K. Genetic diversity in green gram [Vigna radiata (L.)] landraces analyzed by using random amplified polymorphic DNA (RAPD). Afr. J. Biotechnol. 2006, 5, 1214–1219. [Google Scholar]
- Jat, S.L.; Shivay, Y.S.; Parihar, C.M.; Meena, H.N. Evaluation of summer legumes for their economic feasibility, nutrient accumulation and soil fertility. J. Food Legumes 2012, 25, 240–243. [Google Scholar]
- Singh, C.M.; Pratap, A.; Kumar, H.; Singh, S.; Singh, B.K.; Prasad, D.; Dhaliwal, I.; Kumar, M. Recent advances in omics approaches for mungbean improvement. In Technologies in Plant Biotechnology and Breeding of Field Crops; Kamaluddin, Kiran, U., Abdin, M.Z., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insects pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Tutlani, A.; Banshidhar, P.J.; Janeja, H.S. Abiotic and biotic stresses and their effect on Vigna radiata L. Pharma Innov. J. 2022, 11, 230–237. [Google Scholar]
- Qazi, J.; Ilyas, M.; Mansoor, S.; Briddon, R.W. Legume yellow mosaic viruses: Genetically isolated begomoviruses. Mol. Plant Pathol. 2007, 8, 343–348. [Google Scholar] [CrossRef]
- Ilyas, M.; Qazi, J.; Mansoor, S.; Briddon, R.W. Genetic diversity and phylogeography of begomoviruses infecting legumes in Pakistan. J. Gen. Virol. 2010, 91, 2091–2101. [Google Scholar] [CrossRef]
- Akhtar, K.P.; Kitsanachandee, R.; Srinives, P.; Abbas, G.; Asghar, M.; Shah, T.; Atta, B.; Chatchawankanphanich, O.; Sarwar, G.; Ahmad, M.; et al. Field Evaluation of Mungbean Recombinant Inbred Lines against Mungbean Yellow Mosaic Disease Using New Disease Scale in Thailand. Plant Pathol. J. 2009, 25, 422–428. [Google Scholar] [CrossRef]
- Cayalvizhi, B.; Nagarajan, P.; Raveeendran, M.; Rabindran, R.; Selvam, N.J.; Bapu, J.K.; Senthil, N. Unraveling the responses of mungbean (Vigna radiata) to mungbean yellow mosaic virus through 2D-protein expression. Physiol. Mol. Plant Pathol. 2015, 90, 65–77. [Google Scholar] [CrossRef]
- Schoelz, J.E.; Harries, P.A.; Nelson, R.S. Intracellular transport of plant viruses: Finding the door out of the cell. Mol. Plant 2011, 4, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Ikegami, M.; Miura, K.-I. The Nucleotide Sequence and Genome Structure of Mung Bean Yellow Mosaic Geminivirus. Microbiol. Immunol. 1993, 37, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Bos, L. Plant Viruses, Unique and Intriguing Pathogens: A Textbook of Plant Virology; Backhuys Publishers: Leiden, The Netherlands, 1999. [Google Scholar]
- Ahmad, M.; Harwood, R.F. Studies on a whitefly-transmitted yellow mosaic of urd bean (Phaseolus mungo). Plant Dis. Rep. 1973, 57, 800–802. [Google Scholar]
- Malathi, V.G.; John, P. Mungbean Yellow Mosaic Viruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 364–372. [Google Scholar]
- Sohal, B.S.; Bajaj, K.L. Effects of yellow mosaic virus on polyphenol metabolism in resistant and susceptible mungbean (Vigna radiata L. Wilczek) leaves. Biochem. Physiol. Pflanz 1993, 188, 419–423. [Google Scholar] [CrossRef]
- Conrath, U. Systemic Acquired Resistance. Plant Signal. Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; El-Shazly, H.H.; Badr, A. Role of salicylic acid in biotic and abiotic stress tolerance in plants. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Singapore, 2020; pp. 533–554. [Google Scholar]
- Lee, B.-J.; Kim, S.-K.; Choi, S.B.; Bae, J.; Kim, K.-J.; Kim, Y.-J.; Paek, K.-H. Pathogen-inducible CaUGT1 is involved in resistance response against TMV infection by controlling salicylic acid accumulation. FEBS Lett. 2009, 583, 2315–2320. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.; Murphy, A.M.; Berry, J.O.; Carr, J.P. Salicylic acid can induce resistance to plant virus movement. Mol. Plant Microbe Interact. 1998, 11, 860–868. [Google Scholar] [CrossRef]
- Mohase, L.; van der Westhuizen, A.J. Salicylic acid is involved in resistance responses in the Russian wheat aphid-wheat interaction. J. Plant Physiol. 2002, 159, 585–590. [Google Scholar] [CrossRef]
- Loake, G.; Grant, M. Salicylic acid in plant defence—The players and protagonists. Curr. Opin. Plant Biol. 2007, 10, 466–472. [Google Scholar] [CrossRef]
- Savaliya, A.S.; Chopada, G.B.; Sekhada, M.R.; Bhimani, A.A. Survey of the Powdery Mildew of Mungbean (Vigna radiata (L). Wilczek) in Selected Districts of South Gujarat. Trends Biosci. 2018, 11, 3004–3009. [Google Scholar]
- Shen, Y.M.; Liu, H.L.; Chang, S.T.; Chao, C.H. First report of Anthracnose caused by Colletotrichum acutatum on mung bean sprouts in Taiwan. Plant Dis. 2010, 94, 131. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Souframanien, J.; Chand, R.; Pawar, S.E. Genetic diversity study of Cercospora canescens (Ellis & Martin) isolates, the pathogen of Cercospora leaf spot in legumes. Curr. Sci. 2006, 90, 564–568. [Google Scholar]
- Iqbal, U.; Mukhtar, T. Morphological and Pathogenic Variability among Macrophomina phaseolina Isolates Associated with Mungbean (Vigna radiata L.) Wilczek from Pakistan. Sci. World J. 2014, 2014, 950175. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Burlakoti, R.R.; Kenyon, L.; Nair, R.M. Perspectives and challenges for sustainable management of fungal diseases of mungbean [Vigna radiata (L.) R. Wilczek var. radiata]: A review. Front. Environ. Sci. 2018, 6, 53. [Google Scholar] [CrossRef]
- Kaur, L. Multiple disease resistant sources of mungbean. Acta Hortic. 2006, 752, 423–426. [Google Scholar] [CrossRef]
- Reddy, K.S.; Pawar, S.E.; Bhatia, C.R. Inheritance of powdery mildew (Erysiphe polygoni DC) resistance in mungbean (Vigna radiata L. Wilczek). Theor. Appl. Genet. 1994, 88, 945–948. [Google Scholar] [CrossRef]
- Kumar, H.; Singh, R.B. Genetic analysis of adult plant resistance to powdery mildew in pea (Pisum sativum L.). Euphytica 1981, 30, 147–151. [Google Scholar] [CrossRef]
- Majid, S. Annals report of Department of Agriculture, Assam for year ending 31st March 1/950. II. Grow More Food Campaign 1953, 11, 107. [Google Scholar]
- Sharma, H.C.; Khare, M.N.; Joshi, L.K.; Kumar, S.M. Efficacy of fungicides in the control of diseases of kharif pulses mung and urid. In Proceedings of the All India Workshop on Kharif Pulses, Hissar, India, 18–20 March 1971; p. 2. [Google Scholar]
- Kulkarni, S.A. Epidemiology and Integrated Management of Anthracnose of Greengram. Master’s Thesis, University of Agricultural Sciences, Dharwad, India, 2009. [Google Scholar]
- Koller, W.; Parker, D.M. Purification and characterization of cutinase from Venturia inaequalis. Phytopathology 1989, 79, 278–283. [Google Scholar] [CrossRef]
- Dickman, M.B.; Podila, G.K.; Kolattukudy, P.E. Insertion of cutinase gene into a wound pathogen enables it to infect intact host. Nature 1989, 342, 446–448. [Google Scholar] [CrossRef]
- Skamnioti, P.; Gurr, S.J. Magnaporthe grisea cutinase2 mediates appressorium differentiation and host penetration and is required for full virulence. Plant Cell 2007, 19, 2674–2689. [Google Scholar] [CrossRef] [PubMed]
- Deising, H.; Nicholson, R.L.; Haug, M.; Howard, R.J.; Mendgen, K. Adhesion pad formation and the involvement of cutinase and esterases in the attachment of uredospores to the host cuticle. Plant Cell 1992, 4, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A. Studies on the Diseases Caused by Rhizoctonia Solani Kuhn in Green Gram (Phaseolus aureus Roxb). Ph.D. Thesis, Rani Durgavati Vishwavidyalaya, Jabalpur, India, 1993. [Google Scholar]
- Dwivedi, R.P.; Saksena, H.K. Occurrence of web blight caused by Thanatephorus cucumeris on mung bean. Int. J. Farm Sci. 1974, 2, 100. [Google Scholar]
- Juroszek, P.; Von Tiedemann, A. Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol. 2011, 60, 100–112. [Google Scholar] [CrossRef]
- Selvi, R.; Muthiah, A.R.; Manivannan, N.; Raveendran, T.S.; Manickam, A.; Samiyappan, R. Tagging of RAPD marker for MYMV resistance in mungbean (Vigna radiata (L.) Wilczek). Asian J. Plant Sci. 2006, 5, 277–280. [Google Scholar]
- Singh, S.R.; Emden, H.F. Van Insect pests of grain legumes. Annu. Rev. Entomol. 1979, 24, 255–278. [Google Scholar] [CrossRef]
- Saxena, H.P. Pests of grain legumes and their control in India. In Pests of Grain Legumes: Ecology and Control; Singh, S., Van Emden, H.F., Taylor, T.A., Eds.; Academic Press: London, UK, 1978; pp. 15–23. [Google Scholar]
- Ram, S.; Bhattacharya, A. Consumption of soybean by Diacrisia obliqua Walker. Indian J. Entomol. 1978, 40, 335–336. [Google Scholar]
- Sethi, G.R.; Prasad, S.; Singh, K.M. Population build up of Diacrisia obliqua Walker on sunflower at Delhi. Indian J. Entomol. 1979, 41, 36–38. [Google Scholar]
- Aidbhavi, R.; Pratap, A.; Verma, P.; Lamichaney, A.; Bandi, S.M.; Nitesh, S.D.; Akram, M.; Rathore, M.; Singh, B.; Singh, N.P. Screening of endemic wild Vigna accessions for resistance to three bruchid species. J. Stored Prod. Res. 2021, 93, 101864. [Google Scholar] [CrossRef]
- Talekar, N.S. Biology, Damage, and Control of Bruchid Pests of Mungbean. In Proceedings of the 2nd International Symposium on Mungbean, Bangkok, Thailand, 16–20 November 1987; pp. 329–342. [Google Scholar]
- Somta, P.; Ammaranan, C.; Ooi, P.A.-C.; Srinives, P. Inheritance of seed resistance to bruchids in cultivated mungbean (Vigna radiata, L. Wilczek). Euphytica 2007, 155, 47–55. [Google Scholar] [CrossRef]
- Miah, M.A. Host Preference of Pulse Beetles (Callosobruchus chinensis and C. maculatus) on Different Mungbean (Vigna radiata) Varieties. Curr. Trends Entomol. Zool. Std. 2020, 3, 114–2690. [Google Scholar]
- Singh, I.; Sandhu, J.S.; Gupta, S.K.; Singh, S. Introgression of productivity and other desirable traits from ricebean (Vigna umbellata) into black gram (Vigna mungo). Plant Breed. 2013, 132, 401–406. [Google Scholar] [CrossRef]
- Pratap, A.; Chaturvedi, S.K.; Tomar, R.; Rajan, N.; Malviya, N.; Thudi, M.; Saabale, P.R.; Prajapati, U.; Varshney, R.K.; Singh, N.P. Marker-assisted introgression of resistance to fusarium wilt race 2 in Pusa 256, an elite cultivar of desi chickpea. Mol. Genet. Genom. 2017, 292, 1237–1245. [Google Scholar] [CrossRef]
- Sehrawat, N.; Yadav, M.; Bhat, K.V.; Sairam, R.K.; Jaiwal, P.K. Introgression of mungbean yellow mosaic virus resistance in Vigna mungo (L.) Hepper and purity testing of F1 hybrids using SSRs. Turk. J. Agric. For. 2016, 40, 95–100. [Google Scholar] [CrossRef]
- Pratap, A.; Basu, P.S.; Gupta, S.; Malviya, N.; Rajan, N.; Tomar, R.; Madhavan, L.; Nadarajan, N.; Singh, N.P. Identification and characterization of sources for photo- and thermo-insensitivity in Vigna species. Plant Breed. 2014, 133, 756–764. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, S.K.; Kim, M.Y.; Lestari, P.; Kim, K.H.; Ha, B.-K.; Jun, T.H.; Hwang, W.J.; Lee, T.; Lee, J. Genome sequence of mungbean and insights into evolution within Vigna species. Nat. Commun. 2014, 5, 5443. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Li, X.; Guo, W.; Yuan, X.; Cui, X.; Chen, X. Genome re-sequencing of two accessions and fine mapping the locus of lobed leaflet margins in mungbean. Mol. Breed. 2016, 36, 128. [Google Scholar] [CrossRef]
- Ha, J.; Satyawan, D.; Jeong, H.; Lee, E.; Cho, K.; Kim, M.Y.; Lee, S. A near-complete genome sequence of mungbean (Vigna radiata L.) provides key insights into the modern breeding program. Plant Genome 2021, 14, e20121. [Google Scholar] [CrossRef] [PubMed]
- Jegadeesan, S.; Raizada, A.; Dhanasekar, P.; Suprasanna, P. Draft genome sequence of the pulse crop blackgram [Vigna mungo (L.) Hepper] reveals potential R-genes. Sci. Rep. 2021, 11, 11247. [Google Scholar] [CrossRef]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M. The genome of cowpea (Vigna unguiculata [L.] Walp.). Plant J. 2019, 98, 767–782. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sakai, H.; Yoshitsu, Y.; Muto, C.; Anai, T.; Pandiyan, M.; Senthil, N.; Tomooka, N.; Naito, K. Domesticating Vigna stipulacea: A potential legume crop with broad resistance to biotic stresses. Front. Plant Sci. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Satyawan, D.; Shim, S.; Lee, T.; Lee, J.; Hwang, W.J.; Kim, S.K.; Lestari, P.; Laosatit, K.; Kim, K.H. Draft genome sequence of adzuki bean, Vigna angularis. Sci. Rep. 2015, 5, 8069. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Velmurugan, A.; Gupta, D.S.; Kumar, J.; Kesari, R.; Konda, A.; Singh, N.P.; Roy, S.D.; Biswas, U.; Kumar, R.R.; et al. Draft genome sequence of a less-known wild Vigna: Beach pea (V. marina cv. ANBp-14-03). Crop J. 2019, 7, 660–666. [Google Scholar] [CrossRef]
- Pratap, A.; Das, A.; Kumar, S.; Gupta, S. Current perspectives on introgression breeding in food legumes. Front. Plant Sci. 2021, 11, 589189. [Google Scholar] [CrossRef]
- Pratap, A.; Gupta, S.; Nair, R.M.; Schafleitner, R.; Basu, P.S.; Singh, C.M.; Prajapati, U.; Gupta, A.K.; Nayyar, H.; Mishra, A.K.; et al. Using Plant Phenomics to Exploit the Gains of Genomics. Agronomy 2019, 9, 126. [Google Scholar] [CrossRef]
- Singh, P.; Mishra, A.K.; Singh, C.M. Genome-wide identification and characterization of Lectin receptor-like kinase (LecRLK) genes in mungbean (Vigna radiata L. Wilczek). J. Appl. Genet. 2021, 62, 223–234. [Google Scholar] [CrossRef]
- Singh, C.M.; Singh, P.; Tiwari, C.; Purwar, S.; Kumar, M.; Pratap, A.; Singh, S.; Chugh, V.; Mishra, A.K. Improving drought tolerance in Mungbean (Vigna radiata L. Wilczek): Morpho-physiological, biochemical and molecular Perspectives. Agronomy 2021, 11, 1534. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, D.S.; Anjum, T.K.; Pratap, A.; Kumar, J. Inheritance and molecular tagging of MYMIV resistance gene in blackgram (Vigna mungo L. Hepper). Euphytica 2013, 193, 27–37. [Google Scholar] [CrossRef]
- Sudha, M.; Anusuya, P.; Mahadev, N.G.; Karthikeyan, A.; Nagarajan, P.; Raveendran, M.; Senthil, N.; Pandiyan, M.; Angappan, K.; Balasubramanian, P. Molecular studies on mungbean (Vigna radiata (L.) Wilczek) and ricebean (Vigna umbellata (Thunb.)) interspecific hybridisation for Mungbean yellow mosaic virus resistance and development of species-specific SCAR marker for ricebean. Arch. Phytopathol. Plant Prot. 2013, 46, 503–517. [Google Scholar] [CrossRef]
- Sai, C.B.; Nagarajan, P.; Raveendran, M.; Rabindran, R.; Kannan Bapu, J.R.; Senthil, N. Understanding the inheritance of mungbean yellow mosaic virus (MYMV) resistance in mungbean (Vigna radiata L. Wilczek). Mol. Breed. 2017, 37, 1–15. [Google Scholar]
- Haq, Q.M.I.; Rouhibakhsh, A.; Ali, A.; Malathi, V.G. Infectivity analysis of a blackgram isolate of Mungbean yellow mosaic virus and genetic assortment with MYMIV in selective hosts. Virus Genes 2011, 42, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Patwa, N.; Chatterjee, C.; Basak, J. Differential responses of Phaseolus vulgaris cultivars following mungbean yellow mosaic India virus infection. Physiol. Mol. Biol. Plants 2020, 26, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.M.; Pratap, A.; Gupta, S.; Biradar, R.S.; Singh, N.P. Association mapping for mungbean yellow mosaic India virus resistance in mungbean (Vigna radiata L. Wilczek). 3 Biotech 2020, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Kamaal, N.; Pratap, A.; Singh, N.P. Resistance status of mungbean (Vigna radiata (L.) Wilczek) advanced breeding materials against mungbean yellow mosaic India virus. Arch. Phytopathol. Plant Prot. 2021, 54, 2533–2546. [Google Scholar] [CrossRef]
- Nair, R.M.; Pandey, A.K.; War, A.R.; Hanumantharao, B.; Shwe, T.; Alam, A.; Pratap, A.; Malik, S.R.; Karimi, R.; Mbeyagala, E.K. Biotic and abiotic constraints in mungbean production—Progress in genetic improvement. Front. Plant Sci. 2019, 10, 1340. [Google Scholar] [CrossRef]
- Kitsanachandee, R.; Somta, P.; Chatchawankanphanich, O.; Akhtar, K.P.; Shah, T.M.; Nair, R.M.; Bains, T.S.; Sirari, A.; Kaur, L.; Srinives, P. Detection of quantitative trait loci for mungbean yellow mosaic India virus (MYMIV) resistance in mungbean (Vigna radiata (L.) Wilczek) in India and Pakistan. Breed. Sci. 2013, 63, 367–373. [Google Scholar] [CrossRef]
- Alam, A.K.M.; Somta, P.; Srinives, P. Identification and confirmation of quantitative trait loci controlling resistance to mungbean yellow mosaic disease in mungbean [Vigna radiata (L.) Wilczek]. Mol. Breed. 2014, 34, 1497–1506. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K. Impact of micronutrients in mitigation of abiotic stresses in soils and plants—A progressive step toward crop security and nutritional quality. Adv. Agron. 2022, 173, 1–78. [Google Scholar]
- Khan, M.G.; Ahmad, W.; Khattak, G.S.S.; Ahmad, H. Mode of inheritance of resistance to mungbean yellow mosaic virus (MYMV) in mungbean (Vigna radiata (L.) Wilczek). Sarhad J. Agric. 2007, 23, 1071. [Google Scholar]
- Khattak, G.S.S.; Saeed, I.; Shah, S.A. Breeding high yielding and disease resistant mungbean (Vigna radiata (L.) Wilczek) genotypes. Pak. J. Bot. 2008, 40, 1411–1417. [Google Scholar]
- Vadivel, K.; Manivannan, N.; Mahalingam, A.; Satya, V.K.; Vanniarajan, C.; Ragul, S. Identification and validation of quantitative trait loci of mungbean yellow mosaic virus disease resistance in blackgram [Vigna mungo (L). Hepper]. Legume Res. Legume Res. Int. J. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Subramaniyan, R.; Narayana, M.; Krishnamoorthy, I.; Natarajan, G.; Gandhi, K. Novel and stable QTL regions conferring resistance to MYMV disease and its inheritance in blackgram (Vigna mungo (L.) Hepper). J. Genet. 2022, 101, 18. [Google Scholar] [CrossRef] [PubMed]
- Mathivathana, M.K.; Murukarthick, J.; Karthikeyan, A.; Jang, W.; Dhasarathan, M.; Jagadeeshselvam, N.; Sudha, M.; Vanniarajan, C.; Karthikeyan, G.; Yang, T.-J.; et al. Detection of QTLs associated with mungbean yellow mosaic virus (MYMV) resistance using the interspecific cross of Vigna radiata × Vigna umbellata. J. Appl. Genet. 2019, 60, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Sathees, N.; Shoba, D.; Mani, N.; Saravanan, S.; Kumari, M.P.; Pillai, M.A. Tagging of SSR markers associated to yellow mosaic virus resistance in black gram (Vigna mungo (L.) Hepper). Euphytica 2022, 218, 23. [Google Scholar] [CrossRef]
- Chankaew, S.; Somta, P.; Sorajjapinun, W.; Srinives, P. Quantitative trait loci mapping of Cercospora leaf spot resistance in mungbean, Vigna radiata (L.) Wilczek. Mol. Breed. 2011, 28, 255–264. [Google Scholar] [CrossRef]
- Yundaeng, C.; Somta, P.; Chen, J.; Yuan, X.; Chankaew, S.; Chen, X. Fine mapping of QTL conferring Cercospora leaf spot disease resistance in mungbean revealed TAF5 as candidate gene for the resistance. Theor. Appl. Genet. 2021, 134, 701–714. [Google Scholar] [CrossRef]
- Choudhary, S.; Kaurav, H.; Chaudhary, G. Vaibidang (Embelia ribes): A Potential Herbal Drug in Ayurveda with Anthelmintic Property. Int. J. Res. Appl. Sci. Biotechnol. 2021, 8, 237–243. [Google Scholar] [CrossRef]
- Kasettranan, W.; Somta, P.; Srinives, P. Mapping of quantitative trait loci controlling powdery mildew resistance in mungbean (Vigna radiata (L.) Wilczek). J. Crop Sci. Biotechnol. 2010, 13, 155–161. [Google Scholar] [CrossRef]
- Humphry, M.E.; Magner, T.; McIntyre, C.L.; Aitken, E.A.B.; Liu, C.J. Identification of a major locus conferring resistance to powdery mildew (Erysiphe polygoni DC) in mungbean (Vigna radiata L. Wilczek) by QTL analysis. Genome 2003, 46, 738–744. [Google Scholar] [CrossRef]
- Tantasawat, P.A.; Poolsawat, O.; Kativat, C.; Arsakit, K.; Papan, P.; Chueakhunthod, W.; Pookhamsak, P. Inheritance and identification of ISSR-RGA markers associated with powdery mildew resistance in mungbean for marker-assisted breeding. Chil. J. Agric. Res. 2022, 82, 3–9. [Google Scholar] [CrossRef]
- Wu, X.; Wang, B.; Lu, Z.; Wu, X.; Li, G.; Xu, P. Identification and mapping of a powdery mildew resistance geneVu-Pm1in the Chinese asparagus bean landrace ZN016. Legume Res. Int. J. 2014, 37, 32–36. [Google Scholar] [CrossRef]
- Sun, L.; Cheng, X.-Z.; Wang, S.-H.; Wang, L.-X.; Liu, C.-Y.; Mei, L.; Xu, N. Heredity Analysis and Gene Mapping of Bruchid Resistance of a Mungbean Cultivar V2709. Agric. Sci. China 2008, 7, 672–677. [Google Scholar] [CrossRef]
- Chen, T.; Hu, L.; Wang, S.; Wang, L.; Cheng, X.; Chen, H. Construction of High-Density Genetic Map and Identification of a Bruchid Resistance Locus in Mung Bean (Vigna radiata L.). Front. Genet. 2022, 13, 903267. [Google Scholar] [CrossRef]
- Kaewwongwal, A.; Chen, J.; Somta, P.; Kongjaimun, A.; Yimram, T.; Chen, X.; Srinives, P. Novel alleles of two tightly linked genes encoding polygalacturonase-inhibiting proteins (VrPGIP1 and VrPGIP2) associated with the Br locus that confer bruchid (Callosobruchus spp.) resistance to mungbean (Vigna radiata) accession V2709. Front. Plant Sci. 2017, 8, 1692. [Google Scholar] [CrossRef] [PubMed]
- Amkul, K.; Wang, L.; Somta, P.; Wang, S.; Cheng, X. Construction of a high density linkage map and genome dissection of bruchid resistance in zombi pea (Vigna vexillata (L.) A. Rich). Sci. Rep. 2019, 9, 11719. [Google Scholar] [CrossRef] [PubMed]
- Souframanien, J.; Gopalakrishna, T. Source for bruchid resistance and its inheritance in Trombay wild urdbean (Vigna mungo var. silvestris). J. Food Legumes 2007, 20, 19. [Google Scholar]
- Somta, P.; Chen, J.; Yundaeng, C.; Yuan, X.; Yimram, T.; Tomooka, N.; Chen, X. Development of an SNP-based high-density linkage map and QTL analysis for bruchid (Callosobruchus maculatus F.) resistance in black gram (Vigna mungo (L.) Hepper). Sci. Rep. 2019, 9, 3930. [Google Scholar] [CrossRef]
- Bhanu, A.N.; Singh, M.N.; Srivastava, K. Crossability studies of interspecific hybridization among Vigna species. Biomed. J. 2018, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Pratap, A.; Gupta, S.; Malviya, N.; Tomar, R.; Maurya, R.; Joseph John, K.; Madhavan, L.; Singh, N.P. Genome scanning of Asiatic Vigna species for discerning population genetic structure based on microsatellite variation. Mol. Breed. 2015, 35, 178. [Google Scholar] [CrossRef]
- Kumari, G.; Pratap, A.; Lavanya, R.G.; Akram, M.; Rathore, M.; Madhavan, L.; Singh, Y.; Singh, N.P. Potential resistant donors for yellow mosaic disease identified from endemic wild Vigna species. J. Food Legumes 2021, 34, 10–16. [Google Scholar]
- Ragul, S.; Manivannan, N.; Ganapathy, N.; Karthikeyan, G. Screening and biochemical analysis on blackgram genotypes for resistance against storage pest bruchine [Callosobruchus maculatus (F.)]. Legume Res. Int. J. 2022, 45, 371–378. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, K.; Tewari, K.; Sagar, P.; Pandey, J.; Ps, S.; Rathore, M.; Kumar, V.; Akram, M.; Singh, A.; et al. Gene expression and biochemical profiling of contrasting Vigna mungo genotypes against Mungbean Yellow Mosaic India Virus (MYMIV). J. Food. Legumes 2022, 35, 107–116. [Google Scholar]
- Shamim, M.Z.; Pandey, A. Identification of yellow mosaic virus (YMV) resistant black gram (Vigna mungo L.) genotypes for cultivation in Northern India. J. Agroecol. Nat. Resour. Manag. 2014, 1, 48–50. [Google Scholar]
- Rangaiah, S. Evaluation of blackgram (Vigna mungo L. Hepper) genotypes for root traits as a measure of drought tolerance. Int. J. Trop. Agric. 2015, 33, 1463–1468. [Google Scholar]
- Samyuktha, S.M.; Malarvizhi, D.; Mariyammal, I.; Karthikeyan, A.; Seram, D.; Dhasarathan, M.; Juliet Hepziba, S.; Sheela, V.; Thanga Hemavathy, A.; Kavithamani, D. The Hunt for Mungbean (Vigna radiata (L.) Wilczek) Genotypes and Breeding Lines Resistance to South Indian Bruchid Strain. Agriculture 2022, 12, 1050. [Google Scholar] [CrossRef]
- Suman, S.; Rani, B.; Sharma, V.K.; Kumar, H.; Shahi, V.K. SSR marker based profiling and diversity analysis of mungbean [Vigna radiata (L.) Wilczek] genotypes. Legume Res. An. Int. J. 2019, 42, 585–594. [Google Scholar]
- Banni, K.; Moe, K.T.; Park, Y.-J. Assessing genetic diversity, population structure and gene flow in the Korean red bean [Vigna angularis (Willd.) Ohwi & Ohashi] using SSR markers. Plant Genet. Resour. 2012, 10, 74–82. [Google Scholar]
- Kaewwongwal, A.; Kongjaimun, A.; Somta, P.; Chankaew, S.; Yimram, T.; Srinives, P. Genetic diversity of the black gram [Vigna mungo (L.) Hepper] gene pool as revealed by SSR markers. Breed. Sci. 2015, 65, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Isemura, T.; Kaga, A.; Tabata, S.; Somta, P.; Srinives, P.; Shimizu, T.; Jo, U.; Vaughan, D.A.; Tomooka, N. Construction of a genetic linkage map and genetic analysis of domestication related traits in mungbean (Vigna radiata). PLoS ONE 2012, 7, e41304. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wang, L.; Zhang, D. Molecular identification of mung bean accessions (Vigna radiata L.) from Northeast China using capillary electrophoresis with fluorescence-labeled SSR markers. Food Energy Secur. 2020, 9, e182. [Google Scholar] [CrossRef]
- Noble, T.J.; Tao, Y.; Mace, E.S.; Williams, B.; Jordan, D.R.; Douglas, C.A.; Mundree, S.G. Characterization of linkage disequilibrium and population structure in a mungbean diversity panel. Front. Plant Sci. 2018, 8, 2102. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Islam, A.S.M.F.; Limpot, N.; Mackasmiel, L.; Mierzwa, J.; Cortés, A.J.; Blair, M.W. Genome-wide Snp identification and association mapping for seed mineral concentration in mung bean (Vigna Radiata L.). Front. Genet. 2020, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Gupta, S.; Tomar, R.; Malviya, N.; Maurya, R.; Pandey, V.R.; Mehandi, S.; Singh, N.P. Cross-genera amplification of informative microsatellite markers from common bean and scarlet runner bean for assessment of genetic diversity in mungbean (Vigna radiata). Plant Breed. 2016, 135, 499–505. [Google Scholar] [CrossRef]
- He, G.; Woullard, F.E.; Marong, I.; Guo, B.Z. Transferability of soybean SSR markers in peanut (Arachis hypogaea L.). Peanut Sci. 2006, 33, 22–28. [Google Scholar] [CrossRef]
- Kaul, T.; Eswaran, M.; Thangaraj, A.; Meyyazhagan, A.; Nehra, M.; Raman, N.M.; Bharti, J.; Badapanda, C.; Balamurali, B. Rice Bean (Vigna umbellata) draft genome sequence: Unravelling the late flowering and unpalatability related genomic resources for efficient domestication of this underutilized crop. bioRxiv 2019. [Google Scholar] [CrossRef]
- Ambreen, H.; Oraon, P.K.; Wahlang, D.R.; Satyawada, R.R.; Katiyar-Agarwal, S.; Agarwal, M.; Jagannath, A.; Kumar, A.; Budhwar, R.; Shukla, R.N.; et al. Long-read-based draft genome sequence of Indian black gram IPU-94-1 ‘Uttara’: Insights into disease resistance and seed storage protein genes. Plant Genome 2022, 15, e20234. [Google Scholar] [CrossRef]
- Jaiswal, V.; Mir, R.R.; Mohan, A.; Balyan, H.S.; Gupta, P.K. Association mapping for pre-harvest sprouting tolerance in common wheat (Triticum aestivum L.). Euphytica 2012, 188, 89–102. [Google Scholar] [CrossRef]
- Nie, X.; Huang, C.; You, C.; Li, W.; Zhao, W.; Shen, C.; Zhang, B.; Wang, H.; Yan, Z.; Dai, B. Genome-wide SSR-based association mapping for fiber quality in nation-wide upland cotton inbreed cultivars in China. BMC Genom. 2016, 17, 352. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, V.; BS, G.; Rathi, P.; Shukla, S.; RK, S. Linkage mapping of Mungbean yellow mosaic India virus (MYMIV) resistance gene in soybean. Breed. Sci. 2017, 67, 95–100. [Google Scholar] [CrossRef]
- Kumar, J.; Gupta, D.S.; Gupta, S.; Dubey, S.; Gupta, P.; Kumar, S. Quantitative trait loci from identification to exploitation for crop improvement. Plant Cell Rep. 2017, 36, 1187–1213. [Google Scholar]
- Langridge, P.; Lagudah, E.S.; Holton, T.A.; Appels, R.; Sharp, P.J.; Chalmers, K.J. Trends in genetic and genome analyses in wheat: A review. Aust. J. Agric. Res. 2001, 52, 1043–1077. [Google Scholar] [CrossRef]
- Mariyammal, I.; Seram, D.; Samyuktha, S.M.; Karthikeyan, A.; Dhasarathan, M.; Murukarthick, J.; Kennedy, J.S.; Malarvizhi, D.; Yang, T.-J.; Pandiyan, M. QTL mapping in Vigna radiata × Vigna umbellata population uncovers major genomic regions associated with bruchid resistance. Mol. Breed. 2019, 39, 110. [Google Scholar] [CrossRef]
- Venkataramana, P.B.; Gowda, R.; Somta, P.; Ramesh, S.; Mohan Rao, A.; Bhanuprakash, K.; Srinives, P.; Gireesh, C.; Pramila, C.K. Mapping QTL for bruchid resistance in rice bean (Vigna umbellata). Euphytica 2016, 207, 135–147. [Google Scholar] [CrossRef]
- Chotechung, S.; Somta, P.; Chen, J.; Yimram, T.; Chen, X.; Srinives, P. A gene encoding a polygalacturonase-inhibiting protein (PGIP) is a candidate gene for bruchid (Coleoptera: Bruchidae) resistance in mungbean (Vigna radiata). Theor. Appl. Genet. 2016, 129, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, L.; Wang, L.; Wang, S.; Cheng, X. Identification of a candidate gene for bruchid resistance by combining fine mapping and transcriptome profiling in mung bean (Vigna radiata L.). ResearchSquare 2021. [Google Scholar] [CrossRef]
- Agarwal, P.; Parida, S.K.; Mahto, A.; Das, S.; Mathew, I.E.; Malik, N.; Tyagi, A.K. Expanding frontiers in plant transcriptomics in aid of functional genomics and molecular breeding. Biotechnol. J. 2014, 9, 1480–1492. [Google Scholar] [CrossRef]
- Wang, B.; Kumar, V.; Olson, A.; Ware, D. Reviving the transcriptome studies: An insight into the emergence of single-molecule transcriptome sequencing. Front. Genet. 2019, 10, 384. [Google Scholar] [CrossRef]
- Baruah, I.K.; Panda, D.; M.V., J.; Das, D.J.; Acharjee, S.; Sen, P.; Sarmah, B.K. Bruchid egg induced transcript dynamics in developing seeds of black gram (Vigna mungo). PLoS ONE 2017, 12, e0176337. [Google Scholar] [CrossRef]
- Lin, W.-J.; Ko, C.-Y.; Liu, M.-S.; Kuo, C.-Y.; Wu, D.-C.; Chen, C.-Y.; Schafleitner, R.; Chen, L.-F.O.; Lo, H.-F. Transcriptomic and proteomic research to explore bruchid-resistant genes in mungbean isogenic lines. J. Agric. Food Chem. 2016, 64, 6648–6658. [Google Scholar] [CrossRef]
- Liu, M.-S.; Kuo, T.C.-Y.; Ko, C.-Y.; Wu, D.-C.; Li, K.-Y.; Lin, W.-J.; Lin, C.-P.; Wang, Y.-W.; Schafleitner, R.; Lo, H.-F. Genomic and transcriptomic comparison of nucleotide variations for insights into bruchid resistance of mungbean (Vigna radiata [L.] R. Wilczek). BMC Plant Biol. 2016, 16, 46. [Google Scholar] [CrossRef]
- Das, D.; Baruah, I.K.; Panda, D.; Paswan, R.R.; Acharjee, S.; Sarmah, B.K. Bruchid beetle ovipositioning mediated defense responses in black gram pods. BMC Plant Biol. 2021, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Gupta, D.S.; Kesari, R.; Verma, R.; Murugesan, S.; Basu, P.S.; Soren, K.R.; Gupta, S.; Singh, N.P. Comprehensive RNAseq analysis for identification of genes expressed under heat stress in lentil. Physiol. Plant. 2021, 173, 1785–1807. [Google Scholar] [CrossRef] [PubMed]
- Salgotra, R.K.; Stewart, C.N. Functional markers for precision plant breeding. Int. J. Mol. Sci. 2020, 21, 4792. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Gopalakrishna, T. Development of unigene-derived SSR markers in cowpea (Vigna unguiculata) and their transferability to other Vigna species. Genome 2010, 53, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Bansal, R.; Gopalakrishna, T. Development and characterization of genic SSR markers for mungbean (Vigna radiata (L.) Wilczek). Euphytica 2014, 195, 245–258. [Google Scholar] [CrossRef]
- Souframanien, J.; Gopalakrishna, T. ISSR and SCAR markers linked to the mungbean yellow mosaic virus (MYMV) resistance gene in blackgram [Vigna mungo (L.) Hepper]. Plant Breed. 2006, 125, 619–622. [Google Scholar] [CrossRef]
- Dhole, V.J.; Reddy, K.S. Genetic analysis of resistance to mungbean yellow mosaic virus in mungbean (Vigna radiata). Plant Breed. 2012, 131, 414–417. [Google Scholar] [CrossRef]
- Zhang, J.; Panthee, D.R. Next-generation sequencing-based bulked segregant analysis without sequencing the parental genomes. G3 2022, 12, jkab400. [Google Scholar] [CrossRef]
- Feng, S.; Zhu, Y.; Yu, C.; Jiao, K.; Jiang, M.; Lu, J.; Shen, C.; Ying, Q.; Wang, H. Development of species-specific SCAR markers, based on a SCoT analysis, to authenticate Physalis (Solanaceae) species. Front. Genet. 2018, 9, 192. [Google Scholar] [CrossRef]
- Gore, P.G.; Tripathi, K.; Pratap, A.; Bhat, K.V.; Umdale, S.D.; Gupta, V.; Pandey, A. Delineating taxonomic identity of two closely related Vigna species of section Aconitifoliae: V. trilobata (L.) Verdc. and V. stipulacea (Lam.) Kuntz in India. Genet. Resour. Crop Evol. 2019, 66, 1155–1165. [Google Scholar] [CrossRef]
- Zheng, K.; Cai, Y.; Chen, W.; Gao, Y.; Jin, J.; Wang, H.; Feng, S.; Lu, J. Development, identification, and application of a germplasm specific SCAR Marker for Dendrobium officinale Kimura et Migo. Front. Plant Sci. 2021, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, Q.; Lin, Y.; Chen, J.; Somta, P.; Yan, Q.; Xue, C.; Liu, J.; Chen, X.; Yuan, X. Marker-Assisted Backcross Breeding for Improving Bruchid (Callosobruchus spp.) Resistance in Mung Bean (Vigna radiata L.). Agronomy 2022, 12, 1271. [Google Scholar] [CrossRef]



| Stress | Crop | Population | Approach | Genetics | Marker Used | Reference |
|---|---|---|---|---|---|---|
| MYMIV | Mungbean | RILs of KPS 2 × NM 10-12-1 | Linkage mapping | QTL | SSR | [76] |
| BM6 × BM1 | Linkage mapping | QTL | SSR | [77] | ||
| A panel of 130 genotypes | Association mapping | QTL | SSR | [73] | ||
| Black gram | AKU 9904 × DPU 88-31 | BSA | Single dominant gene | SSR | [68] | |
| Black gram × ricebean | KUG253 × Mash114 | QTL-seq | Major QTL | SNP | [78] | |
| MYMV | Mungbean | KMG 189 × VBN (Gg) 2 | Segregation analysis | Single recessive gene | -- | [69] |
| KMG 189 × VBN (Gg) 2 | Segregation analysis | Single recessive gene/linkage mapping | SCAR | [70] | ||
| NM92 × VC2272, 6601 × VC2272, 6601 × Pusa Baisakhi, VC3902A × NM92, VC3902A × ML-5, NM92 × Pusa Baisaki, VC 1560D × 6601, VC 1560D × NM92 | F2 segregation analysis | -- | -- | [79] | ||
| NM 92 × NM 98 | F2 segregation analysis | -- | -- | [80] | ||
| VBN(Gg)2 × KMG189 | F2 segregation analysis | Single recessive gene | -- | [70] | ||
| Black gram | MDU1 × Mash 1008 | Linkage mapping | QTLs | SSR | [81] | |
| MDU 1 × TU 68 | Linkage mapping | Major QTL | SSR | [82] | ||
| Ricebean × mungbean | TNAU Red × VRM (Gg) 1 | Segregation analysis | Single recessive gene | -- | [69] | |
| Mungbean × ricebean | VRM (Gg) 1 × TNAU RED | GBS approach | One major QTL and three minor QTLs | SNP | [83] | |
| YMV (causal virus not identified) | Black gram | IC436656 × KKB14045 | Segregation analysis and linkage mapping | Single recessive gene | SSR | [84] |
| Cercospora leaf spot | Mungbean | KPS1×V4718 | Linkage mapping | QTL | SSR | [85] |
| Kamphaeng Saen 1 × V4718 | Fine mapping | QTL | SNP | [86] | ||
| Kopergaon × HUM12; Kopergaon × ML 1720 | GMA | Quantitative inheritance | -- | [87] | ||
| KPS1 × V4718 | GBS | QTL | SNP | [86] | ||
| Powdery mildew | Mungbean | Kamphaeng Saen 1 × VC6468-11-1A (RILs) | Linkage mapping | Two QTLs | SSR | [88] |
| Berken × ATF 3640 | Linkage mapping | Major QTL | RFLP | [89] | ||
| Chai Nat 72 × V4758 | Segregation analysis and BSA | QTL | ISSR-RGA | [90] | ||
| Cowpea | ZN 016 × Zhijiang282 | Linkage mapping | Major QTL | SSR and SNP | [91] | |
| Bruchid (C. chinansis) | Mungbean | V2709 | -- | Single dominant gene | RAPD/SSR/STS | [92] |
| Callosobruchus spp. | Mungbean × V. sublobata | VC2778A × TC 1966 | Comparative genomics | Major QTL | SNP | [93] |
| C. Chinansis; C. maculatus | KPS1 × V2709 | Segregation analysis and linkage mapping | Major QTL | SNP | [94] | |
| V. vexillata | TVNu 240 × TVNu 1623 | SLAF sequencing | QTL | SNP | [95] | |
| Bruchid (C. maculatus) | Urdbean × Vigna mungo var. sylvestris | TU 94-2 × Vigna mungo var. sylvestris | Segregation analysis | QTLs | RAPD, ISSR, SSR | [96] |
| Urdbean × Vigna mungo var. sylvestris | BC48 × TC2210 | Linkage mapping | Two QTLs | SNP | [97] | |
| Urdbean | MDU1 × TU 68 | Linkage mapping | QTL | SSR | [82] |
| Disease/Pest | Donor Genotypes | Species | Method | References |
|---|---|---|---|---|
| MYMIV | IC277021 | V. sylvestris | Field screening | [100] |
| IC248326, IC248326, IC248343 | V. vexillata | Field screening | ||
| LRM/13-43, LRM/13-32, LRM/13-34, IC276983, IC331436, IC331454, IC331456, Trichy local, Kumur local, IIPRW17-3 | V. trilobata | Field screening | ||
| RBL-50, IC251445, PRR 2007-2, PRR 2008-2, RB-5-1, IC251439, IC251442, IC251446, IC251447, IC528878, IC197812, IIPRW 17-1, | V. umbellata | Field screening | ||
| PRR 2008-2 | V. umbellata | Field screening | ||
| LRM/13-11, LRM/13-33, TMV-1, LRM/13-26, LRM/13-37, LRM/13-38, LRM/13-36, | V. aconitifolia | Field screening | ||
| IC331450 | V. hainiana | Field screening | ||
| Trichy Local-1, Trichy Local-2 | V. stipulaceae | Field screening | ||
| TCR-20 | V. glaberesense | Field screening | ||
| TCR-7 | V. umbellata | Field screening | ||
| TU-68 | V. mungo | Bioassay and GC–MS analysis | [101] | |
| TCR-79,TCR-82, TCR-239, | V. radiata | Field screening | [66] | |
| TCR-7, TCR-238,JAP/10-36,TCR-110,JAP/10-47,TCR-160, TCR-88,JAP/10-51, NSB 007, | V. sublobata | Field screening | ||
| TCR-64,LRM/13-43, LRM/13-34, LRM/13-32, LRM/13-24, LRM/13-30, ZAP/10-5, ZAP/10-7, ZAP/10-9,TCR-192, TCR-305, TCR-319, TCR-320, TCR-513,LRM/13-44, LRM/13-33, LRM/13-26, LRM/13-38, LRM/13-36, LRM/13-37, | V. trilobata | Field screening | ||
| TCR-254,TCR-390, | V. sylvestris | Field screening | ||
| TCR-314,TCR-315,TCR-24, TCR-29 | V. haniana | Field screening | ||
| TCR-20 | V. glabrascens | Field screening | ||
| TLC1, TLC2 | V. stipulaceae | Field screening | ||
| RBL-1, TCR-93, PRR-2007-2, PRR-2008-2, RB-5-1, TCR-91, TCR-87, TCR-90, TCR-94, TCR-95, TCR-279, | V. umbellata | Field screening | ||
| MYMIV | IC-546453, IPU 11-02, IC-548278, IC-43647, COBG-653, Pant-Urd-19, UR-218, Shekhar-2, STY-2289, VBG-04-008, IPU 31-1, PDU-19, PDU-3, IPU 99-211, PLU-110, DPU88-31 | V. mungo | Field screening | [102] |
| UPU 8335, IPU 99-205, PGRU 95004, SPS 43 | Field screening | [103] | ||
| Powdery mildew | LBG 645, LBG 17, IC-281977 | V. mungo | Field and artificial screening | [104] |
| Callosobruchus maculatus | V2802BG, V2709, BSR-GG-1-49-3-1, BSRGG-1-56-2-2, BSR-GG-1-160-5-3, BSR-GG-1-170-2-4, BSR-GG-1-198-1-4 | V. radiata | Bioassay | [105] |
| Bruchid (C. analis) | IC251439, IC251442, PRR 2007-2, IC251440, TCR 279 | V. Umbellata | Bioassay | [48] |
| JAP/10-51 | V. trinervia | |||
| IC251435, IC553527, IC553526, JAP/10-7,IC349701 | V. trilobata | |||
| TMV 1, LRM 13-44 | V. aconitifolia | |||
| Trichy Local 1 | V. stipulacea | |||
| Kumur Local | V. khandalensis | |||
| Mung seed 1, IC571775, IC251434 | V. radiata | |||
| IC251390IC251387 | V. mungo | |||
| IC210580 | V. pilosa | |||
| Bruchid (C. maculatus) | Mung Seed-1, IC251426A, IC251426B | V. radiata | Bioassay | [48] |
| IC247408 | V. dalzelliana | |||
| IC248326, IC248343 | V. vexillata | |||
| Kumur Local | V. khandalensis | |||
| JAP/10-51 | V. trinervia | |||
| IC210575 | V. pilosa | |||
| IC251394, IC251390, IC251385, IC251387 | V. mungo | |||
| JAP/10-5, IC251435, IC553527 | V. trilobata | |||
| IC251439, IC251442, PRR 2007-2, IC251440 | V. umbellata | |||
| Bruchid (C. chinesis) | Kumur Local | V. khandalensis | Bioassay | [48] |
| IC251397, IC251390, IC251385, IC251387 | V. mungo | |||
| IC247408 | V. dalzelliana | |||
| IC248326, IC248343 | V. vexillata | |||
| IC251439, IC251442, PRR 2007-2, IC251440 | V. umbellata | |||
| JAP/10-51 | V. trinervia | |||
| IC247407 | V. trinervia var. bournei | |||
| IC210575 | V. pilosa | |||
| Mung seed-1 | V. radiata |
| Gene Name | LG | Stress | Crop | Functional Characterization | Reference |
|---|---|---|---|---|---|
| Vradi04g06770 | 04 | MYMV | Mungbean × ricebean | Protein kinase superfamily protein (serine/threonine kinase activity) | [83] |
| Vradi04g06840 | 04 | Small GTP-binding protein (disease resistance protein)/ leucine-rich repeat/P-loop containing nucleoside triphosphate hydrolase | |||
| Vradi04g06900 | 04 | Zinc finger, RING/FYVE/PHD-type (RING finger protein 165-like | |||
| Vradi04g06950 | 04 | Receptor-like kinase/leucine-rich repeats | |||
| Vradi04g06960 | 04 | Zinc finger, RING/FYVE/PHD-type (U-box domain-containing protein 15-like) | |||
| Vradi04g07000 | 04 | Protein kinase superfamily protein (serine/threonine kinase activity) | |||
| Vradi04g07100 | 04 | WRKY family transcription factor | |||
| Vradi04g07130 | 04 | WRKY family transcription factor | |||
| Vradi04g07220 | 04 | MYB transcription factor MYB64 | |||
| Vradi04g07240 | 04 | Transcription factor bHLH79-like (basic helix–loop–helix (bHLH) domain) | |||
| Vradi04g0727 | 04 | MYB transcription factor MYB183 | |||
| Vradi04g07290 | 04 | DNA/RNA helicase, DEAD/DEAH box type, N-terminal/P-loop containing nucleoside triphosphate hydrolase | |||
| Vradi04g07440 | 04 | Zinc finger, RING/FYVE/PHD-type (U-box domain-containing protein 38-like) | |||
| Vradi04g07450 | 04 | Jasmonic acid carboxyl methyltransferase (SAM-dependent carboxyl methyltransferase)/ methyltransferase activity | |||
| Vradi04g07490 | 04 | Zinc finger, RING/FYVE/PHD-type (RING-H2 finger protein 2B) | |||
| Vradi04g07540 | 04 | Cytochrome P450 (oxidation-reduction process) | |||
| Vradi05g09450 | 05 | Bruchid | WRKY family transcription factor | [122] | |
| Vradi05g09480 | 05 | Kelch repeat F-box protein | |||
| Vradi05g09650 | 05 | Aminoacyl-tRNA synthetase | |||
| Vradi05g09830 | 05 | Flavin-binding monooxygenase family protein | |||
| Vradi05g09990 | 05 | Cellulose synthase family protein | |||
| Vradi05g10080 | 05 | Ethylene-responsive transcription factor (ERF) | |||
| Vradi05g10110 | 05 | F-box family protein (leucine-rich repeat) | |||
| Vradi05g10130 | 05 | Ascorbate peroxidase (Peroxidase activity) | |||
| Vradi05g10140 | 05 | Receptor-like serine/threonine-protein kinase | |||
| Vradi05g10200 | 05 | Chloroplastic ATP synthase | |||
| Vradi05g10210 | 05 | Protein kinase superfamily protein/protein kinase activity/ATP binding/protein phosphorylation | |||
| Vradi05g10410 | 05 | Cellulose synthase family protein | |||
| Vradi05g10460 | 05 | Protein kinase superfamily protein/concanavalin A-like lectin/glucanase/protein phosphorylation | |||
| Vradi05g10480 | 05 | Ethylene-responsive transcription factor (ERF) | |||
| Vradi05g10500 | 05 | Zinc finger protein 1-like (zinc finger, C2H2) | |||
| Vradi05g10560 | 05 | Calmodulin-binding transcription activator 4-like isoform X5 | |||
| Vradi05g10580 | 05 | Calmodulin-binding transcription activator 4 isoform X3 protein | |||
| Vigan.05G027700 | 05 | C. maculatus | Black gram | Zinc finger RING/FYVE/PHD-type, CTLH/CRA C-terminal to LisH motif | [82] |
| Vigan.05G028300 | 05 | Leucine-rich repeat—N-terminal, protein kinase family | |||
| Vigan.05G029200 | 05 | Pathogenesis-related protein 1- like/cysteine-rich secretory protein allergen V5/Tpx-1 family | |||
| Vigan.05G030000 | 05 | Myc-type basic helix–loop–helix (bHLH) typ | |||
| Vigan.05G030500 | 05 | Zinc finger proteins (C2H2 type) | |||
| Vigan.05G031900 | 05 | Protein kinase family (serine–threonine/tyrosine-protein kinase catalytic), Concanavalin A-like lectin/glucanase subgroup | |||
| Vigan.05G035600 | 05 | F-box family protein | |||
| Vigan.05G036000 | 05 | Diacylglycerol kinase catalytic protein, ATP-NAD kinase-lik | |||
| Vigan.05G036200 | 05 | Target SNARE site (coiled-coil structure), syntaxin N-terminal | |||
| Vigan.05G038400 | 05 | Toll/interleukin-1 receptor homology (TIR) | |||
| Vigan.05G042200 | 05 | Ubiquitin-conjugating enzyme E2/RWD-like | |||
| Vigan.05G044400 | 05 | Leucine-rich repeat (malectin-like carbohydrate binding) | |||
| Vigan.05G046400 | 05 | Glutaredoxin-like protein/Thioredoxin-like fold | |||
| Vigan.05G046700 | 05 | NB-LRR family proteins | |||
| Vigan.05G048300 | 05 | Pathogenesis-related genes transcriptional activator (PTI5) | |||
| Vigan.05G049800 | 05 | Chloramphenicol acetyltransferase-like | |||
| Vigan.05G056900 | 05 | Ankyrin repeat-containing protein | |||
| Vigan.05G066300 | 05 | F-box proteins/Kelch repeat type 1 | |||
| Vigan.05G075700 | 05 | F-box/kelch-repeat protein At3g23880-like isoform X1 | |||
| Vigan.08G002100 | 08 | Bi-functional inhibitor/seed storage helical protein | |||
| Vigan.08G002200 | 08 | Lipid-transfer protein DIR1 family | |||
| Vigan.08G002600 | 08 | Myc-type basic helix–loop–helix (bHLH) type | |||
| Vigan.08G003600 | 08 | Zinc finger CCCH-type K homology protein | |||
| Vigan.08G003900 | 08 | Cytochrome P450 conserved protein | |||
| Vigan.08G004400 | 08 | Protein kinase super family (serine–threonine-dual specificity protein)/Concanavalin A-like lectin/glucanase subgroup | |||
| Vigan.08G004700 | 08 | Basic-leucine zipper protein/transcription factor TGA-like | |||
| VrPGIP1, VrPGIP 2 | 05 | Callosobruchus chinensis and Callosobruchus maculatus | Green gram | Polygalacturonase-inhibiting protein | [94,124] |
| Vradi04g00919 | 04 | C. chinensis | polygalacturonase inhibitor | [125] | |
| Vradi05g03810 | 05 | Callosobruchus spp. | V. radiata >× V. sublobata | Resistant-specific protein-1 | [93] |
| Vradi05g03830 | 05 | Resistant-specific protein-2 | |||
| Vradi05g03840 | 05 | Resistant-specific protein-2 | |||
| Vradi05g03860 | 05 | Resistant-specific protein-2 | |||
| Vradi05g03870 | 05 | Resistant-specific protein-1 | |||
| Vradi05g03880 | 05 | Resistant-specific protein-1 | |||
| Vradi05g03930 | 05 | Resistant-specific protein-2 | |||
| Vradi05g03940 | 05 | Polygalacturonase inhibitor | |||
| Vradi05g03950 | 05 | Polygalacturonase inhibitor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, P.; Pandey, B.; Pratap, A.; Gyaneshwari, U.; Nair, R.M.; Mishra, A.K.; Singh, C.M. Genetic and Genomics Resources of Cross-Species Vigna Gene Pools for Improving Biotic Stress Resistance in Mungbean (Vigna radiata L. Wilczek). Agronomy 2022, 12, 3000. https://doi.org/10.3390/agronomy12123000
Singh P, Pandey B, Pratap A, Gyaneshwari U, Nair RM, Mishra AK, Singh CM. Genetic and Genomics Resources of Cross-Species Vigna Gene Pools for Improving Biotic Stress Resistance in Mungbean (Vigna radiata L. Wilczek). Agronomy. 2022; 12(12):3000. https://doi.org/10.3390/agronomy12123000
Chicago/Turabian StyleSingh, Poornima, Brijesh Pandey, Aditya Pratap, Upagya Gyaneshwari, Ramakrishnan M. Nair, Awdhesh Kumar Mishra, and Chandra Mohan Singh. 2022. "Genetic and Genomics Resources of Cross-Species Vigna Gene Pools for Improving Biotic Stress Resistance in Mungbean (Vigna radiata L. Wilczek)" Agronomy 12, no. 12: 3000. https://doi.org/10.3390/agronomy12123000
APA StyleSingh, P., Pandey, B., Pratap, A., Gyaneshwari, U., Nair, R. M., Mishra, A. K., & Singh, C. M. (2022). Genetic and Genomics Resources of Cross-Species Vigna Gene Pools for Improving Biotic Stress Resistance in Mungbean (Vigna radiata L. Wilczek). Agronomy, 12(12), 3000. https://doi.org/10.3390/agronomy12123000







